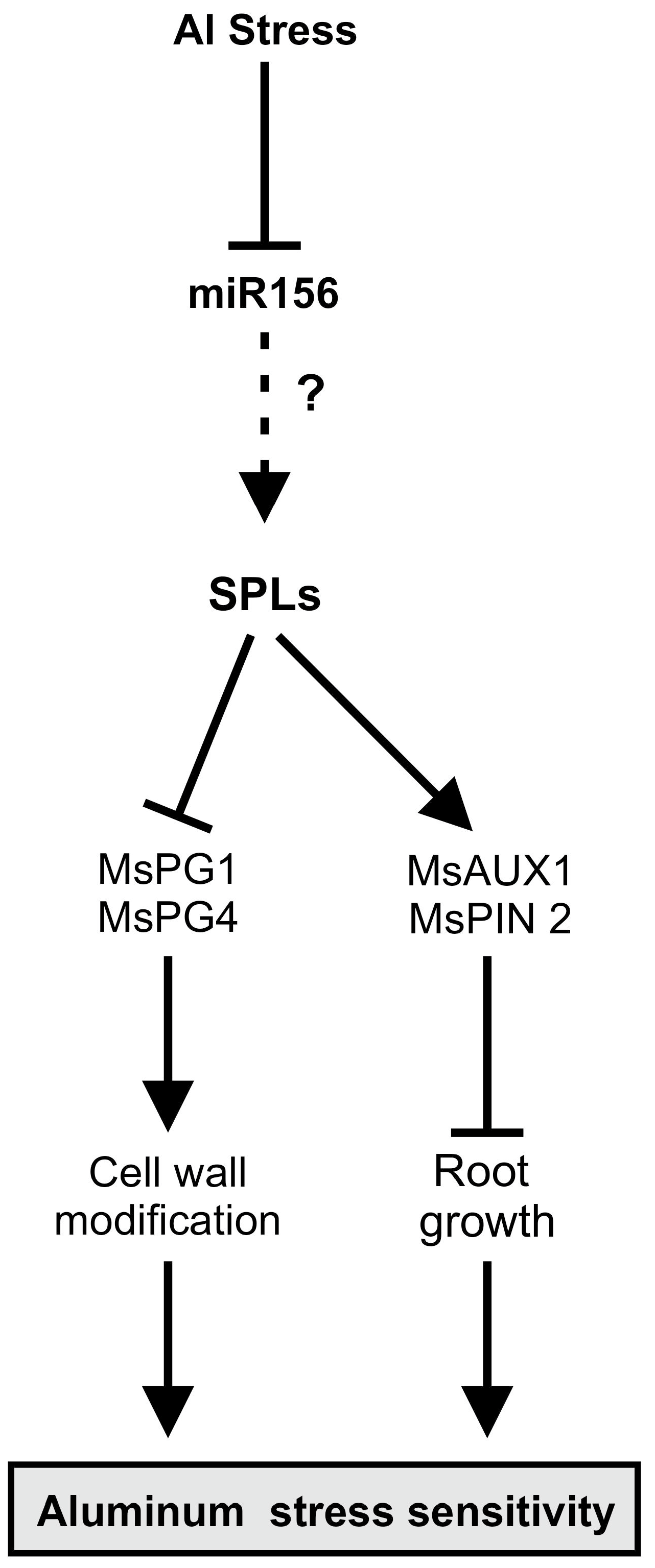
miR156 Is a Negative Regulator of Aluminum Response in Medicago sativa
Abstract
1. Introduction
2. Results
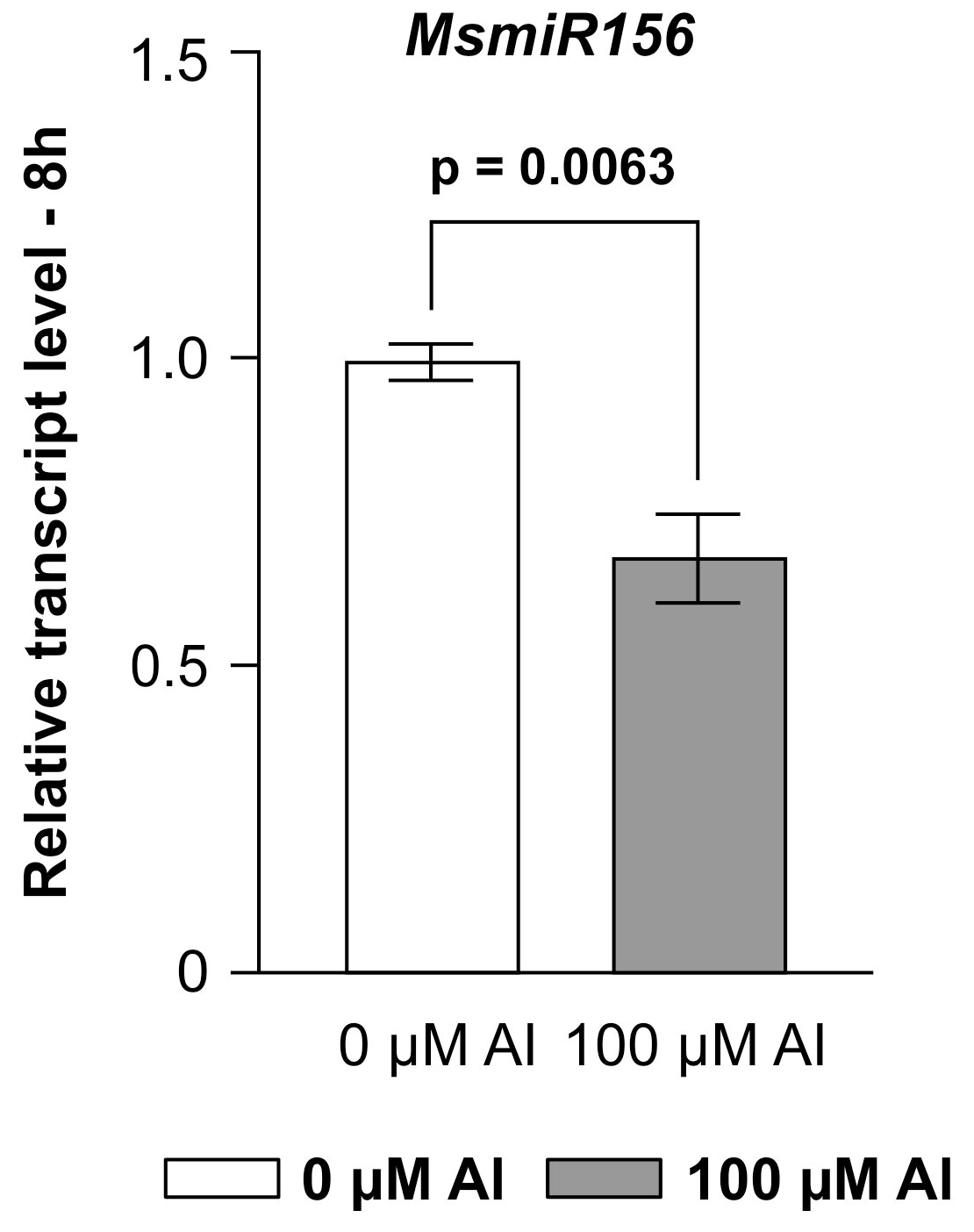
2.1. miR156 Is a Negative Regulator of Al Response in Alfalfa
2.2. Silencing of MsmiR156d Alters Expression of SPL Genes
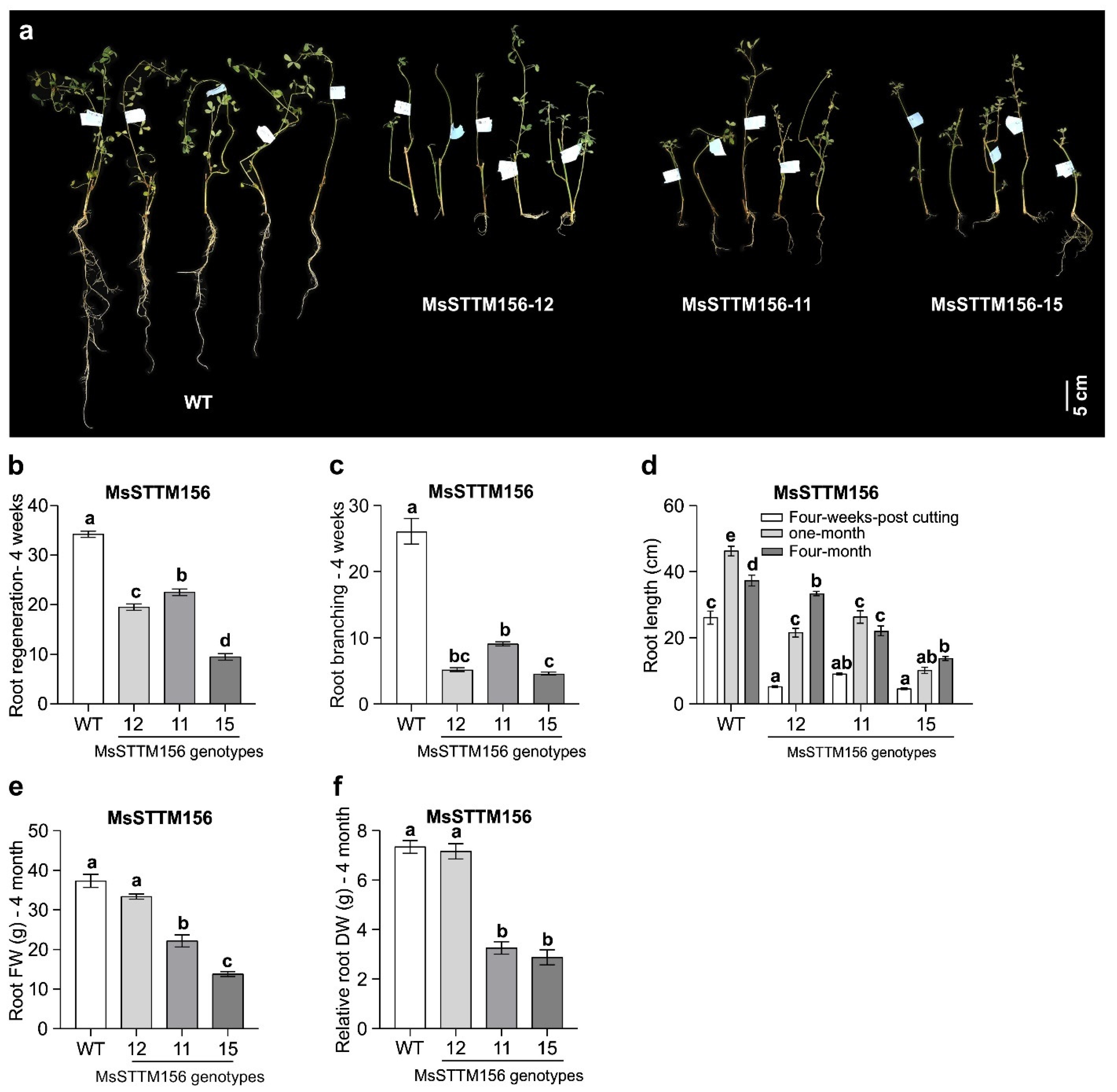
2.3. Morphological Characterization of MsSTTM156 Transgenic Alfalfa
2.4. MsmiR156 Negatively Regulates Al Response by Altering Root Architecture
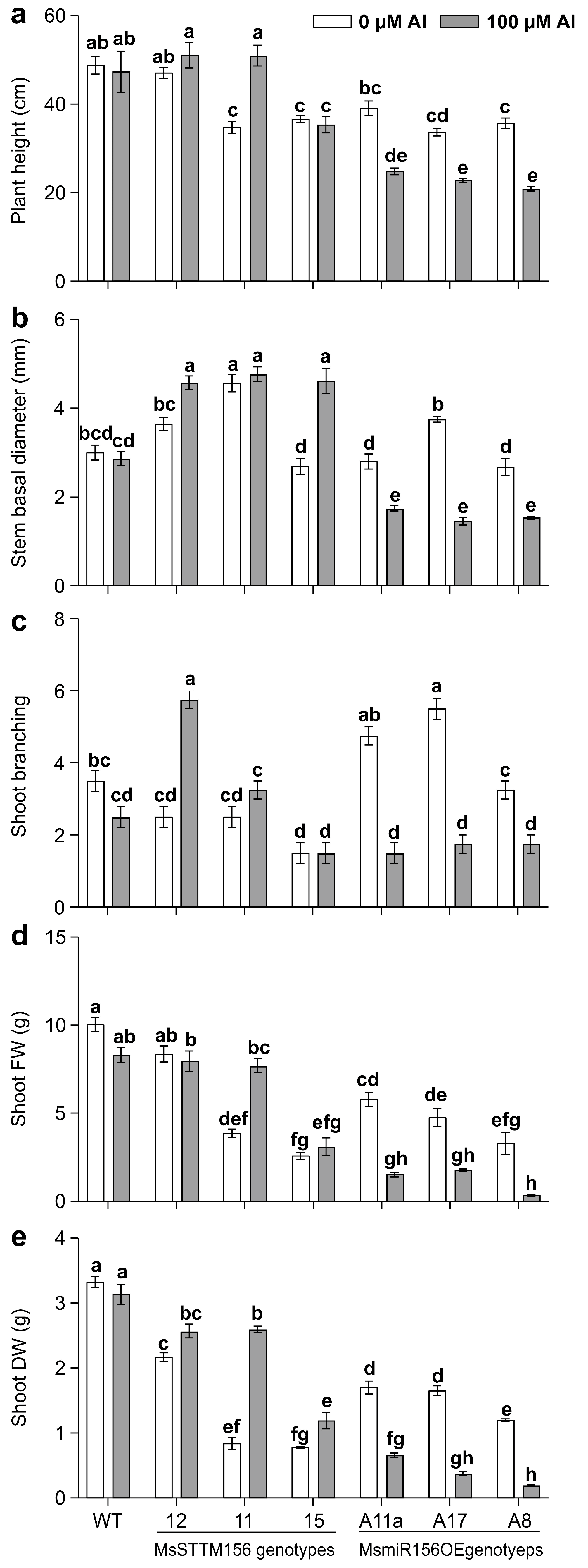
2.5. MsmiR156 Regulates Above-Ground Traits Under Al Stress
2.6. MsmiR156 Modulates Physiological Changes in Response to Al Stress
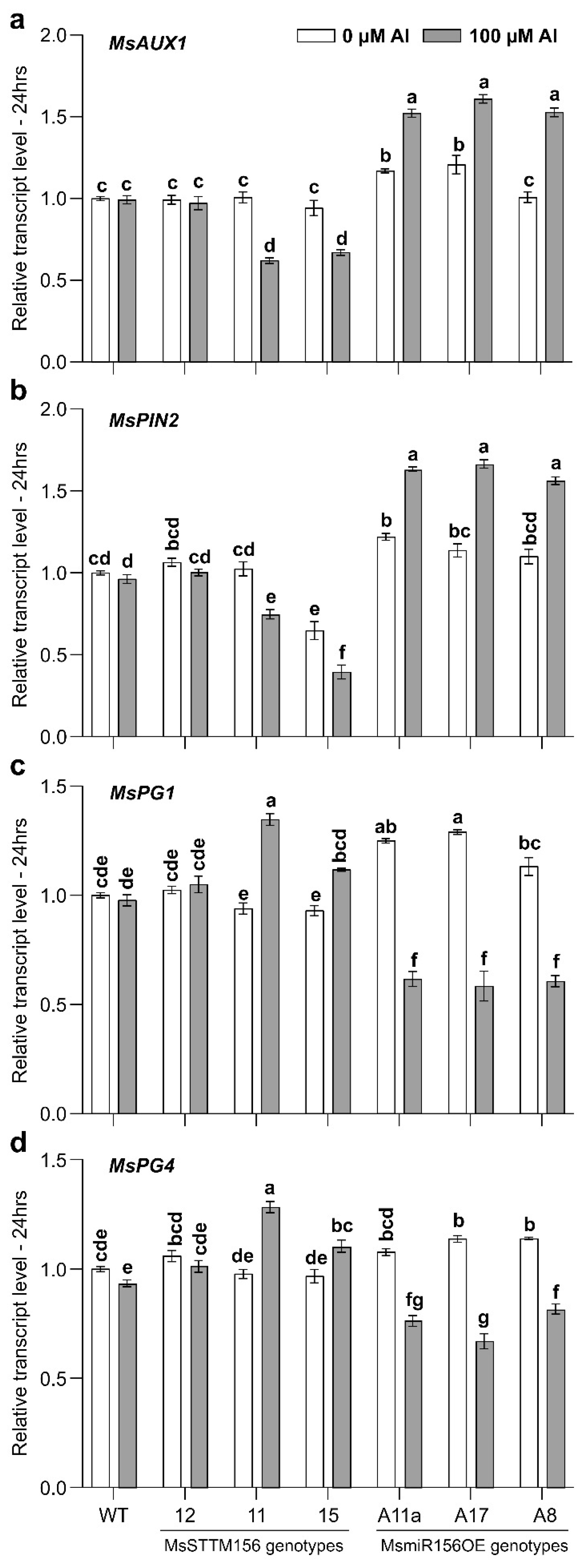
2.7. MsmiR156 Regulates IAA Transport Genes Under Al Stress
2.8. Expression of MsPG Genes Is Affected by miR156 Under Al Stress
3. Discussion
3.1. miR156 Silencing Modulates SPL Genes in Transgenic Alfalfa
3.2. miR156 Silencing Reduces Root Growth in Alfalfa
3.3. miR156 Affects Leaf Pigments in Alfalfa
3.4. miR156 Silencing Alters Flowering in Alfalfa
3.5. miR156 Role in Root Growth Under Al Stress
3.6. miR156 Regulates Root Browning Under Al Stress in Alfalfa
3.7. MsmiR156 Regulates Shoot Architecture and Biomass Traits Under Al Stress
3.8. MsmiR156’s Influences on Water Dynamics and Chlorophyll Content Under Al Stress
3.9. MsmiR156 Regulates IAA Transport in the Roots Under Al Stress
3.10. miR156 Modulates Cell Wall Extensibility and Al Tolerance in Alfalfa Roots
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Generation of MsSTT156 Constructs and Alfalfa Transformation
4.3. Vegetative Propagation of Alfalfa Plants by Stem Cuttings
4.4. Morphological Characterization of Alfalfa Plants
4.5. Al Treatment
4.6. Analysis of Relative Water and Chlorophyll Contents
4.7. RNA Extraction and Reverse Transcription-Quantitative PCR
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, F.; Zhang, J.; Ha, X.; Ma, H. Genome-wide identification and expression analysis of the Auxin-Response factor (ARF) gene family in Medicago sativa under abiotic stress. BMC Genom. 2023, 24, 498. [Google Scholar] [CrossRef] [PubMed]
- Kamran, M.; Yan, Z.; Jia, Q.; Chang, S.; Ahmad, I.; Ghani, M.U.; Hou, F. Irrigation and nitrogen fertilization influence on alfalfa yield, nutritive value, and resource use efficiency in an arid environment. Field Crops Res. 2022, 284, 108587. [Google Scholar] [CrossRef]
- Comas, L.H.; Becker, S.R.; Cruz, V.M.V.; Byrne, P.F.; Dierig, D.A. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef]
- Tucak, M.; Horvat, D.; Čupić, T.; Krizmanić, G.; Ravlić, M. Assessment of Alfalfa Populations for Forage Productivity and Seed Yield Potential under a Multi-Year Field Trial. Agronomy 2023, 13, 349. [Google Scholar] [CrossRef]
- Ferguson, B.J.; Indrasumunar, A.; Hayashi, S.; Lin, M.H.; Lin, Y.H.; Reid, D.E.; Gresshoff, P.M. Molecular analysis of legume nodule development and autoregulation. J. Integr. Plant Biol. 2010, 52, 61–76. [Google Scholar] [CrossRef]
- Sheaffer, C.C.; Seguin, P. Forage Legumes for Sustainable Cropping Systems. J. Crop Prod. 2003, 8, 187–216. [Google Scholar] [CrossRef]
- Francioni, M.; Trozzo, L.; Baldoni, N.; Toderi, M.; Bianchini, M.; Kishimoto-Mo, A.W.; D’ottavio, P. Management of a Mediterranean Forage/Cereal-Based Cropping System: An Ecosystem Service Multisectoral Analysis in the Perspective of Climate Change. Atmosphere 2022, 13, 487. [Google Scholar] [CrossRef]
- Khu, D.-M.; Reyno, R.; Brummer, E.C.; Monteros, M.J. Screening Methods for Aluminum Tolerance in Alfalfa. Crop Sci. 2012, 52, 161–167. [Google Scholar] [CrossRef]
- Annicchiarico, P.; Barrett, B.; Brummer, E.C.; Julier, B.; Marshall, A.H. Achievements and Challenges in Improving Temperate Perennial Forage Legumes. Crit. Rev. Plant Sci. 2015, 34, 327–380. [Google Scholar] [CrossRef]
- Narasimhamoorthy, B.; Bouton, J.H.; Olsen, K.M.; Sledge, M.K. Quantitative trait loci and candidate gene mapping of aluminum tolerance in diploid alfalfa. Theor. Appl. Genet. 2007, 114, 901–913. [Google Scholar] [CrossRef]
