Application of Salicylic Acid Improves the Production of Medicinal Components in Mucuna macrocarpa Wall by Regulating Endogenous Hormone and Nutrient Balance
Abstract
1. Introduction
2. Results
2.1. Effect of SA Concentrations on the Growth and Biomass of M. macrocarpa
2.2. Effect of SA Concentrations on Endogenous Hormone Content in Leaves of M. macrocarpa
2.2.1. Content of IAA, ABA, GA3, and ZR
2.2.2. Ratio of Endogenous Hormones
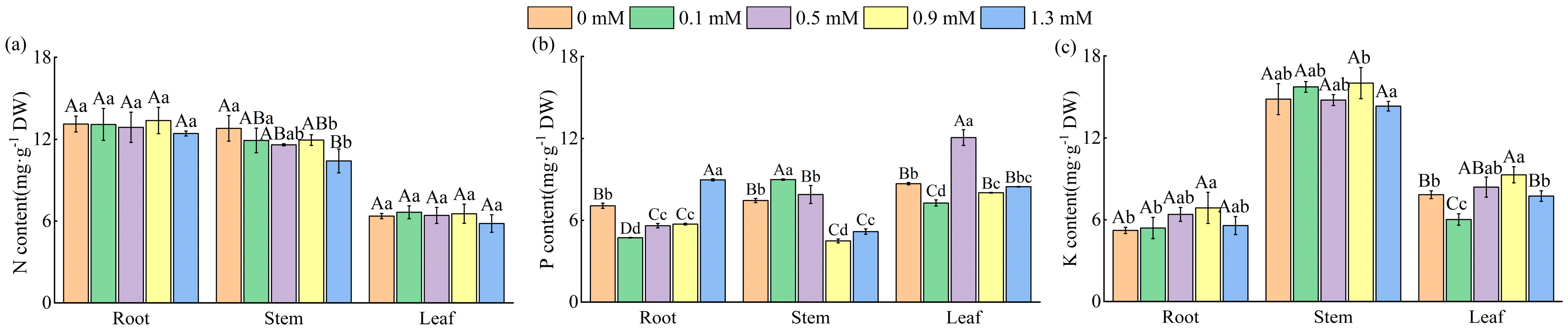
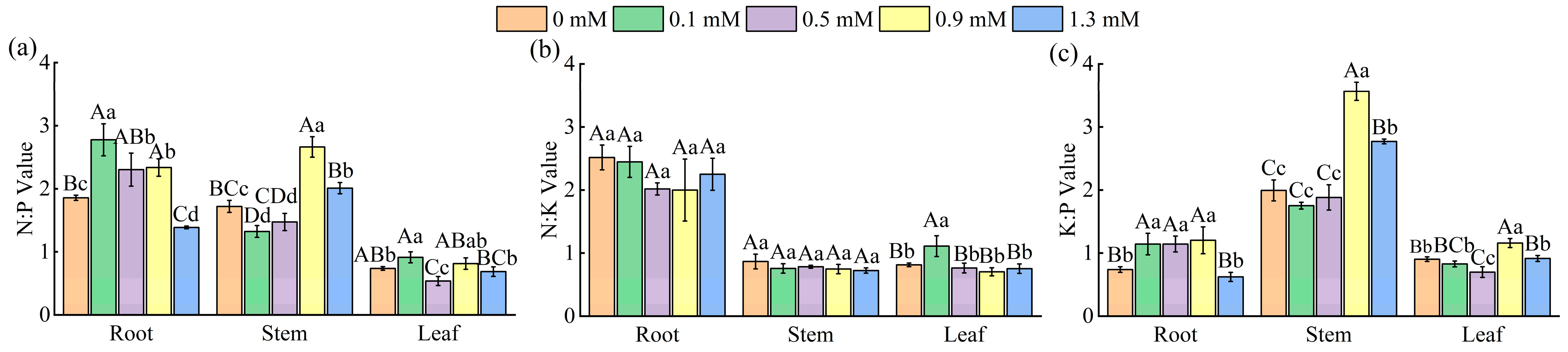
2.3. Effect of SA Concentrations on Nutrient Content and on the Stoichiometric Ratios of M. macrocarpa
2.3.1. N, P, and K Content
2.3.2. Ratio of N:P, N:K, and K:P
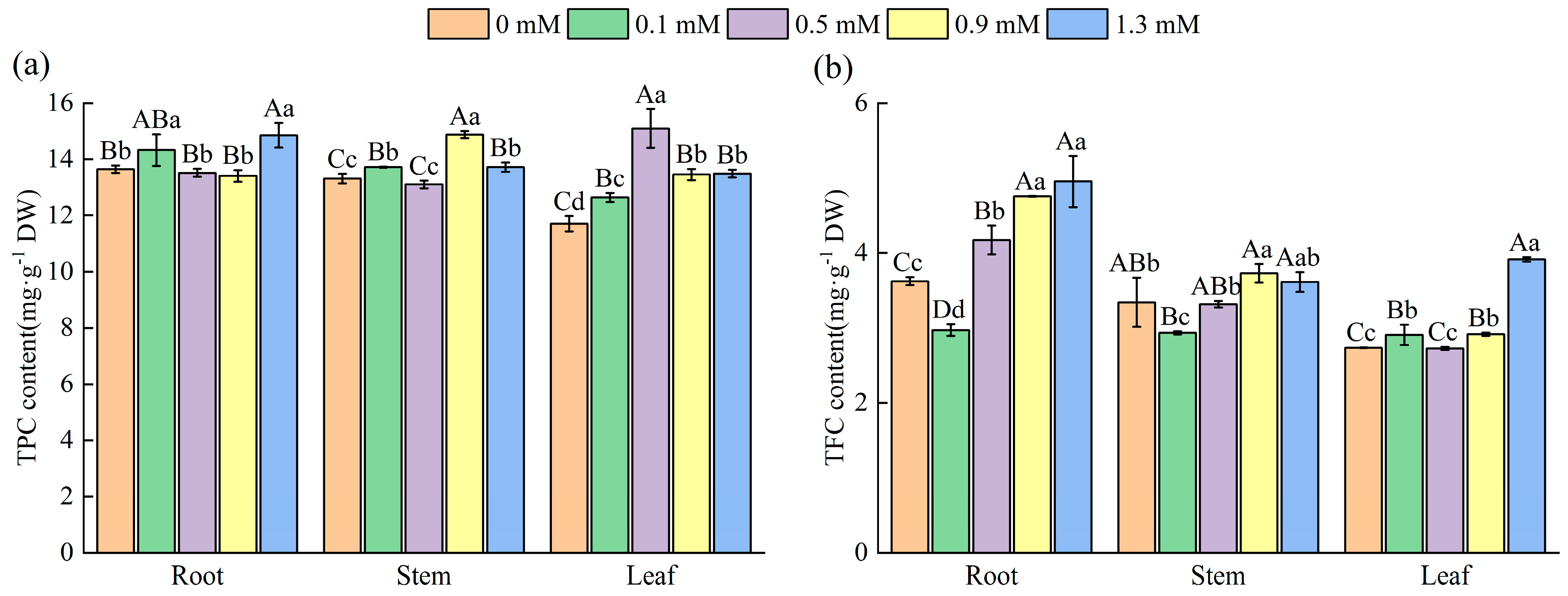
2.4. Effect of SA Concentrations on the Medicinal Components of M. macrocarpa
2.4.1. Total Phenolic Content (TPC) and Total Flavonoid Content (TFC) in Various Organs
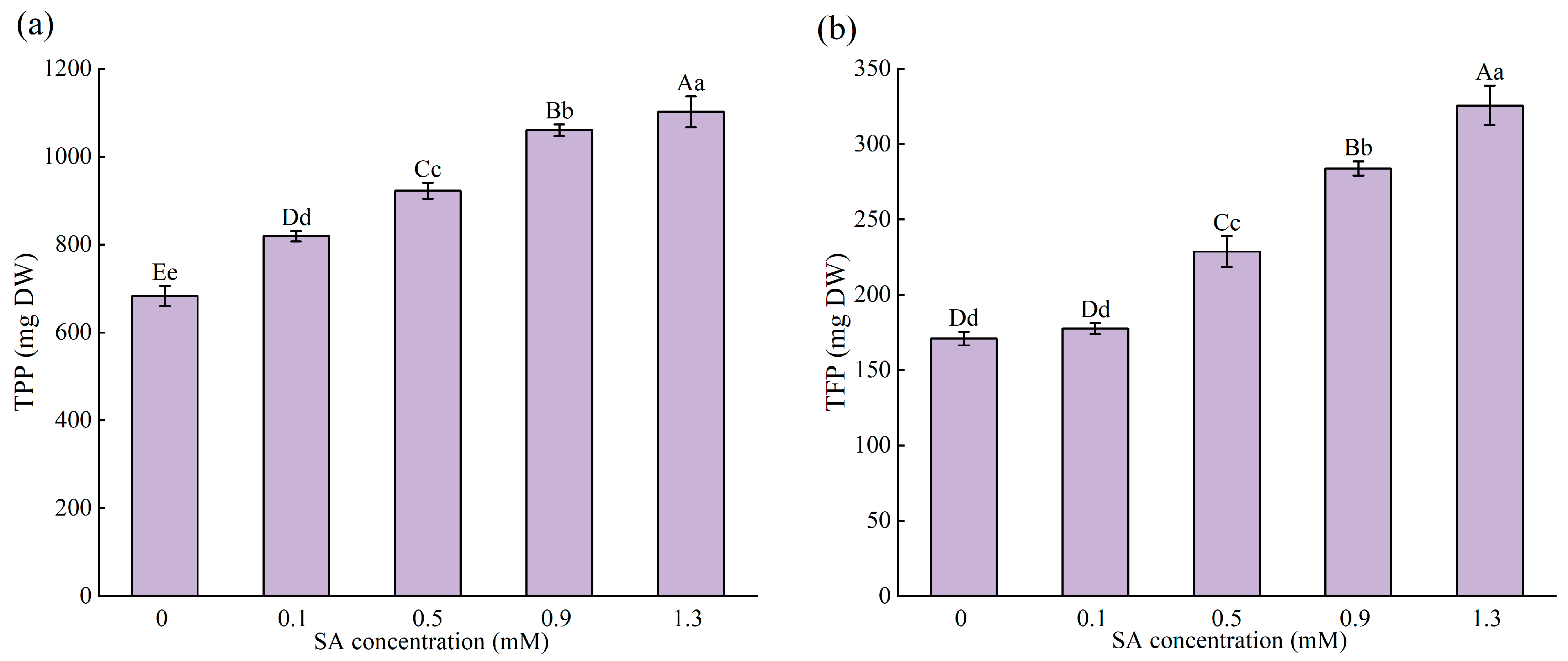
2.4.2. Total Phenolic Production (TPP) and Total Flavonoid Production (TFP) of the Whole Plant
2.5. Correlation Analysis
2.6. Membership Function Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. SA Treatments
4.3. Experimental Materials
4.3.1. Growth and Biomass Measurements
4.3.2. Endogenous Hormone Measurements
4.3.3. Measurements of N, P, and K Content
4.3.4. Analysis of Total Phenolic and Total Flavonoid Content
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kusumoto, B.; Enoki, T. Contribution of a liana species, Mucuna macrocarpa Wall., to litterfall production and nitrogen input in a subtropical evergreen broad-leaved forest. J. For. Res.-Jpn. 2017, 13, 35–42. [Google Scholar] [CrossRef]
- Dong, L.; Zhu, J.; Wang, Y.F.; Liu, J. Chemical constituents of Xue teng(Mucuna). J. Beijing Univ. Tradit. Chin. Med. 2009, 32, 846–848. [Google Scholar]
- Hu, W.; Luo, S.; Cai, J. Studies on the chemical components of Daguoyoumateng (Mucuna macrocarpa). Chin. Tradit. Herb. Drugs 1994, 25, 59–63. [Google Scholar]
- Jiang, W.; Ni, Z.; Hua, X. Effect of qianglisheng Injection on Rat’s Gastrocne mi us Fatigue in vivo Induced by Electric Stimulation. Chin. J. Sports Med. 2002, 4, 274–277. [Google Scholar] [CrossRef]
- Liang, H.; Sun, H.; Shao, C.; Zhang, Y. Role and Regulatory Mechanism of Plant Hormones in Ginsenoside biosynthesis. J. Jilin Agric. Univ. 2023, 45, 685–692. [Google Scholar] [CrossRef]
- Yu, T.; Xin, Y.; Liu, P. Exogenous abscisic acid (ABA) improves the filling process of maize grains at different ear positions by promoting starch accumulation and regulating hormone levels under high planting density. BMC Plant Biol. 2024, 24, 80. [Google Scholar] [CrossRef]
