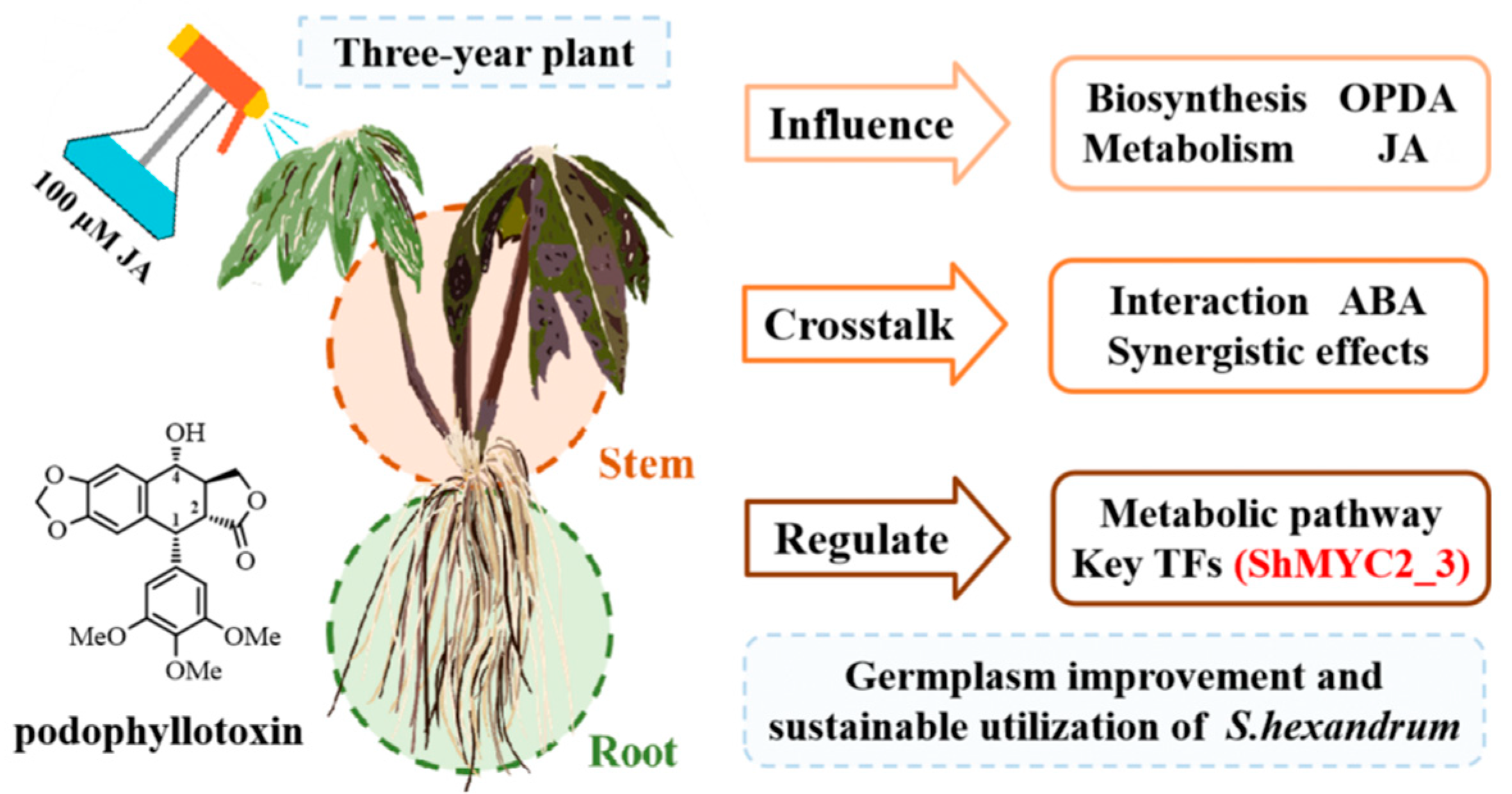
The Dynamic Changes in Biosynthesis and Spatiotemporal Distribution of Phytohormones Under Jasmonic Acid Treatment Provide Insights into Hormonal Regulation in Sinopodophyllum hexandrum
Abstract
1. Introduction
2. Results
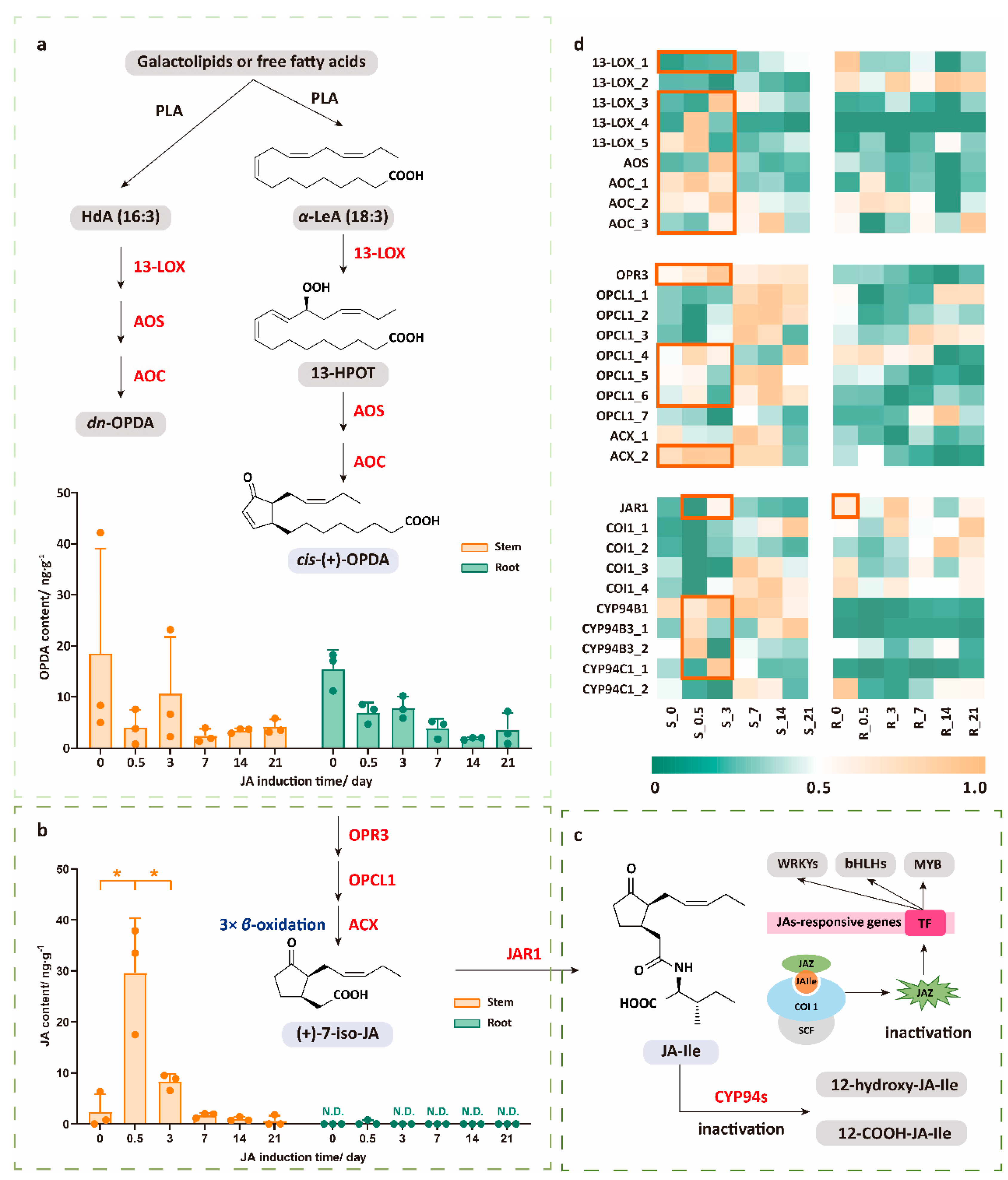
2.1. Dynamic Response Characteristics of JA Pathway Under JA Treatment
2.2. The High Expression Level of JAR1 Mainly Contributes to the Rapid Decline in JA Levels, Which Transfers to JA-Ile and Rapidly Oxidizes to Become Inactivated
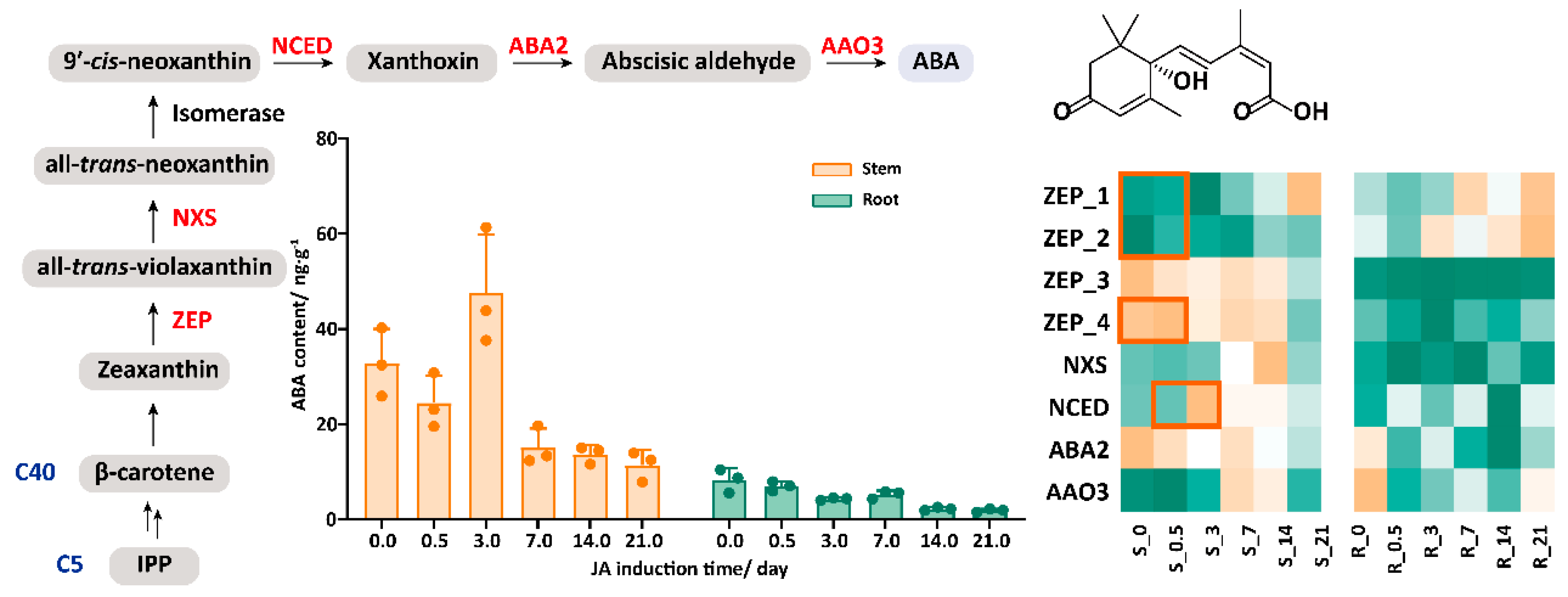
2.3. The Synergistic Effect of JA and ABA in Response to JA Treatment
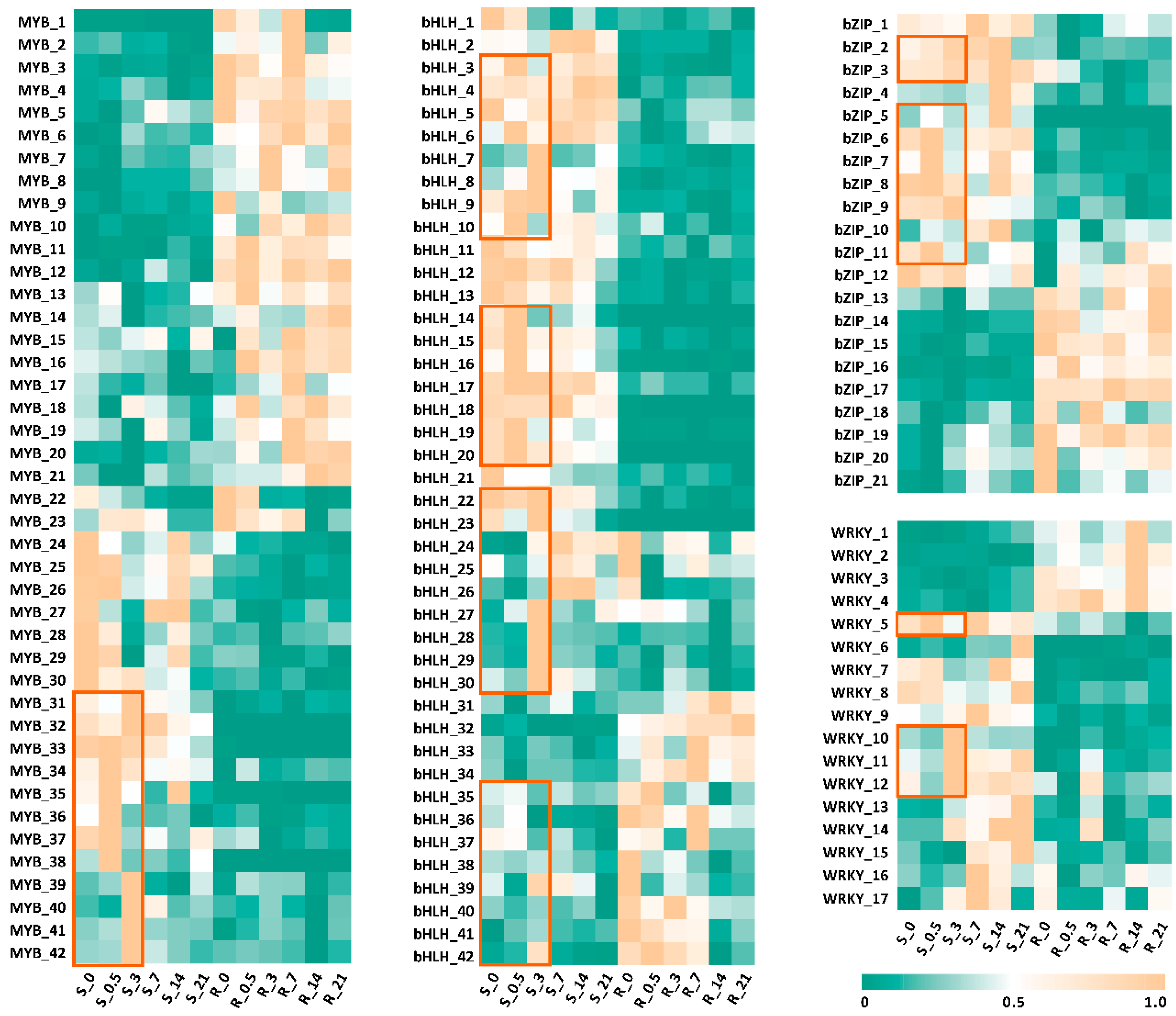
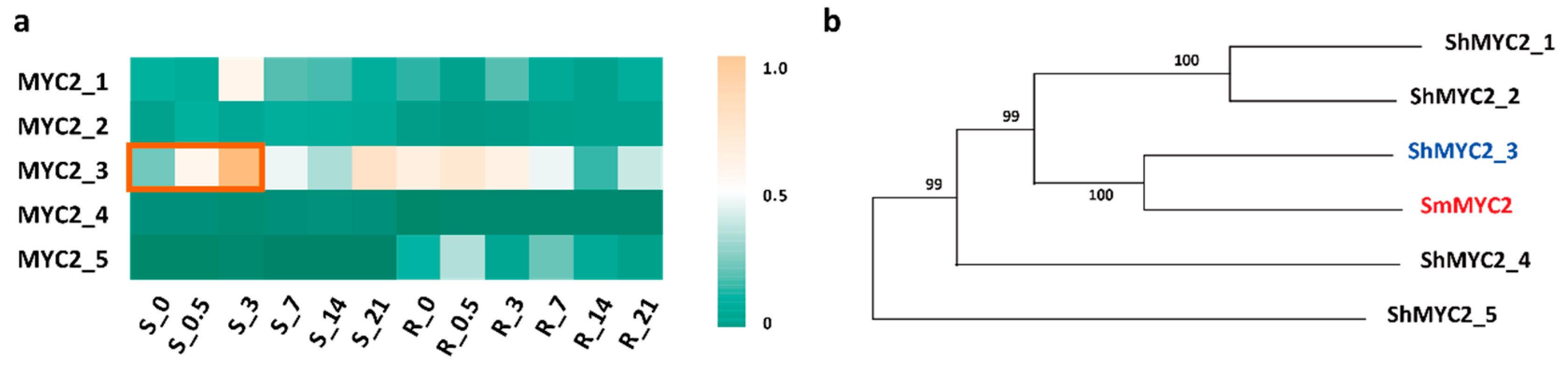
2.4. ShMYC2_3 May Serve as a Key Regulator Within a Complex Network That Orchestrates the Plant’s Response to JA Signals While Mediating the Production of Secondary Metabolites in S. hexandrum
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Sample Collection
4.2. Extraction of Phytohormones
4.3. LC-MS Conditions for Phytohormone Detection and Standard Curve Plotting
4.4. mRNA Sequencing and Library Construction
4.5. Quantification of Gene Expression Using FPKM in Transcriptome Sequencing
4.6. RNA-Seq Data Analysis, De Novo Transcriptome Assembly, and Annotation
4.7. Differential Gene Expression Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, L.; Sun, P.; Li, M.F. Chilling temperature stimulates growth, gene over-expression and podophyllotoxin biosynthesis in Podophyllum hexandrum Royle. Plant Physiol. Biochem. PPB 2016, 107, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Su, H.Y.; Li, M.L.; Yang, D.L.; Yao, R.Y.; Li, M.F.; Wei, J.H. Effect of UV-B radiation on growth, flavonoid and podophyllotoxin accumulation, and related gene expression in Sinopodophyllum hexandrum. Plant Biol. 2020, 23, 202–209. [Google Scholar] [CrossRef]