- Sade, H.; Meriga, B.; Surapu, V.; Gadi, J.; Sunita, M.S.L.; Suravajhala, P.; Kavi Kishor, P.B. Toxicity and tolerance of aluminum in plants: Tailoring plants to suit to acid soils. BioMetals 2016, 29, 187–210. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Tian, Q.Y.; Chen, J.; Zhang, W.H. Aluminium-induced inhibition of root elongation in Arabidopsis is mediated by ethylene and auxin. J. Exp. Bot. 2010, 61, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.F.; Ferreira, B.G.; dos Santos Isaias, R.M.; Alexandre, S.S.; França, M.G.C. Immunocytochemistry and Density Functional Theory evidence the competition of aluminum and calcium for pectin binding in Urochloa decumbens roots. Plant Physiol. Biochem. 2020, 153, 64–71. [Google Scholar] [CrossRef]
- Shi, H.; Sun, G.; Gou, L.; Guo, Z. Rhizobia–Legume Symbiosis Increases Aluminum Resistance in Alfalfa. Plants 2022, 11, 1275. [Google Scholar] [CrossRef]
- Liu, C.; Cheng, H.; Wang, S.; Yu, D.; Wei, Y. Physiological and Transcriptomic Analysis Reveals That Melatonin Alleviates Aluminum Toxicity in Alfalfa (Medicago sativa L.). Int. J. Mol. Sci. 2023, 24, 17221. [Google Scholar] [CrossRef]
- Guo, C.; Xu, Y.; Shi, M.; Lai, Y.; Wu, X.; Wang, H.; Zhu, Z.; Poethig, R.S.; Wu, G. Repression of miR156 by miR159 regulates the timing of the juvenile-to-adult transition in Arabidopsis. Plant Cell 2017, 29, 1293–1304. [Google Scholar] [CrossRef]
- Zhang, J.; Zeng, B.; Mao, Y.; Kong, X.; Wang, X.; Yang, Y.; Zhang, J.; Xu, J.; Rengel, Z.; Chen, Q. Melatonin alleviates aluminium toxicity through modulating antioxidative enzymes and enhancing organic acid anion exudation in soybean. Funct. Plant Biol. 2017, 44, 961–968. [Google Scholar] [CrossRef]
- Cheng, X.; Fang, T.; Zhao, E.; Zheng, B.; Huang, B.; An, Y.; Zhou, P. Protective roles of salicylic acid in maintaining integrity and functions of photosynthetic photosystems for alfalfa (Medicago sativa L.) tolerance to aluminum toxicity. Plant Physiol. Biochem. 2020, 155, 570–578. [Google Scholar] [CrossRef]
- Guo, P.; Qi, Y.P.; Cai, Y.T.; Yang, T.Y.; Yang, L.T.; Huang, Z.R.; Chen, L.S. Aluminum effects on photosynthesis, reactive oxygen species and methylglyoxal detoxification in two Citrus species differing in aluminum tolerance. Tree Physiol. 2018, 38, 1548–1565. [Google Scholar] [CrossRef]
- Su, L.; Xie, J.; Wen, W.; Li, J.; Zhou, P.; An, Y. Interaction of zinc and IAA alleviate aluminum-induced damage on photosystems via promoting proton motive force and reducing proton gradient in alfalfa. BMC Plant Biol. 2020, 20, 1–17. [Google Scholar] [CrossRef]
- Ofoe, R.; Thomas, R.H.; Asiedu, S.K.; Wang-Pruski, G.; Fofana, B.; Abbey, L. Aluminum in plant: Benefits, toxicity and tolerance mechanisms. Front. Plant Sci. 2023, 13, 1085998. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y. Aluminum toxicity in plant cells: Mechanisms of cell death and inhibition of cell elongation. Soil Sci. Plant Nutr. 2019, 65, 41–55. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, Z.; Zhao, X.; Cao, H.; Wang, L.; Liu, S.; Wang, C.; Liu, M.; Wang, L.; Liu, Z. Superstar microRNA, miR156, involved in plant biological processes and stress response: A review. Sci. Hortic. 2023, 316, 112010. [Google Scholar] [CrossRef]
- Gou, J.-Y.; Felippes, F.F.; Liu, C.-J.; Weigel, D.; Wang, J.-W. Negative regulation of anthocyanin biosynthesis in Arabidopsis by a miR156-targeted SPL transcription factor. Plant Cell 2011, 23, 1512–1522. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Ju, Y.; Seo, P.J.; Lee, J.H.; Park, C.M. The SOC1-SPL module integrates photoperiod and gibberellic acid signals to control flowering time in Arabidopsis. Plant J. 2012, 69, 577–588. [Google Scholar] [CrossRef]
- Cui, J.-G.; Shan, J.X.; Shi, M.; Gao, J.P.; Lin, H.X. The miR156-SPL9-DFR pathway coordinates the relationship between development and abiotic stress tolerance in plants. Plant J. 2014, 80, 1108–1117. [Google Scholar] [CrossRef]