- Samota, M.K.; Sasi, M.; Awana, M.; Yadav, O.P.; Mithra, S.V.A.; Tyagi, A.; Kumar, S.; Singh, A. Elicitor-Induced Biochemical and Molecular Manifestations to Improve Drought Tolerance in Rice (Oryza sativa L.) through Seed-Priming. Front. Plant Sci. 2017, 8, 934. [Google Scholar] [CrossRef]
- Wu, M.; Su, C.; Cao, S.; Sun, Y.; Niu, N.; Chen, L.; Tang, Z. Design of a pseudo-color-assisted biocompatible supramolecular sensing probe for "lighting up" endogenous salicylic acid in plants. Biosens. Bioelectron. 2025, 271, 117088. [Google Scholar] [CrossRef]
- Ali, H.H.; Shehzadi, N.; Zaheer, M.S.; Seleiman, M.F.; Aldhuwaib, K.J.; Din Khan, W.u.; Raza, A. Exploring the Impact of Salicylic Acid and Farmyard Manure on Soil Rhizospheric Properties and Cadmium Stress Alleviation in Maize (Zea mays L.). Plants 2023, 12, 3115. [Google Scholar] [CrossRef]
- Mohd, S.; Qazi, F.; Tibor, J. Multifaceted Role of Salicylic Acid in Combating Cold Stress in Plants: A Review. J. Plant Growth Regul. 2020, 40, 464–485. [Google Scholar] [CrossRef]
- Zamani, F.; Hosseini, N.M.; Oveisi, M.; Arvin, K.; Rabieyan, E.; Torkaman, Z.; Rodriguez, D. Rhizobacteria and Phytohormonal interactions increase Drought Tolerance in Phaseolus vulgaris through enhanced physiological and biochemical efficiency. Sci. Rep. 2024, 14, 30761. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Zhang, Y.; Cui, J.; Zhang, H.; Wang, H.; Jin, H.; Lu, P.; He, L.; Zhou, Q.; Yu, J.; et al. An Enhanced Interaction of Graft and Exogenous SA on Photosynthesis, Phytohormone, and Transcriptome Analysis in Tomato under Salinity Stress. Int. J. Mol. Sci. 2024, 25, 10799. [Google Scholar] [CrossRef]
- Keya, S.S.; Mostofa, M.G.; Rahman, M.; Das, A.K.; Sultana, S.; Ghosh, P.K.; Anik, T.R.; Ahsan, S.M.; Rahman, A.; Jahan, N.; et al. Salicylic Acid Application Improves Photosynthetic Performance and Biochemical Responses to Mitigate Saline Stress in Cotton. J. Plant Growth Regul. 2023, 42, 5881–5894. [Google Scholar] [CrossRef]
- Bagautdinova, Z.Z.; Omelyanchuk, N.; Tyapkin, A.V.; Kovrizhnykh, V.V.; Lavrekha, V.V.; Zemlyanskaya, E.V. Salicylic Acid in Root Growth and Development. Int. J. Mol. Sci. 2022, 23, 2228. [Google Scholar] [CrossRef] [PubMed]
- Yadav, T.; Kumar, A.; Yadav, R.K.; Yadav, G.; Kumar, R.; Kushwaha, M. Salicylic acid and thiourea mitigate the salinity and drought stress on physiological traits governing yield in pearl millet-wheat. Saudi J. Biol. Sci. 2020, 27, 2010–2017. [Google Scholar] [CrossRef] [PubMed]
