Resource Characteristics of Common Reed (Phragmites australis) in the Syr Darya Delta, Kazakhstan, by Means of Remote Sensing and Random Forest
Abstract
1. Introduction
2. Results
2.1. Plot-Level Above-Ground and Above-Water Surface Standing Biomass of Reed Beds and Relationships with Remote Sensing Variables
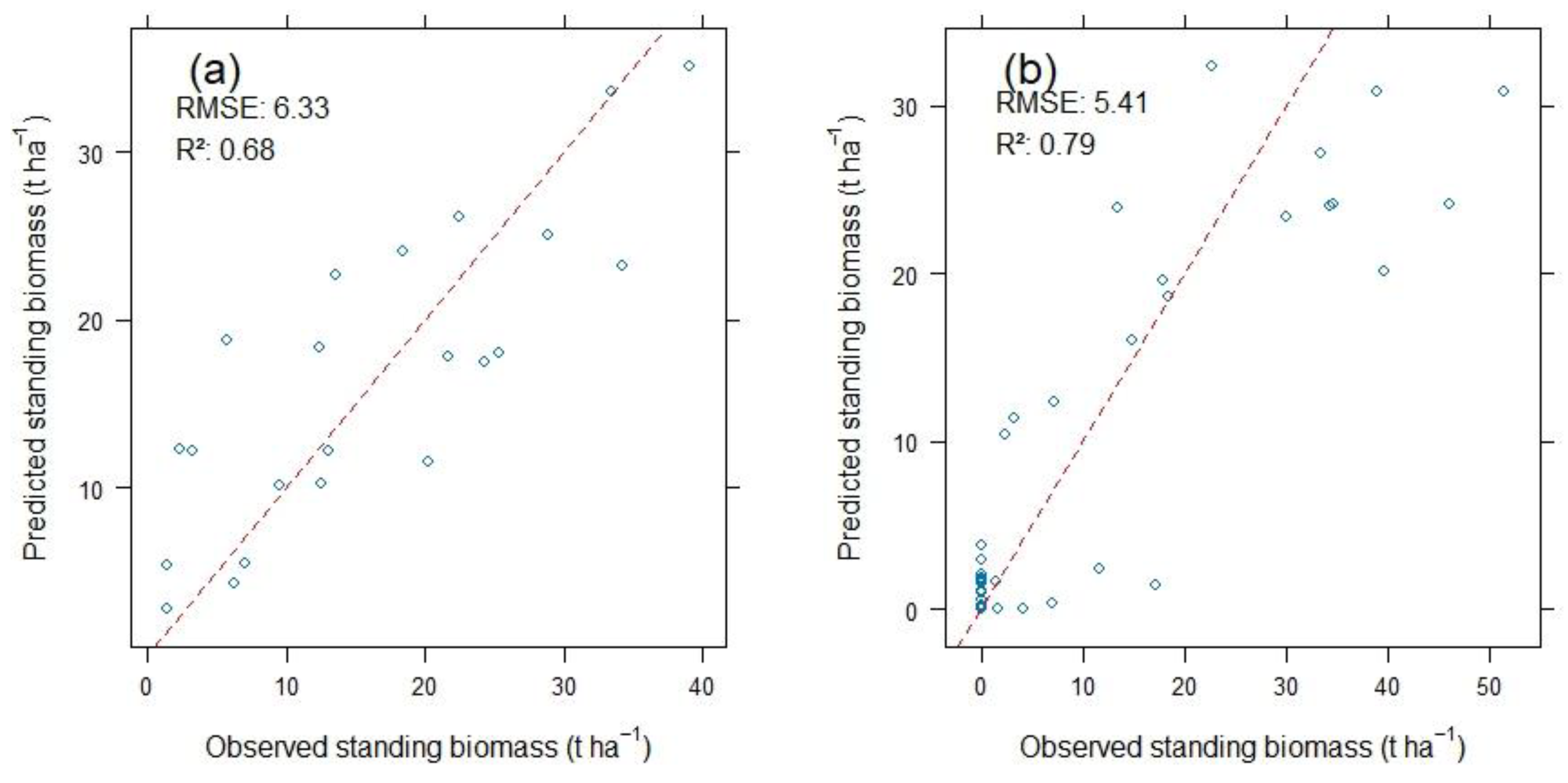
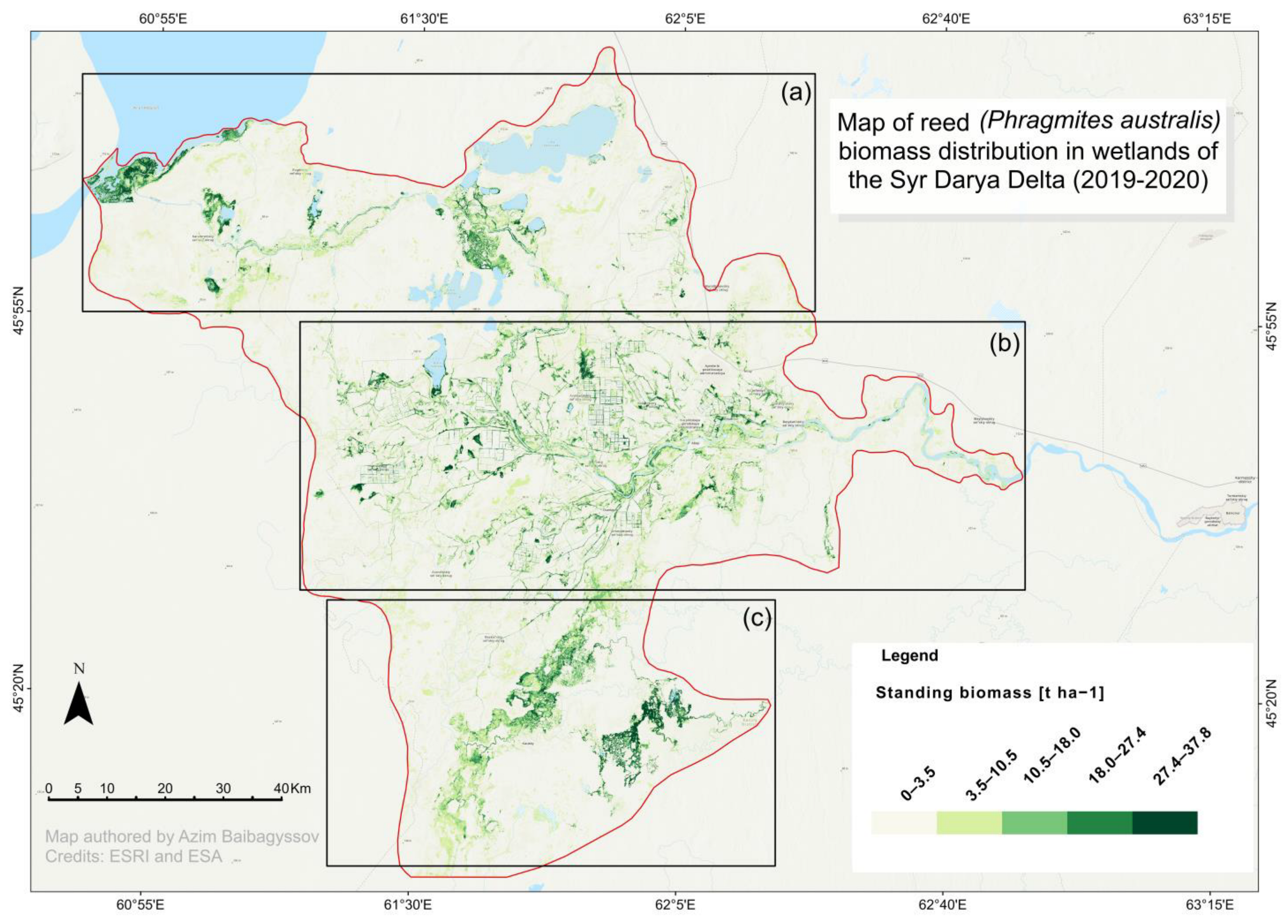
2.2. The Random Forest Model Performance and Mapping of Above-Ground and Above-Water Surface Reed Biomass and Spatial Distribution Patterns
2.3. Reed Bed Area Assessment and Biomass Resource Quantification
3. Discussion
3.1. Estimated Reed Biomass and Its Spatial Distribution in the Syr Darya Delta, Kazakhstan
3.2. Implications for Management Strategies and Sustainable Use of Reed Biomass Resources
3.3. Limitations of Above-Ground and Above-Water Surface Wetland Biomass Assessment Using Random Forest Predictive Modeling and Satellite Data
4. Materials and Methods
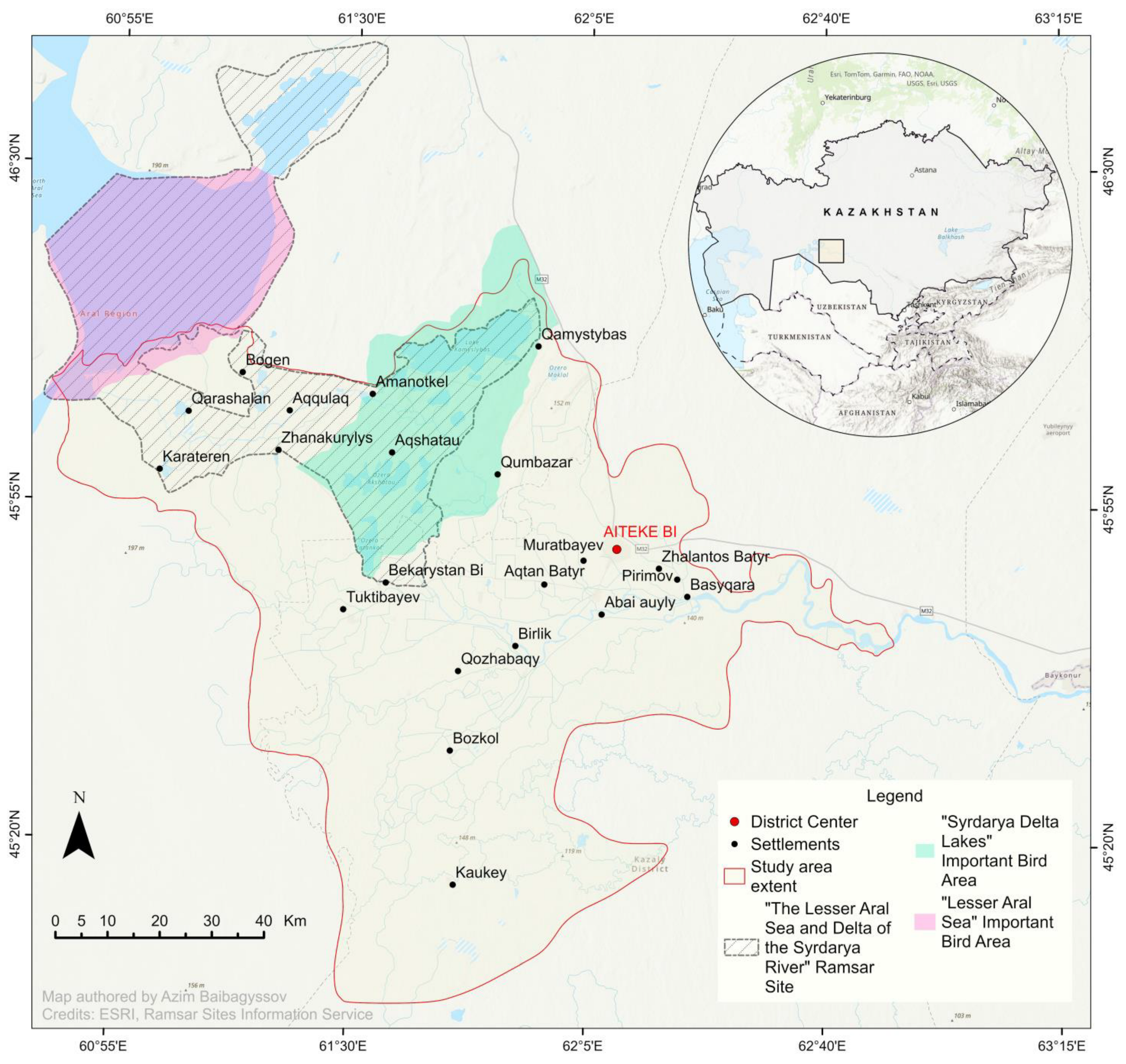
4.1. Study Area
