Multivariate Adaptability of Tropical Wheat Cultivars to Drought and Salinity Stresses
Abstract
1. Introduction
2. Material and Methods
2.1. Plant Material and Stress Treatments
2.2. Germination and Growth Conditions
2.3. Measurement of Germination, Plant Growth and Tolerance Indices
2.4. Experimental Design and Statistical Analyses
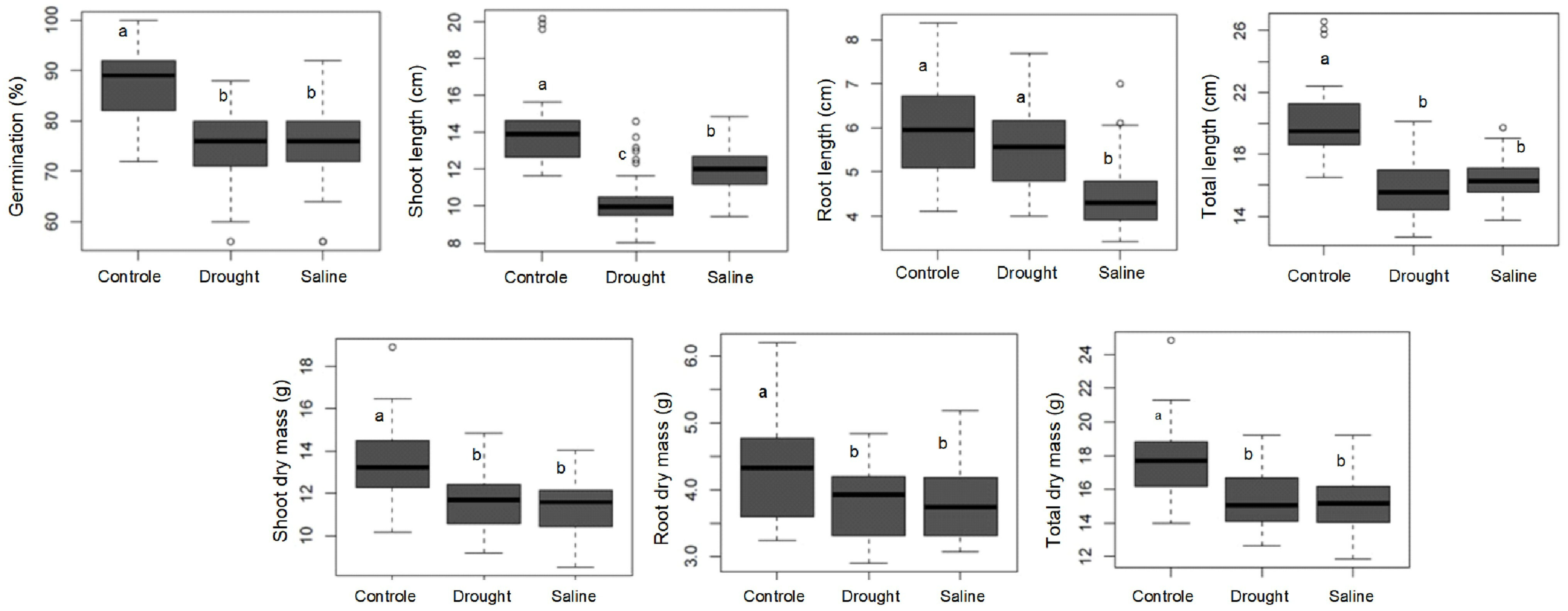
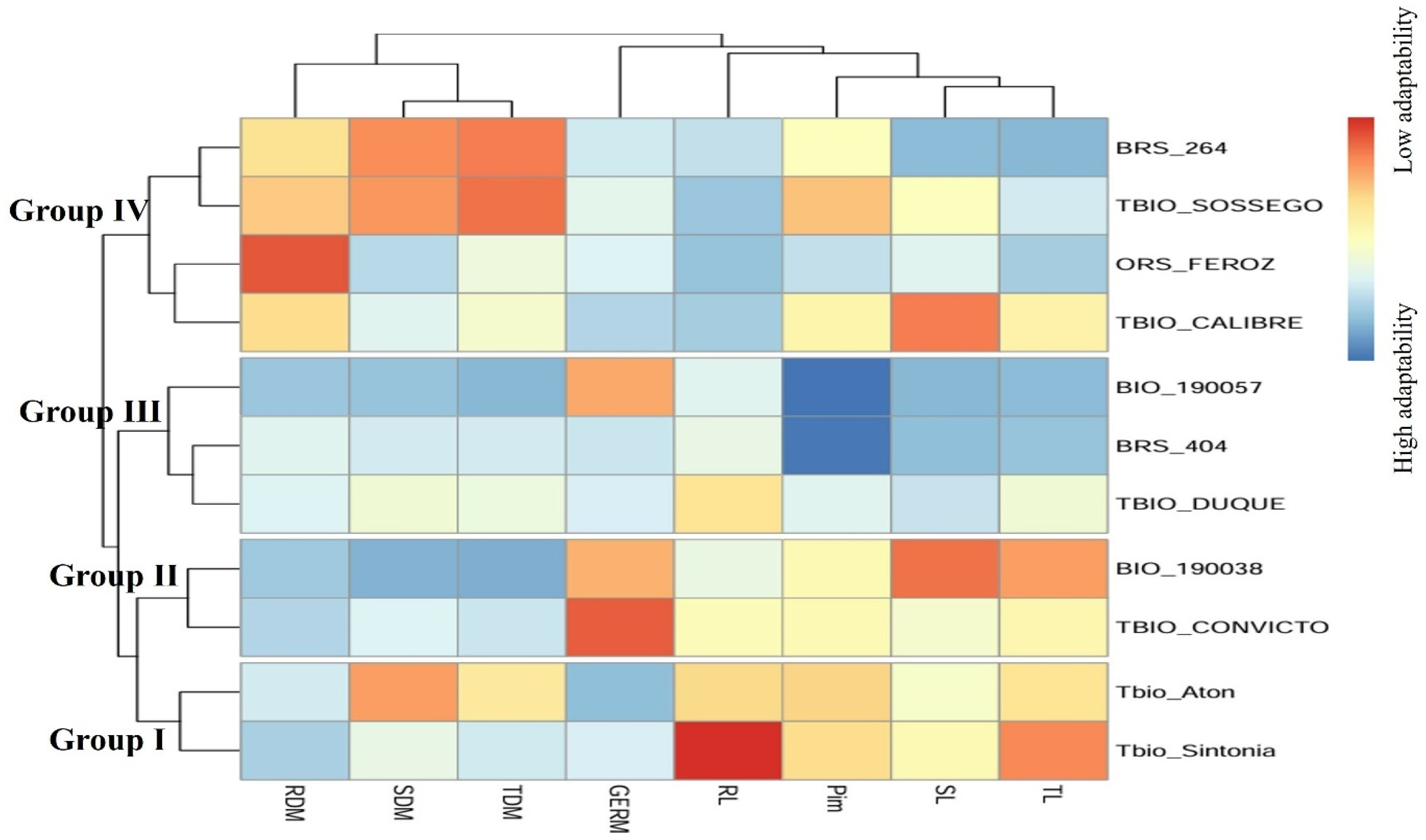
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pereira, J.F.; Cunha, G.R.; Moresco, E.R. Improved drought tolerance in wheat is required to unlock the production potential of the Brazilian Cerrado. Crop Breed. Appl. Biotechnol. 2019, 19, 217–225. [Google Scholar] [CrossRef]
- Oliveira, M.E.A.S.; Alves, T.D.O.; Gutkoski, L.C.; Miranda, M.Z.; Larraz Ferreira, M.S.; Takeiti, C.Y. Brazilian Cerrado wheat: Technological quality of genotypes grown in tropical locations. J. Food Process. Preserv. 2022, 46, e16228. [Google Scholar] [CrossRef]
- Naeem, M.K.; Ahmad, M.; Kamran, M.; Shah, M.K.N.; Iqbal, M.S. Physiological responses of wheat (Triticum aestivum L.) to drought stress. Int. J. Plant Soil Sci. 2015, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nyaupane, S.; Poudel, M.R.; Panthi, B.; Dhakal, A.; Paudel, H.; Bhandari, R. Drought stress effect, tolerance, and management in wheat—A review. Cogent Food Agric. 