- Li, M.; Ge, K.; Sun, T.; Xing, P.; Yang, H. High-elevation cultivation increases anti-cancer podophyllotoxin accumulation in Podophyllum hexandrum. Ind. Crops Prod. 2018, 121, 338–344. [Google Scholar] [CrossRef]
- Shen, S.; Tong, Y.; Luo, Y.; Huang, L.; Gao, W. Biosynthesis, total synthesis, and pharmacological activities of aryltetralin-type lignan podophyllotoxin and its derivatives. Nat. Prod. Rep. 2022, 39, 1856–1875. [Google Scholar] [CrossRef]
- Cao, X.; Li, M.; Li, J.; Song, Y.; Zhang, X.; Yang, D.; Li, M.; Wei, J. Co-expression of hydrolase genes improves seed germination of Sinopodophyllum hexandrum. Ind. Crops Prod. 2021, 164. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, M.; Jin, L.; Wei, J. Changes in growth characteristics and secondary metabolites in Sinopodophyllum hexandrum with increasing age. Ind. Crops Prod. 2023, 196, 116509. [Google Scholar] [CrossRef]
- Wei, H.; Jing, Y.; Zhang, L.; Kong, D. Phytohormones and their crosstalk in regulating stomatal development and patterning. J. Exp. Bot. 2021, 72, 2356–2370. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Liu, Q.; Wang, B.; Yuan, F. Roles of Phytohormones and Their Signaling Pathways in Leaf Development and Stress Responses. J. Agric. Food Chem. 2021, 69, 3566–3584. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Tong, Y.R.; Wang, T.L.; Zhou, X.T.; Zhou, J.H.; Ge, Y.; Zheng, H.; Yu, M.Y.; Luo, Y.F.; Ji, R.F. Challenges of Continuous Cropping Obstacles in Panax ginseng: Formation and Response Mechanisms. Sci. Tradit. Chin. Med. [CrossRef]