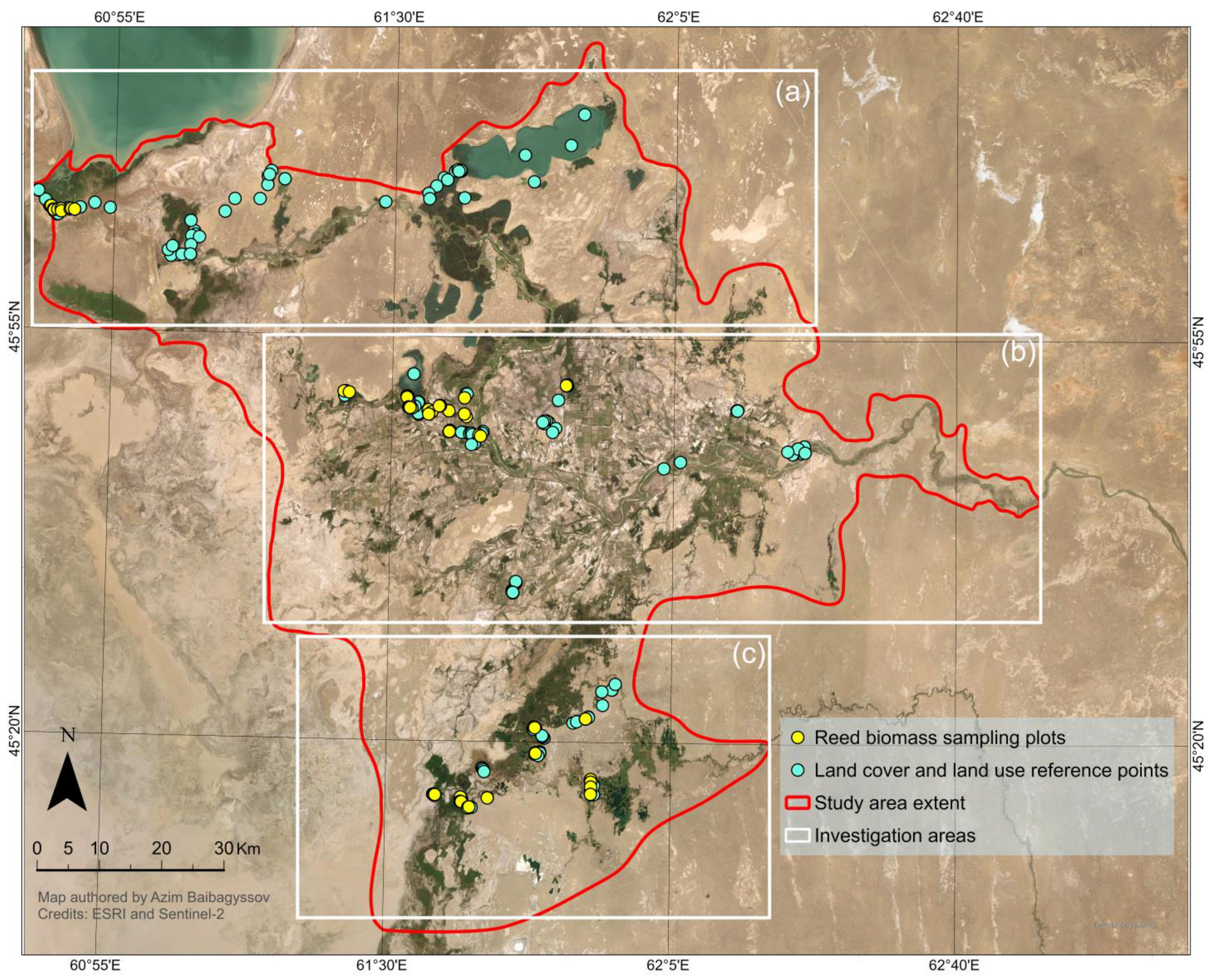
4.2. Field Surveying and In Situ Above-Ground and Above-Water Surface Reed Biomass Sampling
4.3. Satellite Data Acquisition and Spectral Index Calculation
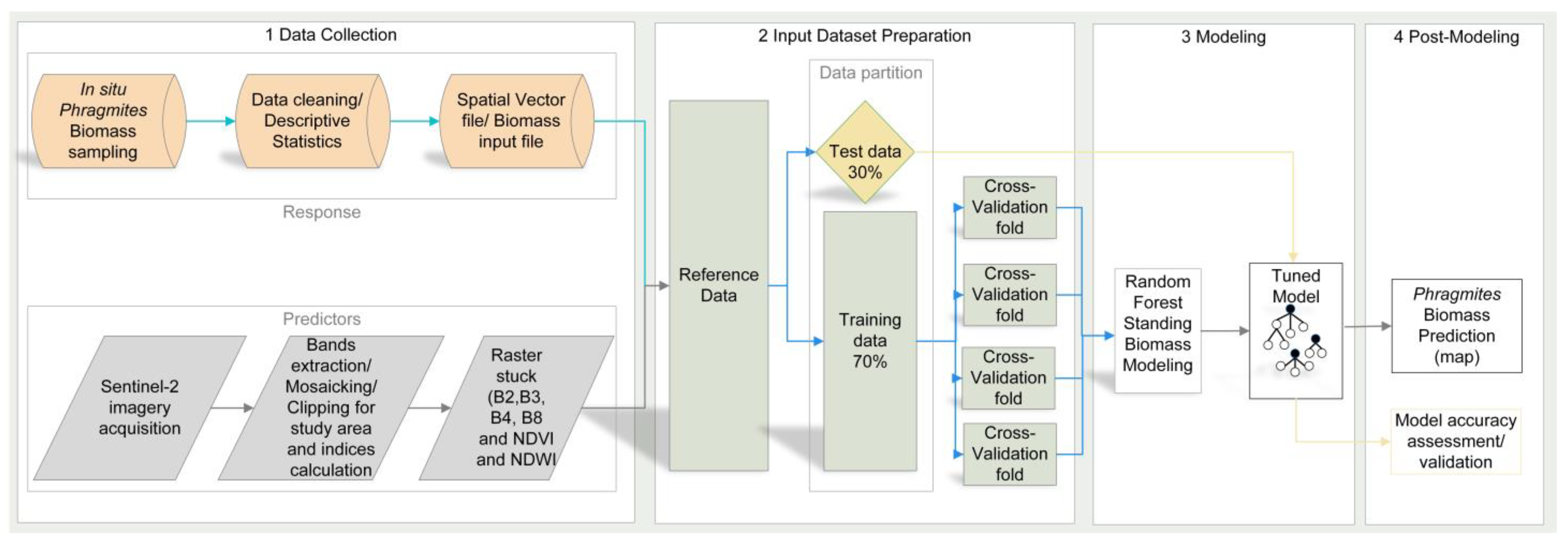
4.4. Overview of the Data and the Study Workflow
4.5. The Random Forest Modeling, Application, and Performance Assessment
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| RF | Random Forest |
| NDVI | Normalized Difference Vegetation Index |
| NDWI | Normalized Difference Water Index |
| RMSE | Root mean square error |
| NIR | Near-infrared |
| WGS 84 | World Geodetic System 1984 |
| UTM | Universal Transverse Mercator |
| 41N | The Northern hemisphere zone 41 |
| GIS | Geographic Information System |
| EPSG | European Petroleum Survey Group |
| UAV | Unmanned Aerial Vehicle |
| LiDAR | Light Detection and Ranging |
| IBA | Important Bird Area |
| NAS | North Aral Sea |
| SYNAS | Syr Darya control and Northern Aral Sea |
| GPS | Global Positioning System |
