Histopathology of Thecaphora frezzii Colonization: A Detailed Analysis of Its Journey Through Peanut (Arachis hypogaea L.) Tissues
Abstract
1. Introduction
2. Results
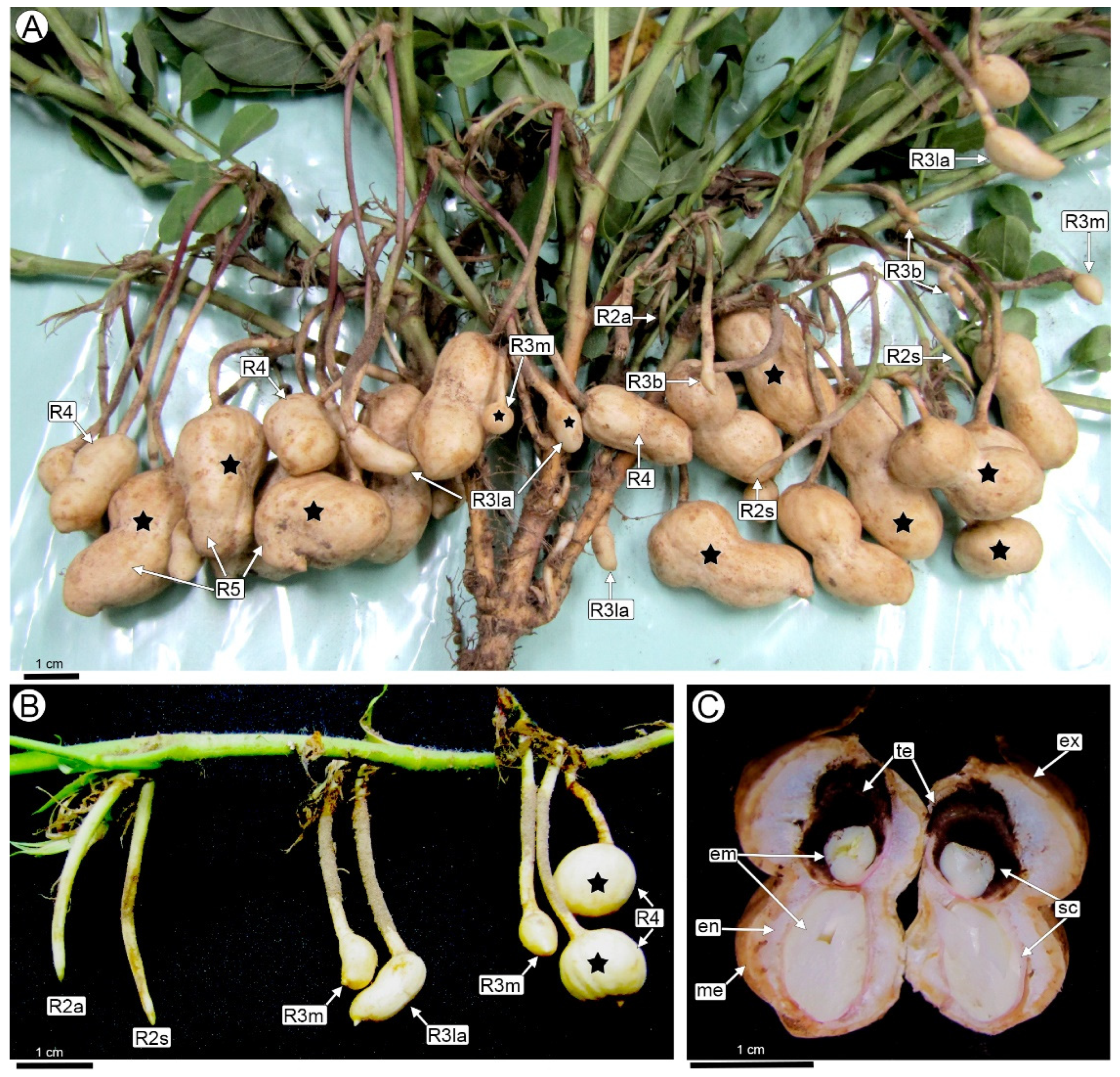
2.1. Developmental Stages of Peanut Fruit and Seed Associated with T. frezzii Colonization
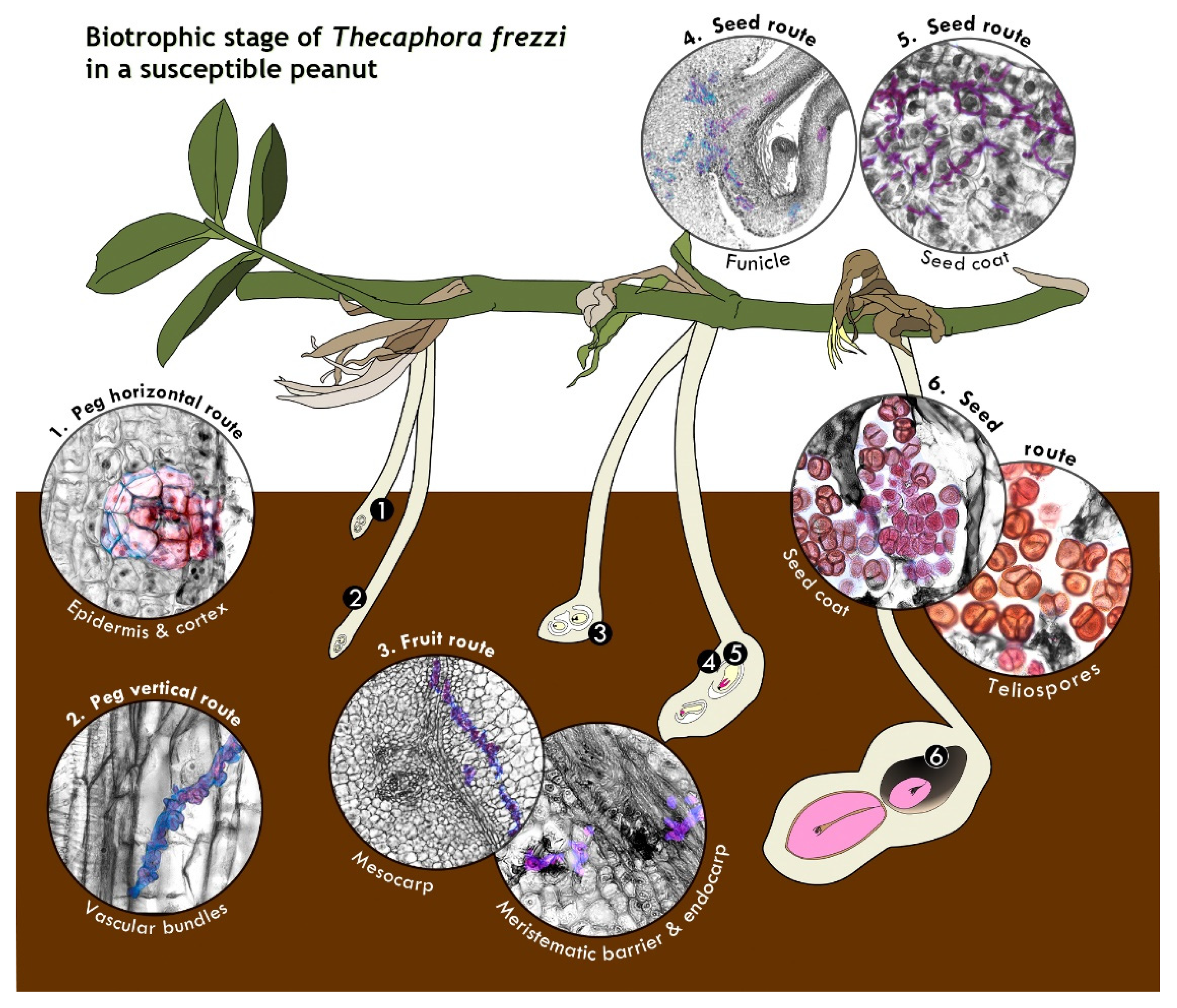
2.2. Anatomy of the Biotrophic Stage of Infection of T. frezzii
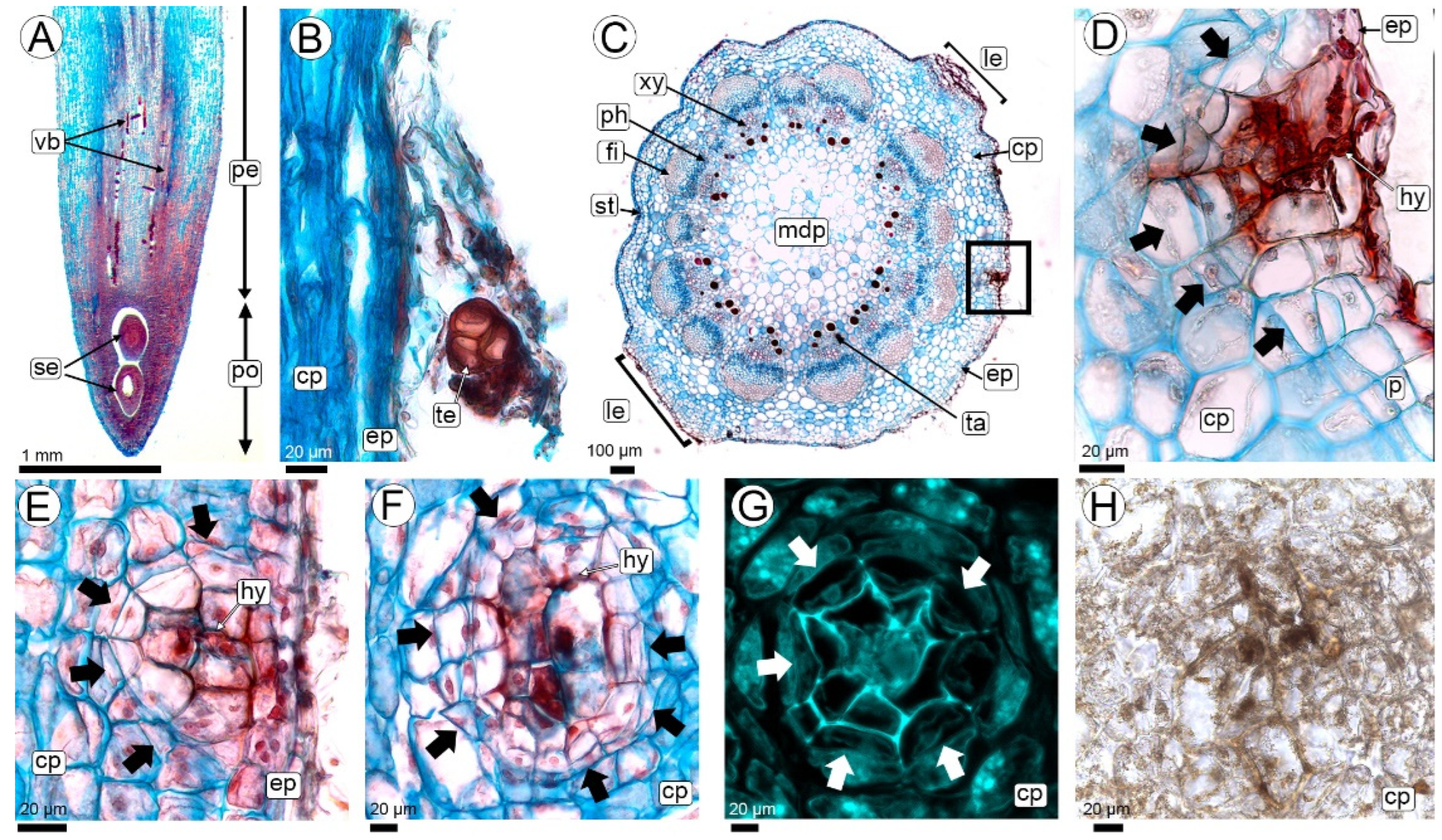
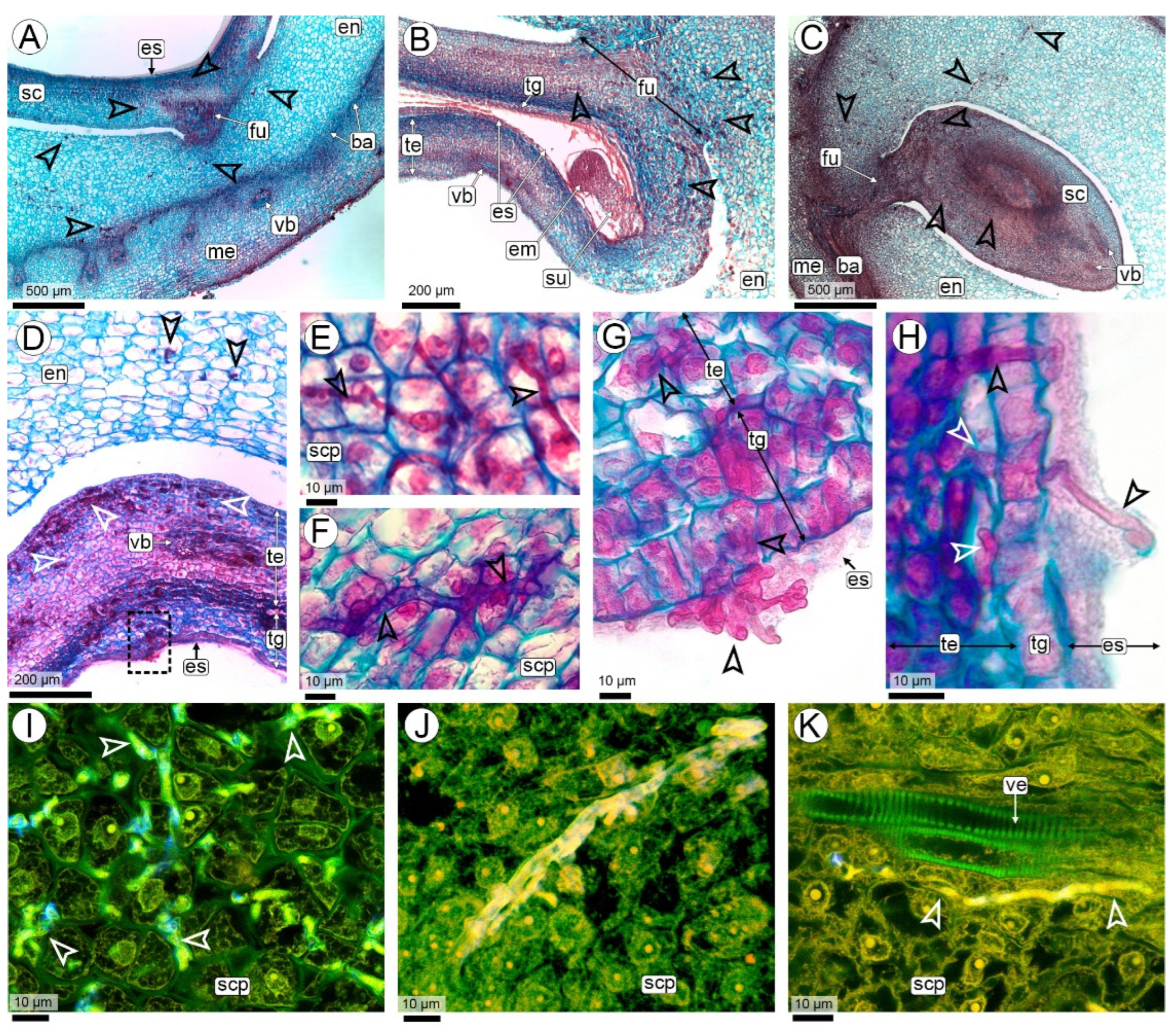
2.2.1. Fungal Colonization in the Peg (R2-Aerial Peg Stage to R3-Beginning Pod Stage)
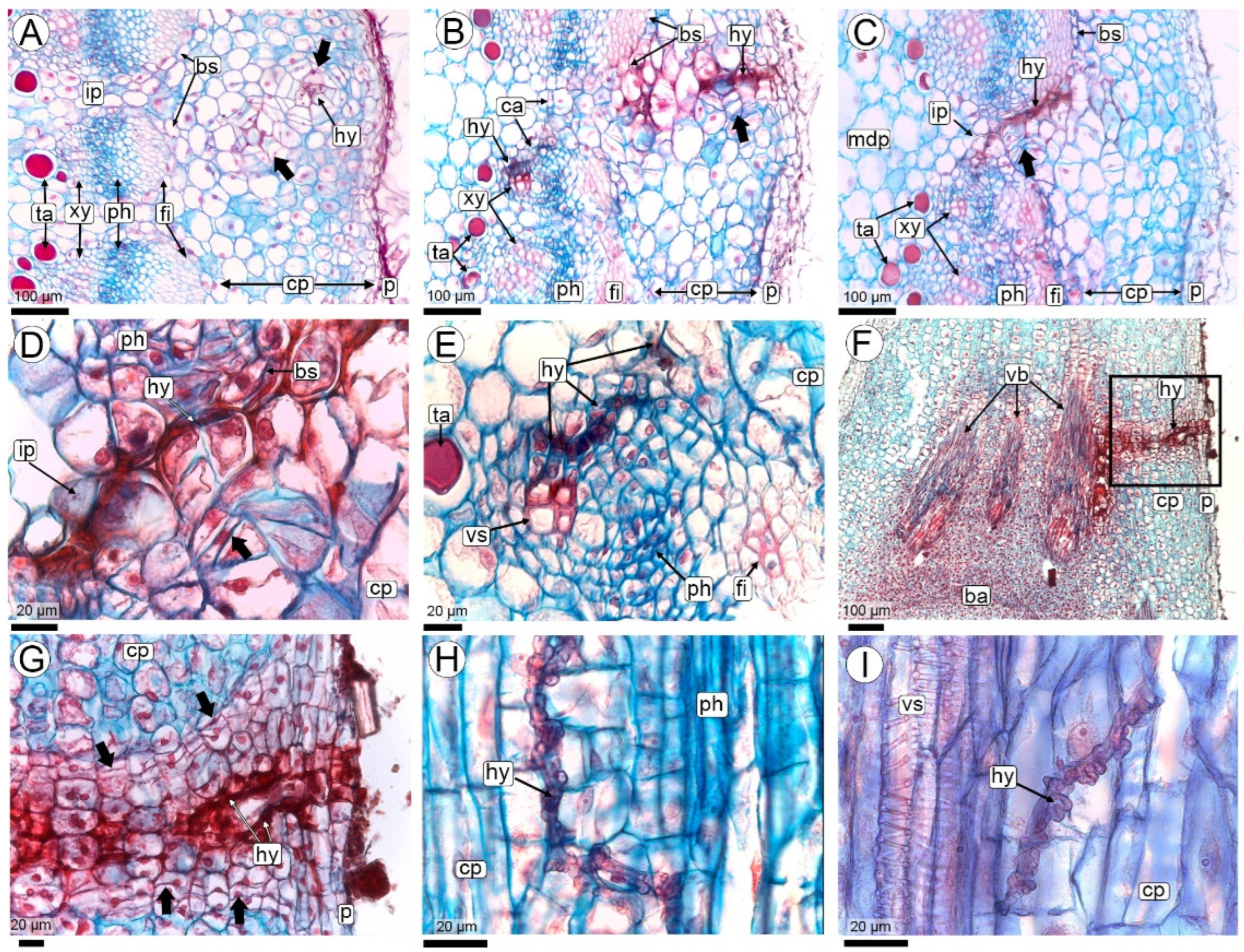
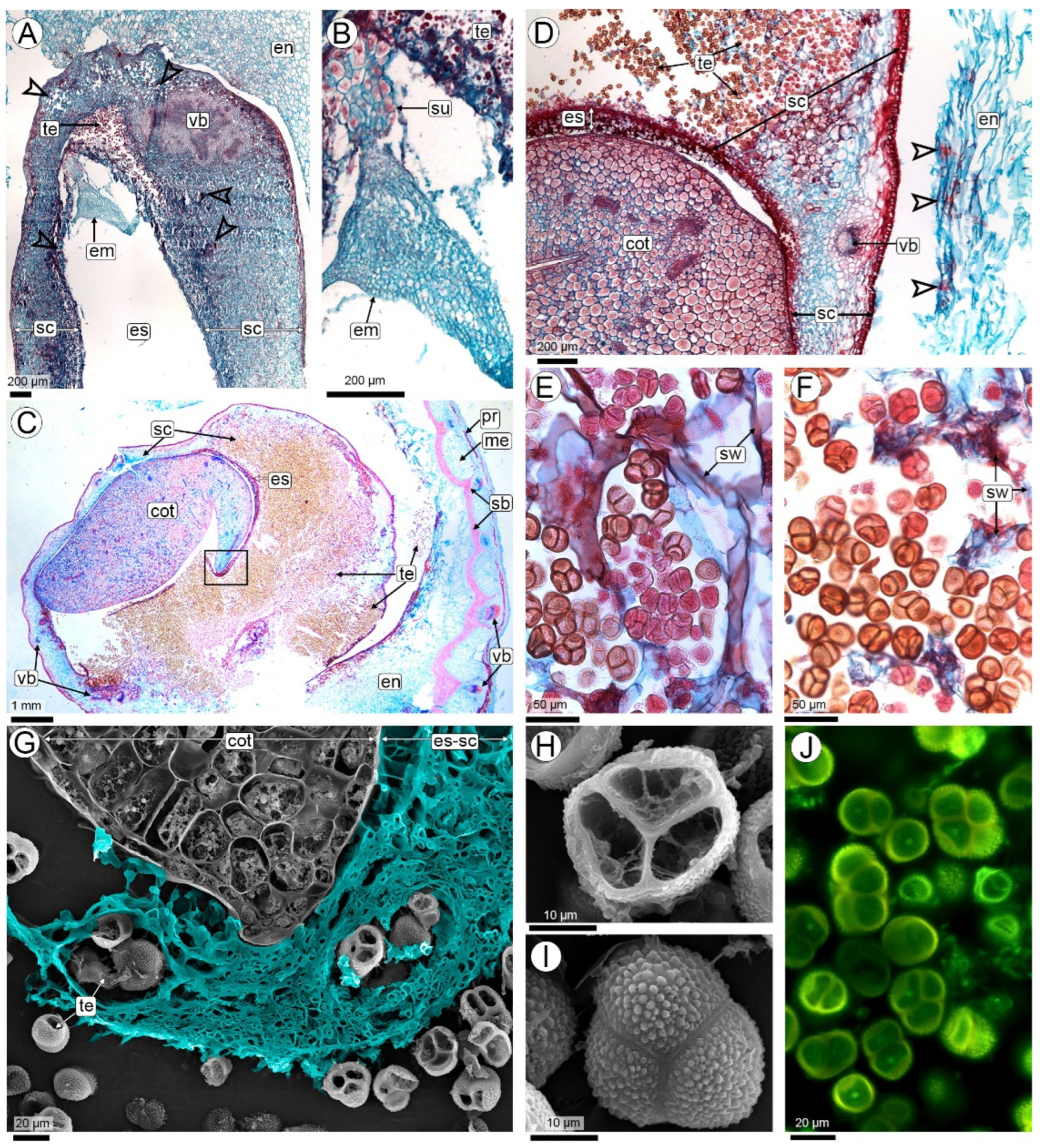
2.2.2. Fungal Colonization of the Pod (R3-Beginning to R3-Medium Pod Stages)
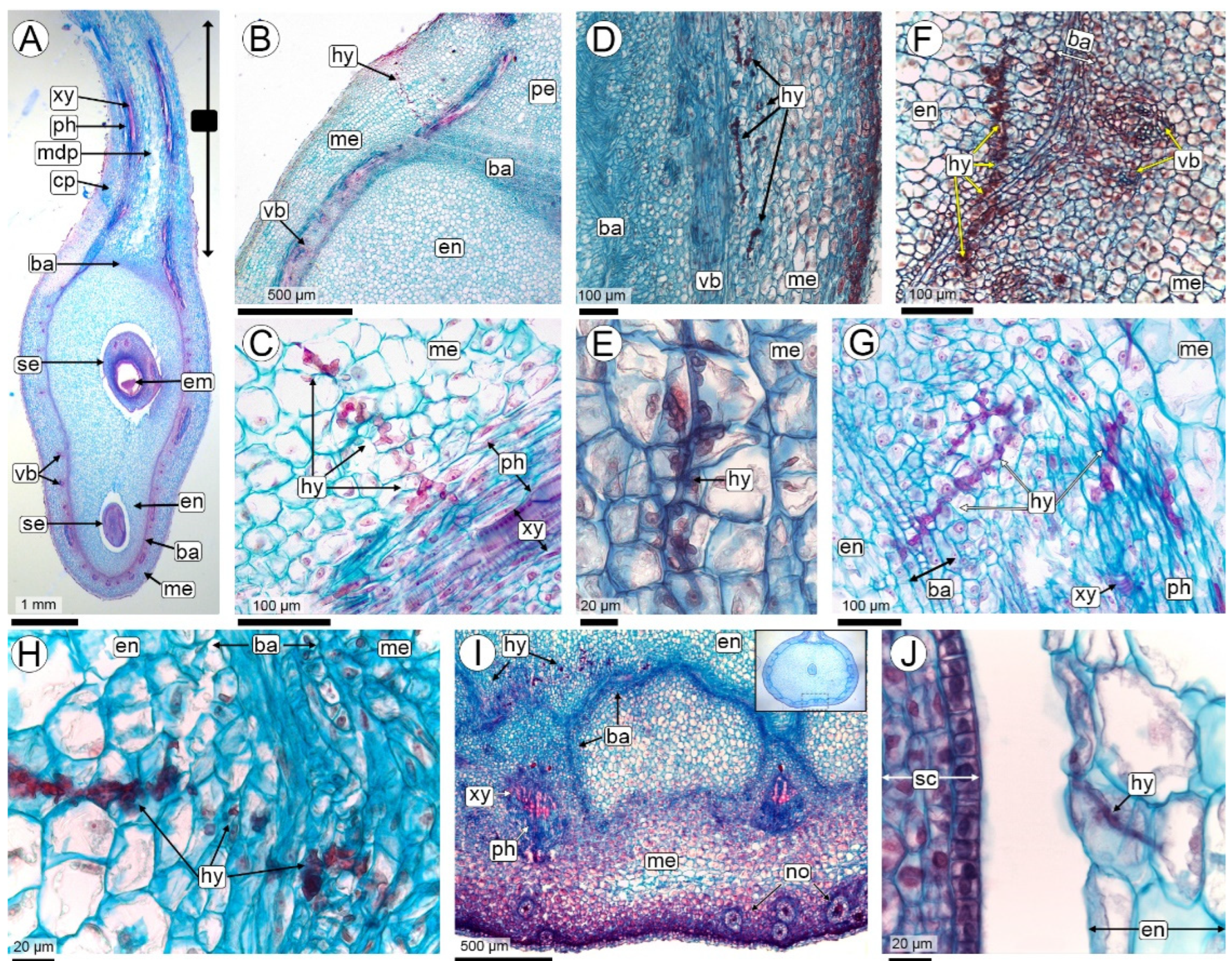
2.2.3. Seed Colonization (R3-Medium Pod to R3-Late Pod Stages)
2.2.4. The Sporulation Site (R4 Stage—Full Pod Stage and Subsequent Stages)
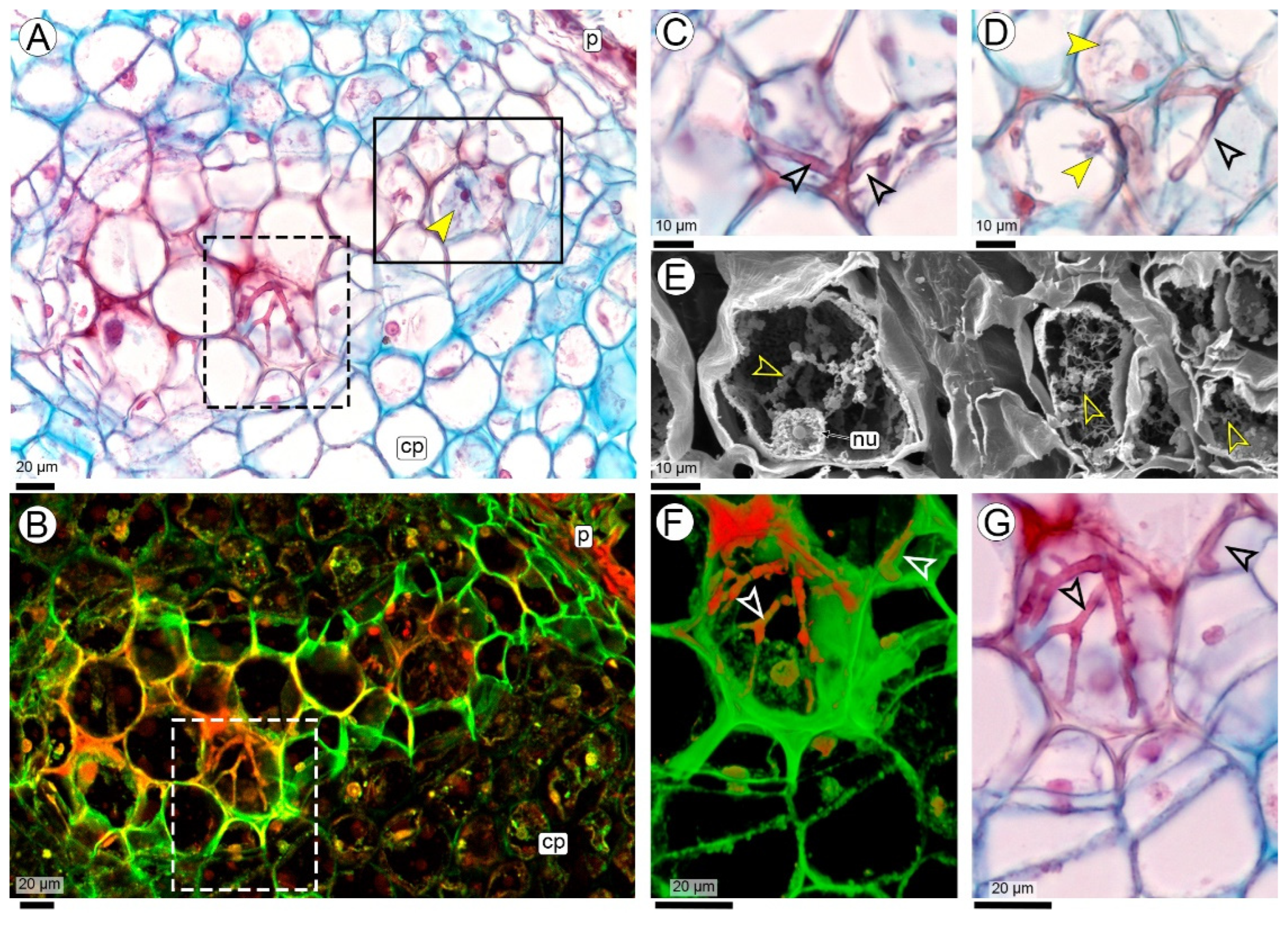
2.3. Hyphal Characteristics
3. Discussion
3.1. Hyphae Entry and Colonization of Peanut Pegs by T. frezzii
3.2. First Challenges: Overcoming Peanut Plant Defenses
3.3. Second Challenge: Acquiring Nutrients from the Host During Colonization
3.4. Fruit Colonization
3.5. Seed Colonization
3.6. Hyphal and Teliospores Morphology
4. Conclusions
5. Materials and Methods
5.1. Plant Material
5.2. Light Microscopy (LM)
5.3. Confocal Laser Scanning Microscopy (CLSM)
5.4. Scanning Electron Microscopy (SEM)
5.5. Measurements
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krapovickas, A.; Gregory, W.C. Taxonomía del género Arachis (Leguminosae). Bonplandia 1994, 8, 1–186. [Google Scholar] [CrossRef]
- Seijo, J.G.; Lavia, G.I.; Fernández, A.; Krapovickas, A.; Ducasse, D.A.; Moscone, E.A. Genomic relationships between the cultivated peanut (Arachis hypogaea—Leguminosae) and its wild relatives revealed by FISH and GISH. Am. J. Bot. 2007, 94, 1963–1971. [Google Scholar] [CrossRef] [PubMed]
- Grabiele, M.; Chalup, L.; Robledo, G.; Seijo, G. Genetic and geographic origin of domesticated peanut as evidenced by 5S rDNA and chloroplast DNA sequences. Plant Syst. Evol. 2012, 298, 1151–1165. [Google Scholar] [CrossRef]
- Bertioli, D.J.; Jenkins, J.; Clevenger, J.; Dudchenko, O.; Gao, D.; Seijo, G.; Leal-Bertioli, S.C.M.; Ren, L.; Farmer, A.D.; Pandey, M.K.; et al. The genome sequence of segmental allotetraploid peanut (Arachis hypogaea) Nat. Genet. 2019, 51, 877–884. [Google Scholar] [CrossRef]
- Bertioli, D.J.; Abernathy, B.; Seijo, G.; Clevenger, J.; Cannon, S. Evaluating two different models of Peanut’s origin. Nat. Genet. 2020, 52, 557–559. [Google Scholar] [CrossRef]
- Davis, J.P.; Dean, L.L. Peanut composition, flavor and nutrition. In Peanuts; Stalker, H.T., Wilson, R.F., Eds.; AOCS Press: Urbana, IL, USA, 2016; pp. 289–345. [Google Scholar] [CrossRef]