- Pirasteh-Anosheh, H.; Ranjbar, G.; Hasanuzzaman, M.; Khanna, K.; Bhardwaj, R.; Ahmad, P. Salicylic Acid-Mediated Regulation of Morpho-Physiological and Yield Attributes of Wheat and Barley Plants in Deferring Salinity Stress. J. Plant Growth Regul. 2022, 41, 1291–1303. [Google Scholar] [CrossRef]
- Colak, N.; Kurt-Celebi, A.; Roth, M.R.; Welti, R.; Torun, H.; Ayaz, F.A. Salicylic acid priming before cadmium exposure increases wheat growth but does not uniformly reverse cadmium effects on membrane glycerolipids. Plant Biol. 2025, 27, 79–91. [Google Scholar] [CrossRef]
- Shi, L.; Tan, Y.; Sun, Z.; Ren, A.; Zhu, J.; Zhao, M. Exogenous Salicylic Acid (SA) Promotes the Accumulation of Biomass and Flavonoid Content in Phellinus igniarius (Agaricomycetes). Int. J. Med. Mushrooms 2019, 21, 955–963. [Google Scholar] [CrossRef]
- Yin, Y.; Hu, M.; Yang, Z.; Zhu, J.; Fang, W. Salicylic acid promotes phenolic acid biosynthesis for the production of phenol acid-rich barley sprouts. J. Sci. Food Agric. 2024, 104, 5350–5359. [Google Scholar] [CrossRef]
- Dai, W.; Pan, M.; Peng, L.; Zhang, D.; Ma, Y.; Wang, M.; Wang, N. Integrated Transcriptome and Metabolome Analysis Reveals Insights into Flavone and Flavonol Biosynthesis in Salicylic Acid-Induced Citrus Huanglongbing Tolerance. J. Agric. Food Chem. 2025, 73, 919–937. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Li, S.; Zhai, Y.; Zhao, Y.; Zhong, F.; Wang, Z.; Zhao, M.; Li, S. Methyl jasmonate and salicylic acid enhance the total flavonoid, phenolics, and cannabidiol contents of Cannabis sativa L. adventitious roots. Plant Cell Tissue Organ Cult. (PCTOC) 2024, 159, 49. [Google Scholar] [CrossRef]
- Yousefvand, P.; Sohrabi, Y.; Mastinu, A.; Heidari, G.; Weisany, W. Optimizing growth, yield, and water use efficiency of Allium hirtifolium with salicylic acid under water stress condiions. Heliyon 2025, 11, e41550. [Google Scholar] [CrossRef] [PubMed]
- Veloso, L.L.D.S.; Lima, G.S.D.; Silva, A.A.R.D.; Souza, L.D.P.; Lacerda, C.N.D.; Silva, I.J.D.; Chaves, L.H.G.; Fernandes, P.D. Attenuation of salt stress on the physiology and production of bell peppers by treatment with salicylic acid. Semin. Ciências Agrárias 2021, 42, 2751–2768. [Google Scholar] [CrossRef]
- Arif, Y.; Sami, F.; Siddiqui, H.; Hayat, S. Salicylic acid in relation to other phytohormones in plant: A study towards physiology and signal transduction under challenging environment. Environ. Exp. Bot. 2020, 175, 104040. [Google Scholar] [CrossRef]