| ESA | European Space Agency |
| BOA | Bottom-of-Atmosphere |
Appendix A
| Date | Dataset | Explanatory Variables | RF (No) | % of Variation Explained | Ten-Fold Cross-Validation | |
|---|---|---|---|---|---|---|
| R2 | RMSE | |||||
| 13 July 2019 | 78 biomass data | B2, B3, B4, B8, NDVI and NDWI | rf1 | 61.2 | 0.61 | 8.11 |
| 78 biomass data | NDVI, NDWI, B4 and B2 | rf2 | 59.8 | 0.59 | 7.98 | |
| 78 biomass data | NDVI and NDWI | rf3 | 55.4 | 0.55 | 8.26 | |
| 78 biomass data and 205 land cover points | B2, B3, B4, B8, NDVI and NDWI | rf4 | 78.5 | 0.78 | 4.46 | |
| 78 biomass data and 205 land cover points | NDWI, NDVI, B2 and B8 | rf5 | 78.3 | 0.78 | 4.53 | |
| 78 biomass data and 205 land cover points | NDWI and NDVI | rf6 | 74.3 | 0.74 | 4.94 | |
| 2 August 2019 | 78 biomass data | B2, B3, B4, B8, NDVI and NDWI | rf7 | 61.3 | 0.61 | 8.02 |
| 78 biomass data | B4, B2, NDVI and B8 | rf8 | 62.7 | 0.62 | 7.78 | |
| 78 biomass data | NDVI and NDWI | rf9 | 55.4 | 0.55 | 8.94 | |
