Discovery of Arbuscular Mycorrhizae in Mosses of the Pottiaceae Family from the Chaco Serrano (Tucumán, Argentina)
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
- Plant sampling
- Plants description
- Gertrudiella uncinicoma (Müll. Hal.) G.M. Suárez & Schiavone
- Pleurochaete luteola (Besch.) Thér.
- AMF prospection in mosses
- Cytological studies using scanning electron microscopy of the AMF-infected stem
- Molecular identification of AMF
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, B.; Qiu, Y.-L. Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 2006, 16, 299–363. [Google Scholar] [CrossRef] [PubMed]
- Pressel, S.; Bidartondo, M.I.; Ligrone, R.; Duckett, J.G. Fungal symbioses in bryophytes: New insights in the twenty first century. Phytotaxa 2010, 9, 238–253. [Google Scholar] [CrossRef]
- Brundrett, M.C. Coevolution of roots and mycorrhizas of land plants. New Phytol. 2002, 154, 275–304. [Google Scholar] [CrossRef] [PubMed]
- Pirozynski, K.; Malloch, D. The origin of land plants: A matter of mycotrophism. Biosystems 1975, 6, 153–164. [Google Scholar] [CrossRef]
- van der Heijden, M.G.A.; Martin, F.M.; Selosse, M.-A.; Sanders, I.R. Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef] [PubMed]
- Wijayawardene, N.N.; Hyde, K.D.; Al-Ani, L.K.T.; Tedersoo, L.; Haelewaters, D.; Rajeshkumar, K.C.; Zhao, R.; Aptroot, A.; Leontyev, D.; Saxena, R. Outline of Fungi and fungus-like taxa. Mycosphere Online J. Fungal Biol. 2020, 11, 1060–1456. [Google Scholar] [CrossRef]
- Wang, B.; Yeun, L.H.; Xue, J.-Y.; Liu, Y.; Ané, J.-M.; Qiu, Y.-L. Presence of three mycorrhizal genes in the common ancestor of land plants suggests a key role of mycorrhizas in the colonization of land by plants. New Phytol. 2010, 186, 514–525. [Google Scholar] [CrossRef]
- Field, K.J.; Pressel, S.; Duckett, J.G.; Rimington, W.R.; Bidartondo, M.I. Symbiotic options for the conquest of land. Trends Ecol. Evol. 2015, 30, 477–486. [Google Scholar] [CrossRef]
- Rayner, M. Note on the ecology of mycorrhiza. J. Ecol. 1928, 16, 418–419. [Google Scholar] [CrossRef]
- Kelley, A.P. Mycotrophy in plants. In Mycotrophy in Plants. Lectures on the Biology of Mycorrhizae and Related Structures; Chronica Botánica Co.: Waltham, MA, USA, 1950; pp. 1–223. [Google Scholar]
- Gerdemann, J. Vesicular-arbuscular mycorrhiza and plant growth. Annu. Rev. Phytopathol. 1968, 6, 397–418. [Google Scholar] [CrossRef]
- Schüßler, A. Glomus claroideum forms an arbuscular mycorrhiza-like symbiosis with the hornwort Anthoceros punctatus. Mycorrhiza 2000, 10, 15–21. [Google Scholar] [CrossRef]
- Russell, J.; Bulman, S. The liverwort Marchantia foliacea forms a specialized symbiosis with arbuscular mycorrhizal fungi in the genus Glomus. New Phytol. 2005, 165, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Albornoz, P.; Catania, M. Presencia de micorrizas arbusculares y endofitos septados en Plagiochasma rupestre (Aytoniaceae, Marchantiophyta) del Chaco Serrano (Tucumán, Argentina). Lilloa 2022, 59, 189–197. [Google Scholar] [CrossRef]
- Butler, E.J. The occurrences and systematic position of the vesicular-arbuscular type of mycorrhizal fungi. Trans. Br. Mycol. Soc. 1939, 22, 274–301. [Google Scholar] [CrossRef]
- Dowding, E.S. Ecology of endogone. Trans. Br. Mycol. Soc. 1959, 42, 449–457. [Google Scholar] [CrossRef]
- Parke, J.L.; Linderman, R. Association of vesicular–arbuscular mycorrhizal fungi with the moss Funaria hygrometrica. Can. J. Bot. 1980, 58, 1898–1904. [Google Scholar] [CrossRef]
- Rabatin, S.C. The occurrence of the vesicular-arbuscular-mycorrhizal fungus Glomus tenuis with moss. Mycologia 1980, 72, 191–195. [Google Scholar] [CrossRef]
- Valdés, F.E.; Peralta, D.F.; Velázquez, M.S.; Covacevich, F.; Becerra, A.G.; Cabello, M.N. On the occurrence of arbuscular mycorrhizal fungi in a Bryophyte community of Punta Lara Natural Reserve, Buenos Aires, Argentina. Diversity 2023, 15, 442. [Google Scholar] [CrossRef]