- Wang, J.; Wang, M.; Wang, Y.; Lv, S.; Fu, S.; Liu, J.; Wu, R. Effects of salicylic acid concentration on accumulation of biomass, total flavonoids and polysaccharide in Astragalus membranaceus. Jiangsu Agric. Sci. 2018, 46, 120–123. [Google Scholar] [CrossRef]
- Humbal, A.; Pathak, B. Influence of exogenous elicitors on the production of secondary metabolite in plants: A review (“VSI: Secondary metabolites”). Plant Stress 2023, 8, 100166. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, Y.H.; Zhang, L.; Li, Y.; Sun, Y.D.; Li, Z.Y. Changes in the distribution of endogenous hormones in Phyllostachys edulis ‘Pachyloen’ during bamboo shooting. PLoS ONE 2020, 15, e0241806. [Google Scholar] [CrossRef]
- Wang, H.Y.; Cui, K.; He, C.Y.; Zeng, Y.F.; Liao, S.X.; Zhang, J.G. Endogenous hormonal equilibrium linked to bamboo culm development. Genet. Mol. Res. 2015, 14, 11312–11323. [Google Scholar] [CrossRef]
- Chen, H.; Song, L.; Zhou, H.; Yao, T.; Zhang, Z.; Zhang, H.; Meng, L.; Zhang, H. ABA signal transduction and ROS metabolic balance play a key role in the drought resistance of safflower. Plant Growth Regul. 2025, 105, 273–294. [Google Scholar] [CrossRef]
- Yang, M.; Wang, L.; Belwal, T.; Zhang, X.; Lu, H.; Chen, C.; Li, L. Exogenous Melatonin and Abscisic Acid Expedite the Flavonoids Biosynthesis in Grape Berry of Vitis vinifera cv. Kyoho. Molecules 2020, 25, 12. [Google Scholar] [CrossRef]
- Guan, L.; Bai, L.; Pang, Y.; Yu, F.; Yuan, C.; Chen, X.; Xie, X. Effects of Exogenous Salicylic Acid on Active Components, Antioxidant Enzyme Activities and Endogenous Hormone Contents in Blumea balsamifera. J. Chin. Med. Mater. 2019, 42, 490–494. [Google Scholar] [CrossRef]
- Niu, Y.; Ye, L.; Wang, Y.; Shi, Y.; Luo, A. Salicylic acid mitigates ‘Hayward’ kiwifruit chilling injury by regulating hormone and proline metabolism, as well as maintaining cellular structure. Food Biosci. 2024, 57, 103573. [Google Scholar] [CrossRef]
- Song, Z.; Lai, X.; Chen, H.; Wang, L.; Pang, X.; Hao, Y.; Lu, W.; Chen, W.; Zhu, X.; Li, X. Role of MaABI5-like in abscisic acid-induced cold tolerance of ‘Fenjiao’ banana fruit. Hortic. Res.—Eng. 2022, 9, uhac130. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Chen, G.; Zheng, D.; Feng, N. Transcriptomic and metabolomic analyses reveal that ABA increases the salt tolerance of rice significantly correlated with jasmonic acid biosynthesis and flavonoid biosynthesis. Sci. Rep. 2023, 13, 20365. [Google Scholar] [CrossRef]
- He, H.; Li, Q.; Fang, L.; Yang, W.; Xu, F.; Yan, Y.; Mao, R. Comprehensive analysis of NAC transcription factors in Scutellaria baicalensis and their response to exogenous ABA and GA3. Int. J. Biol. Macromol. 2023, 244, 125290. [Google Scholar] [CrossRef]
