‘BRS Vitoria’ Grapes Across Four Production Cycles: Morphological, Mineral, and Phenolic Changes
Abstract
1. Introduction
2. Results and Discussion
2.1. Qualitative and Quantitative Profile of Anthocyanins and Mineral Composition of ‘BRS Vitoria’ Grapes from PC1
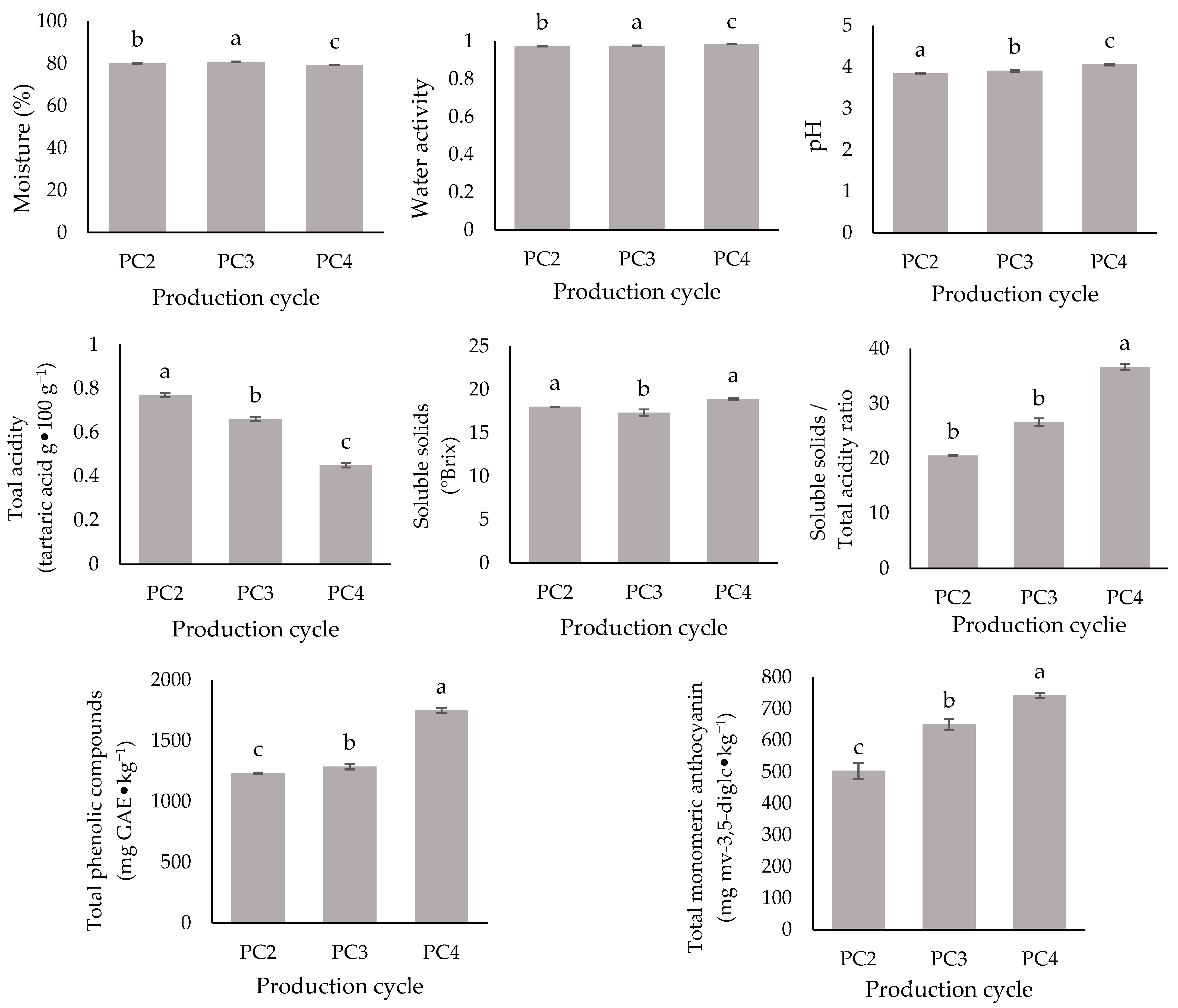
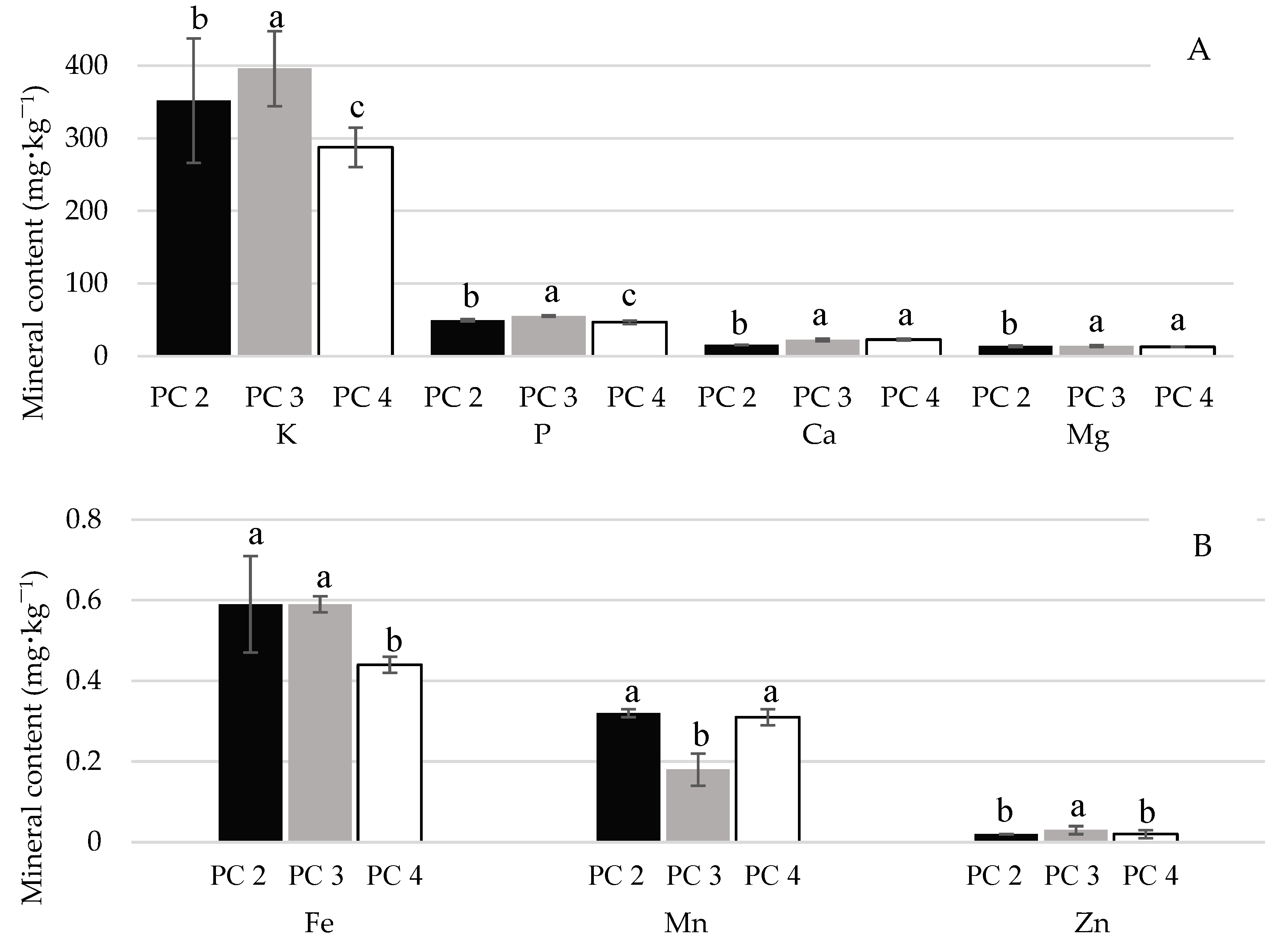
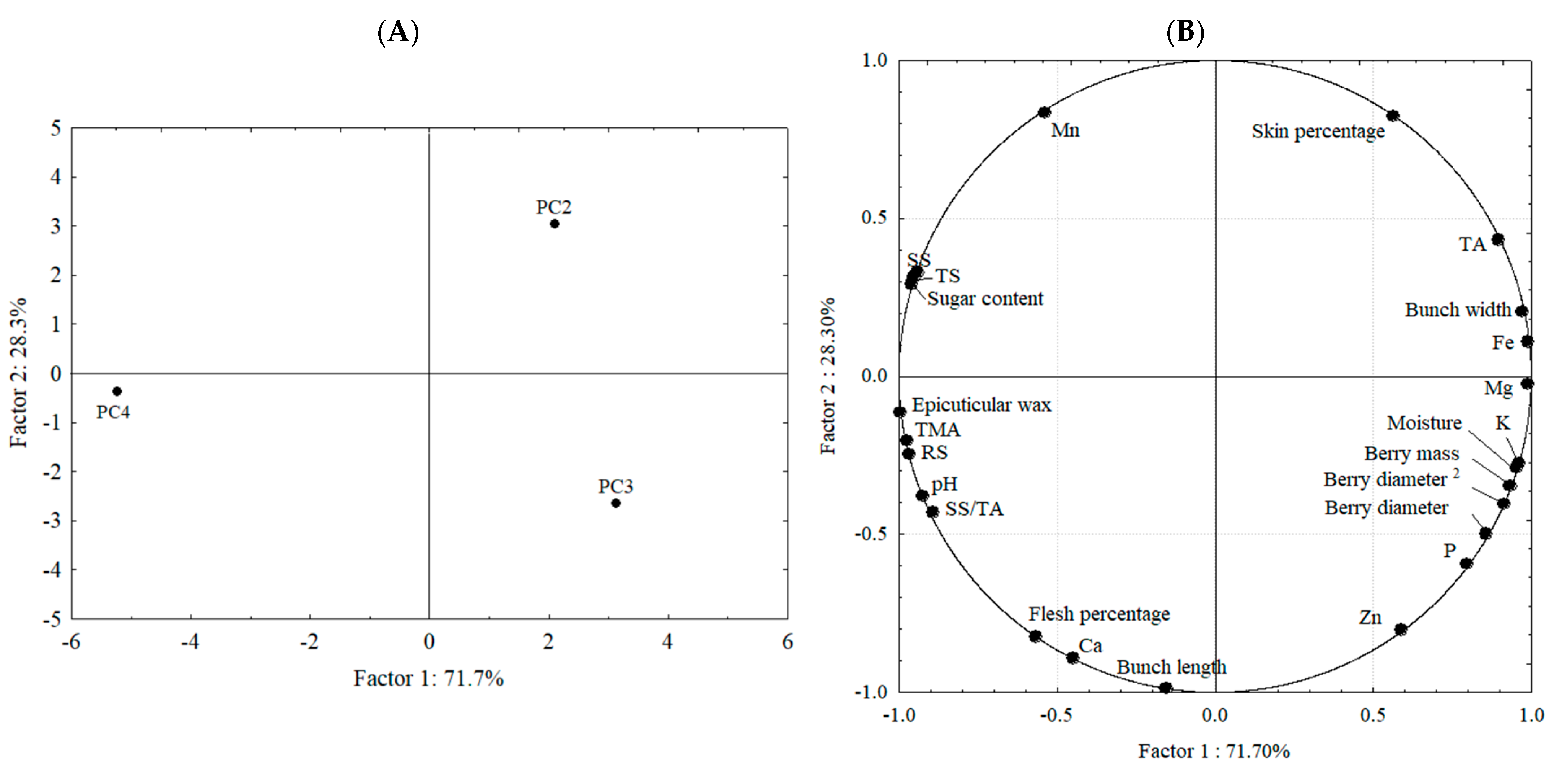
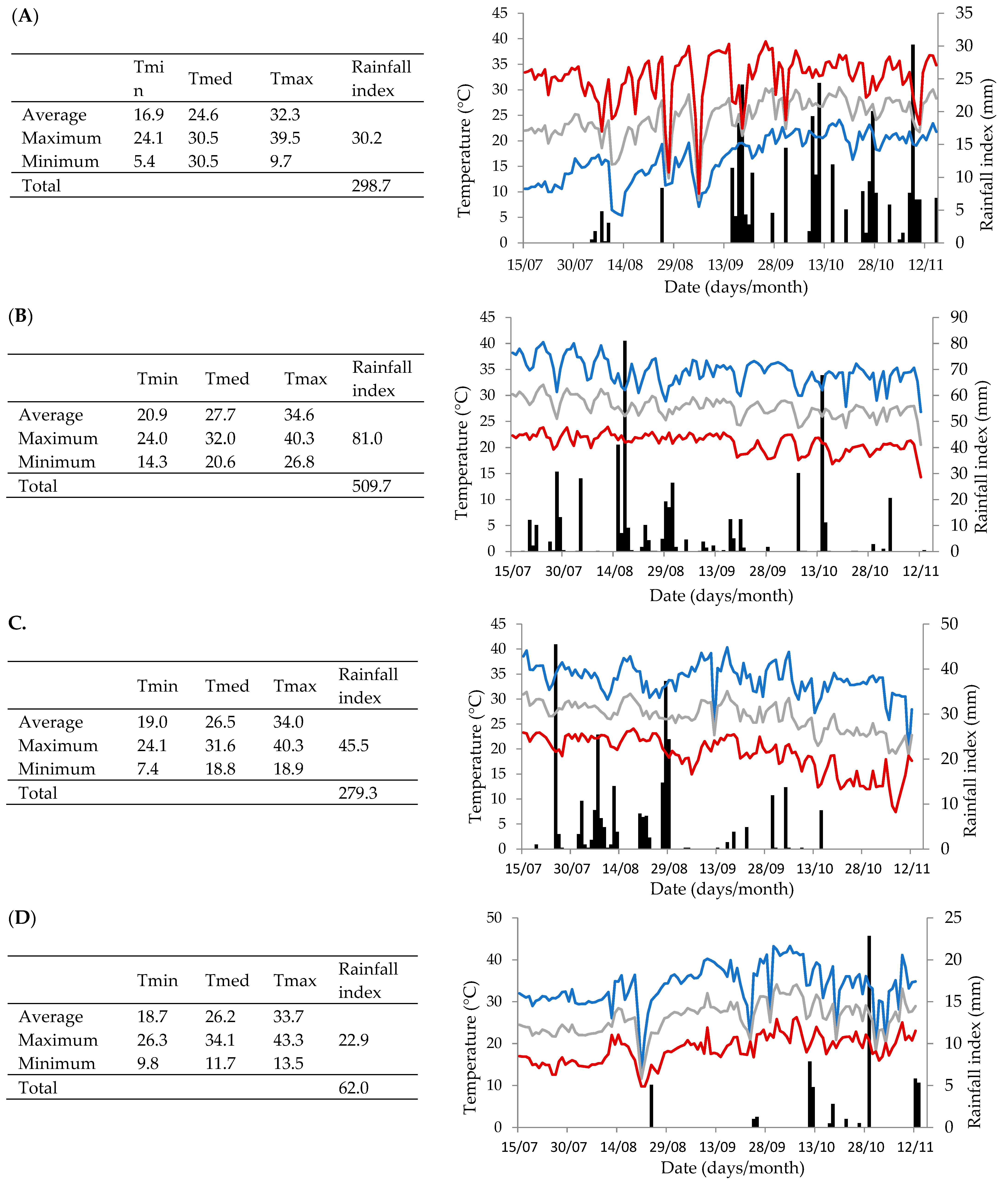
2.2. Physicochemical, Morphological, and Mineral Characterization of ‘BRS Vitoria’ Grapes Across Different PCs
| n° | Descriptor | Categories | PC2 | PC3 | PC4 |
|---|---|---|---|---|---|
| 502 | Bunch mass (g) 1 | 1: Very low (≤100 g); 3: Low (≈300 g); 5: Medium (≈500 g); 7: High (≈700 g); 9: Very high (≥900). | 221.40 b ± 37.94 (3) | 295.05 a ± 22.55 (3) | 206.27 b ± 32.92 (3) |
| 202 | Bunch length (mm) 1 | 1: Very short (≤80 mm); 3: Short (≈120 mm); 5: Medium (≈160 mm); 7: Long (≈200 mm); 9: Very long (≥240 mm). | 134.34 b ± 14.49 (3) | 161.93 a ± 15.17 (5) | 154.68 a ± 10.81 (5) |
| 203 | Bunch width (mm) 1 | 1: Very narrow (≤40 mm); 3: Narrow (≈80 mm); 5: Medium (≈120 mm); 7: Wide (≈160 mm); 9: Very wide (≥200 mm). | 68.37 a ± 6.73 (3) | 67.25 a ± 4.93 (3) | 57.13 b ± 4.59 (3) |
| - | Bunch compactness 3 | 1: Very loose; 3: Loose; 5: Full; 7: Moderately compact; 9: Very compact. | 9 | 9 | 9 |
| - | Bunch shape 4 | 1: Short tapered; 2: Tapered with shoulders; 3: Tapered; 4: Cylindrical; 5: Cylindrical winged; 6: Winged with double bunches. | 4 | 4 | 4 |