- Nguyen, T.H.; Goossens, A.; Lacchini, E. Jasmonate: A hormone of primary importance for plant metabolism. Curr. Opin. Plant Biol. 2022, 67, 102197. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Wang, H.; You, Q.; Cao, R.; Sun, G.; Yu, D. Jasmonate-regulated seed germination and crosstalk with other phytohormones. J. Exp. Bot. 2023, 74, 1162–1175. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, Y.; Wang, S.; Qi, T.; Song, S. Jasmonate action and crosstalk in flower development and fertility. J. Exp. Bot. 2023, 74, 1186–1197. [Google Scholar] [CrossRef]
- Li, C.; Xu, M.; Cai, X.; Han, Z.; Si, J.; Chen, D. Jasmonate Signaling Pathway Modulates Plant Defense, Growth, and Their Trade-Offs. Int. J. Mol. Sci. 2022, 23, 3945. [Google Scholar] [CrossRef] [PubMed]
- Staswick, P.E.; Yuen, G.Y.; Lehman, C.C. Jasmonate signaling mutants of Arabidopsis are susceptible to the soil fungus Pythium irregulare. Plant J. Cell Mol. Biol. 1998, 15, 747–754. [Google Scholar] [CrossRef]
- Dicke, M. Specificity of herbivore-induced plant defences. Novartis Found. Symp. 1999, 223, 43–54, discussion 54–49, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Liu, Y.; Cai, P.; Duan, X.; Sang, S.; Qiu, Z. Jasmonic acid pretreatment improves salt tolerance of wheat by regulating hormones biosynthesis and antioxidant capacity. Front. Plant Sci. 2022, 13, 968477. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Ma, X.; Qiu, Y.; Lu, Y.; Shen, S.; Li, Y.; Gao, H.; Chen, K.; Zhou, J.; Hu, T.; et al. Structural and Catalytic Insight into the Unique Pentacyclic Triterpene Synthase TwOSC. Angew. Chem. (Int. Ed. Engl.) 2023, 62, e202313429. [Google Scholar] [CrossRef]
- Tong, Y.; Ma, X.; Hu, T.; Chen, K.; Cui, G.; Su, P.; Xu, H.; Gao, W.; Jiang, T.; Huang, L. Structural and mechanistic insights into the precise product synthesis by a bifunctional miltiradiene synthase. Plant Biotechnol. J. 2023, 21, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Luo, T.; Guo, X.; Zou, X.; Zhou, D.; Afrin, S.; Li, G.; Zhang, Y.; Zhang, R.; Luo, Z. PgMYB2, a MeJA-Responsive Transcription Factor, Positively Regulates the Dammarenediol Synthase Gene Expression in Panax Ginseng. Int. J. Mol. Sci. 2019, 20, 2219. [Google Scholar] [CrossRef]