- Gao, X.R.; Zhang, H.; Li, X.; Bai, Y.W.; Peng, K.; Wang, Z.; Dai, Z.R.; Bian, X.F.; Zhang, Q.; Jia, L.C.; et al. The B-box transcription factor IbBBX29 regulates leaf development and flavonoid biosynthesis in sweet potato. Plant Physiol. 2023, 191, 496–514. [Google Scholar] [CrossRef]
- Fang, X.; Zhou, Y.; Wang, Y.; Guan, Z.; Chen, S.; Fang, W.; Chen, F.; Zhao, S. Effects of different nitrogen, phosphorus and potassium application amounts on the growth, nutrient absorption and distribution of chrysanthemum. J. Nanjing Agric. Univ. 2020, 43, 1015–1023. Available online: https://link.cnki.net/urlid/32.1148.S.20200929.0934.002 (accessed on 10 March 2025).
- Shinde, M.; Khan, V.; Umar, S.; Iqbal, N. Salicylic Acid with Phosphorus Strengthens Antioxidative System, Secondary Metabolite Accumulation, and Limited Resource Enrichment to Enhance Photosynthesis Under Drought in Mustard. J. Soil. Sci. Plant Nut. 2024, 24, 956–974. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, S.; Wang, P.; Hou, J.; Qian, J.; Ao, Y.; Lu, J.; Li, L. Salicylic acid involved in the regulation of nutrient elements uptake and oxidative stress in Vallisneria natans (Lour.) Hara under Pb stress. Chemosphere 2011, 84, 136–142. [Google Scholar] [CrossRef]
- Liu, R.; Li, B.; Wang, Z. Research progress on molecular mechanisms of phosphate starvation response in plants. J. Zhejiang Univ. (Agric. Life Sci.) 2024, 50, 455–468. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, L.; Chen, C.; Guo, L.; Zhao, T.; Liu, D. Effects of phosphorus stress on the growth and secondary metabolism of Artemisia argyi. J. Plant Res. 2023, 136, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Donovan, L.A.; Maherali, H.; Caruso, C.M.; Huber, H.; Kroon, H.D. The evolution of the worldwide leaf economics spectrum. Trends Ecol. Evol. 2011, 26, 88–95. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Y.; Bing, D.; Zhang, K.; Huang, D.; Wang, D. Effect of nitrogen and phosphorus addition on seedling growth and ecological stoichiometry of Pinus armandii. J. Northwest A F Univ. (Nat. Sci. Ed.) 2025, 53, 1–10. [Google Scholar] [CrossRef]
- Xu, Y.; Li, W.; Luo, M.; Ling, Z.; Yang, M.; Chen, Z. The Total Flavonoid and Total Phenol Content in Different Organs of Caesalpinia sappan and Their Relationship with Nutrient Content. Mol. Plant Breed. 2024, 1–14. Available online: http://kns.cnki.net/kcms/detail/46.1068.s.20240327.0958.002.html (accessed on 10 March 2025).