- Fletcher, S.M.; Shi, Z. An overview of world peanut markets. In Peanuts; Stalker, H.T., Wilson, R.F., Eds.; AOCS Press: Urbana, IL, USA, 2016; pp. 267–287. [Google Scholar] [CrossRef]
- Marinelli, A.; March, G.J.; Oddino, C. Aspectos biológicos y epidemiológicos del carbón del maní (Arachis hypogaea L.) causado por Thecaphora frezii Carranza & Lindquist. Agriscientia 2008, 25, 1–5. [Google Scholar] [CrossRef]
- Rago, A.M.; Cazón, L.I.; Paredes, J.A.; Edwards Molina, J.P.; Conforto, E.C.; Bisonard, E.M.; Oddino, C. Peanut smut: From an emerging disease to an actual threat to Argentine peanut production. Plant Dis. 2017, 101, 400–408. [Google Scholar] [CrossRef]
- Marinelli, A.; March, G.; Rago, A. El carbón del maní Thecaphora frezii sobre Arachis hypogaea L. In Proceedings of the 7° Congreso de Micología y 17° Jornadas Argentinas de Micología, Rosario, Santa Fe, Argentina, 14–16 June 1995; p. 134. [Google Scholar]
- Marraro Acuña, F.; Wiemer, A.P.; Cosa, M.T. Carbón del maní bajo la lupa, anatomía de la infección. In Proceedings of the XXVII Jornada Nacional del Maní, General Cabrera, Córdoba, Argentina, 20 September 2012; pp. 52–54. [Google Scholar]
- Marraro Acuña, F.; Cosa, M.T.; Wiemer, A.P. Carbón del maní: Histopatología, incidencia y severidad. In Proceedings of the XXVIII Jornada Nacional del Maní, General Cabrera, Córdoba, Argentina, 19 September 2013; pp. 1–2. [Google Scholar]
- Astiz Gasso, M.M.; Lovisolo, M.; Molla Kralj, A. Histopatología del carbón Thecaphora frezii en Arachis hypogaea. In Proceedings of the XXV Jornada Nacional del Maní, General Cabrera, Córdoba, Argentina, 16 September 2010; pp. 1–2. [Google Scholar]
- Mary, V.S.; Otaiza, S.; Di Paola, R.; Yunes, P.; Wunderling, D.; Rubinstein, H.; Theumer, M.G. Estudio del perfil químico de extractos de clavo de maní, inductores de germinación de teliosporas de Thecaphora frezii. In Proceedings of the IV Congreso Argentino de Fitopatología, Mendoza, Argentina, 19–21 April 2017. [Google Scholar]
- Carranza, J.M.; Lindquist, J.C. Thecaphora frezii n. sp., parásita de Arachis sp. Bol. Soc. Argent. Bot. 1962, 10, 11–18. [Google Scholar]
- Soave, J.A.; Bianco, C.; Burgoa, R.; Montaño, R.; Rago, A.M.; Cazón, L.I.; Paredes, J.; Buteler, M.; Faustinelli, P.; Soave, S.; et al. First detection of peanut smut (Thecaphora frezii) in Bolivia. In Proceedings of the 3° Congreso Argentino de Fitopatología, Córdoba, Argentina, 4–6 June 2014; p. 211. [Google Scholar]
- Paredes, J.A.; Edwards Molina, J.P.; Cazón, L.I.; Asinari, F.; Monguillot, J.H.; Morichetti, S.A.; Torres, A.M. Relationship between incidence and severity of peanut smut and its regional distribution in the main growing region of Argentina. Trop. Plant Pathol. 2022, 47, 1–12. [Google Scholar] [CrossRef]
