Abstract
Geoffroea decorticans (Gill. ex Hook. & Arn) Burk. is a native tree of the dry areas of Northwestern and Central Argentina. Its seeds are considered waste material. The flour of seeds was analyzed as a source of nutritional and bioactive compounds. It has a low carbohydrate content, containing about 9% protein and between 10 and 14% fat. Approximately 82–84% of the fatty acids were unsaturated (oleic and linoleic acids). A high polyphenol and dietary fiber content was detected. Flavonoids and condensed tannins were the dominant phenolics. Polyphenol-enriched extracts were obtained from seed flour. The HPLC–ESI-MS/MS analysis of these concentrated extracts allowed for the identification of six compounds including C-glycosyl flavones (vitexin and isovitexin), type A procyanidins (dimer and trimer), and epicatequin gallate. Polyphenolic extracts showed antioxidant capacity and were able to inhibit enzymes (α-glucosidase and α-amylase) related to carbohydrate metabolism and (lipoxygenase) pro-inflammatory enzymes and were not toxic. Flour and polyphenolic extract from chañar seeds could be considered as new alternative ingredients for the formulation of functional foods, nutraceuticals, or food supplements. The use of the seed flour in addition to the pulp of the fruit along with the rest of the plant would encourage the propagation of this species resistant to extreme arid environments for commercial and conservation purposes to boost the regional economies of vulnerable areas of South America.
Keywords:
chañar; nutritional; functional properties; antioxidant; antilipoxygenase; α-amylase; α-glucosidase 1. Introduction
Geoffroea decorticans (Gill ex Hook & Arn.) Burkart (Fabaceae), traditionally known as “chañar”, is a medicinal and food plant species []. It is native and widely distributed in Argentina, from Jujuy to Patagonia, and associated with algarrobo (Neltuma ex Prosopis) and mistol (Sarcomphalus mistol ex Ziziphus mistol). It is a part of the group of the so-called fruits of the Monte region or dry Chaco native forest region in Argentina. Additionally, its presence has been documented in arid areas of Paraguayan and Bolivian Chaco, as well as in northern Chile. Currently, in Argentina, these native forests are undergoing significant decline due to the expansion of agricultural activities, primarily for soybean production intended for export. In this context, conducting studies that enhance the value of this species is crucial to promoting its expansion, preservation, and sustainable management in arid regions. Fruits are drupes of 1.5 to 3 cm diameter. Its reddish and sweet fleshy pulp is used to obtain a jam named “chañar arrope”, flour, and a fermented alcoholic drink []. Raw and cooked seeds were used as food for the Tobas, Wichis, and Ranqueles communities [,]. In general, during the production of commercial products from chañar, the seeds are discarded because they constitute a waste or byproduct of food supply chain. Currently, there are several studies that indicate the potential of fruits waste as natural resources of bioactive compounds, mainly seeds and peels [], e.g., polyphenolic compounds obtained from fruit seeds. Previous studies suggest a high content of crude fiber and other phytochemicals such as ascorbic acid, carotenoids, and polyphenols, mainly non-flavonoids, in chañar fruit flour without seeds [,]. Polyphenols from chañar fruit pulp without seeds showed antioxidant activity and the ability to inhibit enzymes linked to metabolic syndrome, such as lipase, α-glucosidase, α-amylase, and hydroxy methyl glutaryl CoA reductase []. Studies have demonstrated that the polyphenols found in chañar fruit pulps have anticancerogenic properties and inhibit pro-inflammatory enzymes such as phospholipase A2, lipoxygenase, and cyclooxygenase-1 and -2 [,]. It has been shown that “chañar arrope” and the aqueous extract have antinociceptive properties []. Seeds of fruits obtained from Córdoba, San Juan, and Tucumán, Argentina, comprise high amounts of crude fiber and lipid content [,,]. It also contains tocopherols, tocotrienols, and phytosterols []. There are evidence of anticoagulant and antioxidant activities of peptide isolates from chañar seeds and polyphenol compounds [,]. The oil extracted from these seeds has potential for biodiesel production, while the woody husk can be utilized to manufacture densified solid fuels (pellets), both of which are valuable biofuels [].
The objective of this study was to characterize the polyphenolic profile and nutritional composition of chañar seed flour, as well as to evaluate the effectiveness of its polyphenolic extracts in inhibiting enzymes associated with oxidative stress, hyperglycemia, dyslipidemia, and inflammatory processes.
2. Results and Discussion
Ripe fruits of “chañar” were collected from wild populations in two localities in the NWA, Fernández in Santiago del Estero (Chaco seco eco-region at 700 m a.s.l.) and Colalao del Valle in Tucumán (Monte de Sierras y Bolsones eco-region at 1500 m a.s.l.) (Figure 1). Both are semi-arid regions but with differences in soil conditions. Seeds were extracted from the collected fruits.
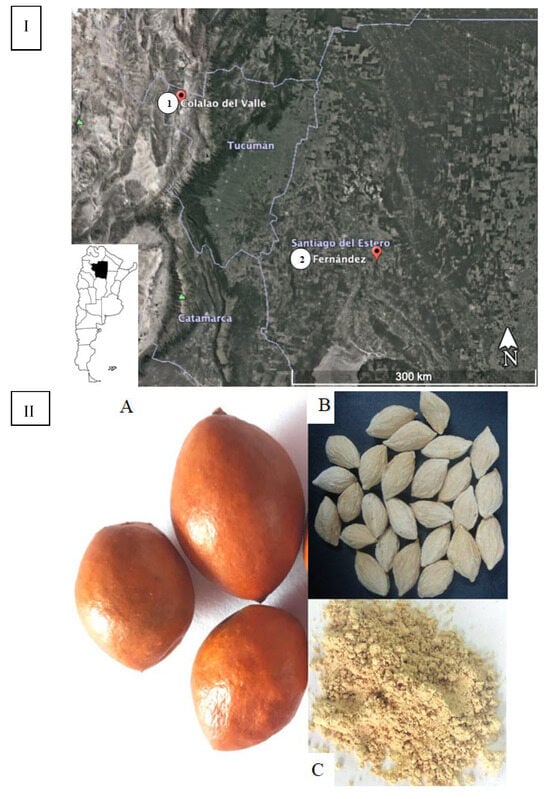
Figure 1.
(I) Map of Argentina showing the collection places of chañar fruits: (1) Colalao del Valle- Tucumán province; (2) Fernández in Santiago del Estero province. (II) (A) Chañar fruits; (B) chañar seeds; (C) chañar seed flour.
In this study, it was determined that this waste material (seeds) represents about 30-35% of fruit weight. Considering that each tree produces about 30 kg of fruits that fall when they are ripe [] and that each person can harvest in 1 h up to 25 kg of fruits [], the seed yield that was determined in this study could be considered high. Therefore, it is important to revalue this waste material.
The color parameters were determined (CIELAB). The flour registers a high luminosity (L* = 67.95 ± 0.15). The values of parameter a* (green to red) were positive (a* = 10.04 ± 0.25), indicating a preponderance of red over green. The values of parameter b* (blue to yellow) were positive (b* = 35.24 ± 0.21), suggesting primacy of yellow over blue. The manifestation and intensity of this parameter is associated with the accumulation of natural pigments, such as anthocyanins, betalains, carotenoids, alkaloids, flavonoids, and chlorophylls. Color is a key sensory attribute in consumer perception and represents one of the determining factors in food selection []. The chañar seed flours with different particle size showed different yield percentage, 0.5%, 98%, 0%, and 1% to F1 (>840 µm), F2 (>500 µm), F3 (>140 µm), and F4 (>105), respectively. Chañar flour meets the granulometry required by the Argentine Food Code for corn flour []. On the other hand, a high uniformity of particle size was found. As a result, this characteristic allows the finished product to have better flavor, texture, and appearance since it can absorb water uniformly. No differences were observed in the color parameters or granulometry of the flours from the different regions.