| 227 | Pruine 1 | 1: None or very low; 3: Low; 5: Medium; 7: High; 9: Very high. | 7 | 7 | 9 |
| 503 | Berry mass (g)1 | 1: Very low (up to about 1 g); 3: Low (about 3 g); 5: Medium (about 5 g); 7: High (about 7 g); 9: Very high (about 9 g or more). | 3.77 b ± 0.28 (3) | 4.57 a ± 0.29 (5) | 2.86 c ± 0.20 (3) |
| - | Berry shape 3 | 1: Spherical; 2: Flat; 3: Ellipsoid; 4: Elongated; 5: Ovoid; 6: Oval; 7: Obovoid; 8: Elongated curved. | 3 | 3 | 3 |
| 220 | Berry length (mm) 1 | 1: Very short (up to about 8 mm); 3: Short (about 13 mm); 5: Medium (about 18 mm); 7: Long (about 23 mm); 9: Very long (about 28 mm or more). | 21.42 b ± 0.63 (7) | 22.80 a ± 0.53 (7) | 19.22 c ± 1.39 (5) |
| 221 | Berry diameter (mm) 1 | 1: Very narrow (up to about 8 mm); 3: Narrow (about 13 mm); 5: Medium (about 18 mm); 7: Wide (about 23 mm); 9: Very wide (about 28 mm or more). | 16.45 b ± 0.41 (5) | 18.15 a ± 0.67 (5) | 15.32 c ± 0.41 (3) |
| - | Berry diameter (mm) 2 | 10: <12 mm; 12: 12–14 mm; 14: 14–16 mm; 16: 16–18 mm; 18: 18–20 mm; 20: 20–22 mm; 22: 22–24 mm; 24: 24–26 mm; 26: 26–28 mm; 28: 28–30 mm; 30: 30–32 mm; 32: ≥32 mm. | 16 | 18 | 14 |
| 222 | Uniformity of berry size 1 | 1: Not uniform; 2: Uniform. | 2 | 2 | 2 |
| 225 | Skin color 1 | 1: Green-yellow; 2: Rose; 3: Red; 4: Grey; 5: Dark red violet; 6: Blue-black. | 5 | 5 | 5 |
| 226 | Uniformity of skin color 1 | 1: Not uniform; 2: Uniform. | 2 | 2 | 2 |