- Oddino, C.; Marinelli, A.; March, G.; García, J.; Tarditi, L.; D’Eramo, L.; Ferrari, S. Relación entre el potencial inóculo de Thecaphora frezii, la intensidad de carbón de maní y el rendimiento del cultivo. In Proceedings of the XXV Jornada Nacional de Maní, General Cabrera, Córdoba, Argentina, 16 September 2010; pp. 24–26. [Google Scholar]
- Paredes, J.A.; Cazón, L.I.; Conforto, E.C.; Rago, A. Peanut Smut in Argentina: An analysis of the disease, advances, and challenges. Plant Dis. 2024, 108, 2593–2606. [Google Scholar] [CrossRef]
- Paredes, J.A.; Cazón, L.I.; Osella, A.; Peralta, V.; Alcalde, M.; Kearney, M.I.; Zuza, M.S.; Rago, A.M.; Oddino, C. Relevamiento regional del carbono del maní y estimaciones de pérdidas producidas por la enfermedad. In Proceedings of the XXXI Jornada Nacional de Maní, General Cabrera, Córdoba, Argentina, 22 September 2016; pp. 53–54. [Google Scholar]
- Cazón, L.I.; Paredes, J.A.; Rago, A.M. The biology of Thecaphora frezii smut and its effects on Argentine peanut production. Adv. Plant Pathol. 2018, 31, 31–46. [Google Scholar] [CrossRef][Green Version]
- Astiz Gasso, M.; Leis, R.; Marinelli, A. Evaluación de incidencia y severidad del carbón de maní (Thecaphora frezii) en infecciones artificiales, sobre cultivares comerciales de maní. In Proceedings of the 1° Congreso Argentino de Fitopatología, Córdoba, Argentina, 28–30 May 2008; p. 161. [Google Scholar]
- Paredes, J.A.; Cazón, L.I.; Oddino, C.; Monguillot, J.H.; Rago, A.M.; Molina, J.E. Efficacy of fungicides against peanut smut in Argentina. Crop Prot. 2021, 140, 105403. [Google Scholar] [CrossRef]
- Bressano, M.; Massa, A.N.; Arias, R.S.; De Blas, F.; Oddino, C.; Faustinelli, P.C.; Soave, S.; Soave, J.; Pérez, M.A.; Sobolev, V.S.; et al. Introgression of peanut smut resistance from landraces to elite peanut cultivars (Arachis hypogaea L.). PLoS ONE 2019, 14, e0211920. [Google Scholar] [CrossRef]
- de Blas, F.J.; Bressano, M.; Teich, I.; Balzarini, M.G.; Arias, R.S.; Manifesto, M.M.; Costero, B.P.; Oddino, C.; Soave, S.J.; Soave, J.; et al. Identification of smut resistance in wild Arachis species and its introgression into peanut elite lines. Crop Sci. 2019, 59, 1657–1665. [Google Scholar] [CrossRef]
- de Blas, F.J.; Bruno, C.I.; Arias, R.S.; Taborda, C.B.; Mamani, E.; Oddino, C.; Rosso, M.; Costero, B.P.; Bressano, M.; Soave, J.A.; et al. Genetic mapping and QTL analysis for peanut smut resistance. BMC Plant Biol. 2021, 21, 312. [Google Scholar] [CrossRef]
- BICON. Australian Biosecurity Import Conditions. Australian Department of Agriculture, Fisheries and Forestry, 2017. Available online: https://bicon.agriculture.gov.au/BiconWeb4.0 (accessed on 30 January 2025).
- Chamberlin, K.D.; Baldessari, J.; Bennett, R.S.; Clevenger, J.P.; Holbrook, C.C.; Tallury, S.P.; Chu, Y.; Ozias-Akins, P.; Conde, M.B.; Payton, M.E. Identification of germplasm resistant to peanut smut. Peanut Sci. 2022, 49, 1–16. [Google Scholar] [CrossRef]