| 78 biomass data and 205 land cover points | B2, B3, B4, B8, NDVI and NDWI | rf10 | 69.8 | 0.69 | 5.18 | |
| 78 biomass data and 205 land cover points | NDVI, NDWI, B2 and B4 | rf11 | 71.2 | 0.71 | 5.16 | |
| 78 biomass data and 205 land cover points | NDVI and NDWI | rf12 | 62.6 | 0.63 | 6.09 | |
| 21 September 2019 | 78 biomass data | B2, B3, B4, B8, NDVI and NDWI | rf13 | 63.1 | 0.63 | 8.19 |
| 78 biomass data | B2, NDVI, B4 and B8 | rf14 | 62.5 | 0.62 | 8.24 | |
| 78 biomass data | NDVI and NDWI | rf15 | 42.2 | 0.42 | 10.29 | |
| 78 biomass data and 205 land cover points | B2, B3, B4, B8, NDVI and NDWI | rf16 | 69.6 | 0.69 | 5.65 | |
| 78 biomass data and 205 land cover points | NDVI, NDWI, B2 and B4 | rf17 | 70.9 | 0.71 | 5.56 | |
| 78 biomass data and 205 land cover points | NDVI and NDWI | rf18 | 62.5 | 0.62 | 6.22 | |
| 17 July 2020 | 78 biomass data | B2, B3, B4, B8, NDVI and NDWI | rf19 | 54.9 | 0.54 | 8.90 |
| 78 biomass data | B2, B4, B3 and NDVI | rf20 | 57.2 | 0.57 | 8.75 | |