- Zhang, T.-Q.; Lian, H.; Tang, H.; Dolezal, K.; Zhou, C.-M.; Yu, S.; Chen, J.-H.; Chen, Q.; Liu, H.; Ljung, K. An intrinsic microRNA timer regulates progressive decline in shoot regenerative capacity in plants. Plant Cell 2015, 27, 349–360. [Google Scholar] [CrossRef]
- Ioannidi, E.; Rigas, S.; Tsitsekian, D.; Daras, G.; Alatzas, A.; Makris, A.; Tanou, G.; Argiriou, A.; Alexandrou, D.; Poethig, S. Trichome patterning control involves TTG1 interaction with SPL transcription factors. Plant Mol. Biol. 2016, 92, 675–687. [Google Scholar] [CrossRef]
- Preston, J.C.; Jorgensen, S.A.; Orozco, R.; Hileman, L.C. Paralogous SQUAMOSA PROMOTER BINDING PROTEIN-LIKE (SPL) genes differentially regulate leaf initiation and reproductive phase change in petunia. Planta 2016, 243, 429–440. [Google Scholar] [CrossRef]
- Yu, S.; Galvão, V.C.; Zhang, Y.C.; Horrer, D.; Zhang, T.Q.; Hao, Y.H.; Feng, Y.Q.; Wang, S.; Schmid, M.; Wang, J.W. Gibberellin regulates the Arabidopsis floral transition through miR156-targeted SQUAMOSA PROMOTER BINDING-LIKE transcription factors. Plant Cell 2012, 24, 3320–3332. [Google Scholar] [CrossRef]
- Ye, B.B.; Shang, G.D.; Pan, Y.; Xu, Z.G.; Zhou, C.M.; Mao, Y.B.; Bao, N.; Sun, L.; Xu, T.; Wang, J.W. AP2/ERF Transcription Factors Integrate Age and Wound Signals for Root Regeneration[OPEN]. Plant Cell 2020, 32, 226–241. [Google Scholar] [CrossRef] [PubMed]
- Singer, S.D.; Burton Hughes, K.; Subedi, U.; Dhariwal, G.K.; Kader, K.; Acharya, S.; Chen, G.; Hannoufa, A. The CRISPR/Cas9-Mediated Modulation of SQUAMOSA PROMOTER-BINDING PROTEIN-LIKE 8 in Alfalfa Leads to Distinct Phenotypic Outcomes. Front. Plant Sci. 2022, 12, 774146. [Google Scholar] [CrossRef] [PubMed]
- Hanly, A.; Karagiannis, J.; Lu, Q.S.M.; Tian, L.; Hannoufa, A. Characterization of the role of SPL9 in drought stress tolerance in Medicago sativa. Int. J. Mol. Sci. 2020, 21, 6003. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Charagh, S.; Karikari, B.; Sharif, R.; Yadav, V.; Mubarik, M.S.; Habib, M.; Zhuang, Y.; Zhang, C.; Chen, H.; et al. miRNAs for crop improvement. Plant Physiol. Biochem. 2023, 201, 107857. [Google Scholar] [CrossRef]
- Aung; Gruber, M.Y.; Amyot, L.; Omari, K.; Bertrand, A.; Hannoufa, A. MicroRNA156 as a promising tool for alfalfa improvement. Plant Biotechnol. J. 2015, 13, 779–790. [Google Scholar] [CrossRef]
- Wei, S.; Yu, B.; Gruber, M.Y.; Khachatourians, G.G.; Hegedus, D.D.; Hannoufa, A. Enhanced seed carotenoid levels and branching in transgenic Brassica napus expressing the Arabidopsis miR156b gene. J. Agric. Food Chem. 2010, 58, 9572–9578. [Google Scholar] [CrossRef]
- Fu, C.; Sunkar, R.; Zhou, C.; Shen, H.; Zhang, J.Y.; Matts, J.; Wolf, J.; Mann, D.G.; Stewart Jr, C.N.; Tang, Y. Overexpression of miR156 in switchgrass (Panicum virgatum L.) results in various morphological alterations and leads to improved biomass production. Plant Biotechnol. J. 2012, 10, 443–452. [Google Scholar] [CrossRef]
- Bhogale, S.; Mahajan, A.S.; Natarajan, B.; Rajabhoj, M.; Thulasiram, H.V.; Banerjee, A.K. MicroRNA156: A potential graft-transmissible microRNA that modulates plant architecture and tuberization in Solanum tuberosum ssp. andigena. Plant Physiol. 2014, 164, 1011–1027. [Google Scholar] [CrossRef]
- Franco-Zorrilla, J.M.; Valli, A.; Todesco, M.; Mateos, I.; Puga, M.I.; Rubio-Somoza, I.; Leyva, A.; Weigel, D.; García, J.A.; Paz-Ares, J. Target mimicry provides a new mechanism for regulation of microRNA activity. Nat. Genet. 2007, 39, 1033–1037. [Google Scholar] [CrossRef]
- Liu, W.; Ji, X.; Cao, H.; Huo, C.; He, L.; Peng, X.; Yang, Y.; Yang, F.; Xiong, S. Comparative Transcriptome Analysis Reveals the Effect of miR156a Overexpression on Mineral Nutrient Homeostasis in Nicotiana tabacum. Plants 2023, 12, 1739. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, J.; Zhang, N.; Wu, J.; Si, H. Roles of microRNAs in abiotic stress response and characteristics regulation of plant. Front. Plant Sci. 2022, 13, 919243. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.; Feyissa, B.A.; Amyot, L.; Aung, B.; Hannoufa, A. MicroRNA156 improves drought stress tolerance in alfalfa (Medicago sativa) by silencing SPL13. Plant Sci. 2017, 258, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, Y.; Teng, F.; Cen, H.; Yan, J.; Lin, S.; Li, D.; Zhang, W. Heterogeneous expression of Osa-MIR156bc increases abiotic stress resistance and forage quality of alfalfa. Crop J. 2021, 9, 1135–1144. [Google Scholar] [CrossRef]
