Improving Lunar Soil Simulant for Plant Cultivation: Earthworm-Mediated Organic Waste Integration and Plant-Microbe Interactions
Abstract
1. Introduction
2. Results
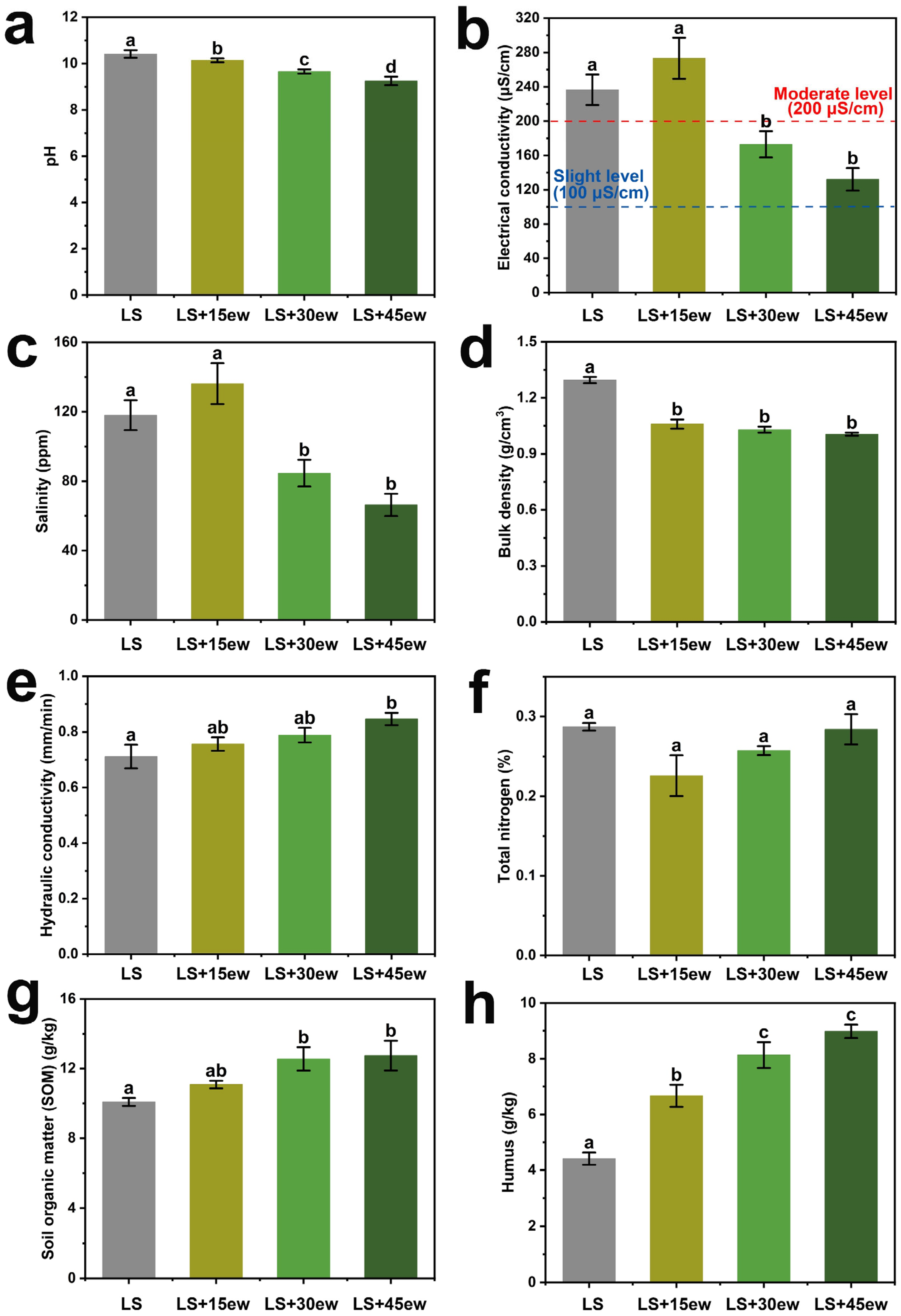
2.1. Earthworms Mitigated the Substrate’s Salinization and Compaction
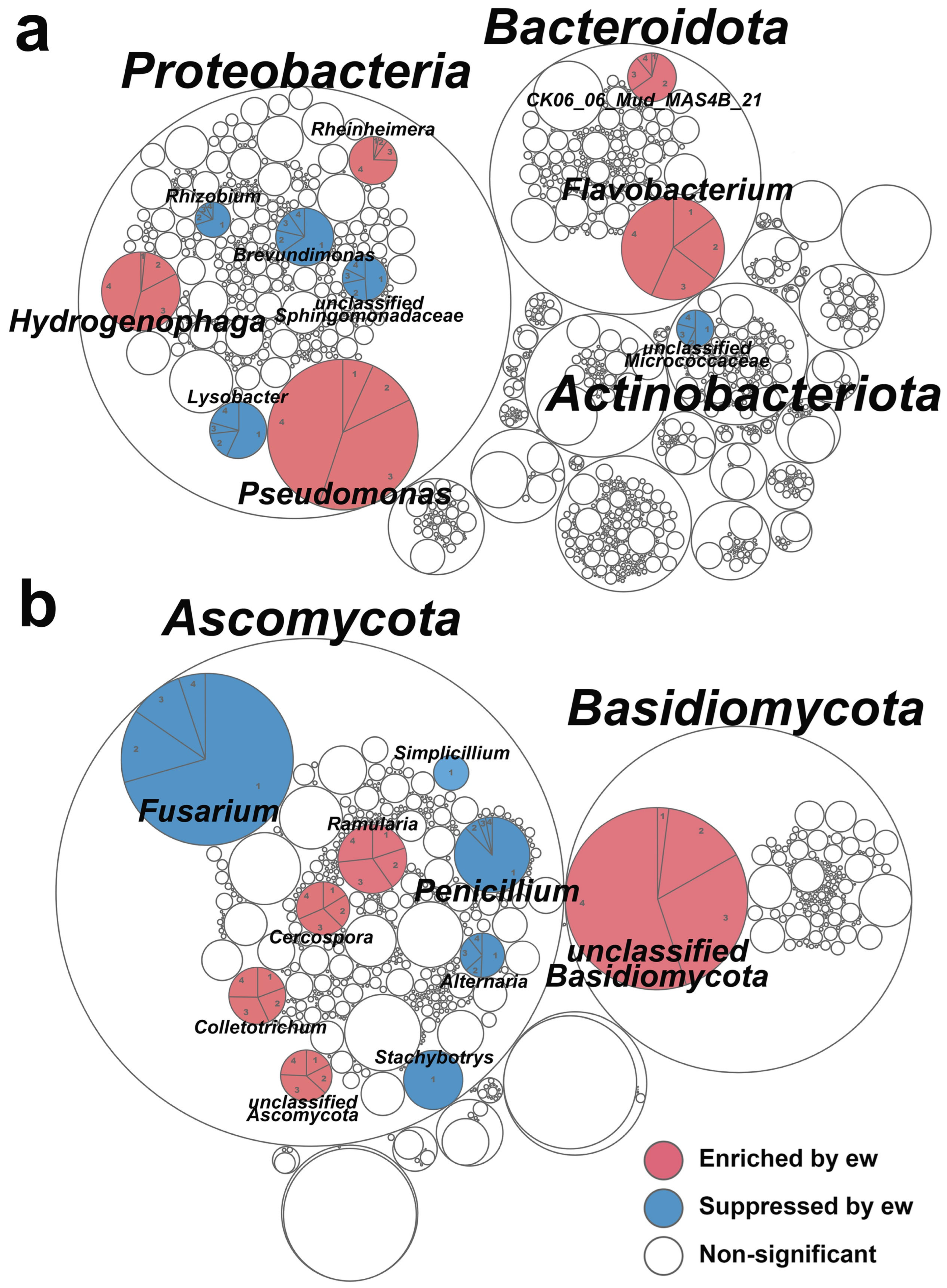
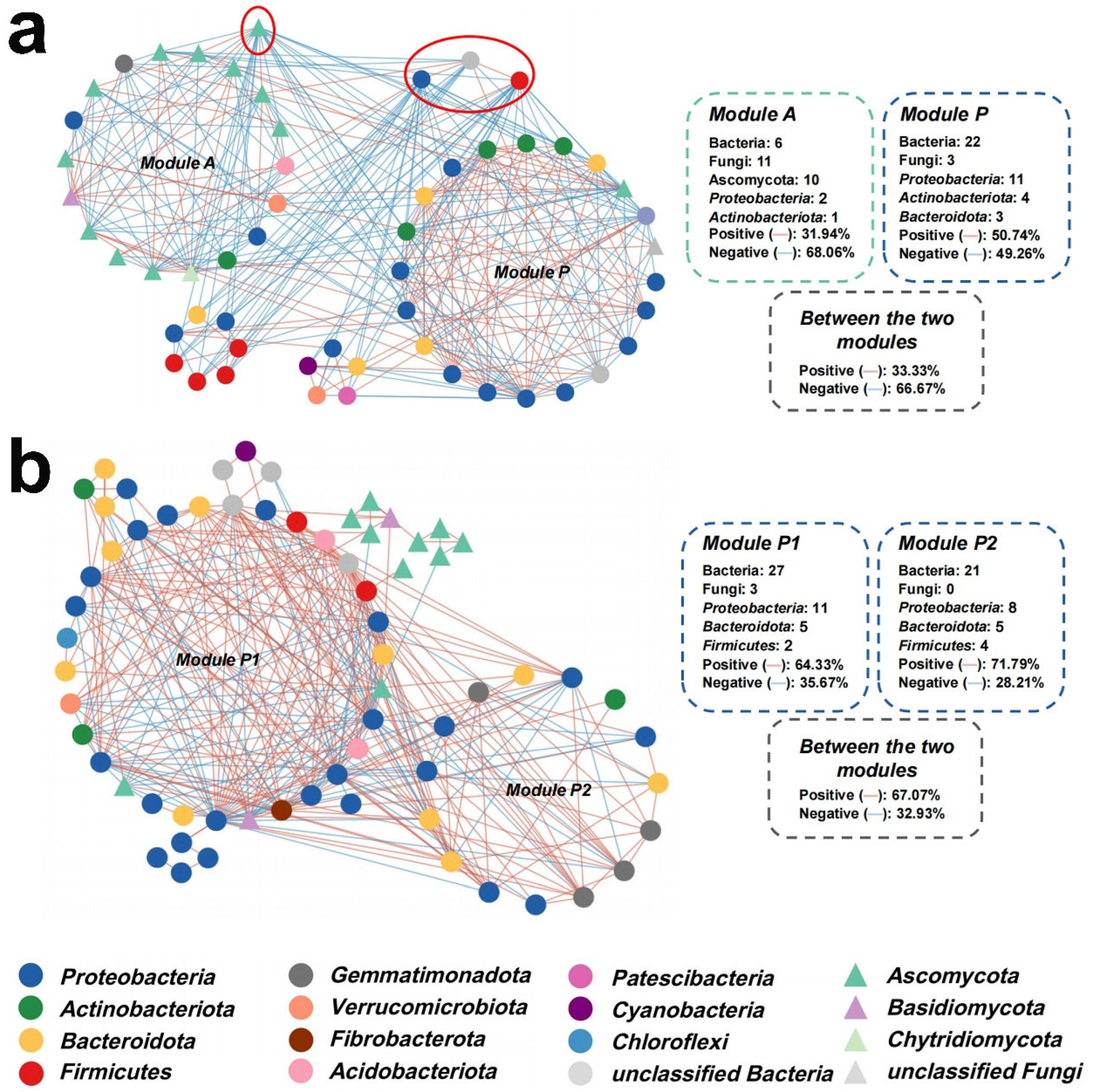
2.2. Substrate Microbial Communities Improved as Probiotic and Cooperative
2.3. The Improved Wheat Growth by Earthworms
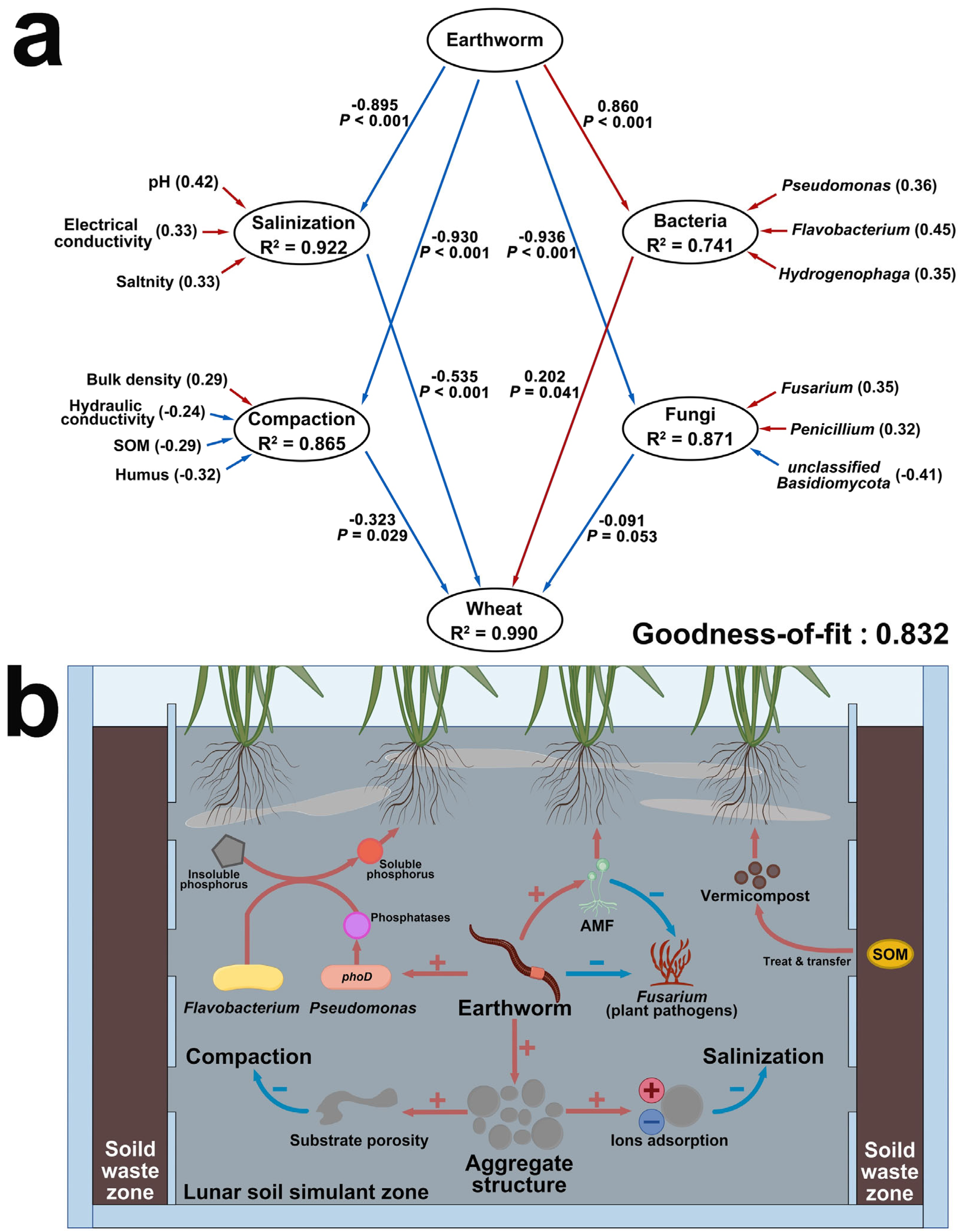
2.4. Improvement Mechanisms Explained by Ecological Models
3. Discussion
4. Materials and Methods
4.1. Lunar Soil Simulant
4.2. Earthworms and Their Feeding
4.3. Wheat Cultivation Experiment
4.4. Wheat Parameters Measurement
4.5. Substrates’ Physical/Chemical Properties
4.6. Sequencing Analysis of the Substrates’ Microbial Community
4.7. Molecular Ecological Network Analysis
4.8. Statistical Analyses
5. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, H.; Yao, Z.; Fu, Y.; Feng, J. Review of research into Bioregenerative Life Support System(s) which can support humans living in space. Life Sci. Space Res. 2021, 31, 113–120. [Google Scholar] [CrossRef]
- Fu, Y.; Li, L.; Xie, B.; Dong, C.; Wang, M.; Jia, B.; Shao, L.; Dong, Y.; Deng, S.; Liu, H. How to establish a Bioregenerative Life Support System for long-term crewed missions to the Moon or Mars. Astrobiology 2016, 16, 925–936. [Google Scholar]
- Meng, C.; Wang, W.; Hao, Z.; Liu, H. Investigation on the influence of isolated environment on human psychological and physiological health. Sci. Total Environ. 2020, 716, 136972. [Google Scholar] [CrossRef]
- Toklu, Y.C.; Akpinar, P. Lunar soils, simulants and lunar construction materials: An overview. Adv. Space Res. 2022, 70, 762–779. [Google Scholar] [CrossRef]