- Ghassemi-Golezani, K.; Farhangi-Abriz, S. Foliar sprays of salicylic acid and jasmonic acid stimulate H+-ATPase activity of tonoplast, nutrient uptake and salt tolerance of soybean. Ecotox Environ. Safe 2018, 166, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Fatma, M.; Gautam, H.; Sehar, Z.; Rasheed, F.; Khan, M.I.R.; Sofo, A.; Khan, N.A. Salicylic Acid Increases Photosynthesis of Drought Grown Mustard Plants Effectively with Sufficient-N via Regulation of Ethylene, Abscisic Acid, and Nitrogen-Use Efficiency. J. Plant Growth Regul. 2022, 41, 1966–1977. [Google Scholar] [CrossRef]
- Larbat, R.; Paris, C.; Le Bot, J.; Adamowicz, S. Phenolic characterization and variability in leaves, stems and roots of Micro-Tom and patio tomatoes, in response to nitrogen limitation. Plant Sci. 2014, 224, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Strissel, T.; Halbwirth, H.; Hoyer, U.; Zistler, C.; Stich, K.; Treutter, D. Growth-promoting nitrogen nutrition affects flavonoid biosynthesis in young apple (Malus domestica Borkh.) leaves. Plant Biol. 2005, 7, 677–685. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, H.; Jiang, X.; Zhang, L.; Qin, Y. Integrated physiological, transcriptomic, and metabolomic analyses reveal that low-nitrogen conditions improve the accumulation of flavonoids in snow chrysanthemum. Ind. Crop Prod. 2023, 197, 116574. [Google Scholar] [CrossRef]
- Wu, Y.; Shen, Y. Dormancy in Tilia miqueliana is attributable to permeability barriers and mechanical constraints in the endosperm and seed coat. Braz. J. Bot. 2021, 44, 725–740. [Google Scholar] [CrossRef]
- Lu, R. Methods of Soil Agrochemical Analysis; Agricultural Science and Technology Press: Beijing, China, 2000; pp. 308–315. [Google Scholar]
- Wei, D.; Sun, W.; Huang, M.; Zeng, X.; Chen, Y.; Yuan, Z. Phenolic Characterization and Antioxidant Activity of Chrysanthemum indicum and Opisthopappus Shih during Different Growth Stages. Chem. Biodivers. 2023, 20, e202300370. [Google Scholar] [CrossRef] [PubMed]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, J.; Zhou, Y.; Yuan, M.; Zhang, Y. Effects of different proportions of a grape branch culture formula on the growth and quality of four edible fungi. J. Gansu Agric. Univ. 2024, 59, 126–135. [Google Scholar] [CrossRef]
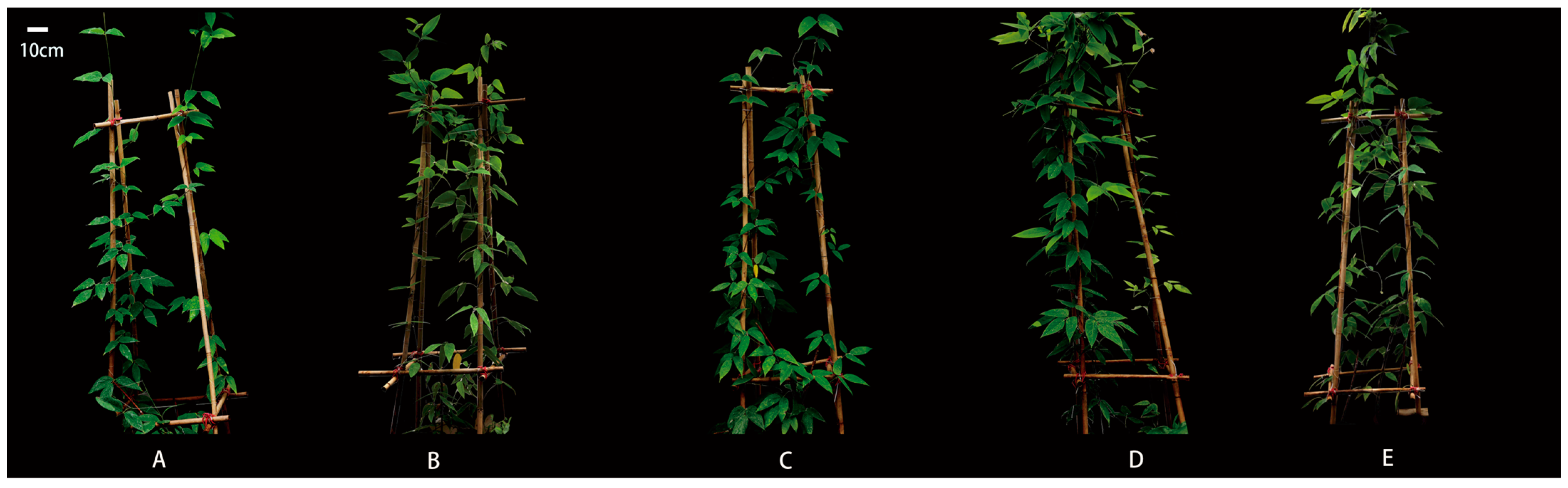
| SA Concentration (mM) | Basal Diameter (mm) | Biomass (g) | Total Biomass (g) | ||
|---|---|---|---|---|---|
| Root | Stem | Leaf | |||