- Chu, Y.; Xiao, S.; Su, H.; Liao, B.; Zhang, J.; Xu, J.; Chen, S. Genome-wide characterization and analysis of bHLH transcription factors in Panax ginseng. Acta Pharm. Sinica B 2018, 8, 666–677. [Google Scholar] [CrossRef]
- Xiu, H.; Nuruzzaman, M.; Guo, X.; Cao, H.; Huang, J.; Chen, X.; Wu, K.; Zhang, R.; Huang, Y.; Luo, J.; et al. Molecular Cloning and Expression Analysis of Eight PgWRKY Genes in Panax ginseng Responsive to Salt and Hormones. Int. J. Mol. Sci. 2016, 17, 319. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Fu, X.; Shao, J.; Tang, Y.; Yu, M.; Li, L.; Huang, L.; Tang, K. Transcriptional regulatory network of high-value active ingredients in medicinal plants. Trends Plant Sci. 2023, 28, 429–446. [Google Scholar] [CrossRef]
- Tong, Y.R.; Chen, K.; Jiang, Z.Q.; Tu, L.C.; Luo, Y.F.; Zheng, H.; Zhao, Y.Q.; Shen, S.Y.; Hu, Y.T.; Gao, W. Spatiotemporal expression analysis of jasmonic acid and saponin-related genes uncovers a potential biosynthetic regulation in Panax notoginseng. J. Sci. Food Agric. 2024, 104, 9772–9781. [Google Scholar] [CrossRef]
- Hazra, S.; Bhattacharyya, D.; Chattopadhyay, S. Methyl Jasmonate Regulates Podophyllotoxin Accumulation in Podophyllum hexandrum by Altering the ROS-Responsive Podophyllotoxin Pathway Gene Expression Additionally through the Down Regulation of Few Interfering miRNAs. Front. Plant Sci. 2017, 8, 164. [Google Scholar] [CrossRef]
- Bhattacharyya, D.; Sinha, R.; Ghanta, S.; Chakraborty, A.; Hazra, S.; Chattopadhyay, S. Proteins differentially expressed in elicited cell suspension culture of Podophyllum hexandrum with enhanced podophyllotoxin content. Proteome Sci. 2012, 10, 34. [Google Scholar] [CrossRef]