- Walkinshaw, C.H.; Venketeswaran, S.; Baur, P.S.; Hall, R.H.; Croley, T.E.; Weete, J.D.; Scholes, V.E.; Halliwell, R.S. Effect of lunar materials on plant tissue culture. Space Life Sci. 1973, 4, 78–89. [Google Scholar] [CrossRef]
- Laseter, J.L.; Weete, J.D.; Baur, P.S.; Walkinshaw, C.H. Lipid composition of slash pine tissue cultures grown with lunar and earth soils. Space Life Sci. 1973, 4, 353–356. [Google Scholar] [CrossRef]
- Baur, P.; Walkinshaw, C.; Halliwell, R.; Scholes, V. Morphology of Nicotiana tabacum cells grown in contact with lunar material. Can. J. Bot. 2011, 51, 151–156. [Google Scholar] [CrossRef]
- Paul, A.; Elardo, S.M.; Ferl, R. Plants grown in Apollo lunar regolith present stress-associated transcriptomes that inform prospects for lunar exploration. Commun. Biol. 2022, 5, 382. [Google Scholar]
- Yao, Z.; Feng, J.; Liu, H. Bioweathering improvement of lunar soil simulant improves the cultivated wheat’s seedling length. Acta Astronaut. 2022, 193, 1–8. [Google Scholar] [CrossRef]
- Liu, T.; Chen, X.; Gong, X.; Lubbers, I.M.; Jiang, Y.; Feng, W.; Li, X.; Whalen, J.K.; Bonkowski, M.; Griffiths, B.S.; et al. Earthworms Coordinate Soil Biota to Improve Multiple Ecosystem Functions. Curr. Biol. 2019, 29, 3420–3429.e5. [Google Scholar] [CrossRef]
- Lu, Y.; Lu, M.; Peng, F.; Wan, Y.; Liao, M.-H. Remediation of polychlorinated biphenyl-contaminated soil by using a combination of ryegrass, arbuscular mycorrhizal fungi and earthworms. Chemosphere 2014, 106, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Romano, D.; Di Giovanni, A.; Pucciariello, C.; Stefanini, C. Turning earthworms into moonworms: Earthworms colonization of lunar regolith as a bioengineering approach supporting future crop growth in space. Heliyon 2023, 9, e14683. [Google Scholar] [CrossRef] [PubMed]