- Read, D.J.; Ducket, J.G.; Francis, R.; Ligron, R.; Russell, A. Symbiotic fungal associations in ‘lower’ land plants. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2000, 355, 815–830. [Google Scholar] [CrossRef]
- Ligrone, R.; Carafa, A.; Lumini, E.; Bianciotto, V.; Bonfante, P.; Duckett, J.G. Glomeromycotean associations in liverworts: A molecular, cellular, and taxonomic analysis. Am. J. Bot. 2007, 94, 1756–1777. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, L.-D. Arbuscular mycorrhizal structure and fungi associated with mosses. Mycorrhiza 2007, 17, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Kodama, K.; Rich, M.K.; Yoda, A.; Shimazaki, S.; Xie, X.; Akiyama, K.; Mizuno, Y.; Komatsu, A.; Luo, Y.; Suzuki, H.; et al. An ancestral function of strigolactones as symbiotic rhizosphere signals. Nat. Commun. 2022, 13, 3974. [Google Scholar] [CrossRef]
- Rich, M.K.; Vigneron, N.; Libourel, C.; Keller, J.; Xue, L.; Hajheidari, M.; Radhakrishnan, G.V.; Le Ru, A.; Diop, S.I.; Potente, G.; et al. Lipid exchanges drove the evolution of mutualism during plant terrestrialization. Science 2021, 372, 864–868. [Google Scholar] [CrossRef]
- Pressel, S.; Bidartondo, M.I.; Field, K.J.; Duckett, J.G. Advances in understanding of mycorrhizal-like associations in bryophytes. Bryophyt. Divers. Evol. 2021, 43, 284–306. [Google Scholar] [CrossRef]
- Radhakrishnan, G.V.; Keller, J.; Rich, M.K.; Vernié, T.; Mbadinga Mbadinga, D.L.; Vigneron, N.; Cottret, L.; Clemente, H.S.; Libourel, C.; Cheema, J.; et al. An ancestral signalling pathway is conserved in intracellular symbioses-forming plant lineages. Nat. Plants 2020, 6, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Renaut, S.; Daoud, R.; Masse, J.; Vialle, A.; Hijri, M. Inoculation with Rhizophagus irregularis does not alter arbuscular mycorrhizal fungal community structure within the roots of corn, wheat, and soybean crops. Microorganisms 2020, 8, 83. [Google Scholar] [CrossRef]
- Strullu-Derrien, C.; Kenrick, P.; Pressel, S.; Duckett, J.G.; Rioult, J.P.; Strullu, D.G. Fungal associations in Horneophyton ligneri from the Rhynie Chert (c. 407 million year old) closely resemble those in extant lower land plants: Novel insights into ancestral plant–fungus symbioses. New Phytol. 2014, 203, 964–979. [Google Scholar] [CrossRef] [PubMed]
- Feijen, F.A.; Vos, R.A.; Nuytinck, J.; Merckx, V.S. Evolutionary dynamics of mycorrhizal symbiosis in land plant diversification. Sci. Rep. 2018, 8, 10698. [Google Scholar] [CrossRef]
- Delaux, P.-M.; Radhakrishnan, G.V.; Jayaraman, D.; Cheema, J.; Malbreil, M.; Volkening, J.D.; Sekimoto, H.; Nishiyama, T.; Melkonian, M.; Pokorny, L. Algal ancestor of land plants was preadapted for symbiosis. Proc. Natl. Acad. Sci. USA 2015, 112, 13390–13395. [Google Scholar] [CrossRef]
- Kelly, K.H.; Coleine, C.; Coshland, C.; Stajich, J.E. Novel Glomeromycotina-moss associations identified in California dryland biocrusts. bioRxiv 2024. [Google Scholar] [CrossRef]
- Johnson, P. Mycorrhizal endogonaceae in a New Zealand forest. New Phytol. 1977, 78, 161–170. [Google Scholar] [CrossRef]
- Rimington, W.R.; Duckett, J.G.; Field, K.J.; Bidartondo, M.I.; Pressel, S. The distribution and evolution of fungal symbioses in ancient lineages of land plants. Mycorrhiza 2020, 30, 23–49. [Google Scholar] [CrossRef] [PubMed]
- Schübler, A.; Schwarzott, D.; Walker, C. A new fungal phylum, the Glomeromycota: Phylogeny and evolution. Mycol. Res. 2001, 105, 1413–1421. [Google Scholar] [CrossRef]
- Bowman, J.L.; Kohchi, T.; Yamato, K.T.; Jenkins, J.; Shu, S.; Ishizaki, K.; Yamaoka, S.; Nishihama, R.; Nakamura, Y.; Berger, F. Insights into land plant evolution garnered from the Marchantia polymorpha genome. Cell 2017, 171, 287–304.e15. [Google Scholar] [CrossRef]
