The Effects of Drought Stress Intensity and Duration on the Dynamics of Nonstructural Carbohydrates in Pinus yunnanensis Seedlings
Abstract
1. Introduction
2. Results
2.1. Effect of Drought Stress on NSC
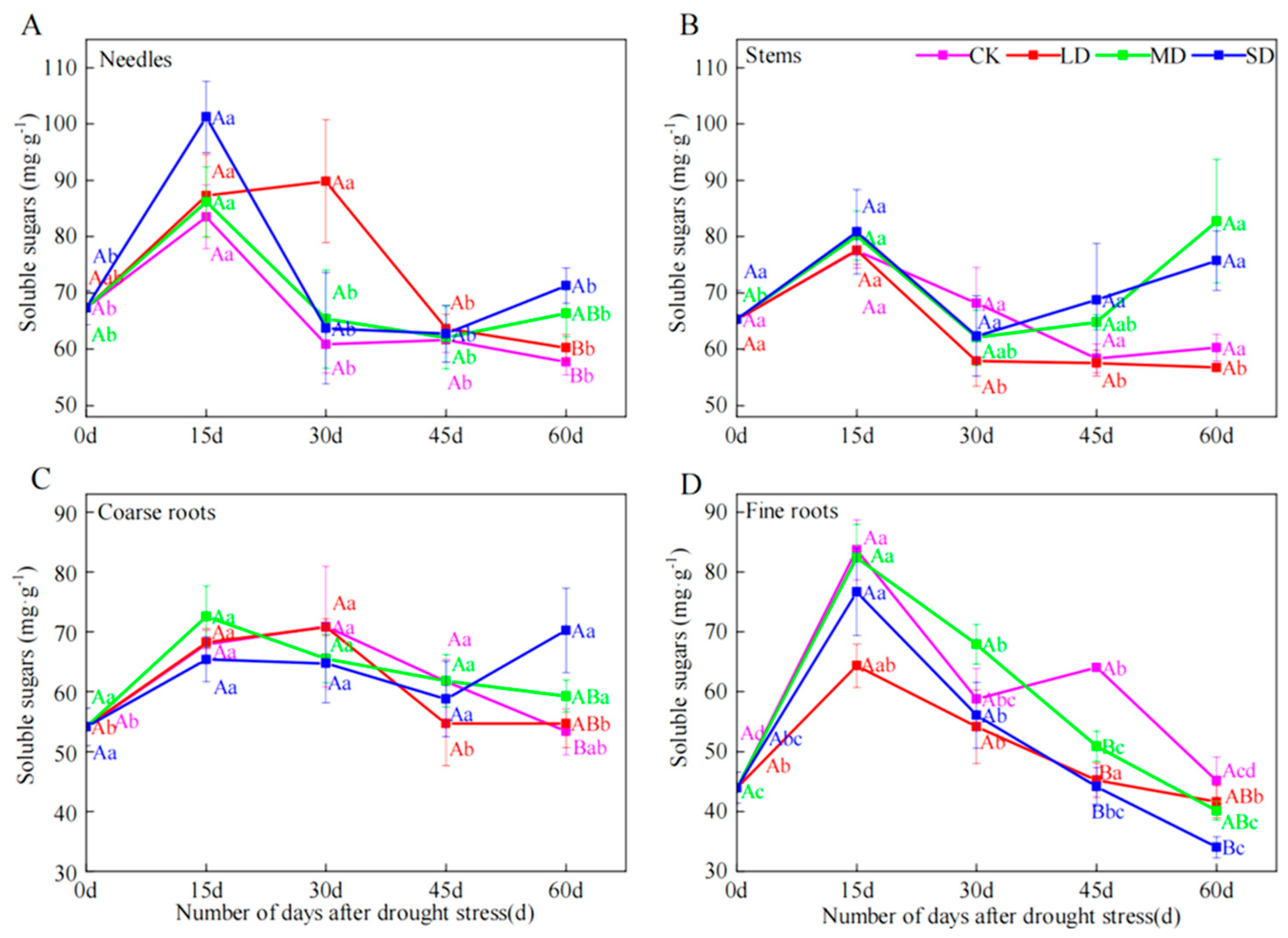
2.2. Soluble Sugar Concentrations
2.3. Starch Concentrations
2.4. NSC Concentrations
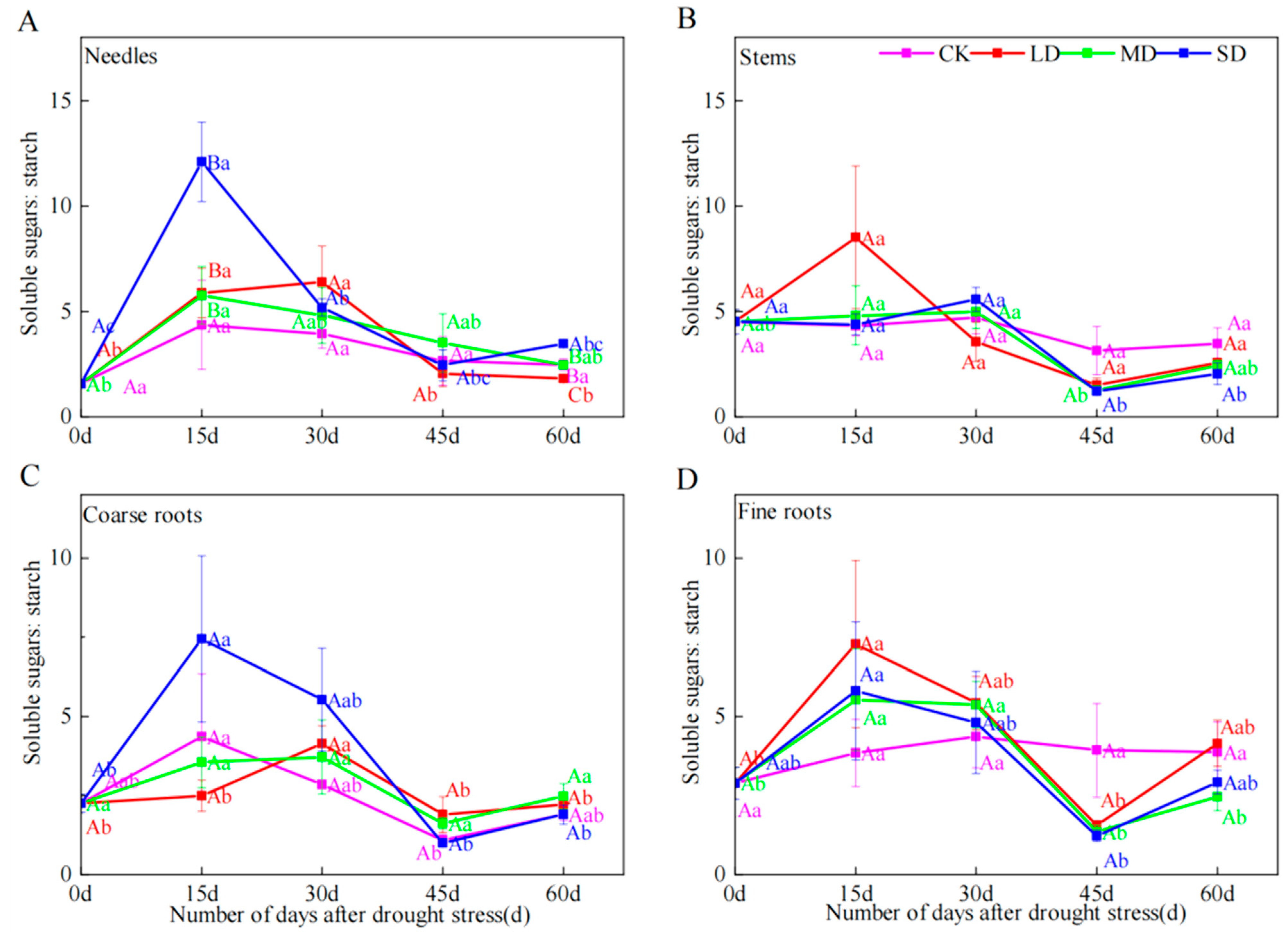
2.5. Soluble Sugar-to-Starch Ratio
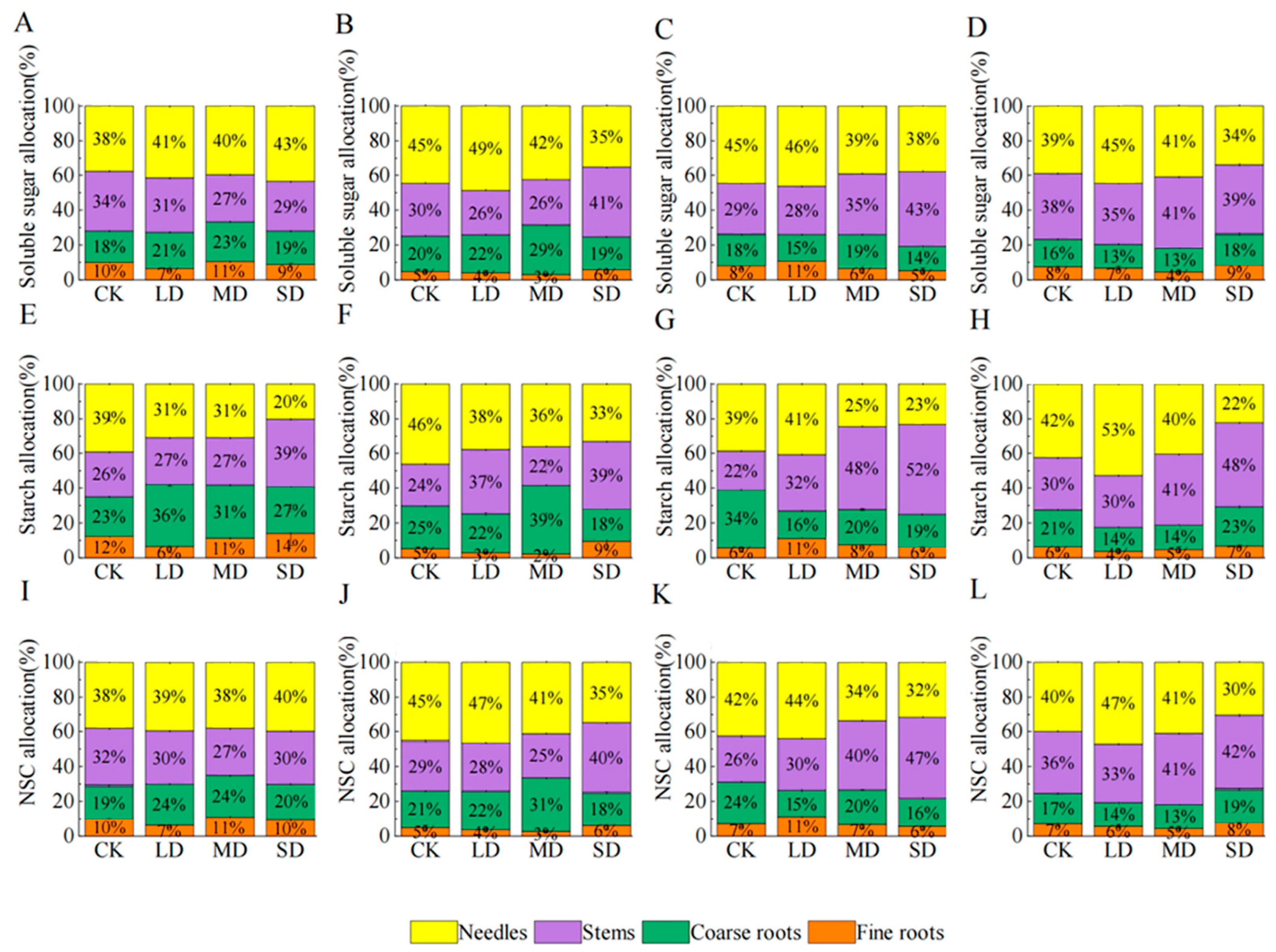
2.6. NSC Distribution Pattern
3. Materials and Methods
3.1. Experimental Site
3.2. Seedling Preparation
3.3. Application of Drought Treatments
3.4. Measurements and Sampling
3.5. Statistical Analysis
4. Discussion
4.1. Effects of Drought Intensity on NSC Dynamics
4.2. Effects of Drought Duration on NSC Dynamics
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Piao, S.; Wang, K.; Wang, X.; Wang, T.; Ciais, P.; Chen, A.; Lian, X.; Peng, S.; Peñuelas, J. Temporal trade-off between gymnosperm resistance and resilience increases forest sensitivity to extreme drought. Nat. Ecol. Evol. 2020, 4, 1075–1083. [Google Scholar] [PubMed]
- O’Brien, M.J.; Leuzinger, S.; Philipson, C.D.; Tay, J.; Hector, A. Drought survival of tropical tree seedlings enhanced by non-structural carbohydrate levels. Nat. Clim. Change 2014, 4, 710–714. [Google Scholar] [CrossRef]
- Tixier, A.; Guzmán-Delgado, P.; Sperling, O.; Amico Roxas, A.; Laca, E.; Zwieniecki, M.A. Comparison of phenological traits, growth patterns, and seasonal dynamics of non-structural carbohydrate in Mediterranean tree crop species. Sci. Rep. 2020, 10, 347. [Google Scholar]
- Shen, J.; Li, Z.; Gao, C.; Li, S.; Huang, X.; Lang, X.; Su, J. Radial growth response of Pinus yunnanensis to rising temperature and drought stress on the Yunnan Plateau, southwestern China. Forest Ecol. Manag. 2020, 474, 118357. [Google Scholar]