| 78 biomass data | NDVI and NDWI | rf21 | 34.1 | 0.34 | 10.93 | |
| 78 biomass data and 205 land cover points | B2, B3, B4, B8, NDVI and NDWI | rf22 | 71.7 | 0.72 | 5.26 | |
| 78 biomass data and 205 land cover points | NDWI, NDVI, B2 and B3 | rf23 | 72.1 | 0.72 | 5.22 | |
| 78 biomass data and 205 land cover points | NDWI and NDVI | rf24 | 62.8 | 0.63 | 6.43 | |
| 31 August 2020 | 78 biomass data | B2, B3, B4, B8, NDVI and NDWI | rf25 | 61.5 | 0.61 | 7.75 |
| 78 biomass data | NDVI, NDWI, B4 and B3 | rf26 | 59 | 0.59 | 7.96 | |
| 78 biomass data | NDVI and NDWI | rf27 | 60.7 | 0.60 | 7.84 | |
| 78 biomass data and 205 land cover points | B2, B3, B4, B8, NDVI and NDWI | rf28 | 67.4 | 0.67 | 5.52 | |
| 78 biomass data and 205 land cover points | NDWI, NDVI, B4 and B8 | rf29 | 64.9 | 0.64 | 5.69 | |
| 78 biomass data and 205 land cover points | NDWI and NDVI | rf30 | 60.6 | 0.60 | 5.93 | |
| 20 September 2020 | 78 biomass data | B2, B3, B4, B8, NDVI and NDWI | rf31 | 67.8 | 0.68 | 7.29 |