- Vánky, K. Smut Fungi of the World; American Phytopathological Society: St. Paul, MN, USA, 2012. [Google Scholar]
- van der Linde, K.; Göhre, V. How do smut fungi use plant signals to spatiotemporally orientate on and in planta? J. Fungi 2021, 7, 107. [Google Scholar] [CrossRef]
- Arias, S.L.; Mary, V.S.; Velez, P.A.; Rodriguez, M.G.; Otaiza-González, S.N.; Theumer, M.G. Where does the peanut smut pathogen, Thecaphora frezii, fit in the spectrum of smut diseases? Plant Dis. 2021, 105, 2268–2280. [Google Scholar] [CrossRef]
- Xia, W.; Yu, X.; Ye, Z. Smut fungal strategies for the successful infection. Microb. Pathog. 2020, 142, 104039. [Google Scholar] [CrossRef]
- Andrade, O.; Munoz, G.; Galdames, R.; Duran, P.; Honorato, R. Characterization, in vitro culture, and molecular analysis of Thecaphora solani, the causal agent of potato smut. Phytopathology 2004, 94, 875–882. [Google Scholar] [CrossRef]
- Frantzeskakis, L.; Courville, K.J.; Plücker, L.; Kellner, R.; Kruse, J.; Brachmann, A.; Feldbrügge, M.; Göhre, V. The plant-dependent life cycle of Thecaphora thlaspeos: A smut fungus adapted to Brassicaceae. Mol. Plant Microbe Interact. 2017, 30, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Brefort, T.; Doehlemann, G.; Mendoza-Mendoza, A.; Reissmann, S.; Djamei, A.; Kahmann, R. Ustilago maydis as a pathogen. Annu. Rev. Phytopathol. 2009, 47, 423–445. [Google Scholar] [CrossRef]
- Jose, R.C.; Goyari, S.; Louis, B.; Waikhom, S.D.; Handique, P.J.; Talukdar, N.C. Investigation on the biotrophic interaction of Ustilago esculenta on Zizania latifolia found in the Indo-Burma biodiversity hotspot. Microb. Pathog. 2016, 98, 6–15. [Google Scholar] [CrossRef]
- Jacobs, W.P. The development of the gynophore of the peanut plant, Arachis hypogaea L. I. The distribution of mitoses, the region of greatest elongation, and the maintenance of vascular continuity in the intercalary meristem. Am. J. Bot. 1947, 34, 361–370. [Google Scholar] [CrossRef]
- Smith, B.W. Arachis hypogaea: Aerial flower and subterranean fruit. Am. J. Bot. 1950, 37, 802–815. [Google Scholar] [CrossRef]
- Smith, B.W. Arachis hypogaea: Embryogeny and the effect of peg elongation upon embryo and endosperm growth. Am. J. Bot. 1956, 43, 233. [Google Scholar] [CrossRef]
- Moctezuma, E. The peanut gynophore: A developmental and physiological perspective. Can. J. Bot. 2003, 81, 183–190. [Google Scholar] [CrossRef]
- Oddino, C.M.; Giordano, D.F.; Rosso, M.; Soave, S.J.; Bressano, M.; de Blas, F.J.; Giuggia, J.A.; Crenna, A.C.; Mortigliengo, S. Comportamiento de genotipos de maní frente a distintas fuentes de inóculo de Thecaphora frezii. In Proceedings of the XXXVII Jornada Nacional del Maní, General Cabrera, Argentina, 15 September 2022; pp. 1–4. [Google Scholar]