- McDowell, N.G.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- Nathalie, B.; Roland, H.; André, G.; Erwin, D. Temperate forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes and long-term consequences. Ann. Forest Sci. 2006, 63, 625–644. [Google Scholar]
- McDowell, N.G. Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiol. 2011, 155, 1051–1059. [Google Scholar] [CrossRef]
- Raessler, M.; Wissuwa, B.; Breul, A.; Unger, W.; Grimm, T. Chromatographic analysis of major non-structural carbohydrates in several wood species—An analytical approach for higher accuracy of data. Anal. Methods 2010, 2, 532–538. [Google Scholar] [CrossRef]
- Hartmann, H.; Adams, H.D.; Hammond, W.M.; Hoch, G.; Landhäusser, S.M.; Wiley, E.; Zaehle, S. Identifying differences in carbohydrate dynamics of seedlings and mature trees to improve carbon allocation in models for trees and forests. Environ. Exp. Bot. 2018, 152, 7–18. [Google Scholar]
- Zhang, P.; McDowell, N.G.; Zhou, X.; Wang, W.; Leff, R.T.; Pivovaroff, A.L.; Zhang, H.; Chow, P.S.; Ward, N.D.; Indivero, J.; et al. Declining carbohydrate content of Sitka-spruce treesdying from seawater exposure. Plant Physiol. 2021, 185, 1682–1696. [Google Scholar]
- He, W.; Liu, H.; Qi, Y.; Liu, F.; Zhu, X. Patterns in nonstructural carbohydrate contents at the tree organ level in response to drought duration. Glob. Change Biol. 2020, 26, 3627–3638. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, H.; Ziegler, W.; Trumbore, S. Lethal drought leads to reduction in nonstructural carbohydrates in Norway spruce tree roots but not in the canopy. Funct. Ecol. 2013, 27, 413–427. [Google Scholar] [CrossRef]
- O’Brien, M.J.; Burslem DF, R.P.; Caduff, A.; Tay, J.; Hector, A. Contrasting nonstructural carbohydrate dynamics of tropical tree seedlings under water deficit and variability. New Phytol. 2015, 205, 1083–1094. [Google Scholar] [CrossRef]