| 78 biomass data | NDVI, NDWI, B4 and B3 | rf32 | 65.3 | 0.65 | 7.59 | |
| 78 biomass data | NDVI and NDWI | rf33 | 57.1 | 0.57 | 8.69 | |
| 78 biomass data and 205 land cover points | B2, B3, B4, B8, NDVI and NDWI | rf34 | 70.4 | 0.70 | 5.24 | |
| 78 biomass data and 205 land cover points | NDWI, NDVI, B3 and B2 | rf35 | 65.7 | 0.66 | 5.76 | |
| 78 biomass data and 205 land cover points | NDWI and NDVI | rf36 | 60.4 | 0.60 | 6.26 | |
| Mean 2019 | 78 biomass data | mean B2, mean B3, mean B4, mean B8, mean NDVI and mean NDWI | rf37 | 76.6 | 0.77 | 6.81 |
| 78 biomass data | mean NDVI, mean NDWI, mean B2 and mean B4 | rf38 | 75.1 | 0.75 | 7.04 | |
| 78 biomass data | NDVI and NDWI | rf39 | 65.7 | 0.66 | 7.93 | |
| 78 biomass data and 205 land cover points | mean B2, mean B3, mean B4, mean B8, mean NDVI and mean NDWI | rf40 | 80.9 | 0.81 | 4.11 | |
| 78 biomass data and 205 land cover points | mean NDVI, mean NDWI, mean B2 and mean B8 | rf41 | 81 | 0.81 | 4.08 | |
| 78 biomass data and 205 land cover points | mean NDVI and mean NDWI | rf42 | 76.8 | 0.77 | 4.46 | |