2024, 10, e2296094. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, X.; Zhao, Y.; Tang, G.; Nan, R.; Zhang, Y.; Zhang, C. Evaluation of wheat drought resistance using hyperspectral and chlorophyll fluorescence imaging. Plant Physiol. Biochem. 2025, 219, e109415. [Google Scholar] [CrossRef]
- Guizani, A.; Askri, H.; Amenta, M.L.; Defez, R.; Babay, E.; Bianco, C.; Rapaná, N.; Finetti-Sialer, M.; Gharbi, F. Drought responsiveness in six wheat genotypes: Identification of stress resistance indicators. Front. Plant Sci. 2023, 13, e1232583. [Google Scholar] [CrossRef]
- Khan, R.A.M.; Khan, A.S.; Sadaqat, H.A.; Saeed, F. Combining ability assessments of some morpho-physiological traits for improved grain production in bread wheat under water deficit stress conditions. Pak. J. Agric. Sci. 2020, 57, 1011–1019. [Google Scholar] [CrossRef]
- Bavandpouri, F.; Farshadfar, E.; Cheghamirza, K.; Farshadfar, M.; Bihamta, M.R.; Mahdavi, A.M.; Jelodar, N. Identification of molecular markers associated with genomic regions controlling agronomic traits in bread wheat genotypes under different moisture conditions. Plant Mol. Biol. Report. 2024, 1–21. [Google Scholar] [CrossRef]