- Earles, J.M.; Stevens, J.T.; Sperling, O.; Orozco, J.; North, M.P.; Zwieniecki, M.A. Extreme mid-winter drought weakens tree hydraulic-carbohydrate systems and slows growth. New Phytol. 2018, 219, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Körner, C. Carbon limitation in trees. J. Ecol. 2003, 91, 4–17. [Google Scholar] [CrossRef]
- Hsiao, T.C. Plant Responses to Water Stress. Ann. Rev. Plant Physiol. 1973, 24, 519–570. [Google Scholar] [CrossRef]
- Li, W.B.; Hartmann, H.; Adams, H.D.; Zhang, H.X.; Jin, C.J.; Zhao, C.Y.; Guan, D.X.; Wang, A.Z.; Yuan, F.H.; Wu, J.B. The sweet side of global change-dynamic responses of non-structural carbohydrates to drought, elevated CO2 and nitrogen fertilization in tree species. Tree Physiol. 2018, 38, 1706–1723. [Google Scholar] [CrossRef]
- Rodríguez-Calcerrada, J.; Li, M.; López, R.; Cano, F.J.; Oleksyn, J.; Atkin, O.K.; Pita, P.; Aranda, I.; Gil, L. Drought-induced shoot dieback starts with massive root xylem embolism and variable depletion of nonstructural carbohydrates in seedlings of two tree species. New Phytol. 2016, 213, 597–610. [Google Scholar] [CrossRef]
- Adams, H.D.; Zeppel MJ, B.; Anderegg WR, L.; Hartmann, H.; Landhäusser, S.M.; Tissue, D.T.; Allen, C.D. A multi-species synthesis of physiological mechanisms in drought-induced tree mortality. Nat. Ecol. Evol. 2017, 1, 1285–1291. [Google Scholar] [CrossRef]
- Sun, H.L.; Li, S.C.; Xiong, W.L.; Yang, Z.R.; Cui, B.S.; Yang, T. Influence of slope on root system anchorage of Pinus yunnanensis. Ecol. Eng. 2008, 32, 60–67. [Google Scholar] [CrossRef]