| 231 | Intensity of flesh coloration 1 | 1: None or very weak; 3: Weak; 5: Medium; 7: Strong; 9: Very strong. | 1 | 1 | 1 |
| 241 | Formation of seeds 1 | 1: None; 2: Rudimentary (incomplete embryo development); 3: Complete (perfectly developed seeds). | 1 | 1 | 1 |
| 505 | Sugar content 1 | 1: Very low (≤12%); 3: Low (≈15%); 5: Medium (≈18%); 7: High (≈21%); 9: Very high (≥24%). | 14.59 a ± 0.27 (3) | 14.47 a ± 0.14 (3) | 14.75 a ± 0.39 (3) |
| 506 | Total acidity (g of tartaric acid⋅L−1) 1 | 1: Very low (3 g⋅L−1); 3: Low (≈6 g⋅L−1); 5: Medium (≈9 g⋅L−1); 7: High (≈15 g⋅L−1); 9: Very high (≥15 g⋅L−1). | 7.69 a ± 0.05 (5) | 6.55 b ± 0.01 (3) | 4.51 c ± 0.81 (3) |
3. Materials and Methods
3.1. Reagents and Standards
3.2. Grape Samples
3.3. Physicochemical Characterization and Profiling of Minerals and Anthocyanins of ‘BRS Vitoria’ Grape
3.4. Determination of Physicochemical and Morphological Characteristics and Mineral Compounds of ‘BRS Vitoria’ Grapes in Three PCs
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PC | Production cycle |
| SS | Soluble solids |
| TA | Total acidity |
| Aw | Water activity |
| TS | Total sugar |
| RS | Reducing sugar |
| TMA | Total monomeric anthocyanin |
| TPC | Total phenolic compound |
| mv | Malvidin |
| pn | Peonidin |
| pt | Petunidin |
| dp | Delphinidin |
| cy | Cyanidin |
| glc | Glucoside |
| K | Potassium |
| P | Phosphorus |
| Ca | Calcium |
| Mg | Magnesium |
| Mn | Manganese |
| Zn | Zinc |
| Fe | Iron |
References