- Mellers, G.; Aguilera, J.G.; Bird, N.; Variani Bonato, A.L.; Bonow, S.; Caierão, E.; Consoli, L.; Santana, F.M.; Simmonds, J.; Steed, A.; et al. Genetic characterization of a wheat association mapping panel relevant to Brazilian breeding using a high-density single-nucleotide polymorphism array. G3-Genes Genomes Genet. 2020, 10, 2229–2239. [Google Scholar] [CrossRef]
- Nazir, M. Salinity stress resistance in wheat. World J. Biol. Biotechnol. 2021, 6, 27–31. [Google Scholar] [CrossRef]
- Shahzad, A.; Iqbal, M.; Asif, M.; Hirani, A.H.; Goyal, A. Growing wheat on saline lands: Can a dream come true? Aust. J. Crop Sci. 2013, 7, 515–524. [Google Scholar]
- Ilyas, M.; Ahad, A.; Batool, T.S.; Jabbar, W.; Ejaz, M.; Gul, A.; Ozturk, M. Salinity induced stress in wheat. J. Cereal Res. 2022, 14, 67–82. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Aslam, M.T.; Alhammad, B.A.; Hassan, M.U.; Maqbool, R.; Chattha, M.U.; Khan, I.; Gitari, H.I.; Uslu, O.S.; Roy, R.; et al. Salinity stress in wheat: Effects, mechanisms and management strategies. Phyton 2022, 91, e017365. [Google Scholar] [CrossRef]
- Miransari, M.; Smith, D. Sustainable wheat (Triticum aestivum L.) production in saline fields: A review. Crit. Rev. Biotechnol. 2019, 39, 999–1014. [Google Scholar] [CrossRef]
- Ministério da Agricultura, Pecuária e Abastecimento. Regras para Análise de Sementes; MAPA/ACS: Brasília, DF, Brasil, 2009.