- Trifilò, P.; Casolo, V.; Raimondo, F.; Petrussa, E.; Boscutti, F.; Lo Gullo, M.A.; Nardini, A. Effects of prolonged drought on stem non-structural carbohydrates content and post-drought hydraulic recovery in Laurus nobilis L. the possible link between carbon starvation and hydraulic failure. Plant Physiol. Bioch. 2017, 120, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Zheng, H.Z.; Huang, Z.H.; Wang, J.; Zhu, J.M. Non-structural carbohydrate dynamics in leaves and branches of Pinus massoniana (Lamb.) following 3-year rainfall exclusion. Forests 2018, 9, 315. [Google Scholar] [CrossRef]
- Ivanov, Y.V.; Kartashov, A.V.; Zlobin, I.E.; Sarvin, B.; Stavrianidi, A.N.; Kuznetsov, V.V. Water deficit-dependent changes in non-structural carbohydrate profiles, growth and mortality of pine and spruce seedlings in hydroculture. Environ. Exp. Bot. 2018, 157, 151–160. [Google Scholar] [CrossRef]
- Camarero, J.J.; Sangüesa-Barreda, G.; Vergarechea, M. Prior height, growth, and wood anatomy differently predispose to drought-induced dieback in two Mediterranean oak speciesk. Ann. Forest Sci. 2016, 73, 341–351. [Google Scholar] [CrossRef]
- Deng, X.X.; Xiao, W.F.; Shi, Z.; Zeng, L.X.; Lei, L. Combined effects of drought and shading on growth and non-structural carbohydrates in pinus massoniana lamb. seedlings. Forests 2020, 11, 18. [Google Scholar] [CrossRef]
- Sala, A.; Woodruff, D.R.; Meinzer, F.C. Carbon dynamics in trees: Feast or famine? Tree Physiol. 2012, 32, 764–775. [Google Scholar] [CrossRef]
- Piper, F.I.; Fajardo, A. Carbon dynamics of Acer pseudoplatanus seedlings under drought and complete darkness. Tree Physiol. 2016, 36, 1400–1408. [Google Scholar]
- Wiley, E.; Huepenbecker, S.; Casper, B.B.; Helliker, B.R. The effects of defoliation on carbon allocation: Can carbon limitation reduce growth in favour of storage? Tree Physiol. 2013, 33, 1216–1228. [Google Scholar] [CrossRef]
- Correia, B.; Hancock, R.D.; Amaral, J.; Gomez-Cadenas, A.; Valledor, L.; Pinto, G. Combined drought and heat activates protective responses in eucalyptus globulus that are not activated when subjected to drought or heat stress alone. Front. Plant Sci. 2018, 9, 819. [Google Scholar] [CrossRef]