- Brady, N.C.; Weil, R.R.; Weil, R.R. The Nature and Properties of Soils; Prentice Hall: Upper Saddle River, NJ, USA, 2008; Volume 13. [Google Scholar]
- Daniells, I.G. Hardsetting soils: A review. Soil Res. 2012, 50, 349–359. [Google Scholar] [CrossRef]
- Yang, X.; Shang, G.; Wang, X. Biochemical, transcriptomic, gut microbiome responses and defense mechanisms of the earthworm Eisenia fetida to salt stress. Ecotoxicol. Environ. Saf. 2022, 239, 113684. [Google Scholar] [CrossRef]
- Stătescu, F.; Zaucă, D.C.; Pavel, L.V. Soil structure and water-stable aggregates. Environ. Eng. Manag. J. 2013, 12, 741–746. [Google Scholar] [CrossRef]
- Yudina, A.; Kuzyakov, Y. Dual nature of soil structure: The unity of aggregates and pores. Geoderma 2023, 434, 116478. [Google Scholar] [CrossRef]
- Khan, K.Y.; Pozdnyakov, A.; Son, B. Structure and stability of soil aggregates. Eurasian Soil Sci. 2007, 40, 409–414. [Google Scholar] [CrossRef]
- Tan, L.; Zeng, W.; Xiao, Y.; Li, P.; Gu, S.; Wu, S.; Zhai, Z.; Feng, K.; Deng, Y.; Hu, Q. Fungi-bacteria associations in wilt diseased rhizosphere and endosphere by interdomain ecological network analysis. Front. Microbiol. 2021, 12, 722626. [Google Scholar] [CrossRef]
- Durán, P.; Thiergart, T.; Garrido-Oter, R.; Agler, M.; Kemen, E.; Schulze-Lefert, P.; Hacquard, S. Microbial Interkingdom Interactions in Roots Promote Arabidopsis Survival. Cell 2018, 175, 973–983.e14. [Google Scholar] [CrossRef]