- Majeed, U.; Shafi, A.; Majeed, H.; Akram, K.; Liu, X.; Ye, J.; Luo, Y. Grape (Vitis vinifera L.) phytochemicals and their biochemical protective mechanisms against leading pathologies. Food Chem. 2023, 405, 134762. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of phenolic compounds in human disease: Current knowledge and future prospects. Molecules 2022, 27, 233. [Google Scholar] [CrossRef]
- Colombo, R.C.; Roberto, S.R.; Da Cruz, M.A.; Carvalho, D.U.; Yamamoto, L.Y.; Nixdorf, S.L.; Pérez-Navarro, J.; Gómez-Alonso, S.; Shahab, M.; Ahmed, S.; et al. Characterization of the phenolic ripening development of ‘BRS Vitoria’ seedless table grapes using HPLC-DAD-ESI-MS/MS. J. Food Compos. Anal. 2021, 95, 103693. [Google Scholar] [CrossRef]
- IBGE (Brazilian Institute of Geography and Statistics). Grape Production in Brazil. Available online: https://www.ibge.gov.br/explica/producao-agropecuaria/uva/br (accessed on 5 March 2025).
- OIV (International Organisation of Vine and Wine). Annual Assessment 2023. Available online: https://www.oiv.int/sites/default/files/documents/Annual_Assessment_2023.pdf (accessed on 5 March 2025).
- Leão, P.C.S.; Nunes, B.T.G.; Do-Nascimento, J.H.B.; De Souza, M.C.; Rego, J.I.S. ‘BRS Vitoria’: A new seedless table-grape cultivar for the São Francisco Valley, northeast Brazil. Acta Hortic. 2019, 1248, 275–280. [Google Scholar] [CrossRef]