- Liu, H.Y.; Shangguan, H.L.; Zhou, M.; Airebule, P.; Zhao, P.W.; He, W.Q.; Xiang, C.L.; Wu, X.C. Differentiated responses of nonstructural carbohydrate allocation to climatic dryness and drought events in the Inner Asian arid timberline. Agric. Forest Meteorol. 2019, 271, 355–361. [Google Scholar] [CrossRef]
- Adams, H.D.; Germino, M.J.; Breshears, D.D.; Barron-Gafford, G.A.; Guardiola-Claramonte, M.; Zou, C.B.; Huxman, T.E. Nonstructural leaf carbohydrate dynamics of Pinus edulis during drought-induced tree mortality reveal role for carbon metabolism in mortality mechanism. New Phytol. 2013, 197, 1142–1151. [Google Scholar] [PubMed]
- Jin, Y.Q.; Li, J.; Liu, C.G.; Liu, Y.T.; Zhang, Y.P.; Sha, L.Q.; Wang, Z.; Song, Q.H.; Lin, Y.X.; Zhou, R.W.; et al. Carbohydrate dynamics of three dominant species in a Chinese savanna under precipitation exclusion. Tree Physiol. 2018, 38, 1371–1383. [Google Scholar] [PubMed]
- Fatichi, S.; Leuzinger, S.; Körner, C. Moving beyond photosynthesis: From carbon source to sink-driven vegetation modeling. New Phytol. 2014, 201, 1086–1095. [Google Scholar] [PubMed]
- McDowell, N.G.; Sevanto, S. The mechanisms of carbon starvation: How, when, or does it even occur at all? New Phytol. 2010, 186, 264–266. [Google Scholar]
- Jacquet, J.S.; Bosc, A.; O’Grady, A.; Jactel, H. Combined effects of defoliation and water stress on pine growth and non-structural carbohydrates. Tree Physiol. 2014, 34, 367–376. [Google Scholar]


| Fixed Factors | Soluble Sugar | Starch | NSC | Soluble Sugar: Starch | |
|---|---|---|---|---|---|
| Needles | Drought intensity | 2.10 | 2.04 | 2.46 | 3.66 * |
| Drought duration | 10.60 ** | 24.81 ** | 10.88 ** | 18.76 ** | |