2.1. Chañar Seed Flour Nutritional Characterization
No significant differences were observed in carbohydrate or proteins content of seed flours obtained from fruits collected in two provinces from NWA (Tucumán and Santiago del Estero, Monte region, and Chaco seco region). The carbohydrate content of chañar seed flour (9.63–8.46 g GE/100 g) (Table 1) was lower than that of the chañar pulp flour (19.5 g GE/100 g) [], but higher than mistol (S. mistol) seed flour (2.1%), another wild food plant species from NWA []. Hence, when compared with flours from cereals of greater consumption, such as rice, corn, and wheat (84.7, 81.1, and 78.4% respectively), we can see that said content was the lowest []. The protein content of chañar seed flour from both samples collected in NWA (8.80–9.17 g/100 g) was higher than that reported in chañar fruit seeds collected in Cordoba and San Luis, two provinces of the Argentine Center [,,]. The differences in protein content among samples from different environments in Argentina may be attributed to environmental factors, since the collection areas in the northwest of Argentina are more arid than the central area of the country. The protein content in chañar seeds was higher than previously reported for chañar pulp []. A similar protein content was found in mistol seed flour [,], as well as in corn and rice flour []. In contrast, wheat flour had the highest protein content (14%) [].

Table 1.
Chemical characterization of chañar seed flour.
The fat content of chañar seed flour from Fernández (14.00 g/100 g) was higher than that from Colalao del Valle (10.95 g/100 g) (Table 1). The fat content was lower than mistol seed flour [], but it was significantly higher than the fat content in rice, corn, and wheat (2.2, 4.7, and 2.3%, respectively) []. The composition of fatty acids is a key characteristic that defines the identity of fats and oils, playing a crucial role in their stability and nutritional quality. The chañar seed oils were characterized to be highly unsaturated with oleic acid (around 40%) and linoleic acid (43%). Linoleic acid, an essential fatty acid, cannot be synthesized by the human body and must be acquired through diet. It has been associated with various physiological benefits, including anticarcinogenic, antiadipogenic, antidiabetogenic, and antiatherosclerotic properties [,]. The total content of linoleic acid of chañar seed flour was comparable to that found in soy and amaranth flour [,]. Saturated fatty acids of chañar seeds were mainly composed of palmitic acid and stearic acid (Table 1).
The total crude fiber content was very high with significant differences between samples from the two provinces (51.65 and 57.41 g/100). The total crude fiber of mistol seed flour was lower than the chañar seed flour []. The soluble dietary fiber content in chañar seed flour from Fernández and Colalao del Valle (8.2 and 9.5%, respectively) (Table 1) was close to that of barley and wheat bran (9.2 and 9.82%, respectively), higher than those registered for quinoa (2.73%), and lower than that of oat bran (11.29%) [,,]. Several studies have associated the benefits to health with the consumption of foods rich in soluble dietary fiber and have shown that the intake of dietary fiber can reduce the risk of cardiovascular disease and type II diabetes []. Chañar seed flour from Fernández and Colalao del Valle could also be considered as a source of insoluble fiber (19.8 and 19.5%, respectively) according to Regulation (EC) No. 1924/2006 of the European Parliament. Therefore, fiber today plays an important role in nutrition and is a functional ingredient widely used by the food industry.
The different in fat and fiber content between seed flours of chañar collected at different regions, Tucuman and Santiago del Estero in Argentina, could be due to differences in the altitudinal level or the hydric conditions of the collection sites. Both regions have arid soil, but in Tucumán, the chañar populations are more isolated than in Santiago del Estero and grow in the wetter valleys from the Calchaquies Valleys in Colalao del Valle (Figure 2).

Figure 2.
(A) Chañar tree in Fernández (Santiago del Estero). (B) Chañar tree in Colalao del Valle (Tucumán). (C) Fruits in soil.
2.2. Phytochemical Composition
The total polyphenol content of seed flour of Fernández fruits was lower than that of Colalao del Valle fruit seeds (400.60 and 587.34 mg GAE/100 g of flour, respectively). These values were lower than those reported for chañar pulp (1240 mg/100 g) [] and similar to those obtained in mistol seed flour [].
The flavonoid content of seed flour showed a different behavior. So, the level of flavonoids was higher in Fernández seed flour than Colalao del Valle seed flour (830.55 and 575 mg QE/100 g seed flour, respectively) and higher than those registered for the chañar pulp (70 mg QE/100 g) [], algarrobo seeds (396 mg EQ/100 g) [], and mistol seed flour (444 mg EQ/100 g) [] from NWA.
Condensed tannin content (191.73 and 127.78 mg procyanidin B2/100 g flour from Fernández and Colalao del Valle, respectively) (Table 1) was comparable to that registered for algarrobo seeds (175 mg procyanidin B2/100 g) [] and mistol seed (153.4 mg procyanidin B2/100 g) [], but lower than chañar pulp (844 mg procyanidin B2/100 g) []. Condensed tannins contribute to both sensorial characteristics and quality of food. These compounds are known to be powerful antioxidants with potential health-promoting properties. They also inhibit enzymes linked to metabolic syndrome, such as amylase, glucosidase, and lipase [,]. The hydrolysable tannins and carotenoids were not detected in seeds flour in any of the samples analyzed.
The specialized metabolites have a highly complex and intricate regulatory network []. The variability observed in the content of specialized metabolites in flour from different provinces could be attributed to different environmental conditions, some of which may promote increased levels of these compounds. On the other hand, although Geoffroea decorticans is a native species adapted to ecologically limiting conditions in extremely arid areas, its genetic variability has not yet been evaluated.
2.3. Identification of Phenolic Compounds
Six peaks were tentatively identified for the first time in ethanolic extracts of chañar seed flour by HPLC-ESI-MS/MS (Table 2, Figure 3). Most of these compounds exhibited a maximum absorbance around 278 nm, according to UV-visible analysis in the range of 200 to 800 nm, corresponding to the absorbance spectrum of flavan-3-ols. Three A-type procyanidins were found in the chañar seed flour: Peak 1 (Rt = 16.5 min, λ max = 277 nm) displayed a molecular ion signal ([M-H]) at m/z 863.1744, along with fragment ions at m/z 711, 573, 451, and 239, indicating a representative cleavage model of A-type procyanidin trimer. Peaks 2 and 4 (Rt = 20.3 min and 25.9 min., λ max = 276 nm) exhibited a negative molecular ion peak [M-H]− at m/z 575.1, with MS2 fragmentation data suggesting the presence of A-type procyanidin dimer (Table 2). This structure contains two fewer protons than a B-type procyanidin dimer, indicating an additional C–O–C linkage. Peak 5 corresponded to gallate A type procyanidin, and peak 3 corresponded to epicatechin gallate. A-type procyanidins are much less prevalent than the B-type procyanidins. Several studies have reported that these compounds offer health benefits, including antioxidant activities, such as scavenging ability of ABTS•+, O2•, HO• [], immunomodulatory effects [], hypoglycemic activities [], and the ability to inhibit the adhesion of uropathogenic bacteria (in vitro assays) []. Major plant sources of A-type procyanidins are from cinnamon, cranberry juice, litchi pericarp, and peanut skins [,,]. C-glycosyl flavones (vitexin and isovitexin) in peak 6 were also found in chañar seed flour. These compounds were previously described from P. alba mesocarp and seed and Prosopis pod syrups [,,]. Vitexin was also found in chañar seed ethanolic extracts []. C-glycosyl flavonoids have demonstrated various biological activities, including anti-inflammatory, antioxidant, hypoglycemic, anticancer, and antiplatelet effects. Furthermore, these compounds exhibit angiotensin-converting enzyme (ACE) inhibitory activity, neuroprotective effects, cardiovascular protection, benefits against endocrine and metabolic diseases, and antimicrobial and antiviral properties [,,,].

Table 2.
Identification of nine phenolic compounds in chañar seed flour by HPLC-DAD-ESI-MS data.
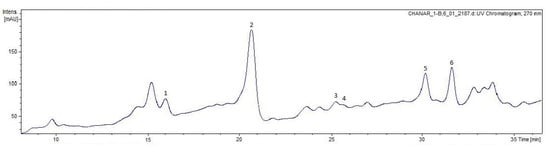
Figure 3.