- Michel, B.E.; Kaufmann, M.R. The osmotic potential of polyethylene glycol 6000. Plant Physiol. 1973, 51, 914–916. [Google Scholar] [CrossRef]
- Hillel, D. Soil and Water: Physical Principles and Process; Academic Press: New York, NY, USA, 1971. [Google Scholar]
- Zuffo, A.M.; Steiner, F.; Sousa, T.O.; Aguilera, J.G.; Teodoro, P.E.; Alcântara-Neto, F.; Ratke, R.F. How does water and salt stress affect the germination and initial growth of Brazilian soya bean cultivars? J. Agron. Crop Sci. 2020, 206, 837–850. [Google Scholar] [CrossRef]
- Lin, C.S.; Binns, M.R. A superiority measure of cultivar performance for cultivar× location data. Can. J. Plant Sci. 1988, 68, 193–198. [Google Scholar] [CrossRef]
- Aguilera, J.G.; Teodoro, P.E.; Silva, J.P.; Pereira, J.F.; Zuffo, A.M.; Consoli, L. Selection of aluminum-resistant wheat genotypes using multienvironment and multivariate indices. Agron. J. 2019, 111, 2804–2810. [Google Scholar] [CrossRef]
- Yan, W.; Tinker, N.A. Biplot analysis of multienvironment trial data: Principles and applications. Can. J. Plant Sci. 2006, 86, 623–645. [Google Scholar] [CrossRef]
- Bhering, L.L. Rbio: A tool for biometric and statistical analysis using the R platform. Crop Breed. Appl. Biotechnol. 2017, 17, 187–190. [Google Scholar] [CrossRef]
- Pereira, J.F.; Barichelo, D.; Ferreira, J.R.; Aguilera, J.G.; Consoli, L.; Silva Júnior, J.P.; Cargnin, A.; Bonow, S. TaALMT1 and TaMATE1B allelic variability in a collection of Brazilian wheat and its association with root growth on acidic soil. Mol. Breed. 2015, 35, 169. [Google Scholar] [CrossRef]
- Aguilera, J.G.; Minozzo, J.A.D.; Barichello, D.; Fogaça, C.M.; Silva, J.P.; Consoli, L.; Pereira, J.F. Alleles of organic acid transporter genes are highly correlated with wheat resistance to acidic soil in field conditions. Theor. Appl. Genet. 2016, 129, 1317–1331. [Google Scholar] [CrossRef] [PubMed]
- Argentel-Martinez, L.; Penuelas-Rubio, O.; Perez-Lopez, L.; Aguilera, J.G.; Steiner, F.; Zuffo, A.M.; Ratke, R.F. Assessing salinity, drought and high temperature stress in maize (Zea mays L.) and wheat (Triticum aestivum L.) varieties: Theoretical combination as multifactorial stress. J. Agron. Crop Sci. 2024, 210, e70001. [Google Scholar] [CrossRef]
- Pereira, J.F. Initial root length in wheat is highly correlated with acid soil tolerance in the field. Sci. Agric. 2018, 75, 79–83. [Google Scholar] [CrossRef]
- Roychoudhury, A.; Paul, S.; Basu, S. Cross-talk between abscisic acid-dependent and abscisic acid-independent pathways during abiotic stress. Plant Cell Rep. 2013, 32, 985–1006. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.P.O.; Sousa, D.J.P.; Carvalho, C.G.; Willmann, G.O.; Dias, D.C.F.S.; Nardino, M. Wheat genotypes selection via multitrait for abiotic stresses. Ciência Rural 2024, 54, e20230280. [Google Scholar] [CrossRef]
- Steiner, F.; Zuffo, A.M.; Zoz, T.; Zoz, A.; Zoz, J. Drought tolerance of wheat and black oat crops at early stages of seedling growth. Rev. Ciênc. Agrár. 2017, 40, 576–586. [Google Scholar] [CrossRef]