| Drought intensity × progression | 1.67 | 0.60 | 0.99 | 2.69 ** | |
| Stems | Drought intensity | 2.00 | 3.88 * | 4.05 * | 1.56 |
| Drought duration | 4.08 ** | 16.80 ** | 5.45 ** | 9.98 ** | |
| Drought intensity × progression | 1.75 | 1.54 | 1.44 | 1.87 | |
| Coarse roots | Drought intensity | 1.73 | 1.07 | 1.53 | 1.58 |
| Drought duration | 6.26 ** | 9.45 ** | 4.31 ** | 6.76 ** | |
| Drought intensity × progression | 1.58 | 1.40 | 2.12 * | 1.13 | |
| Fine roots | Drought intensity | 5.77 ** | 1.95 | 5.83 ** | 0.55 |
| Drought duration | 42.82 ** | 7.48 ** | 19.15 ** | 6.86 ** | |
| Drought intensity × progression | 1.76 | 1.16 | 0.69 | 1.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, X.; Chen, X.; Li, T.; Zhang, H.; Bao, Y.; Yang, J.; Zheng, L.; Lan, P.; Wu, J. The Effects of Drought Stress Intensity and Duration on the Dynamics of Nonstructural Carbohydrates in Pinus yunnanensis Seedlings. Plants 2025, 14, 980. https://doi.org/10.3390/plants14060980
Deng X, Chen X, Li T, Zhang H, Bao Y, Yang J, Zheng L, Lan P, Wu J. The Effects of Drought Stress Intensity and Duration on the Dynamics of Nonstructural Carbohydrates in Pinus yunnanensis Seedlings. Plants. 2025; 14(6):980. https://doi.org/10.3390/plants14060980
Chicago/Turabian StyleDeng, Xin, Xin Chen, Tianyu Li, Hang Zhang, Yun Bao, Jingwen Yang, Li Zheng, Ping Lan, and Junwen Wu. 2025. "The Effects of Drought Stress Intensity and Duration on the Dynamics of Nonstructural Carbohydrates in Pinus yunnanensis Seedlings" Plants 14, no. 6: 980. https://doi.org/10.3390/plants14060980
APA StyleDeng, X., Chen, X., Li, T., Zhang, H., Bao, Y., Yang, J., Zheng, L., Lan, P., & Wu, J. (2025). The Effects of Drought Stress Intensity and Duration on the Dynamics of Nonstructural Carbohydrates in Pinus yunnanensis Seedlings. Plants, 14(6), 980. https://doi.org/10.3390/plants14060980