- Oddino, C.; Rosso, M.; Soave, J.; Soave, S.; Mendoza, M.; Giordano, D.F.; Bressano, M.; de Blas, F.; Mortigliengo, S.; Buteler, M. Comportamiento de variedades de maní resistentes a carbón a través de los años. In Proceedings of the XXXVIII Jornada Nacional del Maní, General Cabrera, Córdoba, Argentina, 21 September 2023; pp. 1–2. [Google Scholar]
- Boote, K.J. Growth stages of peanut (Arachis hypogaea L.). Peanut Sci. 1982, 9, 35–40. [Google Scholar]
- Stevenson, W.R.; Loria, R.; Franc, G.D.; Weingartner, D.P. (Eds.) Compendium of Potato Diseases, 2nd ed.; APS Press: Saint Paul, MN, USA, 2001; pp. 43–44. [Google Scholar]
- Astiz Gasso, M.M.; Marinelli, A. Cultivo in vitro de Thecaphora frezii (Ustilaginales), carbón de maní (Arachis hypogaea). In Proceedings of the 29° Jornadas Argentinas de Botánica & 15° Reunión Anual de la Sociedad Botánica de Chile, San Luis, Argentina, 19–23 October 2003; p. 256. [Google Scholar]
- Moctezuma, E. Gravitropic Mechanisms of the Peanut Gynophore. Ph.D. Thesis, University of California, Berkeley, CA, USA, 1998. [Google Scholar]
- Periasamy, K.; Sampoornam, C. The morphology and anatomy of ovule and fruit development in Arachis hypogaea L. Ann. Bot. 1984, 53, 399–412. [Google Scholar] [CrossRef]
- Bhatla, S.C.; Lal, M.A. Source-to-Sink translocation of photoassimilates. In Plant Physiology, Development and Metabolism; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Dubrovsky, J.G.; Rodríguez-Alonso, G. The root apical meristem. In Plant Roots: The Hidden Half, 5th ed.; Beeckman, T., Esheltalker, A., Eds.; CRC Press: Boca Raton, FL, USA, 2024; pp. 33–58. [Google Scholar] [CrossRef]
- Kahmann, R.; Steinberg, G.; Basse, C.; Feldbrügge, M.; Kämper, J. Ustilago maydis, the causative agent of corn smut disease. In Fungal Pathology; Kronstad, J.W., Ed.; Springer: Dordrecht, The Netherlands, 2000; pp. 347–371. [Google Scholar] [CrossRef]
- Martínez-Espinoza, A.D.; García-Pedrajas, M.D.; Gold, S.E. The Ustilaginales as plant pests and model systems. Fungal Genet. Biol. 2002, 35, 1–20. [Google Scholar] [CrossRef]
- Appezzato-da-Glória, B.; Albernas, M.C.C.; Amorim, L. Structural characteristics of buds of sugarcane cultivars with different levels for resistance to smut. J. Plant Dis. 1995, 102, 502–508. [Google Scholar]
- Marques, J.P.; Hoy, J.W.; Appezzato-da-Glória, B.; Viveros, A.F.; Vieira, M.L.; Baisakh, N. Sugarcane cell wall-associated defense responses to infection by Sporisorium scitamineum. Front. Plant Sci. 2018, 9, 698. [Google Scholar] [CrossRef]
- Tanaka, S.; Brefort, T.; Neidig, N.; Djamei, A.; Kahnt, J.; Vermerris, W.; Koenig, S.; Feussner, K.; Feussner, I.; Kahmann, R. A secreted Ustilago maydis effector promotes virulence by targeting anthocyanin biosynthesis in maize. eLife 2014, 3, e01355. [Google Scholar] [CrossRef] [PubMed]
- Matei, A.; Ernst, C.; Günl, M.; Thiele, B.; Altmüller, J.; Walbot, V.; Usadel, B.; Doehlemann, G. How to make a tumour: Cell type specific dissection of Ustilago maydis-induced tumour development in maize leaves. New Phytol. 2018, 217, 1681–1695. [Google Scholar] [CrossRef]