- Jimenez Aleman, G.H.; Thirumalaikumar, V.P.; Jander, G.; Fernie, A.R.; Skirycz, A. OPDA, more than just a jasmonate precursor. Phytochemistry 2022, 204, 113432. [Google Scholar] [CrossRef] [PubMed]
- Staswick, P.E.; Tiryaki, I. The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell 2004, 16, 2117–2127. [Google Scholar] [CrossRef]
- Hu, S.; Yu, K.; Yan, J.; Shan, X.; Xie, D. Jasmonate perception: Ligand-receptor interaction, regulation, and evolution. Mol. Plant 2023, 16, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Koo, A.J.; Thireault, C.; Zemelis, S.; Poudel, A.N.; Zhang, T.; Kitaoka, N.; Brandizzi, F.; Matsuura, H.; Howe, G.A. Endoplasmic reticulum-associated inactivation of the hormone jasmonoyl-L-isoleucine by multiple members of the cytochrome P450 94 family in Arabidopsis. J. Biol. Chem. 2014, 289, 29728–29738. [Google Scholar] [CrossRef] [PubMed]
- Kitaoka, N.; Matsubara, T.; Sato, M.; Takahashi, K.; Wakuta, S.; Kawaide, H.; Matsui, H.; Nabeta, K.; Matsuura, H. Arabidopsis CYP94B3 encodes jasmonyl-L-isoleucine 12-hydroxylase, a key enzyme in the oxidative catabolism of jasmonate. Plant Cell Physiol. 2011, 52, 1757–1765. [Google Scholar] [CrossRef] [PubMed]
- Koo, A.J.; Cooke, T.F.; Howe, G.A. Cytochrome P450 CYP94B3 mediates catabolism and inactivation of the plant hormone jasmonoyl-L-isoleucine. Proc. Natl. Acad. Sci. USA 2011, 108, 9298–9303. [Google Scholar] [CrossRef] [PubMed]
- Heitz, T.; Widemann, E.; Lugan, R.; Miesch, L.; Ullmann, P.; Désaubry, L.; Holder, E.; Grausem, B.; Kandel, S.; Miesch, M.; et al. Cytochromes P450 CYP94C1 and CYP94B3 catalyze two successive oxidation steps of plant hormone Jasmonoyl-isoleucine for catabolic turnover. J. Biol. Chem. 2012, 287, 6296–6306. [Google Scholar] [CrossRef]