- Arshad, M.; Gruber, M.Y.; Wall, K.; Hannoufa, A. An insight into microRNA156 role in salinity stress responses of alfalfa. Front. Plant Sci. 2017, 8, 356. [Google Scholar] [CrossRef]
- Matthews, C.; Arshad, M.; Hannoufa, A. Alfalfa response to heat stress is modulated by microRNA156. Physiol. Plant. 2019, 165, 830–842. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, H.; Jiang, H.; Wang, H.; Chen, K.; Duan, J.; Feng, S.; Wu, G. Regulation of cadmium tolerance and accumulation by miR156 in Arabidopsis. Chemosphere 2020, 242, 125168. [Google Scholar] [CrossRef]
- Lu, L.; Chen, X.; Chen, J.; Zhang, Z.; Zhang, Z.; Sun, Y.; Wang, Y.; Xie, S.; Ma, Y.; Song, Y.; et al. MicroRNA-encoded regulatory peptides modulate cadmium tolerance and accumulation in rice. Plant Cell Environ. 2024, 47, 1452–1470. [Google Scholar] [CrossRef]
- Kouhi, F.; Sorkheh, K.; Ercisli, S. MicroRNA expression patterns unveil differential expression of conserved miRNAs and target genes against abiotic stress in safflower. PLoS ONE 2020, 15, e0228850. [Google Scholar] [CrossRef]
- Qiu, Z.; Hai, B.; Guo, J.; Li, Y.; Zhang, L. Characterization of wheat miRNAs and their target genes responsive to cadmium stress. Plant Physiol. Biochem. 2016, 101, 60–67. [Google Scholar] [CrossRef]
- Zubair, M.; Khan, M.Z.; Rauf, I.; Raza, A.; Shah, A.H.; Hassan, I.; Amin, I.; Mansoor, S. Artificial micro RNA (amiRNA)-mediated resistance against whitefly (Bemisia tabaci) targeting three genes. Crop Prot. 2020, 137, 105308. [Google Scholar]
- Wang, Z.; Zhu, F. Different roles of a novel shrimp microRNA in white spot syndrome virus (WSSV) and Vibrio alginolyticus infection. Dev. Comp. Immunol. 2018, 79, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Fei, Q.; Li, R.; Liu, B.; Xia, R.; Char, S.N.; Meyers, B.C.; Yang, B. Disruption of miRNA sequences by TALENs and CRISPR/Cas9 induces varied lengths of miRNA production. Plant Biotechnol. J. 2020, 18, 1526–1536. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Gu, Y.; Jia, X.; Kang, W.; Pan, S.; Tang, X.; Chen, X.; Tang, G. Effective small RNA destruction by the expression of a short tandem target mimic in Arabidopsis. Plant Cell 2012, 24, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Othman, S.M.I.S.; Mustaffa, A.F.; Che-Othman, M.H.; Samad, A.F.A.; Goh, H.H.; Zainal, Z.; Ismail, I. Overview of Repressive miRNA Regulation by Short Tandem Target Mimic (STTM): Applications and Impact on Plant Biology. Plants 2023, 12, 669. [Google Scholar] [CrossRef]
- Teotia, S.; Tang, G. Silencing of stress-regulated miRNAs in plants by short tandem target mimic (STTM) approach. Methods Mol. Biol. 2017, 1631, 337–348. [Google Scholar] [CrossRef]
- Peng, T.; Qiao, M.; Liu, H.; Teotia, S.; Zhang, Z.; Zhao, Y.; Wang, B.; Zhao, D.; Shi, L.; Zhang, C.; et al. A Resource for Inactivation of MicroRNAs Using Short Tandem Target Mimic Technology in Model and Crop Plants. Mol. Plant 2018, 11, 1400–1417. [Google Scholar] [CrossRef]
- Li, Q.; Shen, H.; Yuan, S.; Dai, X.; Yang, C. miRNAs and lncRNAs in tomato: Roles in biotic and abiotic stress responses. Front. Plant Sci. 2023, 13, 1094459. [Google Scholar] [CrossRef]
- Zhang, A.; Zhang, S.; Wang, F.; Meng, X.; Ma, Y.; Guan, J.; Zhang, F. The roles of microRNAs in horticultural plant disease resistance. Front. Genet. 2023, 14, 1137471. [Google Scholar] [CrossRef]
- Wang, S.; Ren, X.; Huang, B.; Wang, G.; Zhou, P.; An, Y. Aluminium-induced reduction of plant growth in alfalfa (Medicago sativa) is mediated by interrupting auxin transport and accumulation in roots. Sci. Rep. 2016, 6, 30079. [Google Scholar] [CrossRef]