- Gaudet, D.A.; Wang, Y.; Penniket, C.; Lu, Z.X.; Bakkeren, G.; Laroche, A. Morphological and molecular analyses of host and nonhost interactions involving barley and wheat and the covered smut pathogen Ustilago hordei. Mol. Plant Microbe Interact. 2010, 23, 1619–1634. [Google Scholar] [CrossRef]
- Doonan, J.; Sablowski, R. Walls around tumours—Why plants do not develop cancer. Nat. Rev. Cancer 2010, 10, 794–802. [Google Scholar] [CrossRef]
- Harris, M.O.; Pitzschke, A. Plants make galls to accommodate foreigners: Some are friends, most are foes. New Phytol. 2020, 225, 1852–1872. [Google Scholar] [CrossRef]
- Gorshkov, V.; Tsers, I. Plant susceptible responses: The underestimated side of plant–pathogen interactions. Biol. Rev. 2022, 97, 45–66. [Google Scholar] [CrossRef]
- Van Bel, A.J.; Helariutta, Y.; Thompson, G.A.; Ton, J.; Dinant, S.; Ding, B.; Patrick, J.W. Phloem: The integrative avenue for resource distribution, signaling, and defense. Front. Plant Sci. 2013, 4, 471. [Google Scholar] [CrossRef]
- Braun, D.M. Phloem loading and unloading of sucrose: What a long, strange trip from source to sink. Annu. Rev. Plant Biol. 2022, 73, 553–584. [Google Scholar] [CrossRef]
- Singh, N.; Somai, B.M.; Pillay, D. Smut disease assessment by PCR and microscopy in inoculated tissue cultured sugarcane cultivars. Plant Sci. 2004, 167, 987–994. [Google Scholar] [CrossRef]
- Fullerton, R.A. A histological study of the grass Heteropogon contortus infected by the smut Sorosporium caledonicum. Aust. J. Bot. 1975, 23, 51–54. [Google Scholar] [CrossRef]
- Yan, H.C.; Leu, L.S. Formation and histopathology of galls induced by Ustilago esculenta in Zizania latifolia. Phytopathology 1978, 68, 1572–1576. [Google Scholar]
- Billett, E.E.; Burnett, J.H. The host-parasite physiology of the maize smut fungus, Ustilago maydis II. Translocation of 14C-labelled assimilates in smutted maize plants. Physiol. Plant Pathol. 1978, 12, 103–112. [Google Scholar]
- Johri, B.M. (Ed.) Embryology of Angiosperms; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Weber, H.; Borisjuk, L.; Wobus, U. Molecular physiology of legume seed development. Annu. Rev. Plant Biol. 2005, 56, 253–279. [Google Scholar] [CrossRef] [PubMed]
- Langdon, R.F.N.; Fullerton, R.A. Sorus ontogeny and sporogenesis in some smut fungi. Aust. J. Bot. 1975, 23, 915–930. [Google Scholar] [CrossRef]
- Radchuk, V.; Borisjuk, L. Physical, metabolic, and developmental functions of the seed coat. Front. Plant Sci. 2014, 5, 510. [Google Scholar] [CrossRef]
- Aguirre, M.; Kiegle, E.; Leo, G.; Ezquer, I. Carbohydrate reserves and seed development: An overview. Plant Reprod. 2018, 31, 263–290. [Google Scholar] [CrossRef]