HPLC-UV270 chromatogram of ethanolic extract of chañar seed flour. The numbers above the peaks correspond to compounds identified in the extract.
The chemical composition of chañar seed flour was very different from the chemical composition of chañar pulp []. While the pulp contained mainly phenolic acid (caffeic acid derivates, protocatechuic acid, vanillic acid) and O glycosyl flavones, the seed with tegument contained condensed tannins and C glycosyl flavones.
2.4. Inhibition of Digestive Enzymes (α-Glucosidase, α-Amylase, and Lipase) by Polyphenolic Extracts Obtained from Seed Flour
Digestive enzymes such as glucosidase, amylase, and lipase are responsible for the degradation of carbohydrates and lipids and, consequently, their absorption in the gastrointestinal tract [,]. Polyphenols can inhibit carbohydrate metabolism enzymes and glucose absorption in the intestine, stimulate insulin secretion, decrease hepatic glucose production, and mitigate endothelial dysfunction, thereby slowing vascular complications [,]. While there are drugs available, which act as inhibitors of these enzymes, they can trigger side effects, such as diarrhea, abdominal discomfort, and flatulence, so phytochemicals as natural compounds could be a good strategy to control glycemic effects [].
The extracts of chañar seed flours from Fernández and Colalao del Valle were able to inhibit both α-glucosidase with IC50 values of 175.24 and 182.73 µg DW (dry weight of extracts)/mL, respectively and α-amylase with IC50 values of 1092.39 and 621.28 µg DW/mL, respectively. The inhibitory effect of the extracts on α-glucosidase did not show significant differences between both localities, while the extracts of flour from Colalao del Valle was more active against α-amylase that Fernández extracts. The values of IC50 were higher than that of the reference compound acarbose (25.0 µg/mL) (see Table 3).

Table 3.
Effect of polyphenols enriched extract of chañar seed flour and reference compounds on enzymes related to carbohydrate metabolism, as well as oxidative processes.
The IC50 values of chañar seed extracts against α-glucosidase (7.7–15 µg GAE/mL) and amylase (48–51 µg GAE/mL) expressed as phenolic compound concentration necessary to produce 50% of enzyme inhibition showed that these polyphenols were less effective than the polyphenols from chañar pulp (IC50 values of 0.68 µg GAE/mL) []. Many studies have shown that vitexin and isovitexin, two C-glucosyl flavonoids present in chañar seed, exert a significant inhibitory effect on α-glucosidase and α-amylase []. Additionally, they can slow starch digestion by interacting with α-amylase. Research has also indicated that dietary supplementation with A-type procyanidins contributes to improved glucose homeostasis []. Phenolic extracts obtained from chañar seed flour showed no inhibitory capacity against lipase, while chañar pulp polyphenolic extract showed inhibitory activity on lipase with activity higher than that reported for white and green tea polyphenols []. Furthermore, previous studies have identified chañar pulp flour polyphenols as a natural source of HMG-CoA reductase inhibitors, an enzyme involved in cholesterol metabolism []. According to these results, we suggest that chañar seed and pulp polyphenols could be consumed together to help reduce blood lipids and carbohydrate.
2.5. Inhibition of Pro-Inflammatory Enzymes
Currently, there is a growing need to identify safer anti-inflammatory compounds for managing chronic inflammatory conditions [], such as natural products that can inhibit enzymes involved in the formation of leukotrienes such as 5-lipoxygenase (5-LOX). Inflammation and oxidative stress are the main causes of metabolic complications in diabetes and obesity. The effect of polyphenol extract from chañar seed flours was measured against the pro-inflammatory enzymes LOX. The IC50 values of the chañar seed extracts of Fernández and Colalao del Valle for LOX were 386.89 and 134.00 μg DW/mL, respectively, equivalents to 17 and 11 μg GAE/mL, respectively (Table 3). The extract obtained with seeds of fruits collected in Colalao del Valle were more active than Fernández. In previous papers, the 5-lipoxygenase potential inhibitory effect of vitexin, a component of polyphenolic extract of chañar seed flour, was demonstrated [].
2.6. The Antioxidant Activity of Polyphenolic Extracts Obtained from Seed Flour
H2O2 is generated by the cellular metabolism in the human body and can produce reactive oxygen radicals like HO•, causing damage to the tissues. Epidemiological studies suggest that antioxidants in the diet can have a beneficial effect on many diseases related to a chronic process [].
The phenolic-compound-enriched preparations obtained from seed flour of chañar from Fernández and Colalao del Valle were shown to be tenfold more active in ABTS•+ scavenging (SC50 0.80 µg GAE/mL) than the chañar pulp (SC50 9 µg GAE/mL) []. These extracts were more active as scavengers of hydroxyl radical (SC50 3.76 and 5.75 μg GAE/mL, respectively) than other native fruits such as mistol (SC50 14.13 μg GAE/mL) []. Comparing the potency as dry weight of extracts obtained from chañar seed flours from both locations, it is evident that the seed extracts from Colalao del Valle have greater antioxidant potential (Table 3). Moreover, due to the role played by reactive oxygen species in inflammatory processes, the free radical scavenging capacity of seed extracts could be relevant to attenuating inflammatory processes.
Both extracts showed the protection capacity of oxidative hemolysis of red blood cells with IC50 values between 0.19 and 0.38 μg GAE/mL (4.32–4.63 μg DW/mL). Ethanolic extracts of chañar seed flour were also able to protect lipids from oxidation in the β-carotene system (IC50 28–35 μg GAE/mL) like the chañar pulp (IC50 23 μg GAE/mL) [].
2.7. Toxicity
Different countries have made considerable progress towards replacing animal testing with alternative models in a very progressive way []. The Artemia salina test has been widely used as a rapid toxicity screening assay, showing a strong correlation with other animal experimental methods. In this work, it was demonstrated that both polyphenolic extracts tested (obtained from chañar seed flours) did not show toxicity towards A. salina, which suggests their potential for safe use. After 24 h of incubation in the presence of the polyphenolic extracts, no alteration was observed in their viability, while the potassium dichromate (positive control) showed a lethal dose 50 (LD50) of 40.00 ± 0.02 μg/mL. Some researchers have conducted comparative studies to assess the correlation between the A. salina assay and conventional toxicity tests in mice [,]. Their findings indicate that an LD50 > 25 μg/mL in the A. salina test corresponds to an in vivo LD50 ranging from 2000 and 5000 mg/kg, aligning with the toxicity thresholds established by the Organization for Economic Cooperation and Development [].
2.8. Microbiological Control
The chañar seed flour meets all the microbiological criteria to ensure their quality: absence of total coliforms, Escherichia coli, Salmonella sp., Staphylococcus, fungi, and yeasts during two years of storage at −18 °C.
3. Materials and Methods
3.1. Chemicals, Reagents, and Materials
Ethanol, phenol, trichloroacetic acid, H2O2, acetone, sodium nitrite, and petroleum ether were purchased from Cicarelli (Santa Fé, Argentina). Folin–Ciocalteau reagent, AlCl3, gallic acid, quercetin, procyanidin B2, β-carotene, linoleic acid, lipooxigenase from Glycine max (LOX), 2,2′-azobis(2-methylpropionamidine) dihydrochloride (AAPH), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), EDTA, 2-thiobarbituric acid, and ascorbic acid were obtained from Sigma-Aldrich (St. Louis, MO, USA). α-Amylase, α-glucosidase, lipase, p-nitrophenyl-α-D-glucopyranoside, p-nitrophenyl palmitate, 4-aminoantipyrine, 2-deoxy-D-ribose, and acarbose were sourced from Sigma-Aldrich (Darmstadt, Germany). FeCl3 was acquired at Biopack (Buenos Aires, Argentina). Amilokit ® was obtained from Wiener Lab Group (Rosario, Argentina, Kit N°. 1504163370). Orlistat was purchased from Elea Laboratory (Buenos Aires, Argentina). Mac Conkey Agar and Molds & Yeasts Agar were supplied by Laboratorios Britania S.A. (Buenos Aires, Argentina). Plate Count Agar was provided by Merck (Darmstadt, Germany). HPLC-grade standards were from Sigma-Aldrich (St. Louis, MI, USA); Fluka Chemical Corp. (Ronkonkoma, NY, USA); and Indofine Chemical Company, Inc. (Hillsborough, NJ, USA).