- Feyissa, B.A.; Amyot, L.; Nasrollahi, V.; Papadopoulos, Y.; Kohalmi, S.E.; Hannoufa, A. Involvement of the miR156/SPL module in flooding response in Medicago sativa. Sci. Rep. 2021, 11, 3243. [Google Scholar] [CrossRef]
- Wei, S.; Gruber, M.Y.; Yu, B.; Gao, M.J.; Khachatourians, G.G.; Hegedus, D.D.; Parkin, I.A.P.; Hannoufa, A. Arabidopsis mutant sk156 reveals complex regulation of SPL15 in a miR156-controlled gene network. BMC Plant Biol. 2012, 12, 169. [Google Scholar] [CrossRef]
- Lima, J.; Arenhart, R.; Margis-Pinheiro, M.; Margis, R. Aluminum triggers broad changes in microRNA expression in rice roots. Genet. Mol. Res. 2011, 10, 2817–2832. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, T.; Zhao, M.; Tian, Q.; Zhang, W.-H. Identification of aluminum-responsive microRNAs in Medicago truncatula by genome-wide high-throughput sequencing. Planta 2012, 235, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.Y.; Yang, C.Y.; Ma, Q.B.; Li, X.P.; Dong, W.W.; Nian, H. Identification of wild soybean miRNAs and their target genes responsive to aluminum stress. BMC Plant Biol. 2012, 12, 1–16. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, M.; Xu, X.; Li, X.; Li, C.; Ding, Z. System analysis of micro RNA s in the development and aluminium stress responses of the maize root system. Plant Biotechnol. J. 2014, 12, 1108–1121. [Google Scholar] [CrossRef]
- Silva; Rosa-Santos, T.M.; de Castro França, S.; Kottapalli, P.; Kottapalli, K.R.; Zingaretti, S.M. Microtranscriptome analysis of sugarcane cultivars in response to aluminum stress. PLoS ONE 2019, 14, e0217806. [Google Scholar] [CrossRef]
- Wu, L.; Yu, J.; Shen, Q.; Huang, L.; Wu, D.; Zhang, G. Identification of microRNAs in response to aluminum stress in the roots of Tibetan wild barley and cultivated barley. BMC Genom. 2018, 19, 1–14. [Google Scholar] [CrossRef]
- Wu, Y.; Cao, F.; Xie, L.; Wu, F.; Zhu, S.; Qiu, C. Comparative Transcriptome Profiling Reveals Key MicroRNAs and Regulatory Mechanisms for Aluminum Tolerance in Olive. Plants 2023, 12, 978. [Google Scholar] [CrossRef]
- Lu, Z.; Yang, Z.; Tian, Z.; Gui, Q.; Dong, R.; Chen, C. Genome-wide analysis and identification of microRNAs in Medicago truncatula under aluminum stress. Front. Plant Sci. 2023, 14, 1137764. [Google Scholar] [CrossRef]
- Kochian, L.V.; Piñeros, M.A.; Liu, J.; Magalhaes, J.V. Plant Adaptation to Acid Soils: The Molecular Basis for Crop Aluminum Resistance. Annu. Rev. Plant Biol. 2015, 66, 571–598. [Google Scholar] [CrossRef]
- Buchanan, B.B.; Gruissem, W.; Jones, R.L. Biochemistry and Molecular Biology of Plants, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 1–1280. [Google Scholar]
- Panda, S.K.; Baluska, F.; Matsumoto, H. Aluminum stress signaling in plants. Plant Signal. Behav. 2009, 4, 592–597. [Google Scholar] [CrossRef]
- Su, C.; Wang, J.; Feng, J.; Jiang, S.; Man, F.; Jiang, L.; Zhao, M. OsAlR3 regulates aluminum tolerance through promoting the secretion of organic acids and the expression of antioxidant genes in rice. BMC Plant Biol. 2024, 24, 618. [Google Scholar] [CrossRef] [PubMed]
- Fan, N.; Wen, W.; Gao, L.; Lv, A.; Su, L.; Zhou, P.; An, Y. MsPG4-mediated hydrolysis of pectins increases the cell wall extensibility and aluminum resistance of alfalfa. Plant Soil 2022, 477, 357–371. [Google Scholar] [CrossRef]
- Li, J.; Su, L.; Lv, A.; Li, Y.; Zhou, P.; An, Y. MsPG1 alleviated aluminum-induced inhibition of root growth by decreasing aluminum accumulation and increasing porosity and extensibility of cell walls in alfalfa (Medicago sativa). Environ. Exp. Bot. 2020, 175, 104045. [Google Scholar] [CrossRef]
- Jeyakumar, J.M.J.; Ali, A.; Wang, W.M.; Thiruvengadam, M. Characterizing the role of the miR156-SPL network in plant development and stress response. Plants 2020, 9, 1206. [Google Scholar] [CrossRef]
- Yu, N.; Niu, Q.W.; Ng, K.H.; Chua, N.H. The role of miR156/SPL s modules in Arabidopsis lateral root development. Plant J. 2015, 83, 673–685. [Google Scholar] [CrossRef]
- Chuck, G.; Cigan, A.M.; Saeteurn, K.; Hake, S. The heterochronic maize mutant Corngrass1 results from overexpression of a tandem microRNA. Nat. Genet. 2007, 39, 544–549. [Google Scholar] [CrossRef]