- Azcón-Aguilar, C.; Jaizme-Vega, M.C.; Calvet, C. The contribution of arbuscular mycorrhizal fungi to the control of soil-borne plant pathogens. In Mycorrhizal Technology in Agriculture: From Genes to Bioproducts; Gianinazzi, S., Schüepp, H., Barea, J.M., Haselwandter, K., Eds.; Birkhäuser Basel: Basel, Switzerland, 2002; pp. 187–197. [Google Scholar]
- Pozo, M.J.; Cordier, C.; Dumas-Gaudot, E.; Gianinazzi, S.; Barea, J.M.; Azcón-Aguilar, C. Localized versus systemic effect of arbuscular mycorrhizal fungi on defence responses to Phytophthora infection in tomato plants. J. Exp. Bot. 2002, 53, 525–534. [Google Scholar] [CrossRef]
- Field, K.J.; Bidartondo, M.I.; Rimington, W.R.; Hoysted, G.A.; Beerling, D.; Cameron, D.D.; Duckett, J.G.; Leake, J.R.; Pressel, S. Functional complementarity of ancient plant-fungal mutualisms: Contrasting nitrogen, phosphorus and carbon exchanges between Mucoromycotina and Glomeromycotina fungal symbionts of liverworts. New Phytol. 2019, 223, 908–921. [Google Scholar] [CrossRef] [PubMed]
- Field, K.J.; Rimington, W.R.; Bidartondo, M.I.; Allinson, K.E.; Beerling, D.J.; Cameron, D.D.; Duckett, J.G.; Leake, J.R.; Pressel, S. First evidence of mutualism between ancient plant lineages (Haplomitriopsida liverworts) and Mucoromycotina fungi and its response to simulated Palaeozoic changes in atmospheric CO2. New Phytol. 2015, 205, 743–756. [Google Scholar] [CrossRef]
- Zander, R.H. Genera of the Pottiaceae: Mosses of harsh environments. Bull. Buffalo Soc. Nat. Sci. 1993, 32, 1–378. [Google Scholar]
- Zander, R.H. Revision of the genus Leptodontium (Musci) in the New World. Bryologist 1972, 75, 213–280. [Google Scholar] [CrossRef][Green Version]
- Suárez, G.M.; Schiavone, M.M. On the presence of Pleurochaete Lindb. (Pottiaceae, Musci) in Argentina. Lindbergia 2005, 30, 93–96. [Google Scholar]
- Jimenez, S.; Suárez, G.M. The genus Gertrudiella Broth. (Pottiaceae, Bryophyta) in Paraguay. Check List 2017, 13, 2039. [Google Scholar] [CrossRef][Green Version]
- Cottet, A.C.; Scervino, J.M.; Messuti, M.I. An improved staining protocol for the assessment of arbuscular mycorrhizal in bryophytes. Bol. Soc. Argent. Bot. 2018, 53, 201–206. [Google Scholar] [CrossRef]
- Morris, J.K. A formaldehyde glutaraldehyde fixative of high osmolality for use in electron microscopy. J. Cell Biol. 1965, 27, 1A–149A. [Google Scholar]
- Dickson, S. The Arum-Paris continuum of mycorrhizal symbioses. New Phytol. 2004, 163, 187–200. [Google Scholar] [CrossRef]
- Schenck, N.C.; Perez-Collins, Y. Manual for the Identification of VA Mycorrhizal Fungi; Synergistic Publications: Gainesville, FL, USA, 1990. [Google Scholar]
- Oehl, F.; Sieverding, E.; Palenzuela, J.; Ineichen, K.; da Silva, G.A. Advances in Glomeromycota taxonomy and classification. IMA Fungus 2011, 2, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Mayjonade, B.; Gouzy, J.; Donnadieu, C.; Pouilly, N.; Marande, W.; Callot, C.; Langlade, N.; Muños, S. Extraction of high-molecular-weight genomic DNA for long-read sequencing of single molecules. Biotechniques 2016, 61, 203–205. [Google Scholar] [CrossRef]
- Wang, O.; Chin, R.; Cheng, X.; Wu, M.K.Y.; Mao, Q.; Tang, J.; Sun, Y.; Anderson, E.; Lam, H.K.; Chen, D. Efficient and unique cobarcoding of second-generation sequencing reads from long DNA molecules enabling cost-effective and accurate sequencing, haplotyping, and de novo assembly. Genome Res. 2019, 29, 798–808. [Google Scholar] [CrossRef]
- Weisenfeld, N.I.; Kumar, V.; Shah, P.; Church, D.M.; Jaffe, D.B. Direct determination of diploid genome sequences. Genome Res. 2017, 27, 757–767. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Bushnell, B. BBMap: A Fast, Accurate, Splice-Aware Aligner; Lawrence Berkeley National Lab. (LBNL): Berkeley, CA, USA, 2014.