3.2. Plant Material
G. decorticans ripe fruits were collected by hand from different trees in February 2020 from wild populations in Fernández, Robles Department, Santiago del Estero, Argentina (27°55′ 25.1′′ S; 63°53′36.7′′ W), and Colalao del Valle, Tafí del Valle Department, Tucumán, Argentina (26°35′33.3′′ S; 65°51′25.0′′ W) (Figure 1I). The fruits were transported immediately to the laboratory. Seeds were separated by hand from fruits and dried at 40 °C in a forced air stove. The moisture content (10%) was determined by measuring the weight difference between the fresh sample and the sample dried at 40 °C until a constant weight. The seed were ground to obtain flour in a high-speed universal disintegrator (Arcano, Argentina). The flour was vacuum packed into oxygen-barrier bags (Multivac, DZ-400, China) and stored at −20 °C. The analysis was performed immediately. The voucher specimen was deposited in the INBIOFIV collection (INBIO 105). The details of fruits, seeds, and flour are shown in Figure 1II.
3.3. Determination of Granulometry and Colour of Flour
The flour was passed through four sieves of 840, 500, 149, and 105 µm, and each fraction was weighed. The test was carried out in triplicate []. Chromatic parameters were assessed using a Chroma meter NR110 (3NH Technology Co., Ltd., Zengcheng, China) colorimeter, following the CIELab system. The chromaticity coordinates L*, a*, and b* (objective parameter) were used to express the results. The sample’s luminance is represented by the L* coordinate, which ranges from 0 to 100. The parameter a* represents the contribution of green or red, while b* indicates the contribution of blue or yellow.
3.4. Determination of Chemical Composition
3.4.1. Sugars, Proteins, and Fats
Each flour sample (1 g) was exhaustively extracted over three cycles using 80% aqueous ethanol (1 g:4 mL) at 75 °C for 10 min. The extracts were then filtered under reduced pressure through Whatman No. 4 filter paper and subsequently combined. Glucose, fructose, sucrose, and total neutral and reducing sugars were quantified according to Costamagna et al. (2013) using spectrophotometric methods []. Total neutral and reducing sugars were determined by the phenol-sulphuric acid and the Somogyi Nelson method, respectively, with results expressed as g glucose/100 g flour. Total lipid content (991.20) and protein content (N × 6.25) (922.06) were analyzed following the AOAC official method [] (AOAC, 1990) using seed flour. The results were expressed in g/100 g flour.
3.4.2. Dietary Fiber
Crude (962.09) and dietary (991.43) fiber contents were determined according to the AOAC method [,].
3.4.3. Fatty Acid Analysis
Fatty acids of seed flour were determined according to the AOAC, 1990 method (969.33) []. Both seed flours (2 g) were digested with HCl 8.3 M and heated at 80 °C for 40 min. The lipids were extracted with ethyl ether and petroleum ether in multiple cycles, then filtered and evaporated to dryness. Fatty acids were turned into fatty acid methyl esters (FAME) by the addition of methanolic NaOH and boron trifluoride reagent, followed by heating and the addition of n-heptane. The methyl ethers were finally analyzed by GC-IT/MS (gas chromatograph, Clarus® 680 GC; PerkinElmer, Hopkinton, MA, USA) under the following conditions: ELITE WAX column (ID: 0.32 mm; length: 30 m DF: 0.25 µm), hydrogen as a carrier gas, column temperature starting at 125 °C with a gradual increase of 5 °C/min, feeder temperature set at 220 °C, injection temperature at 200 °C, and flame ionization detector (FID) temperature at 300 °C.
3.4.4. Polyphenolic Extract Preparation and Total Polyphenols and Flavonoids Determination
Flour (20 g) was extracted four times with 200 mL ethanol/water 70:30 v/v in an ultrasonic bath at 70 W power for 30 min at 25 °C. The temperature was maintained with an ice bath. Each extracted material was filtered through Whatman No. 4 filter paper at reduced pression. Then, the combined extracts were evaporated, and the aqueous fraction was frozen at −80 °C before being lyophilized. Dry extracts were stored frozen at −20 °C until analysis. Total polyphenols were determined using Folin–Ciocalteau reagent [], with results expressed as mg gallic acid equivalents (mg GAE)/100 g of seed flour. Flavonoid content was measured following the report by Costamagna et al. (2013) [] using AlCl3 and NaNO2. Results were expressed as mg quercetin equivalents (QE)/100 g of seed flour. In both determinations, the absorbance was recorded with a UV–visible spectrophotometer (Jasco v-630, Thermo Fisher Scientific, Tokyo, Japan).
3.4.5. Condensed and Hydrolysable Tannins
The content of total proanthocyanidins was determined with 4-dimethylaminocinnamaldehyde (DMAC) solution (0.1% in acidified ethanol) []. Absorbance was measured at 640 nm with a UV–visible spectrophotometer (Jasco v-630, Thermo Fisher Scientific, Tokyo, Japan) after 20 min at 25 °C. Results were expressed as mg of procyanidin B2 equivalents per 100 g of seed flour (mg EPB2/100 g seed flour). Hydrolysable tannins were quantified following the method described by Costamagna et al. (2013) []. The samples were hydrolyzed using 2 N H2SO4 at 100 °C for 26 h, and the gallic acid released was determined using rhodamine (0.667% in methanol). Absorbance was recorded at 520 nm using a UV–visible spectrophotometer (Jasco v-630, Thermo Fisher Scientific, Tokyo, Japan). Results were expressed as mg gallic acid equivalent (GAE)/100 g of seed flour.
3.4.6. Identification of Phenolic Compounds
The polyphenolic extracts obtained according to 3.4.4 were analyzed by the HPLC-DAD and HPLC-MS/MS method according to the methodology described by Pérez et al., 2018 []. An Agilent Technologies (Inc., Santa Clara, CA, USA) 1200 Series UPLC system was used, equipped with a gradient pump (Agilent G1312B SL Binary), solvent degasser (Agilent G1379B), and autosampler (Agilent G1367 DSL+WP). Chromatographic separation was achieved on a AXBridgeTM C18 column (4.6 × 150 mm, 5 μm; Waters Corporation, Milford, MA, USA) using a linear gradient solvent system consisting of 0.1% acetic acid in water (A) and 0.1% acetic acid in MeOH (B). The gradient program was as follows: 90% to 43% A over 45 min, followed by 43% to 0% A from 45 to 60 min, maintaining 0% A for 5 min at 35 °C. The flow rate was set at 0.4 mL/min, and the injection volume was 40 μL. Compounds were monitored at 254 and 330 nm, while UV spectra from 200 to 600 nm were recorded for peak characterization using a photodiode array detector (Agilent G1315 C Starligth). The HPLC system was connected subsequently to a QTOF mass spectrometer (microTOF-QII Series, Bruker, Billerica, MA, USA) equipped with an electro spray ionization (ESI) source. Mass spectra were recorded in a negative ion mode over an m/z range of 50 to 1000. Ionization was performed at 4500 V, with nitrogen as a nebulizer gas (4.0 bar) and drying gas (200 °C, 8.0 L/min). Argon was used as the collision gas. The MS detector was programmed to perform MS and alternate MS/MS for the three most abundant ions, with a collision energy of 12 eV. Data acquisition and processing were carried out using Compass Version 3.1 and Data Analysis Version 4.0 software, respectively (Bruker Daltonics, MA, USA). The identification of phenolic compounds was carried out by comparison with reference compound spectral properties (UV and ESI-MS and MS/MS).
3.4.7. Carotenoid
Flour (1 g) was extracted using 10 mL of petroleum ether/acetone (1:1, v/v). The total carotenoid content was determined according to Costamagna et al. (2013) []. The β-carotene absorbance was measured at 450 nm using a UV–visible spectrophotometer (Jasco v-630, Thermo Fisher Scientific, Tokyo, Japan), and results were expressed as g of β-carotene equivalents (g β-CE)/100 g of seed flour.