- Antonini, J.C.A.; Muller, A.G.; Oliveira, A.D.; Ribeiro, F.P.; Chagas, J.H.; Sussel, A.A.B.; Albrecht, J.C. Winter wheat irrigation parameters in the Brazilian Cerrado. Aust. J. Crop Sci. 2024, 18, 99–106. [Google Scholar]
- Yılmaz, P.; Babat, C.F.; Yılmaz, C. Comparison of short-term physiological and biochemical effects of drought stress on two wheat cultivars. Braz. Arch. Biol. Technol. 2023, 66, e23220705. [Google Scholar]
- Teodoro, P.E.; Farias, F.J.C.; Carvalho, L.P.; Ribeiro, L.P.; Nascimento, M.; Azevedo, C.F.; Bhering, L.L. Adaptability and stability of cotton genotypes regarding fiber yield and quality traits. Crop Sci. 2019, 59, 518–524. [Google Scholar] [CrossRef]
- Yan, W.; Hunt, L.A.; Sheng, Q.; Szlavnics, Z. Cultivar evaluation and mega-environment investigation based on GGE biplot. Crop Sci. 2007, 47, 643–653. [Google Scholar] [CrossRef]
- Yan, W. GGE Biplot vs. AMMI graphs for genotypeby-environment data analysis. J. Indian Soc. Agric. Stat. 2011, 65, 181–193. [Google Scholar]
- Ge, M.; Tang, Y.; Guan, Y.; Lv, M.; Zhou, C.; Ma, H.; Lv, J. TaWRKY31, a novel WRKY transcription factor in wheat, participates in regulation of plant drought stress tolerance. BMC Plant Biol. 2024, 24, 27. [Google Scholar] [CrossRef]
- Gudi, S.; Halladakeri, P.; Singh, G.; Kumar, P.; Singh, S.; Alwutayd, K.M.; Abd El-Moneim, D.; Sharma, A. Deciphering the genetic landscape of seedling drought stress tolerance in wheat (Triticum aestivum L.) through genome-wide association studies. Front. Plant Sci. 2024, 15, e1351075. [Google Scholar] [CrossRef]

| Wheat Cultivar | Maturation Cycle | Water Content (%) | 1000-Seed Weight (g) | Seed Germination (%) | Plant Emergence (%) |
|---|---|---|---|---|---|
| TBIO ATON | Medium | 12.7 | 37.88 b | 95 a | 100 a |
| TBIO SINTONIA | Early | 12.5 | 32.55 e | 86 a | 82 d |
| BRS 404 | Early/Medium | 12.8 | 38.73 b | 89 a | 92 b |
| BRS 264 | Super Early | 12.7 | 36.13 d | 92 a | 100 a |
| TBIO CALIBRE | Super Early | 12.5 | 35.05 d | 88 a | 100 a |
| BIO 190057 | Medium/Early | 12.6 | 37.08 c | 83 b | 100 a |
| TBIO DUQUE | Early | 12.9 | 34.68 d | 91 a | 100 a |
| BIO 190038 | Early | 12.7 | 37.20 c | 79 b | 100 a |
| TBIO SOSSEGO | Medium | 12.6 | 34.90 d | 88 a | 100 a |
| ORS FEROZ | Super Early | 12.5 | 40.10 a | 94 a | 100 a |
| TBIO CONVICTO | Medium/Late | 12.8 | 40.33 a | 82 b | 84 c |
| Sources of Variation | DF | Probability > F | ||||||
|---|---|---|---|---|---|---|---|---|
| G | SL | RL | TL | SDM | RDM | TDM | ||
| Genotypes (G) | 10 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Environments (E) | 2 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| G × E | 20 | 0.282 | 0.212 | 0.001 | 0.051 | 0.025 | 0.481 | 0.076 |
| Mean | 79.23 | 2.07 | 5.34 | 17.41 | 12.11 | 3.98 | 16.08 | |
| CV (%) | 7.64 | 9.95 | 11.29 | 7.85 | 9.21 | 9.92 | 8.36 | |
| Cultivar | Pi Values—Lin and Binns (1988) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G | SL | RL | TL | SDM | RDM | TDM | Pim | |||||||||||||||
| D * | S | Pi | D | S | Pi | D | S | Pi | D | S | Pi | D | S | Pi | D | S | Pi | D | S | Pi | ||