- Chuck, G.S.; Tobias, C.; Sun, L.; Kraemer, F.; Li, C.; Dibble, D.; Arora, R.; Bragg, J.N.; Vogel, J.P.; Singh, S.; et al. Overexpression of the maize Corngrass1 microRNA prevents flowering, improves digestibility, and increases starch content of switchgrass. Proc. Natl. Acad. Sci. USA 2011, 108, 17550–17555. [Google Scholar] [CrossRef]
- Aung; Gruber, M.Y.; Amyot, L.; Omari, K.; Bertrand, A.; Hannoufa, A. Ectopic expression of LjmiR156 delays flowering, enhances shoot branching, and improves forage quality in alfalfa. Plant Biotechnol. Rep. 2015, 9, 379–393. [Google Scholar] [CrossRef]
- Zheng, Q.; Liu, J.; Goff, B.M.; Dinkins, R.D.; Zhu, H. Genetic manipulation of miR156 for improvement of biomass production and forage quality in red clover. Crop Sci. 2016, 56, 1199–1205. [Google Scholar] [CrossRef]
- Teotia, S.; Tang, G. To bloom or not to bloom: Role of microRNAs in plant flowering. Mol. Plant 2015, 8, 359–377. [Google Scholar] [CrossRef]
- Kopittke, P.M.; Moore, K.L.; Lombi, E.; Gianoncelli, A.; Ferguson, B.J.; Blamey, F.P.C.; Menzies, N.W.; Nicholson, T.M.; McKenna, B.A.; Wang, P. Identification of the primary lesion of toxic aluminum in plant roots. Plant Physiol. 2015, 167, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Kopittke, P.M.; Asher, C.J.; Blamey, F.P.C.; Auchterlonie, G.J.; Guo, Y.N.; Menzies, N.W. Localization and Chemical Speciation of Pb in Roots of Signal Grass (Brachiaria decumbens) and Rhodes Grass (Chloris gayana). Environ. Sci. Technol. 2008, 42, 4595–4599. [Google Scholar] [CrossRef] [PubMed]
- Rout, G.R.; Samantaray, S.; Das, P. Aluminium toxicity in plants: A review. Agronomie 2001, 21, 3–21. [Google Scholar] [CrossRef]
- Long, S.; Xie, W.; Zhao, W.; Liu, D.; Wang, P.; Zhao, L. Effects of acid and aluminum stress on seed germination and physiological characteristics of seedling growth in Sophora davidii. Plant. Signal. Behav. 2024, 19, 2328891. [Google Scholar] [CrossRef]
- Ling, J.; Tan, J.; Chen, H.; Yang, Z.; Luo, Q.; Jia, J. Physiology, Transcriptome and Root Exudates Analysis of Response to Aluminum Stress in Pinus massoniana. Forests 2023, 14, 1410. [Google Scholar] [CrossRef]
- Mohan Murali Achary, V.; Jena, S.; Panda, K.K.; Panda, B.B. Aluminium induced oxidative stress and DNA damage in root cells of Allium cepa L. Ecotoxicol. Environ. Saf. 2008, 70, 300–310. [Google Scholar] [CrossRef]
- Hayes, K.L.; Mui, J.; Song, B.; Sani, E.S.; Eisenman, S.W.; Sheffield, J.B.; Kim, B. Effects, uptake, and translocation of aluminum oxide nanoparticles in lettuce: A comparison study to phytotoxic aluminum ions. Sci. Total Environ. 2020, 719, 137393. [Google Scholar] [CrossRef]
- Ma, X.L.; Ren, J.; Dai, W.R.; Yang, W.; Bi, Y.F. Effects of aluminium on the root activity, organic acids and free proline accumulation of alfalfa grown in nutrient solution. N. Z. J. Agric. Res. 2020, 63, 341–352. [Google Scholar] [CrossRef]
- Fan, N.; Su, L.; Lv, A.; Wen, W.; Gao, L.; You, X.; Zhou, P.; An, Y. PECTIN ACETYLESTERASE12 regulates shoot branching via acetic acid and auxin accumulation in alfalfa shoots. Plant Physiol. 2024, 195, 518–533. [Google Scholar] [CrossRef]
- Prusinkiewicz, P.; Crawford, S.; Smith, R.S.; Ljung, K.; Bennett, T.; Ongaro, V.; Leyser, O. Control of bud activation by an auxin transport switch. Proc. Natl. Acad. Sci. USA 2009, 106, 17431–17436. [Google Scholar] [CrossRef]
- AbdElgawad, H.; de Soua, A.; Alotaibi, M.O.; Mohammed, A.E.; Schoenaers, S.; Selim, S.; Saleh, A.M. The differential tolerance of C3 and C4 cereals to aluminum toxicity is faded under future CO2 climate. Plant Physiol. Biochem. 2021, 169, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Chahardoli, A.; Karimi, N.; Ma, X.; Qalekhani, F. Effects of engineered aluminum and nickel oxide nanoparticles on the growth and antioxidant defense systems of Nigella arvensis L. Sci. Rep. 2020, 10, 3847. [Google Scholar] [CrossRef] [PubMed]