3.5. Measurement of Antioxidant Capacity
The antioxidant activity of polyphenolic extracts obtained according to Section 3.4.4. was determined by different methodologies.
3.5.1. ABTS•+ Free Radical Scavenging Activity
The antioxidant activity of the chañar seed extracts was evaluated using a modified version of the ABTS•+ assay []. The ABTS radical cation was produced by incubating a 7 mM solution of ABTS in the presence of 2.45 mM potassium persulfate. This mixture was left to react at room temperature for 16 h to ensure complete radical formation. The resulting ABTS•+ solution was then diluted in ethanol until reaching an absorbance of 0.700 ± 0.02 at 734 nm. To assess radical scavenging activity, 100 µL of the diluted ABTS•+ solution was combined with varying concentrations of the extract (1–30 μg GAE/mL). Absorbance was measured at 734 nm using a microplate reader (Thermo Scientific Multiskan GO, Finland) after a reaction time of 6 min. Results are shown as SC50 values (μg GAE/mL or μg DW (dry weight of extract)/mL required to scavenge 50% ABTS•+ free radicals).
3.5.2. Hydroxyl Radical Scavenging
To evaluate the ability to scavenge hydroxyl radical, the technique described by Chobot (2010) [] was followed. The reaction mixture contained phenolic enriched extract (2.00–8.81 μg GAE/mL); 50 μL of 10.4 mM 2-deoxy-D-ribose; and 50 μL of 50 μM FeCl3, with and without 50 μL of 52 μM EDTA. Subsequently, 50 μL of 10 mM H2O2 and 50 μL of 1.0 mM ascorbic acid were introduced to initiate the reaction. The mixture was maintained at 37 °C for 60 min, after which 500 μL of 1% (w/v) 2-thiobarbituric acid prepared in 3% (w/v) trichloroacetic acid was added, followed by heating at 100 °C for 20 min. Absorbance measurements were taken at 532 nm using a Jasco V-630 UV/Vis spectrophotometer (Thermo Fisher Scientific, Tokyo, Japan). Results are presented as SC50 values in μg GAE/mL or μg DW/mL required to inhibit by 50% the degradation of 2-deoxy-D-ribose.
3.5.3. Protection of Lipids Against Oxidative Damage: β-Carotene Bleaching Test
The antioxidant potential of the extracts was assessed using the β-carotene bleaching method, following the procedure described by Ordoñez et al. (2006) []. A β-carotene emulsion (0.2 mg/mL) was prepared, and 1 mL of this solution was mixed with varying concentrations of both extracts (up to 50 μg GAE/mL). The mixture was incubated at 50 °C, and the degradation of β-carotene was monitored by measuring absorbance a at 470 nm using a UV–visible spectrophotometer (Jasco V-630, Thermo Fisher Scientific, Tokyo, Japan) for 120 min. The IC50 value, representing the extract concentration (in μg GAE/mL or μg DW/mL) required to inhibit 50% of β-carotene oxidation, was determined.
3.5.4. H2O2 Scavenging Assay
The capacity of phenolic extracts to scavenge hydrogen peroxide was measured according to Pérez et al. (2018) []. The mixture containing phenolic extract (2–50 µg GAE/mL), H2O2 (0.7 mM), and horseradish peroxidase (1 U/mL) was incubated for 3 min at 37 °C. Then, a solution of phenol (12 mM) and 4-aminoantipyrine (0.5 mM) was added to the mixture. The absorbance of reaction was measured at 504 nm (Spectrophotometer Jasco v-630, Thermo Fisher Scientific, Tokyo, Japan). The concentration (µg GAE/mL or μg DW/mL) required to scavenge 50% of H2O2 was used to obtain the SC50 values. As controls, quercetin and ascorbic acid were employed.
3.5.5. Protection Against Oxidative Hemolysis
Protection against oxidative hemolysis was determined by Mendes et al., 2011 []. Phenolic extracts (0.08–0.56 µg GAE/mL), AAPH reagent dissolved in PBS at a final concentration of 50 mM, and red blood cell (RBC) suspension at 2% were incubated for 1 h at 37 °C []. The reaction mixture was centrifuged (4000× g for 3 min) to separate the erythrocytes. The percentage of hemolysis was then determined by measuring the supernatant’s absorbance (545 nm). The IC50 values were determined as the polyphenol concentration or dry weight of extract necessary to protect the RBC from oxidative hemolysis at 50%. Quercetin served as a reference substance. The RBC used in the assays were discarded material from a pool of samples donated by the clinical analysis laboratory of the National University of Tucumán. This protocol was approved by the institutional ethics committee (INBIOFIV ethic committee).
3.6. Inhibitory Activity of Enzymes Related to Metabolic Syndrome
The enzymatic reaction was carried out with and without different concentrations of polyphenolic extracts from chañar seed flour. Controls of substrate, enzyme, and solvent were carried out.
3.6.1. α-Glucosidase Inhibition
The α-glucosidase inhibition assay was conducted following the methodology described by Costamagna et al. (2016) [], with slight modifications. The reaction system consisted of α-glucosidase enzyme (0.0273U) and phenolic seed extract (2–100 µg GAE/mL), which were pre-incubated in 160 μL of 0.1 M sodium phosphate buffer (pH 6.9) at 4 °C for 10 min. The enzymatic reaction was initiated by adding 5 μL of 25 mM p-nitrophenyl α-D-glucopyranoside. The reaction mixture was then incubated at 37 °C for 15 min, after which 80 μL of 0.2 M sodium carbonate was introduced to stop the reaction. The absorbance was recorded at 405 nm using a microplate reader. The IC50 values denote the concentration of phenolic extract (μg GAE/mL) or μg DW/mL required to inhibit the enzyme by 50%. Acarbose was used as a positive control.
3.6.2. α-Amylase Inhibition
The α-amylase inhibitory potential was assessed using starch as a substrate, based on the method described by Costamagna et al. (2016) []. The assay was conducted with Amilokit. The reaction system included 800 µL of 0.01 M sodium phosphate buffer (pH 7.4), 5 µL of α-amylase, and polyphenolic extract from seed flour (2–100 µg GAE/mL). Prior to enzyme activation, the mixture was pre-incubated at 4 °C for 5 min. The reaction was initiated by introducing 500 µL of reagent A (starch-based substrate), followed by incubation at 37 °C for 7 min. To develop the colorimetric reaction, 500 µL of reagent B (iodine solution) was added, and the total volume was adjusted to 5.8 mL with distilled water. Absorbance readings were taken at 640 nm using a Jasco v-630 spectrophotometer (Thermo Fisher Scientific, Tokyo, Japan). The IC50 values, which represent the concentration of phenolic enriched extract (µg GAE/mL) or μg DW/mL required to achieve 50% enzyme inhibition, were determined. Acarbose was included as a standard inhibitory control.
3.6.3. Lipase Inhibition
The enzymatic activity of lipase was determined by assessing the hydrolysis of p-nitrophenyl palmitate into p-nitrophenol, following the protocol outlined by Costamagna et al. (2016) []. IC50 values were expressed as the concentration of phenolic enriched extract (µg GAE/mL) or μg DW/mL required to inhibit 50% of the enzyme activity. Orlistat was included as a reference inhibitor. A solution of lipase (1.0 mg/mL) was incubated with the polyphenolic extract (2–100 µg GAE/mL) at 4 °C for 5 min before initiating the reaction. Then, it was added to the reaction mixture that contained 330 μL of 0.1 M sodium phosphate buffer 0.1 M (pH 7) supplemented with 0.6% (w/v) Triton X-100, 0.15% (w/v) arabic gum, and 20 μL of 10 mM p-nitrophenyl palmitate. The reaction proceeded at 37 °C for 20 min, and the absorbance was recorded at 400 nm using a BiotekELx808 microplate reader.