- Ma, Y.N.; Xu, D.B.; Yan, X.; Wu, Z.K.; Kayani, S.I.; Shen, Q.; Fu, X.Q.; Xie, L.H.; Hao, X.L.; Hassani, D.; et al. Jasmonate- and abscisic acid-activated AaGSW1-AaTCP15/AaORA transcriptional cascade promotes artemisinin biosynthesis in Artemisia annua. Plant Biotechnol. J. 2021, 19, 1412–1428. [Google Scholar] [CrossRef]
- Kumar, P.; Jaiswal, V.; Pal, T.; Singh, J.; Chauhan, R.S. Comparative whole-transcriptome analysis in Podophyllum species identifies key transcription factors contributing to biosynthesis of podophyllotoxin in P. hexandrum. Protoplasma 2017, 254, 217–228. [Google Scholar] [CrossRef]
- Yang, N.; Zhou, W.; Su, J.; Wang, X.; Li, L.; Wang, L.; Cao, X.; Wang, Z. Overexpression of SmMYC2 Increases the Production of Phenolic Acids in Salvia miltiorrhiza. Front. Plant Sci. 2017, 8, 1804. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wei, X.; Yin, C.; Zhou, H.; Yan, J.; He, W.; Yan, J.; Li, H. ZmEREB57 regulates OPDA synthesis and enhances salt stress tolerance through two distinct signalling pathways in Zea mays. Plant Cell Environ. 2023, 46, 2867–2883. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Flores-Sandoval, E.; Ahtesham, U.; Coleman, A.; Clay, J.M.; Bowman, J.L.; Chang, C. Ethylene-independent functions of the ethylene precursor ACC in Marchantia polymorpha. Nat. Plants 2020, 6, 1335–1344. [Google Scholar] [CrossRef]
- Wasternack, C.; Hause, B. OPDA-Ile—a new JA-Ile-independent signal? Plant Signal. Behav. 2016, 11, e1253646. [Google Scholar] [CrossRef] [PubMed]
- Thurow, C.; Krischke, M.; Mueller, M.J.; Gatz, C. Induction of Jasmonoyl-Isoleucine (JA-Ile)-Dependent JASMONATE ZIM-DOMAIN (JAZ) Genes in NaCl-Treated Arabidopsis thaliana Roots Can Occur at Very Low JA-Ile Levels and in the Absence of the JA/JA-Ile Transporter JAT1/AtABCG16. Plants 2020, 9, 1635. [Google Scholar] [CrossRef] [PubMed]