- Kalvari, I.; Nawrocki, E.P.; Ontiveros-Palacios, N.; Argasinska, J.; Lamkiewicz, K.; Marz, M.; Griffiths-Jones, S.; Toffano-Nioche, C.; Gautheret, D.; Weinberg, Z. Rfam 14: Expanded coverage of metagenomic, viral and microRNA families. Nucleic Acids Res. 2021, 49, D192–D200. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The sequence alignment/map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Online Resource. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 26 June 2023).

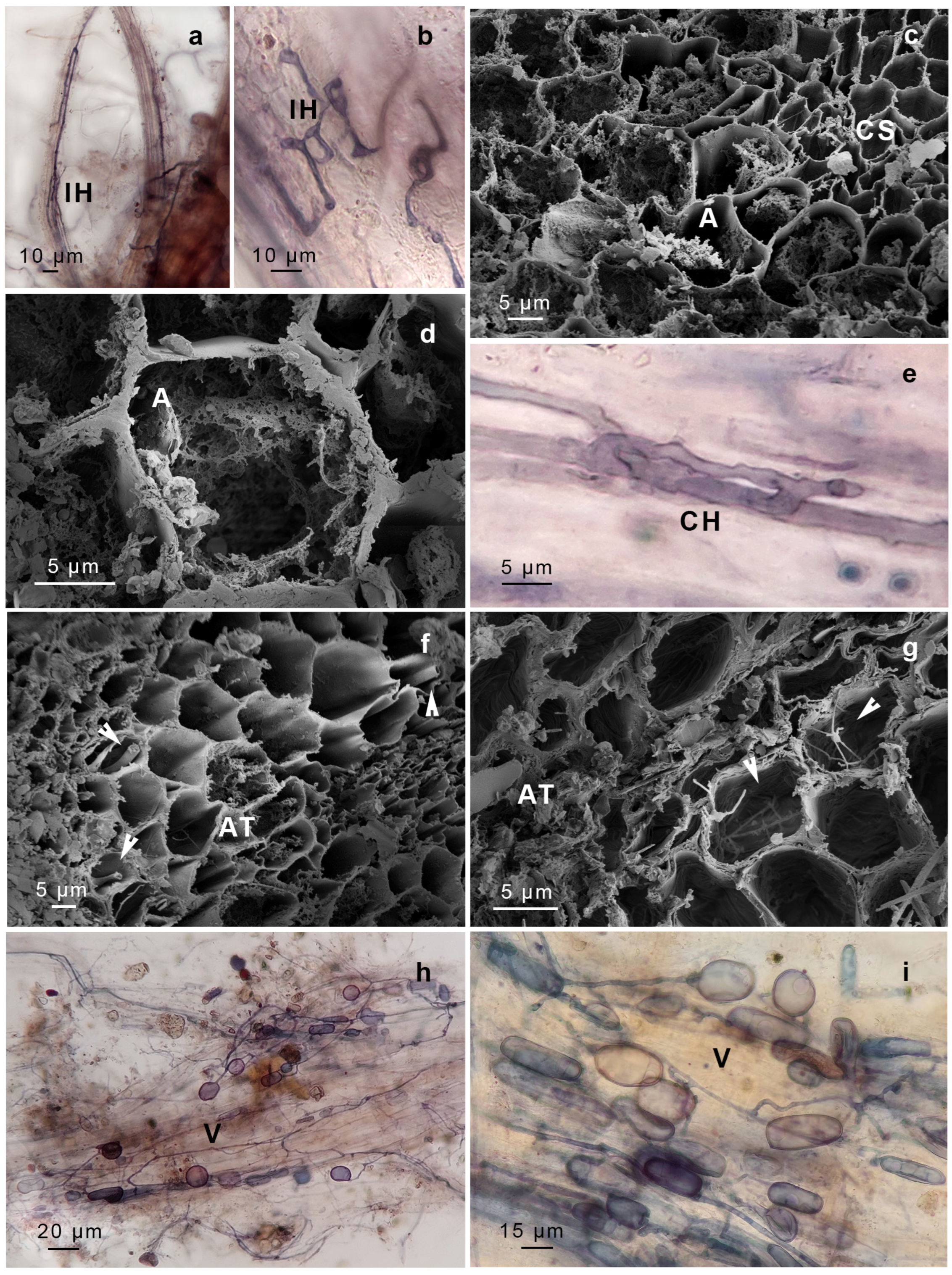

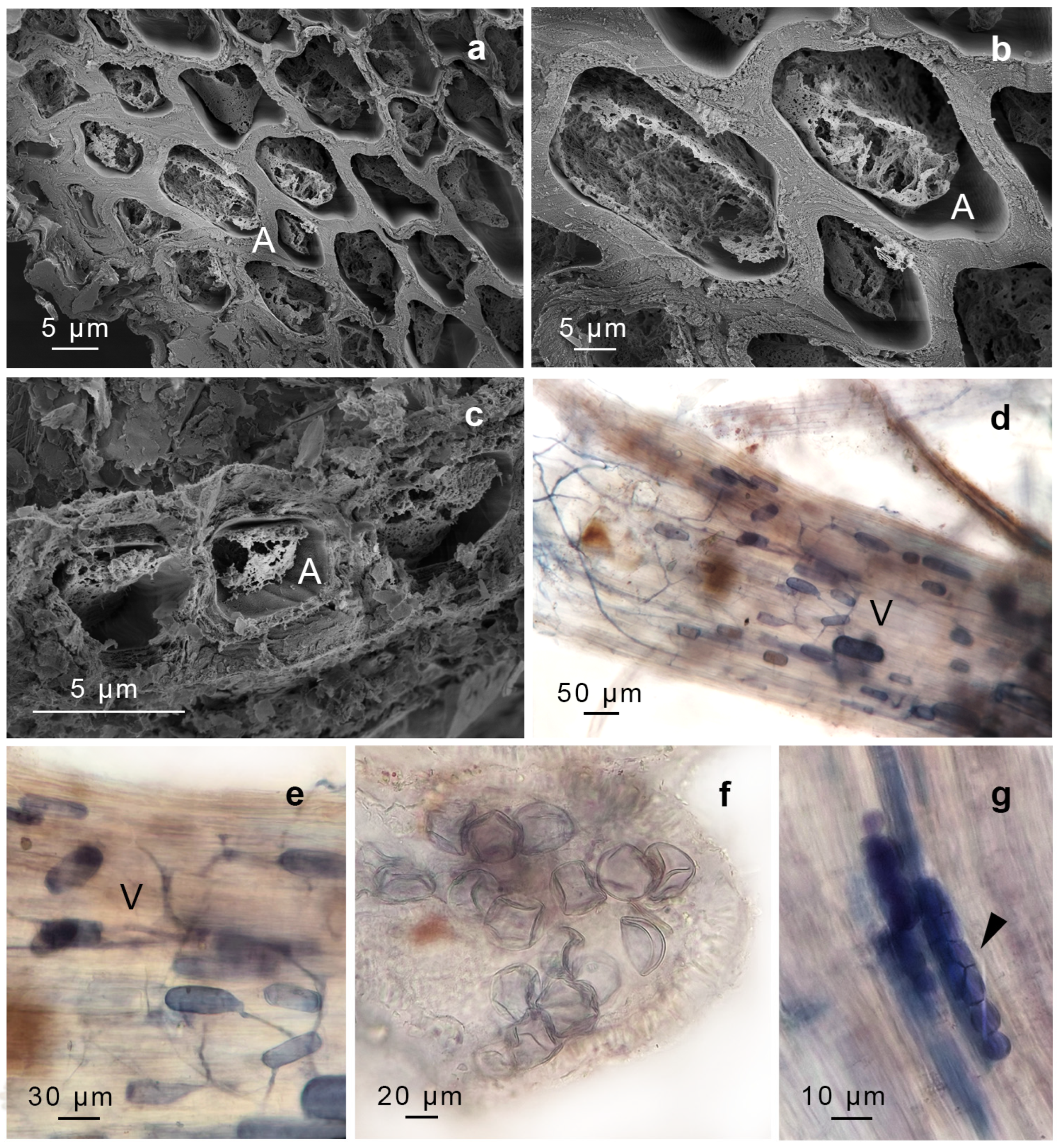
| AMF Structure Observed | Presence in P. luteola (GS1956) | Presence in G. uncinicoma (GS1957) | Molecular Identification (18S rDNA Sequencing) | Congruence |
|---|---|---|---|---|
| Molecular Identification (18S rDNA Sequencing) | Glomeraceae (R. irregularis and Glomus sp.), Gigasporaceae, Acaulosporaceae, Pacisporaceae, Diversisporaceae | Glomeraceae (R. irregularis and Glomus sp.), Claroideoglomeraceae | Matches detected AMF families | High (sequencing supports the identification of R. irregularis and Glomus sp.; other fungal species were not morphologically identified) |
| Arbuscules | Present (stems) | Present (stem, leaves) | ||