- Engel, F.; Cotelle, S.; Somensi, C.A.; Testolin, R.C.; Corrêa, R.; Toumi, H.; Férard, J.F.; Radetski, C.M. A 3D ecotoxi-topological profile: Using concentration-time-response surfaces to show peroxidase activity in Zea mays (L.) exposed to aluminium or arsenic in hydroponic conditions. Chemosphere 2021, 262, 127647. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Riaz, M.; Zhang, M.; Liu, B.; El-Desouki, Z.; Jiang, C. Biochar increases nitrogen use efficiency of maize by relieving aluminum toxicity and improving soil quality in acidic soil. Ecotoxicol. Environ. Saf. 2020, 196, 110531. [Google Scholar] [CrossRef]
- Ren, J.; Yang, X.; Zhang, N.; Feng, L.; Ma, C.; Wang, Y.; Yang, Z.; Zhao, J. Melatonin alleviates aluminum-induced growth inhibition by modulating carbon and nitrogen metabolism, and reestablishing redox homeostasis in Zea mays L. J. Hazard. Mater. 2022, 423, 127159. [Google Scholar] [CrossRef]
- Dawood, M.F.A.; Tahjib-Ul-Arif, M.; Sohag, A.A.M.; Abdel Latef, A.A.H. Fluoride mitigates aluminum-toxicity in barley: Morpho-physiological responses and biochemical mechanisms. BMC Plant Biol. 2022, 22, 287. [Google Scholar] [CrossRef]
- Sami, A.; Shah, F.A.; Abdullah, M.; Zhou, X.; Yan, Y.; Zhu, Z.; Zhou, K. Melatonin mitigates cadmium and aluminium toxicity through modulation of antioxidant potential in Brassica napus L. Plant Biol. 2020, 22, 679–690. [Google Scholar] [CrossRef]
- Sivaguru, M.; Horst, W.J. The distal part of the transition zone is the most aluminum-sensitive apical root zone of maize. Plant Physiol. 1998, 116, 155–163. [Google Scholar] [CrossRef]
- Zelinová, V.; Halušková, L.; Huttová, J.; Illéš, P.; Mistrík, I.; Valentovičová, K.; Tamás, L. Short-term aluminium-induced changes in barley root tips. Protoplasma 2011, 248, 523–530. [Google Scholar] [CrossRef]
- Singh, S.; Tripathi, D.K.; Singh, S.; Sharma, S.; Dubey, N.K.; Chauhan, D.K.; Vaculík, M. Toxicity of aluminium on various levels of plant cells and organism: A review. Environ. Exp. Bot. 2017, 137, 177–193. [Google Scholar] [CrossRef]
- Jones, D.L.; Kochian, L.V. Aluminum Inhibition of the Inositol 1,4,5-Trisphosphate Signal Transduction Pathway in Wheat Roots: A Role in Aluminum Toxicity? Plant Cell 1995, 7, 1913. [Google Scholar] [CrossRef] [PubMed]
- Barceló, J.; Poschenrieder, C. Fast root growth responses, root exudates, and internal detoxification as clues to the mechanisms of aluminium toxicity and resistance: A review. Environ. Exp. Bot. 2002, 48, 75–92. [Google Scholar] [CrossRef]
- Gavassi, M.A.; Dodd, I.C.; Puértolas, J.; Silva, G.S.; Carvalho, R.F.; Habermann, G. Aluminum-induced stomatal closure is related to low root hydraulic conductance and high ABA accumulation. Environ. Exp. Bot. 2020, 179, 104233. [Google Scholar] [CrossRef]
- Ali, S.; Zeng, F.; Qiu, L.; Zhang, G. The effect of chromium and aluminum on growth, root morphology, photosynthetic parameters and transpiration of the two barley cultivars. Biol. Plant. 2011, 55, 291–296. [Google Scholar] [CrossRef]
- Karimaei, M.; Poozesh, V. Effects of aluminum toxicity on plant height, total chlorophyll (Chl a+ b), potassium and calcium. Int. J. Farming Allied Sci. 2016, 5, 76–82. [Google Scholar]
- Zhao, X.; Chen, Q.; Wang, Y.; Shen, Z.; Shen, W.; Xu, X. Hydrogen-rich water induces aluminum tolerance in maize seedlings by enhancing antioxidant capacities and nutrient homeostasis. Ecotoxicol. Environ. Saf. 2017, 144, 369–379. [Google Scholar] [CrossRef]
- Silva, S.; Pinto, G.; Dias, M.C.; Correia, C.M.; Moutinho-Pereira, J.; Pinto-Carnide, O.; Santos, C. Aluminium long-term stress differently affects photosynthesis in rye genotypes. Plant Physiol. Biochem. 2012, 54, 105–112. [Google Scholar] [CrossRef]
- Shen, X.; Xiao, X.; Dong, Z.; Chen, Y. Silicon effects on antioxidative enzymes and lipid peroxidation in leaves and roots of peanut under aluminum stress. Acta Physiol. Plant. 2014, 36, 3063–3069. [Google Scholar] [CrossRef]
- Ribeiro, M.A.Q.; Almeida, A.-A.F.d.; Mielke, M.S.; Gomes, F.P.; Pires, M.V.; Baligar, V.C. Aluminum effects on growth, photosynthesis, and mineral nutrition of cacao genotypes. J. Plant Nutr. 2013, 36, 1161–1179. [Google Scholar] [CrossRef]