3.7. Inhibition of Pro-Inflammatory Enzymes
Lipoxygenase
The anti-inflammatory activity of the extracts was assessed by evaluating their ability to inhibit the lipoxygenase enzyme (LOX) []. This assay is based on the enzymatic oxidation of linoleic acid to its corresponding hydroperoxide. LOX (948 U/mL) and linoleic acid (the substrate, 50 μM in sodium borate buffer 200 mM, pH 9.0) were used to measure the concentration of lipid hydroperoxides generated. The reaction mixture was incubated at 25 °C for 4 min, and absorbance at 234 nm was recorded as a function of time for 3 min. The concentration of phenolic compounds or μg DW (dry weight of extract) required to achieve 50% inhibition of hydroperoxide formation (IC50) was determined from the concentration–inhibition response curve using regression analysis. A molar extinction coefficient of 25 mM−1 cm−1 was applied for hydroperoxide quantification. Naproxen was used as the reference compound.
3.8. Microbiological Stability of Chañar Flour
The numbers (CFU/g flour) of viable aerobic microorganisms, enterobacteria, fungi and yeasts were determined in flour kept at −18 °C for two years by the serial dilution method. Briefly, each sample was homogenized with 9 mL of sterile physiological solution. From this suspension, serial dilutions were made and 100 µL of each dilution was plated on different culture media (Plate Count Agar, Mac Conkey Agar, and Molds & Yeasts Agar). Suitable aliquots obtained were taken at different times and inoculated in the culture media. The number of CFU/g flour was determined at 0 (initial time) and 1, 2, and 3 months [].
3.9. Acute Toxicity Using an Artemia Salina Test
To determine the acute toxicity levels of the extracts (12.5–50 µg GAE/mL), Artemia salina was used as a test organism [,]. After 24 h, the number of shrimps that remained still was recorded, and the mortality rate for each concentration was determined. As a reference drug, potassium dichromate (10–40 mg/mL) was utilized. Negative controls with distilled water were also assayed.
3.10. Statistical Analysis
The data were provided as mean ± standard deviation (SD), and all analyses were performed in triplicate. Statistical analysis was performed by one-way ANOVA followed by Tukey’s multiple comparison test (p < 0.05) using InfoStat (Student Version, 2015; Di Rienzo et al., 2015) [].
4. Conclusions
Geoffroea decorticans is traditionally regarded as a tree that promotes health and energy, with its fruits, leaves, and bark commonly used for medicinal purposes. To date, research has primarily focused on the edible fraction of the fruit (pulp). In this study, the nutritional and functional properties of chañar seed flour were evaluated, highlighting its potential applications. The chemical composition of chañar seed flour differed significantly from that of the pulp. While the pulp primarily contained phenolic acids (caffeic acid derivatives, protocatechuic acid, and vanillic acid) and O-glycosyl flavones, the seeds with tegument were rich in condensed tannins and C-glycosyl flavones, which provides bitterness and astringency. The polyphenolic extracts obtained from the seeds could serve as functional food ingredients or food additives with antioxidant and anti-inflammatory properties, as well as inhibitors of enzymes linked to carbohydrate and lipid metabolism. Furthermore, the fiber, protein, and lipid content and low-carbohydrate content in seed flour make it a valuable food ingredient. These findings could support regional development in arid and vulnerable areas through promoting the conservation of chañar forests, including sustainable development and management for commercial purposes.
Author Contributions
Conceptualization, M.I.I., M.A.R., E.A.M., I.F.R., M.A.M., A.R. and I.C.Z.; methodology, M.I.I., M.A.R., E.A.M., A.R., M.A.M., I.F.R. and I.C.Z.; validation, M.I.I. and M.A.R.; formal analysis, experiments performed: M.I.I., M.A.R., E.A.M., A.R., M.A.M., I.F.R. and I.C.Z.; investigation, experiments performed: M.I.I., M.A.R., E.A.M., A.R., I.F.R., M.A.M. and I.C.Z.; analyzed and interpreted the data M.I.I., M.A.R., E.A.M., A.R., I.F.R., M.A.M. and I.C.Z.; resources, M.I.I. and I.C.Z.; data curation, M.A.R.; writing—original draft preparation, M.I.I. and M.A.R.; writing—review and editing, M.I.I., M.A.R., E.A.M., A.R., I.F.R., M.A.M. and I.C.Z.; supervision, M.I.I.; project administration, M.I.I.; funding acquisition, M.I.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Universidad Nacional de Tucumán (PIUNT 2018-G637 Project), Agencia Nacional de Promoción Científica y Técnica (ANPCyT PICT 2020-3619; PICT-2021-CAT-II-00132), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET-PUE 2018-0011), Fundación Williams 2023. Fondos complementarios para proyectos de investigación con impacto en el territorio argentino and Biolates network “P320RT0186—Sustainable use of Ibero-American vegetable biomass resources in cosmetics” (CYTED).
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors would like to thank Universidad Nacional de Tucumán, Consejo Nacional de Investigaciones Científicas y Técnicas, Ministerio de Ciencia y Técnica, CYTED network “P320RT0186—Sustainable use of Ibero-American vegetable biomass” (BIOLATES) and Fundación Williams 2023. Fondos complementarios para proyectos de investigación con impacto en el territorio argentino. The collection of plant material was carried out according to the Nagoya protocol. The language of the manuscript was checked by Luisa Montivero.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Burkart, A. Las Leguminosas Argentinas, Silvestres y Cultivadas; Acme Agency SRI: Buenos Aires, Argentina, 1952; p. 569. [Google Scholar]
- Saur Pa Figueroa, G.G.; Dantas, M. Recolección, procesamiento y consumo de frutos silvestres en el noroeste semiárido argentino. Casos actuales con implicancias arqueológicas. Zaranda Ideas 2006, 2, 35–50. [Google Scholar]
- Saur Palmieri, V.; Trillo, C.; López, M.L. Rasgos diagnósticos en frutos y residuos secos de la cocción de chañar (Geoffroea decorticans, Fabaceae) para identificar prácticas poscolecta. Intersecc. Antropol. 2019, 20, 167–180. [Google Scholar]
- Deng, G.-F.; Shen, C.; Xu, X.-R.; Kuang, R.-D.; Guo, Y.-J.; Zeng, L.-S.; Gao, L.-L.; Lin, X.; Xie, J.-F.; Xia, E.-Q.; et al. Potential of fruit wastes as natural resources of bioactive compounds. Int. J. Mol. Sci. 2012, 13, 8308–8323. [Google Scholar] [CrossRef] [PubMed]
- Costamagna, M.S.; Ordoñez, R.M.; Zampini, I.C.; Sayago, J.E.; Isla, M.I. Nutritional and antioxidant properties of Geoffroea decorticans, an Argentinean fruit, and derived products (flour, arrope, decoction and hydroalcoholic beverage). Food Res. Int. 2013, 54, 160–168. [Google Scholar] [CrossRef]
- Costamagna, M.S.; Zampini, I.C.; Alberto, M.R.; Cuello, A.S.; Torres, S.; Pérez, J.; Quispe, C.; Schmeda-Hirschmann, G.; Isla, M.I. Polyphenols rich fraction from Geoffroea decorticans fruits flour affects key enzymes involved in metabolic syndrome, oxidative stress and inflammatory process. Food Chem. 2016, 190, 392–402. [Google Scholar] [CrossRef]
- Somaini, G.C.; Aybar, M.J.; Vera, N.R.; Tríbulo, C. Geoffroea decorticans fruit extracts inhibit the wnt/β-catenin pathway, a therapeutic target in cancer. Biochem. Biophys. Res. Commun. 2021, 546, 118–123. [Google Scholar] [CrossRef]
- Reynoso, M.A.; Vera, N.; Aristimuño, M.E.; Daud, A.; Sánchez Riera, A. Antinociceptive activity of fruits extracts and “arrope” of Geoffroea decorticans (Chañar). J. Ethnopharm. 2013, 145, 355–362. [Google Scholar] [CrossRef]
- Lamarque, A.L.; Maestri, D.M.; Zygadlo, J.A.; Guzmán, C.A. Chemical evaluation of Geoffroea decorticans seeds as source of oil and protein. Grasas y Aceites 2000, 51, 241–243. [Google Scholar] [CrossRef]
- Maestri, D.M.; Fortunato, R.H.; Greppi, J.A.; Lamarque, A.L. Compositional Studies of seeds and fruits from two varieties of Geoffroea decorticans. J. Food Compos. Anal. 2001, 14, 585–590. [Google Scholar] [CrossRef]
- Orrabalis, C. Geoffroea Decorticans (Chañar), de la Región Fitogeográfica de la Provincia de Formosa. Ph.D. Thesis, Universidad Nacional de Córdoba, Córdoba, Argentina, 2014. [Google Scholar]
- Masson Salaue, L. Semillas de Frutos Nativos y Cultivados en Chile: Su Aceite Como Fuente de Compuestos Nacionales. Ph.D. Thesis, Universidad Complutense de Madrid, Madrid, Spain, 2012. [Google Scholar]
- Cotabarren, J.; Broitman, D.J.; Quiroga, E.; Obregón, W.D. GdTI, the first thermostable trypsin inhibitor from Geoffroea decorticans seeds. A novel natural drug with potential application in biomedicine. Int. J. Biol. Macromol. 2020, 148, 869–879. [Google Scholar] [CrossRef]
- Silva, A.P.; Cordeiro, M.L.d.S.; Aquino-Martins, V.G.d.Q.; Melo, L.F.d.M.; Paiva, W.d.S.; Naliato, G.F.d.S.; Theodoro, R.C.; Meneses, C.H.S.G.; Rocha, H.A.O.; Scortecci, K.C. Prospecting of the Antioxidant Activity from Extracts Obtained from Chañar (Geoffroea decorticans) Seeds Evaluated In Vitro and In Vivo Using the Tenebrio molitor Model. Nutrients 2024, 16, 2813. [Google Scholar] [CrossRef] [PubMed]
- Santibáñez, C.; Vargas, M. Geoffroea decorticans for Biofuels: A promising feedstock. J. Renew. Energy 2017, 2017, 4216175. [Google Scholar] [CrossRef]
- Cazar Villacís, I.M. Análisis Físico-Químico Para la Determinación de la Calidad de las Frutas. Thesis, Pontificia Universidad Catolica del Ecuador, Quito, Ecuador, 2016. [Google Scholar]
- Argentine Food Code. Cap. IX. Article 694 (Res 794 del 13/12/1994). Available online: http://www.conal.gob.ar/CAA.php (accessed on 1 March 2025).