- Colombo, R.C.; Roberto, S.R.; Nixdorf, S.L.; Pérez-Navarro, J.; Gómez-Alonso, S.; Menta-Morales, A.; García-Romero, E.; Gonçalves, L.S.A.; Cruz, N.A.; Carvalho, D.U.; et al. Analysis of the phenolic composition and yield of ‘BRS Vitoria’ seedless table grape under different bunch densities using HPLC–DAD–ESI-MS/MS. Food Res. Int. 2020, 130, 108955. [Google Scholar] [CrossRef]
- Maia, J.D.G.; Ritschel, P.S.; de Souza, R.T.; Garrido, L.R. ‘BRS Vitoria’—Uva para mesa, sem sementes, de sabor especial e tolerante ao míldio: Recomendações agronômicas para a Região de Campinas, São Paulo. Embrapa Uva e Vinho, Estação Experimental de Viticultura Tropical, Jales, SP. Comun. Técnico 2016, 129, 1–28. Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/145845/1/CirTec129.pdf (accessed on 5 January 2025).
- Leão, P.C.S.; Nascimento, J.H.B.; Moraes, D.S.; Souza, E.R. Rootstocks for the new seedless table grape ‘BRS Vitória’ under tropical semi-arid conditions of São Francisco Valley. Cienc. Agrotec. 2020, 44, e025119. [Google Scholar] [CrossRef]
- Callili, D.; Sánchez, C.A.P.C.; Campos, O.P.; Carneiro, D.C.S.; Scudeletti, A.C.B.; Tecchio, M.A. Phenology, thermal demand, and maturation development of the ‘BRS Vitória’ grape cultivated on diferente rootstocks in subtropical conditions. Radial Basis Funct. 2022, 45, e-999. [Google Scholar] [CrossRef]
- Leão, P.C.S. Rootstocks for Seedless Table Grape Production in the São Francisco Valley. Embrapa Semiárido. 2021. Available online: https://www.infoteca.cnptia.embrapa.br/infoteca/bitstream/doc/1138044/1/Porta-enxertos-uva-de-mesa.-CT129.Patricia.pdf (accessed on 5 March 2025).