- Povilus, R.A.; Gehring, M. Maternal-filial transfer structures in endosperm: A nexus of nutritional dynamics and seed development. Curr. Opin. Plant Biol. 2022, 65, 102121. [Google Scholar] [CrossRef]
- Pegler, J.L.; Grof, C.P.; Patrick, J.W. Sugar loading of crop seeds—A partnership of phloem, plasmodesmal, and membrane transport. New Phytol. 2023, 239, 1584–1602. [Google Scholar] [CrossRef]
- Doll, N.M.; Ingram, G.C. Embryo-endosperm interactions. Annu. Rev. Plant Biol. 2022, 73, 293–321. [Google Scholar] [CrossRef] [PubMed]
- Piepenbring, M. New species of smut fungi from the neotropics. Mycol. Res. 2001, 105, 757–767. [Google Scholar] [CrossRef]
- Ram, B.; Singh, K.P. Smuts of Wheat: A Review. Indian Phytopathol. 2004, 57, 125–134. [Google Scholar]
- Piepenbring, M. Smut fungi (Ustilaginomycetes p.p. and Microbotryales, Basidiomycota); Flora Neotropica Monograph 86; New York Botanical Garden Press: Bronx, NY, USA, 2003; 291p. [Google Scholar]
- Banuett, F.; Herskowitz, I. Discrete developmental stages during teliospore formation in the corn smut fungus, Ustilago maydis. Development 1996, 122, 2965–2976. [Google Scholar] [CrossRef] [PubMed]
- Banuett, F. Pathogenic development in Ustilago maydis: A progression of morphological transitions that results in tumor formation and teliospore production. In Molecular Biology of Fungal Development; Osiewacz, H.D., Ed.; CRC Press: New York, NY, USA, 2002; pp. 349–398. [Google Scholar] [CrossRef]
- Hanna, W.F. Studies in the physiology and cytology of Ustilago zeae and Sorosporium reilianum. Phytopathology 1929, 19, 415–441. [Google Scholar]
- Christensen, J.J. Studies on the genetics of Ustilago zeae. Phytopathology 1931, 4, 124–188. [Google Scholar]
- Sleumer, H.O. Über Sexualität und Zytologie von Ustilago zeae (Beckm.) Unger: Inaugural-Dissertation zur Erlangung der Doktorwürde; Fischer: Jena, Germany, 1932. [Google Scholar]
- Snetselaar, K.M.; Mims, C.W. Light and electron microscopy of Ustilago maydis hyphae in maize. Mycol. Res. 1994, 98, 347–355. [Google Scholar] [CrossRef]
- Bushnell, W.R. Physiology of fungal haustoria. Annu. Rev. Phytopathol. 1972, 10, 151–176. [Google Scholar] [CrossRef]
- Hu, G.G.; Rijkenberg, F.H.J. Ultrastructural studies of the intercellular hypha and haustorium of Puccinia recondita f.sp. tritici. J. Phytopathol. 1998, 146, 39–50. [Google Scholar] [CrossRef]
- Littlefield, L.J.; Li, Y.; Hensley, D.D. Smut and rust diseases. In Plant Pathology: Concepts and Laboratory Exercises; Prentice Hall: Upper Saddle River, NJ, USA, 2004; p. 151. [Google Scholar]
- Cruz-Mireles, N.; Eseola, A.B.; Osés-Ruiz, M.; Ryder, L.S.; Talbot, N.J. From appressorium to transpressorium—Defining the morphogenetic basis of host cell invasion by the rice blast fungus. PLoS Pathog. 2021, 17, e1009779. [Google Scholar] [CrossRef]