| Intercellular aseptate hyphae | Present (stems) | Present (leaves, stems, and rhizoids) | ||
| Intracellular hyphal coils (Paris-type morphology) | Not observed | Present (stems) | ||
| Intracellular hyphal coils (Arum-type morphology) | Present (stems) | Present (leaves, stems) | ||
| Vesicles | Present (stems) | Present (stems, leaves, rhizoids) | ||
| Spores | Present (likely Glomus sp. in stems) | Present (rhizoids, resembling Gigaspora sp.) | Moderate (Gigaspora-like spores observed, but molecular data primarily detected Glomeraceae; Glomus sp. observed and supported with sequencing) | |
| Dark Septate Endophytes (DSE) | Not observed | Present (leaves) | Ascomycota, Basidiomycota (ambiguous) | Low (morphology suggests endophytic fungi, but taxonomic assignment remains uncertain) |
| Unknown Septate Fungi (USH) | Present (stems) | Present (leaves) | Ascomycota, Basidiomycota (ambiguous) | Low (morphology suggests endophytic fungi, but taxonomic assignment remains uncertain) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catania, M.d.V.; Albornoz, P.L.; Rausch, A.O.; Ledesma, T.M.; Dong, S.; Cai, Y.; Zeng, Y.; Liu, Y.; Suárez, G.M.; Moreno, J.E. Discovery