- Bascunán-Godoy, L.; Franck, N.; Zamorano, D.; Sanhueza, C.; Carvajal, D.E.; Ibacache, A. Rootstock effect on irrigated grapevine yield under arid climate conditions are explained by changes in traits related to light absorption of the scion. Sci. Hortic. 2017, 218, 284–292. [Google Scholar] [CrossRef]
- Martínez-Ballesta, M.C.; Alcaraz-López, C.; Muries, B.; Mota-Cadenas, C.; Carvajal, M. Physiological aspects of rootstock-scion interactions. Sci. Hortic. 2010, 127, 112–118. [Google Scholar] [CrossRef]
- Leão, P.C.S.; Nascimento, J.H.B.; Moraes, D.S.; Souza, E.R. Yield components of the new seedless table grape ‘BRS Ísis’ as affected by the rootstock under semi-arid tropical conditions. Sci. Hortic. 2020, 263, 109114. [Google Scholar] [CrossRef]
- Anastasiou, E.; Templalexis, C.; Lentzou, D.; Biniari, K.; Xanthopoulos, G.; Fountas, S. Do Soil and Climatic Parameters Affect Yield and Quality on Table Grapes? Smart Agric. Technology 2023, 13, 100088. [Google Scholar] [CrossRef]
- Olivati, C.; Nishiyama, Y.P.O.; Da-Silva, R.; Gómez-Alonso, S.; Lago-Vanzela, E.S. BRS Clara raisins production: Effect of the pre-treatment and the drying process on the phenolic composition. J. Food Compos. Anal. 2022, 114, 104771. [Google Scholar] [CrossRef]
- Martineli, M.; Mendes, F.T.; Dos Santos, J.R.P.; Maranhão, C.M.A.; Castricini, A. Avaliação sensorial e da qualidade de uvas-passas processadas a partir de três cultivares produzidas no semiárido. Braz. J. Food Technol. 2018, 21, 1–8. [Google Scholar] [CrossRef]
- Nishiyama-Hortense, Y.P.; Olivati, C.; Shimizu-Marin, V.D.; Gonçales, A.C.; Janzantti, N.S.; Da Silva, R.; Lago-Vanzela, E.S.; Gómez-Alonso, S. Structure fruit cube snack of BRS Vitoria grape with gala apple: Phenolic composition and sensory attributes. Molecules 2024, 29, 5205. [Google Scholar] [CrossRef]
- Hao, X.; Goa, F.; Wu, H.; Song, Y.; Zhang, L.; Li, H.; Wang, H. From soil to grape and wine: Geographical variations in elemental profiles in different chinese regions. Foods 2021, 10, 3108. [Google Scholar] [CrossRef]
- Bertoldi, D.; Larcher, R.; Bertamini, M.; Otto, S.; Concheri, G.; Nicolini, G. Accumulation and distribution pattern of macro- and microelements and trace elements in Vitis vinifera L. cv. Chardonnay berries. J. Agric. Food Chem. 2011, 59, 7224–7236. [Google Scholar] [CrossRef]
- Panceri, C.P.; Gomes, T.M.; Gois, J.S.; Borges, D.L.G.; Bordignon-Luiz, M.T. Effect of dehydration process on mineral content, phenolic compounds and antioxidant activity of Cabernet Sauvignon and Merlot grapes. Food Res. Int. 2013, 54, 1343–1350. [Google Scholar] [CrossRef]
- Yan, H.K.; Ma, S.Y.; Lu, X.; Zhang, C.C.; Ma, L.; Li, K.; Wei, Y.C.; Gong, M.S.; Li, S. Response of Wine Grape Quality to Rainfall, Temperature, and Soil Properties in Hexi Corridor. HortScience 2022, 57, 1593–1599. [Google Scholar] [CrossRef]
- OIV (Organisation Internacional De la Vigne et Du Vin). OIV Descriptor List for Grape Varieties and Vitis Species, 2nd ed.; OIV: Paris, France, 2001; Available online: http://www.oiv.int/public/medias/2274/code-2e-edition-finale.pdf (accessed on 11 March 2025).
- Hewitt, S.; Hernández-Montes, E.; Dhingra, A.; Keller, M. Impact of heat stress, water stress, and their combined effects on the metabolism and transcriptome of grape berries. Sci. Rep. 2023, 13, 9907. [Google Scholar] [CrossRef]
- Oliveira, J.B.; Laureano, O.; Castro, R.; Pereira, G.E.; Ricardo-da-Silva, J.M. Rootstock and harvest season affect the chemical composition and sensory analysis of grapes and wines of the Alicante Bouschet (Vitis vinifera L.) grown in a tropical semi-arid climate in Brazil. OENO One 2020, 54, 1021–1039. [Google Scholar] [CrossRef]
- Cheng, J.; Wei, L.; Mei, J.; Wu, J. Effect of rootstock on phenolic compounds and antioxidant properties in berries of grape (Vitis vinifera L.) cv. ‘Red Alexandria’. Sci. Hortic. 2017, 213, 137–144. [Google Scholar] [CrossRef]
- Ramos, M.C.; Ibáñez Jara, M.Á.; Rosillo, L.; Salinas, M.R. Effect of temperature and water availability on grape phenolic compounds and their extractability in Merlot grown in a warm area. Sci. Hortic. 2024, 292, 113475. [Google Scholar] [CrossRef]
- Guan, L.; Dai, Z.; Wu, B.-H.; Wu, J.; Merlin, I.; Hilbert, G.; Renaud, C.; Gomès, E.; Edwards, E.; Li, S.-H.; et al. Anthocyanin biosynthesis is differentially regulated by light in the skin and flesh of white-fleshed and teinturier grape berries. Planta 2015, 242, 23–41. [Google Scholar] [CrossRef]
- Song, J.; Smart, R.; Wang, H.; Dambergs, B.; Sparrow, A.; Qian, M.C. Effect of grape bunch sunlight exposure and uv radiation on phenolics and volatile composition of Vitis vinifera L. cv. Pinot noir wine. Food Chem. 2015, 173, 424–431. [Google Scholar] [CrossRef]
- Zou, L.; Zhong, G.-Y.; Wu, B.; Yang, Y.; Li, S.; Liang, Z. Effects of sunlight on anthocyanin accumulation and associated co-expression gene networks in developing grape berries. Environ. Exp. Botany 2019, 166, 103811. [Google Scholar] [CrossRef]
- BRASIL (Ministério da Agricultura, Pecuária e Abastecimento). Instrução normativa nº.1, de 1 de fevereiro de 2002. Regulamento Técnico de Identidade e de Qualidade para a Classificação da Uva Fina de Mesa. Diário Oficial [da] República Federativa do Brasil 2002. Available online: https://sistemasweb.agricultura.gov.br/sislegis/action/detalhaAto.do?method=visualizarAtoPortalMapa&chave=661183307 (accessed on 11 March 2025).