- Deng, Y.; Jiang, Y.H.; Yang, Y.; He, Z.; Luo, F.; Zhou, J. Molecular ecological network analyses. BMC Bioinform. 2012, 13, 113. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Ayangbenro, A.S.; Glick, B.R.; Babalola, O.O. Plant health: Feedback effect of root exudates-rhizobiome interactions. Appl. Microbiol. Biotechnol. 2019, 103, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Rinnan, R.; Bååth, E. Differential utilization of carbon substrates by bacteria and fungi in tundra soil. Appl. Environ. Microbiol. 2009, 75, 3611–3620. [Google Scholar] [CrossRef]
- Ma, L.-J.; Geiser, D.M.; Proctor, R.H.; Rooney, A.P.; O’Donnell, K.; Trail, F.; Gardiner, D.M.; Manners, J.M.; Kazan, K. Fusarium pathogenomics. Annu. Rev. Microbiol. 2013, 67, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.L.; Lee, I.-J. Endophytic Penicillium funiculosum LHL06 secretes gibberellin that reprograms Glycine max L. growth during copper stress. BMC Plant Biol. 2013, 13, 86. [Google Scholar] [CrossRef]
- Schoch, C.L.; Ciufo, S.; Domrachev, M.; Hotton, C.L.; Kannan, S.; Khovanskaya, R.; Leipe, D.; Mcveigh, R.; O’Neill, K.; Robbertse, B. NCBI Taxonomy: A comprehensive update on curation, resources and tools. Database 2020, 2020, baaa062. [Google Scholar]
- Genre, A.; Lanfranco, L.; Perotto, S.; Bonfante, P. Unique and common traits in mycorrhizal symbioses. Nat. Rev. Microbiol. 2020, 18, 649–660. [Google Scholar]
- Hassen, W.; Neifar, M.; Cherif, H.; Najjari, A.; Chouchane, H.; Driouich, R.C.; Salah, A.; Naili, F.; Mosbah, A.; Souissi, Y. Pseudomonas rhizophila S211, a new plant growth-promoting rhizobacterium with potential in pesticide-bioremediation. Front. Microbiol. 2018, 9, 34. [Google Scholar]
- Li, C.; Hu, H.; Yang, M.F.; Pei, Z.Y.; Zhou, Q.; Ren, X.; Liu, B.; Liu, D.; Zeng, X.; Zhang, G.; et al. Characteristics of the lunar samples returned by the Chang’E-5 mission. Natl. Sci. Rev. 2022, 9, nwab188. [Google Scholar] [CrossRef]