| 0 | 6.89 ± 0.49 Bc | 16.31 ± 0.90 Cd | 19.39 ± 0.73 Bc | 17.22 ± 1.00 Bc | 52.93 ± 1.66 Dd |
| 0.1 | 7.09 ± 0.22 ABbc | 19.46 ± 0.76 Bc | 20.31 ± 0.71 Bc | 20.72 ± 0.99 Bb | 60.49 ± 1.37 Cc |
| 0.5 | 7.27 ± 0.40 ABbc | 21.85 ± 1.17 Bb | 25.10 ± 1.39 Ab | 19.83 ± 1.40 Bb | 66.79 ± 1.97 Bb |
| 0.9 | 8.31 ± 0.22 Aa | 21.73 ± 1.80 Bb | 27.97 ± 1.41 Aa | 26.19 ± 1.94 Aa | 75.89 ± 0.88 Aa |
| 1.3 | 7.85 ± 0.74 ABab | 25.36 ± 1.06 Aa | 27.77 ± 1.30 Aa | 25.51 ± 1.44 Aa | 78.64 ± 2.13 Aa |
| SA Concentration (mM) | IAA (ng·g−1·FW) | ABA (ng·g−1·FW) | GA3 (ng·g−1·FW) | ZR (ng·g−1·FW) |
|---|---|---|---|---|
| 0 | 46.11 ± 3.72 BCc | 156.56 ± 3.97 ABa | 4.84 ± 0.14 Cc | 5.97 ± 0.10 Bb |
| 0.1 | 51.47 ± 2.03 Bb | 126.16 ± 0.24 Cc | 6.10 ± 0.13 Aa | 8.98 ± 0.10 Aa |
| 0.5 | 57.08 ± 0.59 Aa | 160.14 ± 3.84 Aa | 5.57 ± 0.10 Bb | 8.90 ± 0.07 Aa |
| 0.9 | 42.27 ± 1.25 Cd | 154.73 ± 1.38 ABa | 5.66 ± 0.19 Bb | 9.13 ± 0.41 Aa |
| 1.3 | 46.47 ± 1.22 BCc | 148.96 ± 2.99 Bb | 4.90 ± 0.13 Cc | 6.22 ± 0.07 Bb |
| SA Concentration (mM) | IAA:ABA | GA3:ABA | ZR:ABA | (IAA + GA3 + ZR):ABA |
|---|---|---|---|---|
| 0 | 0.2948 ± 0.0283 Ccd | 0.0309 ± 0.0016 Cd | 0.0382 ± 0.0011 Ce | 0.3639 ± 0.0303 Cc |
| 0.1 | 0.4080 ± 0.0165 Aa | 0.0484 ± 0.0011 Aa | 0.0712 ± 0.0006 Aa | 0.5275 ± 0.0171 Aa |
| 0.5 | 0.3566 ± 0.0120 Bb | 0.0348 ± 0.0013 Bbc | 0.0556 ± 0.0015 Bc | 0.4470 ± 0.0146 Bb |
| 0.9 | 0.2732 ± 0.0070 Cd | 0.0366 ± 0.0016 Bb | 0.0590 ± 0.0022 Bb | 0.3688 ± 0.0084 Cc |
| 1.3 | 0.3120 ± 0.0025 Cc | 0.0329 ± 0.0013 BCcd | 0.0418 ± 0.0013 Cd | 0.3867 ± 0.0014 Cc |
| Index | RTPC | STPC | LTPC | RTFC | STFC | LTFC | TPP | TFP |
|---|---|---|---|---|---|---|---|---|
| BD | 0.032 | 0.601 * | 0.289 | 0.578 * | 0.659 ** | 0.354 | 0.671 ** | 0.649 ** |
| TB | 0.243 | 0.544 * | 0.525 * | 0.838 ** | 0.606 * | 0.658 ** | 0.998 ** | 0.961 ** |
| ABA | −0.500 | −0.083 | 0.322 | 0.588 * | 0.584 * | −0.127 | 0.135 | 0.275 |
| IAA | −0.089 | −0.669 ** | 0.525 * | −0.364 | −0.574 * | −0.292 | −0.216 | −0.340 |
| GA3 | −0.190 | 0.286 | 0.258 | −0.426 | −0.439 | −0.410 | 0.024 | −0.259 |
| ZR | −0.348 | 0.349 | 0.485 | −0.161 | −0.186 | −0.464 | 0.198 | −0.080 |
| RN | −0.272 | 0.206 | −0.145 | −0.200 | −0.044 | −0.331 | −0.141 | −0.211 |
| SN | −0.592 * | 0.009 | −0.399 | −0.482 | −0.313 | −0.720 ** | −0.593 * | −0.620 * |
| LN | −0.285 | 0.104 | −0.187 | −0.360 | −0.399 | −0.472 | −0.231 | −0.369 |
| RP | 0.484 | −0.131 | −0.117 | 0.590 * | 0.485 | 0.784 ** | 0.329 | 0.575 * |