- Souza, J.S.I. Uvas para o Brasil, 2nd ed.; FEALQ: Piracicaba, Brazil, 1996. [Google Scholar]
- Weaver, R. Grape Growing; John Wiley and Sons: Hoboken, NJ, USA, 1976. [Google Scholar]
- Koyama, R.; Borges, W.F.S.; Colombo, R.C.; Hussain, I.; Souza, R.T.D.; Roberto, S.R. Phenology and yield of the hybrid seedless grape ‘BRS Melodia’ grown in an annual double cropping system in a subtropical. Horticulturae. 2020, 6, 3. [Google Scholar] [CrossRef]
- Klimek, K.; Kapłan, M.; Najda, A. Influence of rootstock on yield quantity and quality, contents of biologically active compounds and antioxidant activity in regent grapevine fruit. Molecules 2022, 27, 2065. [Google Scholar] [CrossRef]
- Lago-Vanzela, E.S.; Da-Silva, R.; Gomes, E.; García-Romero, E.; Hermosín-Gutiérrez, I. Phenolic composition of the Brazilian seedless table grape varieties BRS Clara and BRS Morena. J. Agric. Food Chem. 2011, 59, 8314–8323. [Google Scholar] [CrossRef]
- Santos, S.D.L.; Santos, L.S.; Ribeiro, V.G.; De Lima, M.A.C.; Souza, E.R.; Shishido, W.K. Influence of gibberellic acid on physiology and quality of vine cv. Sweet Celebration on submedium of São Francisco. Rev. Bras. Frutic. 2015, 37, 827–834. [Google Scholar] [CrossRef]
- Callili, D.; Tecchio, M.A.; Contreras Sánchez, C.A.P.; Campos, O.P.; Teixeira, L.A.J.; Campos, L.S.; Bonfim, F.P.G.; Leonel, S. Rootstocks on yield and on nutrient uptake and extraction in ‘BRS Vitória’ grapevine. Bragantia 2025, 84, e20240213. [Google Scholar] [CrossRef]
- Da-Silva, M.J.R.; Paiva, A.P.M.; Pimental Junior, A.; Sánchez, C.A.P.C.; Callili, D.; Moura, M.F.; Leonel, S.; Tecchio, M.A. ‘Yield performance of new juice grape varieties grafted onto different rootstocks under tropical conditions. Sci. Hortic. 2018, 241, 194–200. [Google Scholar] [CrossRef]
- CIIAGRO—Integrated Center for Agrometeorological Information. Monthly Data. Available online: http://www.ciiagro.org.br/ema/ (accessed on 5 March 2025).
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis of AOAC International, 18rd ed.; AOAC International: Washington, DC, USA, 2005. [Google Scholar]
- Rebello, L.P.G.; Lago-Vanzela, E.S.; Barcia, M.T.; Ramos, A.M.; Stringheta, P.C.; Da-Silva, R.; Castillo-Muñoz, N.; Gómez-Alonso, S.; Hermosín-Gutiérrez, I. Phenolic composition of the berry parts of hybrid grape cultivar BRS Violeta (BRS Rubea × IAC 1398-21) using HPLC-DAD-ESI-MS/MS. Food Res. Int. 2013, 54, 354–366. [Google Scholar] [CrossRef]
- Lago-Vanzela, E.S.; Procópio, D.P.; Fontes, A.D.F.; Ramos, A.M.; Stringheta, P.C.; Da-Silva, R.; Castillo-Muñoz, N.; Hermosín-Gutiérrez, I. Aging of red wines made from hybrid grape cv. BRS Violeta: Effects of accelerated aging conditions on phenolic composition, color and antioxidant activity. Food Res. Int. 2014, 56, 182–189. [Google Scholar] [CrossRef]
- Pacquette, L.H.; Thompson, J.J.; Malaviole, I.; Zywicki, R.; Woltjes, F.; Ding, J.; Mittal, A.; Ikeuchi, Y.; Sadipiralla, B.; Kimura, B.; et al. Minerals and trace elements in milk, milk products, infant formula, and adult/pediatric nutritional formula, ICP-MS Method: Collaborative Study, AOAC Final Action 2015.06, ISO/DIS 21424, IDF 243. J. AOAC Int. 2018, 101, 536–561. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Ribereau-Gayon, P.; Stonestreet, E. Le dosage des anthocyanes dans le vin rouge. Bull. Soc. Chim. Fr. 1965, 9, 2649–2652. [Google Scholar]