of Arbuscular Mycorrhizae in Mosses of the Pottiaceae Family from the Chaco Serrano (Tucumán, Argentina). Plants 2025, 14, 1048. https://doi.org/10.3390/plants14071048
Catania MdV, Albornoz PL, Rausch AO, Ledesma TM, Dong S, Cai Y, Zeng Y, Liu Y, Suárez GM, Moreno JE. Discovery of Arbuscular Mycorrhizae in Mosses of the Pottiaceae Family from the Chaco Serrano (Tucumán, Argentina). Plants. 2025; 14(7):1048. https://doi.org/10.3390/plants14071048
Chicago/Turabian StyleCatania, Myriam del V., Patricia L. Albornoz, Atilio O. Rausch, Tamara M. Ledesma, Shanshan Dong, Yuqing Cai, Yuying Zeng, Yang Liu, Guillermo M. Suárez, and Javier E. Moreno. 2025. "Discovery of Arbuscular Mycorrhizae in Mosses of the Pottiaceae Family from the Chaco Serrano (Tucumán, Argentina)" Plants 14, no. 7: 1048. https://doi.org/10.3390/plants14071048
APA StyleCatania, M. d. V., Albornoz, P. L., Rausch, A. O., Ledesma, T. M., Dong, S., Cai, Y., Zeng, Y., Liu, Y., Suárez, G. M., & Moreno, J. E. (2025). Discovery of Arbuscular Mycorrhizae in Mosses of the Pottiaceae Family from the Chaco Serrano (Tucumán, Argentina). Plants, 14(7), 1048. https://doi.org/10.3390/plants14071048