| SP | −0.022 | −0.677 ** | −0.103 | −0.869 ** | −0.857 ** | −0.514 | −0.737 ** | −0.833 ** |
| LP | −0.350 | −0.553 * | 0.742 ** | 0.193 | 0.055 | −0.254 | 0.012 | 0.005 |
| RK | −0.385 | 0.416 | 0.422 | 0.346 | 0.265 | −0.157 | 0.440 | 0.320 |
| SK | −0.262 | 0.514 * | −0.096 | −0.206 | 0.040 | −0.334 | −0.024 | −0.162 |
| LK | −0.466 | 0.403 | 0.318 | 0.691 ** | 0.652 ** | −0.063 | 0.426 | 0.471 |
| Index | 0 mM | 0.1 mM | 0.5 mM | 0.9 mM | 1.3 mM |
|---|---|---|---|---|---|
| BD | 0.000 | 0.140 | 0.264 | 1.000 | 0.674 |
| TB | 0.000 | 0.294 | 0.539 | 0.893 | 1.000 |
| ABA | 0.895 | 0.000 | 1.000 | 0.841 | 0.671 |
| IAA | 0.259 | 0.621 | 1.000 | 0.000 | 0.284 |
| GA3 | 0.000 | 1.000 | 0.582 | 0.653 | 0.048 |
| ZR | 0.000 | 0.952 | 0.927 | 1.000 | 0.079 |
| RN | 0.267 | 0.302 | 0.523 | 0.000 | 1.000 |
| SN | 0.000 | 0.370 | 0.503 | 0.357 | 1.000 |
| LN | 0.664 | 1.000 | 0.721 | 0.866 | 0.000 |
| RP | 0.553 | 0.000 | 0.208 | 0.236 | 1.000 |
| SP | 0.341 | 0.000 | 0.242 | 1.000 | 0.847 |
| LP | 0.296 | 0.000 | 1.000 | 0.158 | 0.249 |
| RK | 0.000 | 0.105 | 0.708 | 1.000 | 0.217 |
| SK | 0.304 | 0.836 | 0.266 | 1.000 | 0.000 |
| LK | 0.556 | 0.000 | 0.726 | 1.000 | 0.525 |
| Average value | 0.276 | 0.375 | 0.614 | 0.667 | 0.506 |
| Comprehensive sorting | 5 | 4 | 2 | 1 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hua, Y.; Pan, X.; Tian, L.; Xu, Y.; Yang, M.; Deng, R. Application of Salicylic Acid Improves the Production of Medicinal Components in Mucuna macrocarpa Wall by Regulating Endogenous Hormone and Nutrient Balance. Plants 2025, 14, 1023. https://doi.org/10.3390/plants14071023
Hua Y, Pan X, Tian L, Xu Y, Yang M, Deng R. Application of Salicylic Acid Improves the Production of Medicinal Components in Mucuna macrocarpa Wall by Regulating Endogenous Hormone and Nutrient Balance. Plants. 2025; 14(7):1023. https://doi.org/10.3390/plants14071023
Chicago/Turabian StyleHua, Yulan, Xianyuan Pan, Li Tian, Yuanyuan Xu, Mei Yang, and Rongyan Deng. 2025. "Application of Salicylic Acid Improves the Production of Medicinal Components in Mucuna macrocarpa Wall by Regulating Endogenous Hormone and Nutrient Balance" Plants 14, no. 7: 1023. https://doi.org/10.3390/plants14071023
APA StyleHua, Y., Pan, X., Tian, L., Xu, Y., Yang, M., & Deng, R. (2025). Application of Salicylic Acid Improves the Production of Medicinal Components in Mucuna macrocarpa Wall by Regulating Endogenous Hormone and Nutrient Balance. Plants, 14(7), 1023. https://doi.org/10.3390/plants14071023