- Yang, M.; Tan, L.; Xu, Y.; Zhao, Y.; Cheng, F.; Ye, S.; Jiang, W. Effect of low pH and aluminum toxicity on the photosynthetic characteristics of different fast-growing Eucalyptus vegetatively propagated clones. PLoS ONE 2015, 10, e0130963. [Google Scholar] [CrossRef]
- Phukunkamkaew, S.; Tisarum, R.; Pipatsitee, P.; Samphumphuang, T.; Maksup, S.; Cha-Um, S. Morpho-physiological responses of indica rice (Oryza sativa sub. indica) to aluminum toxicity at seedling stage. Environ. Sci. Pollut. Res. 2021, 28, 29321–29331. [Google Scholar]
- Cárcamo, M.P.; Reyes-Díaz, M.; Rengel, Z.; Alberdi, M.; Omena-Garcia, R.P.; Nunes-Nesi, A.; Inostroza-Blancheteau, C. Aluminum stress differentially affects physiological performance and metabolic compounds in cultivars of highbush blueberry. Sci. Rep. 2019, 9, 11275. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.B.; Tabaldi, L.A.; Gonçalves, J.F.; Jucoski, G.O.; Pauletto, M.M.; Weis, S.N.; Nicoloso, F.T.; Borher, D.; Rocha, J.B.T.; Schetinger, M.R.C. Effect of aluminum on δ-aminolevulinic acid dehydratase (ALA-D) and the development of cucumber (Cucumis sativus). Environ. Exp. Bot. 2006, 57, 106–115. [Google Scholar] [CrossRef]
- Haider, S.I.; Kang, W.; Ghulam, J.; Zhango, G. Interaction of cadmium and aluminium toxicity in their effect on growth and physiological parameters in soy beans. J. Zhejiang Univ. Sci. 2006, 8, 181–188. [Google Scholar]
- Badhan, A.; Jin, L.; Wang, Y.; Han, S.; Kowalczys, K.; Brown, D.C.W.; Ayala, C.J.; Latoszek-Green, M.; Miki, B.; Tsang, A.; et al. Expression of a fungal ferulic acid esterase in alfalfa modifies cell wall digestibility. Biotechnol. Biofuels 2014, 7, 1–15. [Google Scholar] [CrossRef]
- Tian, L.; Wang, H.; Wu, K.; Latoszek-Green, M.; Hu, M.; Miki, B.; Brown, D. Efficient recovery of transgenic plants through organogenesis and embryogenesis using a cryptic promoter to drive marker gene expression. Plant Cell Rep. 2002, 20, 1181–1187. [Google Scholar] [CrossRef]
- Boukari, N.; Jelali, N.; Renaud, J.B.; Youssef, R.B.; Abdelly, C.; Hannoufa, A. Salicylic acid seed priming improves tolerance to salinity, iron deficiency and their combined effect in two ecotypes of Alfalfa. Environ. Exp. Bot. 2019, 167, 103820. [Google Scholar] [CrossRef]
- Anderson, J.E.; McNaughton, S.J. Effects of Low Soil Temperature on Transpiration, Photosynthesis, Leaf Relative Water Content, and Growth Among Elevationally Diverse Plant Populations. Ecology 1973, 54, 1220–1233. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper Enzymes in Isolated Chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, 1–12. [Google Scholar] [CrossRef]
- Taylor, S.C.; Nadeau, K.; Abbasi, M.; Lachance, C.; Nguyen, M.; Fenrich, J. The Ultimate qPCR Experiment: Producing Publication Quality, Reproducible Data the First Time. Trends Biotechnol. (Regul. Ed.) 2019, 37, 761–774. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allam, G.; Sakariyahu, S.K.; McDowell, T.; Pitambar, T.A.; Papadopoulos, Y.; Bernards, M.A.; Hannoufa, A. miR156 Is a Negative Regulator of Aluminum Response in Medicago sativa. Plants 2025, 14, 958. https://doi.org/10.3390/plants14060958
Allam G, Sakariyahu SK, McDowell T, Pitambar TA, Papadopoulos Y, Bernards MA, Hannoufa A. miR156 Is a Negative Regulator of Aluminum Response in Medicago sativa. Plants. 2025; 14(6):958. https://doi.org/10.3390/plants14060958
Chicago/Turabian StyleAllam, Gamalat, Solihu K. Sakariyahu, Tim McDowell, Tevon A. Pitambar, Yousef Papadopoulos, Mark A. Bernards, and Abdelali Hannoufa. 2025. "miR156 Is a Negative Regulator of Aluminum Response in Medicago sativa" Plants 14, no. 6: 958. https://doi.org/10.3390/plants14060958
APA StyleAllam, G., Sakariyahu, S. K., McDowell, T., Pitambar, T. A., Papadopoulos, Y., Bernards, M. A., & Hannoufa, A. (2025). miR156 Is a Negative Regulator of Aluminum Response in Medicago sativa. Plants, 14(6), 958. https://doi.org/10.3390/plants14060958