- Dasila, H.; Sah, V.; Jaggi, V.; Kumar, A.; Tewari, L.; Taj, G.; Chaturvedi, S.; Perveen, K.; Bukhari, N.A.; Siang, T.C. Cold-tolerant phosphate-solubilizing Pseudomonas strains promote wheat growth and yield by improving soil phosphorous (P) nutrition status. Front. Microbiol. 2023, 14, 1135693. [Google Scholar] [CrossRef]
- Carrión, V.J.; Perez-Jaramillo, J.; Cordovez, V.; Tracanna, V.; De Hollander, M.; Ruiz-Buck, D.; Mendes, L.W.; van Ijcken, W.F.; Gomez-Exposito, R.; Elsayed, S.S. Pathogen-induced activation of disease-suppressive functions in the endophytic root microbiome. Science 2019, 366, 606–612. [Google Scholar]
- Zhang, Y.-X.; Li, Y.-X.; Zhang, W.; Niu, Y.; Zeng, R.J. Enrichment of biofertilizer-type hydrogen-oxidizing bacteria on urea containing Cu (II). Environ. Res. 2023, 236, 116831. [Google Scholar] [PubMed]
- Shah, S.M.D.M.; Shabbir, G.; Malik, S.I.; Raja, N.I.; Shah, Z.H.; Rauf, M.; Zahrani, Y.A.; Alghabari, F.; Alsamadany, H.; Shahzad, K. Delineation of physiological, agronomic and genetic responses of different wheat genotypes under drought condition. Agronomy 2022, 12, 1056. [Google Scholar] [CrossRef]
- Doube, B.M.; Schmidt, O.; Killham, K.; Correll, R. Influence of mineral soil on the palatability of organic matter for lumbricid earthworms: A simple food preference study. Soil Biol. Biochem. 1997, 29, 569–575. [Google Scholar]
- Neilson, R.; Boag, B. Feeding preferences of some earthworm species common to upland pastures in Scotland. Pedobiologia 2003, 47, 1–8. [Google Scholar]
- Qian, Y.; Xiao, L.; Yin, S.; Zhang, M.; Zhao, S.; Pang, Y.; Wang, J.; Wang, G.; Head, J.W. The regolith properties of the Chang’e-5 landing region and the ground drilling experiments using lunar regolith simulants. Icarus 2020, 337, 113508. [Google Scholar]