- Lascombe, M.B.; Bakan, B.; Buhot, N.; Marion, D.; Blein, J.P.; Larue, V.; Lamb, C.; Prangé, T. The structure of “defective in induced resistance” protein of Arabidopsis thaliana, DIR1, reveals a new type of lipid transfer protein. Protein Sci. Publ. Protein Soc. 2008, 17, 1522–1530. [Google Scholar] [CrossRef] [PubMed]
- Lu, M. Structures of multidrug and toxic compound extrusion transporters and their mechanistic implications. Channels 2016, 10, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Tian, Y.; Zhang, C.; Qin, Y.; Liu, M.; Niu, S.; Li, Y.; Chen, J. The JASMONATE ZIM-domain-OPEN STOMATA1 cascade integrates jasmonic acid and abscisic acid signaling to regulate drought tolerance by mediating stomatal closure in poplar. J. Exp. Bot. 2023, 74, 443–457. [Google Scholar] [CrossRef]
- Yu, Q.; Hua, X.; Yao, H.; Zhang, Q.; He, J.; Peng, L.; Li, D.; Yang, Y.; Li, X. Abscisic acid receptors are involves in the Jasmonate signaling in Arabidopsis. Plant Signal. Behav. 2021, 16, 1948243. [Google Scholar] [CrossRef]
- Zhou, H.; Xie, Y.; Jiang, Y.; Nadeem, H.; Wang, Y.; Yang, N.; Zhu, H.; Tang, C. GhTLP1, a thaumatin-like protein 1, improves Verticillium wilt resistance in cotton via JA, ABA and MAPK signaling pathway-plant pathways. Int. J. Biol. Macromol. 2023, 253, 127388. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Hu, Y.; Wang, H.; Guo, Q.; Chen, Y.; Howe, G.A.; Yu, D. Molecular Mechanism Underlying the Synergetic Effect of Jasmonate on Abscisic Acid Signaling during Seed Germination in Arabidopsis. Plant Cell 2020, 32, 3846–3865. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, L.; Ding, X.; Fan, W.; Liu, J. Transcriptome Analysis Reveals Crosstalk between the Abscisic Acid and Jasmonic Acid Signaling Pathways in Rice-Mediated Defense against Nilaparvata lugens. Int. J. Mol. Sci. 2022, 23, 6319. [Google Scholar] [CrossRef]