| Minerals | Concentration (mg⋅100 g−1) | Minerals | Concentration (mg⋅100 g−1) |
|---|---|---|---|
| Macrominerals | Microminerals | ||
| K | 345.16 ± 104.89 | Fe | 0.54 ± 0.14 |
| P | 50.50 ± 4.76 | Mn | 0.27 ± 0.08 |
| Ca | 20.34 ± 4.10 | Zn | 0.03 ± 0.01 |
| Mg | 13.61 ± 1.41 | ||
| Anthocyanin * | Molecular Ion; Product Ion (m/z) | Molar Ratio (%) |
|---|---|---|
| dp-3,5-diglc | 627; 465, 303 | 0.90 ± 0.05 |
| cy-3,5-diglc | 611; 449, 287 | 0.41 ± 0.04 |
| pt-3,5-diglc | 641; 479; 317 | 1.68 ± 0.15 |
| pn-3,5-diglc | 625; 463; 301 | 6.74 ± 0.52 |
| mv-3,5-diglc | 655; 493; 331 | 16.30 ± 2.06 |
| dp-3-cmglc-5-glc | 773; 611, 465, 303 | 2.43 ± 0.21 |
| cy-3-cmglc-5-glc | 757; 595, 449, 287 | 0.33 ± 0.21 |
| pt-3-cmglc-5-glc | 787; 625;317 | 3.35 ± 1.29 |
| pn-3-cmglc-5-glc | 771; 609; 307 | 2.81 ± 0.72 |
| mv-3-trans-cmglc-5-glc | 801; 639; 331 | 16.94 ± 2.71 |
| mv-3-cis-cmglc-5-glc | 801; 639; 331 | 1.59 ± 1.06 |
| mv-3-acglc-5-glc | 697; 535; 493; 331 | 1.06 ± 0.33 |
| dp-3-trans-glc | 465; 303 | 0.21 ± 0.05 |
| dp-3-cis-glc | 465; 303 | 4.47 ± 1.36 |
| cy-3-glc | 449; 287 | 1.97 ± 0.42 |
| pt-3-trans-glc | 479; 317 | 0.79 ± 0.16 |
| pt-3-cis-glc | 479; 317 | 3.51 ± 0.87 |
| pn-3-glc | 463; 301 | 2.60 ± 0.54 |
| mv-3-glc | 493; 331 | 5.10 ± 1.17 |
| dp-3-acglc | 507; 303 | 0.30 ± 0.13 |
| cy-3-acglc | 491; 287 | 0.48 ± 0.20 |
| pt-3-acglc | 521; 317 | 0.30 ± 0.10 |
| pn-3-acglc | 505; 301 | 0.29 ± 0.12 |
| mv-3-acglc | 535; 331 | 1.64 ± 0.17 |
| dp-3-trans-cmglc | 611; 303 | 6.47 ± 1.02 |
| cy-3-trans-cmglc | 595; 287 | 2.65 ± 0.30 |
| pt-3-trans-cmglc | 625; 317 | 4.58 ± 0.59 |
| pt-3-cis-cmglc | 625; 317 | 0.17 ± 0.01 |
| pn-3-trans-cmglc | 609; 301 | 2.11 ± 0.24 |
| pn-3-cis-cmglc | 609; 301 | 0.12 ± 0.03 |
| mv-3-trans-cmglc | 639; 331 | 7.24 ± 0.88 |
| mv-3-cis-cmglc | 639; 331 | 0.22 ± 0.01 |
| pl-3-cmglc | 433; 271 | 0.02 ± 0.00 |
| Total Anthocyanin Concentration (mv-3-glc) (mg⋅kg−1) | 135.59 ±14.96 | |
| Total Anthocyanin Concentration (mv-3,5-diglc) (mg⋅kg−1) | 202.28 ± 22.31 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Santos, M.d.S.L.; Shimizu-Marin, V.D.; Nishiyama-Hortense, Y.P.; Olivati, C.; Souza, R.T.d.; Silva, F.B.d.; Janzantti, N.S.; Lago-Vanzela, E.S. ‘BRS Vitoria’ Grapes Across Four Production Cycles: Morphological, Mineral, and Phenolic Changes. Plants 2025, 14, 949. https://doi.org/10.3390/plants14060949
Garcia-Santos MdSL, Shimizu-Marin VD, Nishiyama-Hortense YP, Olivati C, Souza RTd, Silva FBd, Janzantti NS, Lago-Vanzela ES. ‘BRS Vitoria’ Grapes Across Four Production Cycles: Morphological, Mineral, and Phenolic Changes. Plants. 2025; 14(6):949. https://doi.org/10.3390/plants14060949
Chicago/Turabian StyleGarcia-Santos, Mariana de Souza Leite, Victoria Diniz Shimizu-Marin, Yara Paula Nishiyama-Hortense, Carolina Olivati, Reginaldo Teodoro de Souza, Francielli Brondani da Silva, Natália Soares Janzantti, and Ellen Silva Lago-Vanzela. 2025. "‘BRS Vitoria’ Grapes Across Four Production Cycles: Morphological, Mineral, and Phenolic Changes" Plants 14, no. 6: 949. https://doi.org/10.3390/plants14060949
APA StyleGarcia-Santos, M. d. S. L., Shimizu-Marin, V. D., Nishiyama-Hortense, Y. P., Olivati, C., Souza, R. T. d., Silva, F. B. d., Janzantti, N. S., & Lago-Vanzela, E. S. (2025). ‘BRS Vitoria’ Grapes Across Four Production Cycles: Morphological, Mineral, and Phenolic Changes. Plants, 14(6), 949. https://doi.org/10.3390/plants14060949