- Orqueda, M.E.; Zampini, I.C.; Torres, S.; Alberto, M.R.; Pino Ramos, L.L.; Schmeda-Hirschmann, G.; Isla, M.I. Chemical and functional characterization of skin, pulp and seed flour from the Argentine native fruit mistol (Ziziphus mistol). Effects of phenolic fractions on key enzymes involved in metabolic syndrome and oxidative stress. J. Funct. Foods 2017, 37, 531–540. [Google Scholar] [CrossRef]
- Naranjo, P. Importantes alimentos aborígenes. Archipielago. Rev. Cult. Nuestra América 2010, 16, 58. [Google Scholar]
- Zhang, J.; Wang, X.; Lu, Y.; Bhusal, S.J.; Song, Q.; Cregan, P.B.; Yen, Y.; Brown, M.; Jiang, G.-L. Genome-wide scan for seed composition provides insights into soybean quality improvement and the impacts of domestication and breeding. Mol. Plant 2018, 11, 460–472. [Google Scholar] [CrossRef]
- Zhang, Z.-S.; Kang, Y.-J.; Che, L. Composition and thermal characteristics of seed oil obtained from Chinese Amaranth. LWT Food Sci. Technol. 2019, 111, 39–45. [Google Scholar] [CrossRef]
- Beloshapka, A.N.; Buff, P.R.; Fahey, G.C.; Swanson, K.S. Compositional Analysis of Whole Grains, Processed Grains, Grain Co-Products, and Other Carbohydrate Sources with Applicability to Pet Animal Nutrition. Foods 2016, 5, 23. [Google Scholar] [CrossRef]
- Repo-Carrasco-Valencia, R.A.-M.; Serna, L.A. Quinoa (Chenopodium quinoa, Willd.) as a source of dietary fiber and other functional components. Food Sci. Technol. 2011, 31, 225–230. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, H.; Cao, X.; Wang, J. Preparation and modification of high dietary fiber flour: A review. Food Res. Int. 2018, 113, 24–35. [Google Scholar] [CrossRef]
- Cattaneo, F.; Costamagna, M.; Zampini, I.; Sayago, J.; Alberto, M.; Chamorro, V.; Pazos, A.; Thomas-Valdés, S.; Schmeda-Hirschmann, G.; Isla, M. Flour from Prosopis alba cotyledons: A natural source of nutrient and bioactive phytochemicals. Food Chem. 2016, 208, 89–96. [Google Scholar] [CrossRef]
- Awika, J.M.; Duodu, K.G. Bioactive polyphenols and peptides in cowpea (Vigna unguiculata) and their health promoting properties: A review. J. Funct. Foods 2017, 38, 686–697. [Google Scholar] [CrossRef]
- Patanè, G.T.; Putaggio, S.; Tellone, E.; Barreca, D.; Ficarra, S.; Maffei, C.; Calderaro, A.; Laganà, G. Catechins and proanthocyanidins involvement in metabolic syndrome. Int. J. Mol. Sci. 2023, 24, 9228. [Google Scholar] [CrossRef] [PubMed]
- Kliebenstein, D.J. Is specialized metabolite regulation specialized? J. Exp. Bot. 2023, 74, 4942–4948. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhao, L.; Wang, K.; Renard, C.M.G.C.; Le Bourvellec, C.; Hu, Z.; Liu, X. A-type proanthocyanidins: Sources, structure, bioactivity, processing, nutrition, and potential applications. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13352. [Google Scholar] [CrossRef]
- Lin, L.-C.; Kuo, Y.-C.; Chou, C.-J. Immunomodulatory Proanthocyanidins from Ecdysanthera utilis. J. Nat. Prod. 2002, 65, 505–508. [Google Scholar] [CrossRef]
- Lu, Z.; Jia, Q.; Wang, R.; Wu, X.; Wu, Y.; Huang, C.; Li, Y. Hypoglycemic activities of A- and B-type procyanidin oligomer-rich extracts from different cinnamon barks. Phytomedicine 2010, 18, 298–302. [Google Scholar] [CrossRef]
- Howell, A.B.; Reed, J.D.; Krueger, C.G.; Winterbottom, R.; Cunningham, D.G.; Leahy, M. A-type cranberry proanthocyanidins and uropathogenic bacterial anti-adhesion activity. Phytochemistry 2005, 66, 2281–2291. [Google Scholar] [CrossRef]
- Anderson, R.A.; Broadhurst, C.L.; Polansky, M.M.; Schmidt, W.F.; Khan, A.; Flanagan, V.P.; Scgoene, N.W.; Graves, D.F. Isolation and characterization of polyphenol type-a polymers from cinnamon with insulin-like biological Activity. J. Agric. Food Chem. 2003, 52, 65–70. [Google Scholar] [CrossRef]
- Appeldoorn, M.M.; Sanders, M.; Vincken, J.-P.; Cheynier, V.; Le Guernevé, C.; Hollman, P.C.; Gruppen, H. Efficient isolation of major procyanidin A-type dimers from peanut skins and B-type dimers from grape seeds. Food Chem. 2009, 117, 713–720. [Google Scholar] [CrossRef]
- Tarascou, I.; Mazauric, J.-P.; Meudec, E.; Souquet, J.-M.; Cunningham, D.; Nojeim, S.; Cheynier, V.; Fulcrand, H. Characterisation of genuine and derived cranberry proanthocyanidins by LC–ESI-MS. Food Chem. 2011, 128, 802–810. [Google Scholar] [CrossRef]
- Cattaneo, F.; Rodríguez, I.F.; Zampini, I.C.; Burgos-Edwards, A.; Schmeda-Hirschmann, G.; Isla, M.I. Neltuma nigracotyledon and seed flour: Nutritional, phytochemical, and techno-functional characterization and nutraceutic potential of polyphenolic enriched extract. Food Funct. 2024, 15, 9446–9456. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.J.; Zampini, I.C.; Alberto, M.R.; Isla, M.I. Prosopis nigra Mesocarp Fine Flour, A Source of Phytochemicals with Potential Effect on Enzymes Linked to Metabolic Syndrome, Oxidative Stress, and Inflammatory Process. J. Food Sci. 2018, 83, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- Courts, F.L.; Williamson, G. The occurrence, fate and biological activities ofc-glycosyl flavonoids in the human diet. Crit. Rev. Food Sci. Nutr. 2015, 55, 1352–1367. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Min, J.-W.; Kong, W.-L.; He, X.-H.; Li, J.-X.; Peng, B.-W. A review on the pharmacological effects of vitexin and isovitexin. Fitoterapia 2016, 115, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Capanoglu, E.; Jassbi, A.R.; Miron, A. Advance on the FlavonoidC-glycosides and Health Benefits. Crit. Rev. Food Sci. Nutr. 2016, 56, 29–45. [Google Scholar] [CrossRef]
- Nazarian-Samani, Z.; Sewell, R.D.E.; Lorigooini, Z.; Rafieian-Kopaei, M. Medicinal Plants with Multiple Effects on Diabetes Mellitus and Its Complications: A Systematic Review. Curr. Diabetes Rep. 2018, 18, 72. [Google Scholar] [CrossRef]