- Jing, L.; Kakati, L.N.; Ao, B.; Kiewhuo, P. Augmentation of plant biomass productivity using epigeic earthworm Perionyx excavatus and Eisenia fetida as soil nutrient facilitators. Sci. Rep. 2023, 13, 18648. [Google Scholar]
- Fu, Y.; Yi, Z.; Du, Y.; Liu, H.; Xie, B.; Liu, H. Establishment of a closed artificial ecosystem to ensure human long-term survival on the moon. bioRxiv 2021. [Google Scholar] [CrossRef]
- Dong, C.; Fu, Y.; Liu, G.; Liu, H. Low light intensity effects on the growth, photosynthetic characteristics, antioxidant capacity, yield and quality of wheat (Triticum aestivum L.) at different growth stages in BLSS. Adv. Space Res. 2014, 53, 1557–1566. [Google Scholar]
- Dong, C.; Hu, D.; Fu, Y.; Wang, M.; Liu, H. Analysis and optimization of the effect of light and nutrient solution on wheat growth and development using an inverse system model strategy. Comput. Electron. Agric. 2014, 109, 221–231. [Google Scholar]
- Sairam, R.K.; Rao, K.V.; Srivastava, G. Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci. 2002, 163, 1037–1046. [Google Scholar]
- Wheatherley, P. Studies in the water relations of cotton plants. I. The field measurement of water deficit in leaves. New Phytol. 1950, 49, 81–87. [Google Scholar]
- Mackinney, G. Absorption of light by chlorophyll solutions. J. Biol. Chem. 1941, 140, 315–322. [Google Scholar]
- Ahmad, S.; Wang, G.-Y.; Muhammad, I.; Chi, Y.-X.; Zeeshan, M.; Nasar, J.; Zhou, X.-B. Interactive effects of melatonin and nitrogen improve drought tolerance of maize seedlings by regulating growth and physiochemical attributes. Antioxidants 2022, 11, 359. [Google Scholar] [CrossRef]
- Qi, P.; Lin, Y.S.; Song, X.J.; Shen, J.B.; Huang, W.; Shan, J.X.; Zhu, M.Z.; Jiang, L.; Gao, J.P.; Lin, H.X. The novel quantitative trait locus GL3.1 controls rice grain size and yield by regulating Cyclin-T1;3. Cell Res. 2012, 22, 1666–1680. [Google Scholar] [CrossRef]