- Zhao, Q.; Dong, M.; Li, M.; Jin, L.; Paré, P.W. Light-Induced Flavonoid Biosynthesis in Sinopodophyllum hexandrum with High-Altitude Adaptation. Plants 2023, 12, 575. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Liu, J.; Ji, R.; Chen, T.; Zhou, X.; Yang, J.; Tong, Y.; Jiang, C.; Zhou, J.; Zhao, Y.; et al. Biogenic Synthesis and Spatial Distribution of Endogenous Phytohormones and Ginsenosides Provide Insights on Their Intrinsic Relevance in Panax ginseng. Front. Plant Sci. 2018, 9, 1951. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, S.; Wu, Y.; Luo, Y.; Li, Y.; Gao, W.; Huang, L.; Hu, Y.; Chen, K.; Tong, Y. The Dynamic Changes in Biosynthesis and Spatiotemporal Distribution of Phytohormones Under Jasmonic Acid Treatment Provide Insights into Hormonal Regulation in Sinopodophyllum hexandrum. Plants 2025, 14, 1001. https://doi.org/10.3390/plants14071001
Shen S, Wu Y, Luo Y, Li Y, Gao W, Huang L, Hu Y, Chen K, Tong Y. The Dynamic Changes in Biosynthesis and Spatiotemporal Distribution of Phytohormones Under Jasmonic Acid Treatment Provide Insights into Hormonal Regulation in Sinopodophyllum hexandrum. Plants. 2025; 14(7):1001. https://doi.org/10.3390/plants14071001
Chicago/Turabian StyleShen, Siyu, Yuqing Wu, Yunfeng Luo, Yang Li, Wei Gao, Luqi Huang, Yating Hu, Kang Chen, and Yuru Tong. 2025. "The Dynamic Changes in Biosynthesis and Spatiotemporal Distribution of Phytohormones Under Jasmonic Acid Treatment Provide Insights into Hormonal Regulation in Sinopodophyllum hexandrum" Plants 14, no. 7: 1001. https://doi.org/10.3390/plants14071001
APA StyleShen, S., Wu, Y., Luo, Y., Li, Y., Gao, W., Huang, L., Hu, Y., Chen, K., & Tong, Y. (2025). The Dynamic Changes in Biosynthesis and Spatiotemporal Distribution of Phytohormones Under Jasmonic Acid Treatment Provide Insights into Hormonal Regulation in Sinopodophyllum hexandrum. Plants, 14(7), 1001. https://doi.org/10.3390/plants14071001