- Santhakumar, A.B.; Battino, M.; Alvarez-Suarez, J.M. Dietary polyphenols: Structures, bioavailability and protective effects against atherosclerosis. Food Chem. Toxicol. 2018, 113, 49–65. [Google Scholar] [CrossRef]
- Silva, C.; Sampaio, G.; Freitas, R.; Torres, E. Polyphenols from guaraná after in vitro digestion: Evaluation of bioacessibility and inhibition of activity of carbohydrate-hydrolyzing enzymes. Food Chem. 2018, 267, 405–409. [Google Scholar] [CrossRef]
- Spínola, V.; Castilho, P.C. Evaluation of Asteraceae herbal extracts in the management of diabetes and obesity. Contribution of caffeoylquinic acids on the inhibition of digestive enzymes activity and formation of advanced glycation end-products (In Vitro). Phytochemistry 2017, 143, 29–35. [Google Scholar] [CrossRef]
- Li, X.; Wu, Q.; Sui, Y.; Li, S.; Xie, B.; Sun, Z. Dietary supplementation of A-type procyanidins from litchi pericarp improves glucose homeostasis by modulating mTOR signaling and oxidative stress in diabetic ICR mice. J. Funct. Foods 2018, 44, 155–165. [Google Scholar] [CrossRef]
- Costa, C.; Tsatsakis, A.; Mamoulakis, C.; Teodoro, M.; Briguglio, G.; Caruso, E.; Tsoukalas, D.; Margina, D.; Dardiotis, E.; Kouretas, D.; et al. Current evidence on the effect of dietary polyphenols intake on chronic diseases. Food Chem. Toxicol. 2017, 110, 286–299. [Google Scholar] [CrossRef]
- Ibrahim, S.R.; Mohamed, G.A.; Alshali, K.Z.; Al Haidari, R.A.; El-Kholy, A.A.; Zayed, M.F. Lipoxygenase inhibitors flavonoids from Cyperus rotundus aerial parts. Rev. Bras. Farm. 2018, 28, 320–324. [Google Scholar] [CrossRef]
- de Ávila, R.I.; Valadares, M.C. Brazil moves toward the replacement of animal experimentation. ATLA-Altern Lab Anim. 2019, 47, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, S.; Kwan, Y.P.; Latha, L.Y.; Rajeh, M.A.B.; Zakaria, Z.; Jothy, S.L. Acute toxicity impacts of Euphorbia hirta L extract on behavior, organs body weight index and histopathology of organs of the mice and Artemia salina. Pharmacogn. Res. 2012, 4, 170–177. [Google Scholar] [CrossRef]
- Parra, A.L.; Yhebra, R.S.; Sardiñas, I.G.; Buela, L.I. Comparative study of the assay of Artemia salina L. and the estimate of the medium lethal dose (LD50 value) in mice, to determine oral acute toxicity of plant extracts. Phytomedicine 2001, 8, 395–400. [Google Scholar] [CrossRef]
- OECD. Guideline for Testing of Chemicals: Acute Oral Toxicity-Fixed Dose Procedure, No. 420. 2001. Available online: https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/oecd/oecd_gl420.pdf (accessed on 1 December 2024).
- Rodriguez, I.F.; Pérez, M.J.; Cattaneo, F.; Zampini, I.C.; Cuello, A.S.; Mercado, M.I.; Ponessa, G.; Isla, M.I. Morphological, histological, chemical and functional characterization of Prosopis alba flours of different particle sizes. Food Chem. 2018, 274, 583–591. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC: Method 969.33. Fatty Acids in Oils and Fats. Preparation of Methyl Esters. Boron Trifluoride Method/AOAC IUPAC Method, 13th ed.; AOAC International: Rockville, MD, USA, 1990. [Google Scholar]
- AOAC. Association of official analytical chemists. In Official Methods of Analysis, 18th ed.; AOAC: Gaithersburg, MD, USA, 1990. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC: Method 991.43. Total Soluble, and Insoluble Dietary Fiber in Foods. Enzymatic-Gravimetric Method, MES-TRIS Buffer; Official Methods of Analysis Association: Arlington, TX, USA, 1992. [Google Scholar]
- Carabajal, M.P.; Isla, M.I.; Borsarelli, C.D.; Zampini, I.C. Influence of In Vitro gastro-duodenal digestion on the antioxidant activity of single and mixed three “Jarilla” species infusions. J. Herb. Med. 2020, 19, 100296. [Google Scholar] [CrossRef]
- Chobot, V. Simultaneous detection of pro- and antioxidative effects in the variants of the deoxyribose degradation assay. J. Agric. Food Chem. 2010, 58, 2088–2094. [Google Scholar] [CrossRef]
- Ordoñez, A.A.L.; Gomez, J.D.; Vattuone, M.A.; Lsla, M.I. Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem. 2006, 97, 452–458. [Google Scholar] [CrossRef]
- Mendes, L.; de Freitas, V.; Baptista, P.; Carvalho, M. Comparative antihemolytic and radical scavenging activities of strawberry tree (Arbutus unedo L.) leaf and fruit. Food Chem. Toxicol. 2011, 49, 2285–2291. [Google Scholar] [CrossRef]
- Torres-Carro, R.; Isla, M.I.; Thomas-Valdes, S.; Jiménez-Aspee, F.; Schmeda-Hirschmann, G.; Alberto, M.R. Inhibition of pro-inflammatory enzymes by medicinal plants from the Argentinean highlands (Puna). J. Ethnopharmacol. 2017, 205, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Uriburu, F.M.C.; Zampini, I.C.; Maldonado, L.M.; Mattson, M.G.; Salvatori, D.; Isla, M.I. Chemical Characterization and Biological Activities of a Beverage of Zuccagnia punctata, an Endemic Plant of Argentina with Blueberry Juice and Lemon Honey. Plants 2024, 13, 3143. [Google Scholar] [CrossRef] [PubMed]
- Svensson, B.-M.; Mathiasson, L.; Mårtensson, L.; Bergström, S. Artemia salina as test organism for assessment of acute toxicity of leachate water from landfills. Environ. Monit. Assess. 2005, 102, 309–321. [Google Scholar] [CrossRef]
- Salay, G.; Lucarelli, N.; Gascón, T.M.; de Carvalho, S.S.; da Veiga, G.R.L.; Reis, B.d.C.A.A.; Fonseca, F.L.A. Acute toxicity assays with the Artemia salina model: Assessment of variables. ATLA-Altern. Lab. Anim. 2024, 52, 142–148. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzales, L.; Tablada, M.; Robledo, C.W. InfoStat Versión 2013. Grupo InfoStat, FCA, Universidad Nacional de Córdova, Argentina. Available online: http://www.infostat.com.ar (accessed on 3 February 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).