- Lee, K.; Ma, H.J.; Rotermund, F.; Kim, D.K.; Park, Y. Non-resonant power-efficient directional Nd: YAG ceramic laser using a scattering cavity. Nat. Commun. 2021, 12, 8. [Google Scholar]
- Sun, Z.; Wang, L.; Zhang, G.; Yang, S.; Zhong, Q. Pepino (Solanum muricatum) metabolic profiles and soil nutrient association analysis in three growing sites on the loess plateau of northwestern China. Metabolites 2022, 12, 885. [Google Scholar] [CrossRef]
- Ding, Z.; Kheir, A.M.; Ali, O.A.; Hafez, E.M.; ElShamey, E.A.; Zhou, Z.; Wang, B.; Ge, Y.; Fahmy, A.E.; Seleiman, M.F. A vermicompost and deep tillage system to improve saline-sodic soil quality and wheat productivity. J. Environ. Manag. 2021, 277, 111388. [Google Scholar]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581. [Google Scholar]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar]
- Maslov, S.; Sneppen, K. Specificity and stability in topology of protein networks. Science 2002, 296, 910–913. [Google Scholar]
- Wu, X.; Zhu, Y.; Yang, M.; Zhang, J.; Lin, D. Earthworms enhance the bioremediation of tris (2-butoxyethyl) phosphate-contaminated soil by releasing degrading microbes. J. Hazard. Mater. 2023, 452, 131303. [Google Scholar]
- Wang, J.; Pan, F.; Soininen, J.; Heino, J.; Shen, J. Nutrient enrichment modifies temperature-biodiversity relationships in large-scale field experiments. Nat. Commun. 2016, 7, 13960. [Google Scholar]

| Property | CUG-1B | Apollo Lunar Soils | CE-5 Lunar Soil |
|---|---|---|---|
| Physical properties | |||
| Equivalent diameter (μm) | 75 | 24–429 | 4.84–432.27 |
| Median grain size (μm) | 60 | 28–493 | 52.54–55.24 |
| Bulk density (g/cm3) | 1.59 | 0.87–1.93 | 1.24 |