- Eseola, A.B.; Ryder, L.S.; Osés-Ruiz, M.; Findlay, K.; Yan, X.; Cruz-Mireles, N.; Molinari, C.; Garduño-Rosales, M.; Talbot, N.J. Investigating the cell and developmental biology of plant infection by the rice blast fungus Magnaporthe oryzae. Fungal Genet. Biol. 2021, 154, 103562. [Google Scholar] [CrossRef]
- Paredes, J.A.; Asinari, F.; Edwards Molina, J.P.; Oddino, C.; Rago, A. Incidencia del carbón del maní en función del inóculo de Thecaphora frezii en el suelo. In Proceedings of the XXXIV Jornada Nacional del Maní, General Cabrera, Córdoba, Argentina, 19 September 2019; pp. 1–3. [Google Scholar]
- Johansen, D.A. Plant Microtechnique; McGraw-Hill Book Co Inc.: New York, NY, USA, 1940. [Google Scholar]
- Gonzalez, A.M.; Cristóbal, C.L. Anatomía y ontogenia de semillas de Helicteres lhostzkyana (Sterculiaceae). Bonplandia 1997, 9, 287–294. [Google Scholar] [CrossRef]
- Bissing, D.R. Haupt’s gelatin adhesive mixed with formalin for affixing paraffin sections to slides. Biotech. Histochem. 1974, 49, 116–117. [Google Scholar]
- Luque, R.H.; Souza, C.; Kraus, J.E. Métodos de coloracão do Roeser (1972) Modificado–E Kropp (1972), visado a substituicão do azul de astra por azul de alcião 8GS on 8GX. Acta Bot. Brasil. 1996, 10, 199–212. [Google Scholar] [CrossRef]
- Ruzin, S.E. Plant Microtechnique and Microscopy; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Hughes, J.; McCully, M.E. The use of an optical brightener in the study of plant structure. Biotech. Histochem. 1975, 50, 319–329. [Google Scholar] [CrossRef]
- Gonzalez, A.M.; Romero, M.F.; Sato, H.A. Exploring hidden connections: Endophytic system and flower meristem development of Pilostyles berteroi (Apodanthaceae) and interaction with its host Adesmia trijuga (Fabaceae). Plants 2024, 13, 3010. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero, M.F.; Samoluk, S.S.; Seijo, J.G.; Gonzalez, A.M. Histopathology of Thecaphora frezzii Colonization: A Detailed Analysis of Its Journey Through Peanut (Arachis hypogaea L.) Tissues. Plants 2025, 14, 1083. https://doi.org/10.3390/plants14071083
Romero MF, Samoluk SS, Seijo JG, Gonzalez AM. Histopathology of Thecaphora frezzii Colonization: A Detailed Analysis of Its Journey Through Peanut (Arachis hypogaea L.) Tissues. Plants. 2025; 14(7):1083. https://doi.org/10.3390/plants14071083
Chicago/Turabian StyleRomero, María Florencia, Sergio Sebastián Samoluk, José Guillermo Seijo, and Ana María Gonzalez. 2025. "Histopathology of Thecaphora frezzii Colonization: A Detailed Analysis of Its Journey Through Peanut (Arachis hypogaea L.) Tissues" Plants 14, no. 7: 1083. https://doi.org/10.3390/plants14071083
APA StyleRomero, M. F., Samoluk, S. S., Seijo, J. G., & Gonzalez, A. M. (2025). Histopathology of Thecaphora frezzii Colonization: A Detailed Analysis of Its Journey Through Peanut (Arachis hypogaea L.) Tissues. Plants, 14(7), 1083. https://doi.org/10.3390/plants14071083