| BIO 190038 | 99 | 109 | 4.70 | 6 | 5 | 5.40 | 1 | 0.5 | 1.47 | 8 | 7 | 4.57 | 0 | 0 | 0.00 | 0.01 | 0.01 | 0.05 | 0 | 0 | 0.00 | 8.31 |
| BIO 190057 | 58 | 128 | 4.11 | 0.03 | 0.03 | 0.03 | 0.6 | 0.5 | 1.17 | 0.03 | 0.1 | 0.04 | 0.4 | 0.4 | 0.35 | 0.002 | 0.001 | 0.01 | 0.4 | 0.4 | 0.21 | 3.08 |
| BRS 264 | 22 | 14 | 0.83 | 0.003 | 0.07 | 0.04 | 0.5 | 0.3 | 0.81 | 0.03 | 0.03 | 0.02 | 7 | 6 | 5.68 | 0.8 | 0.7 | 3.89 | 12 | 11 | 6.04 | 8.72 |
| BRS 404 | 20 | 20 | 0.91 | 0.001 | 0.2 | 0.10 | 1 | 0.5 | 1.47 | 0.2 | 0.3 | 0.15 | 2 | 2 | 1.75 | 0.3 | 0.1 | 1.03 | 3 | 3 | 1.58 | 3.56 |
| ORS Feroz | 36 | 6 | 0.99 | 2 | 1 | 1.48 | 0 | 0.04 | 0.06 | 0.5 | 0.8 | 0.40 | 1 | 1 | 0.88 | 1 | 1 | 5.19 | 4 | 4 | 2.10 | 5.61 |
| TBIO Aton | 0 | 0 | 0.00 | 3 | 3 | 2.94 | 3 | 1 | 3.72 | 7 | 6 | 3.96 | 6 | 5 | 4.80 | 0.2 | 0.1 | 0.77 | 7 | 5 | 3.15 | 9.80 |
| TBIO Calibre | 17 | 14 | 0.71 | 5 | 6 | 5.39 | 0.2 | 0.1 | 0.29 | 5 | 6 | 3.37 | 2 | 2 | 1.75 | 0.8 | 0.7 | 3.89 | 5 | 5 | 2.63 | 9.12 |
| TBIO Convicto | 132 | 116 | 5.64 | 3 | 2 | 2.46 | 2 | 1 | 2.95 | 6 | 3 | 2.71 | 2 | 2 | 1.75 | 0.1 | 0.08 | 0.47 | 3 | 2 | 1.31 | 8.88 |
| TBIO Duque | 3 | 39 | 0.90 | 1 | 1 | 0.98 | 2 | 2 | 4.35 | 4 | 3 | 2.13 | 2 | 1 | 1.29 | 0.2 | 0.2 | 1.04 | 3 | 2 | 1.31 | 6.12 |
| TBIO Sintonia | 24 | 34 | 1.30 | 3 | 4 | 3.43 | 4 | 2 | 5.89 | 9 | 9 | 5.50 | 2 | 3 | 2.22 | 0.05 | 0.03 | 0.21 | 3 | 3 | 1.58 | 10.26 |
| TBIO Sossego | 17 | 44 | 1.34 | 3 | 3 | 2.94 | 0.09 | 0.04 | 0.13 | 2 | 2 | 1.22 | 6 | 6 | 5.26 | 0.9 | 0.8 | 4.41 | 11 | 11 | 5.79 | 10.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuffo, A.M.; Aguilera, J.G.; Silva, F.C.d.S.; Mezzomo, R.; Barrozo, L.M.; Steiner, F.; Oliveira, B.R.d.; Soto, C.A.M.; Morales-Aranibar, C.G.; Linares-Gutiérrez, N.; et al. Multivariate Adaptability of Tropical Wheat Cultivars to Drought and Salinity Stresses. Plants 2025, 14, 1021. https://doi.org/10.3390/plants14071021
Zuffo AM, Aguilera JG, Silva FCdS, Mezzomo R, Barrozo LM, Steiner F, Oliveira BRd, Soto CAM, Morales-Aranibar CG, Linares-Gutiérrez N, et al. Multivariate Adaptability of Tropical Wheat Cultivars to Drought and Salinity Stresses. Plants. 2025; 14(7):1021. https://doi.org/10.3390/plants14071021
Chicago/Turabian StyleZuffo, Alan Mario, Jorge González Aguilera, Francisco Charles dos Santos Silva, Ricardo Mezzomo, Leandra Matos Barrozo, Fábio Steiner, Bruno Rodrigues de Oliveira, César Augusto Masgo Soto, Carlos Genaro Morales-Aranibar, Nataniel Linares-Gutiérrez, and et al. 2025. "Multivariate Adaptability of Tropical Wheat Cultivars to Drought and Salinity Stresses" Plants 14, no. 7: 1021. https://doi.org/10.3390/plants14071021
APA StyleZuffo, A. M., Aguilera, J. G., Silva, F. C. d. S., Mezzomo, R., Barrozo, L. M., Steiner, F., Oliveira, B. R. d., Soto, C. A. M., Morales-Aranibar, C. G., Linares-Gutiérrez, N., & Morales-Aranibar, L. (2025). Multivariate Adaptability of Tropical Wheat Cultivars to Drought and Salinity Stresses. Plants, 14(7), 1021. https://doi.org/10.3390/plants14071021