| True density (g/cm3) | 2.71 | 2.9–3.44 | 3.20 |
| Cohesion (kPa) | 0.14–1.59 | 0.26–1.8 | No data available |
| Internal friction angle (°) | 33.6–35.5 | 25–50 | No data available |
| Void ratio | 0.52–0.95 | 0.67–2.37 | No data available |
| Chemical composition (% w/w) | |||
| SiO2 | 48.11 | 42.2–48.1 | 42.2 |
| TiO2 | 2.64 | 0.53–7.8 | 5.00 |
| Al2O3 | 14.15 | 13.6–27.3 | 10.8 |
| FeOT | 12.45 | 5.1–15.3 | 22.5 |
| MnO | 0.19 | 0.14–0.3 | 0.28 |
| MgO | 5.81 | 5.7–9.4 | 6.48 |
| CaO | 7.42 | 10.7–15.7 | 11.0 |
| Na2O | 4.67 | 0.46–0.7 | 0.26 |
| K2O | 2.55 | 0.16–0.55 | 0.19 |
| P2O5 | 0.92 | 0.05–0.51 | 0.23 |
| Total | 98.92 | 99.48–99.6 | 98.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Hou, S.; Liao, B.; Yao, Z.; Zhu, Y.; Liu, H.; Feng, J. Improving Lunar Soil Simulant for Plant Cultivation: Earthworm-Mediated Organic Waste Integration and Plant-Microbe Interactions. Plants 2025, 14, 1046. https://doi.org/10.3390/plants14071046
Wang Z, Hou S, Liao B, Yao Z, Zhu Y, Liu H, Feng J. Improving Lunar Soil Simulant for Plant Cultivation: Earthworm-Mediated Organic Waste Integration and Plant-Microbe Interactions. Plants. 2025; 14(7):1046. https://doi.org/10.3390/plants14071046
Chicago/Turabian StyleWang, Zhongfu, Sihan Hou, Boyang Liao, Zhikai Yao, Yuting Zhu, Hong Liu, and Jiajie Feng. 2025. "Improving Lunar Soil Simulant for Plant Cultivation: Earthworm-Mediated Organic Waste Integration and Plant-Microbe Interactions" Plants 14, no. 7: 1046. https://doi.org/10.3390/plants14071046
APA StyleWang, Z., Hou, S., Liao, B., Yao, Z., Zhu, Y., Liu, H., & Feng, J. (2025). Improving Lunar Soil Simulant for Plant Cultivation: Earthworm-Mediated Organic Waste Integration and Plant-Microbe Interactions. Plants, 14(7), 1046. https://doi.org/10.3390/plants14071046