| Mean 2020 | 78 biomass data | mean B2, mean B3, mean B4, mean B8, mean NDVI and mean NDWI | rf43 | 66.1 | 0.66 | 7.73 |
| 78 biomass data | mean B4, mean NDWI, mean NDVI and mean B3 | rf44 | 67 | 0.67 | 7.90 | |
| 78 biomass data | mean NDWI and mean NDVI | rf45 | 66.3 | 0.66 | 8.22 | |
| 78 biomass data and 205 land cover points | mean B2, mean B3, mean B4, mean B8, mean NDVI and mean NDWI | rf46 | 76.9 | 0.77 | 4.60 | |
| 78 biomass data and 205 land cover points | mean NDWI, mean NDVI mean B4 and mean B8 | rf47 | 74.7 | 0.75 | 4.86 | |
| 78 biomass data and 205 land cover points | mean NDVI and mean NDWI | rf48 | 72.7 | 0.72 | 5.05 | |
Appendix B
Appendix C
References
- Huston, M.A.; Wolverton, S. The Global Distribution of Net Primary Production: Resolving the Paradox. Ecol. Monogr. 2009, 79, 343–377. [Google Scholar] [CrossRef]
- Convention on Wetlands. Global Wetland Outlook: Special Edition 2021; Secretariat of the Convention on Wetlands: Gland, Switzerland, 2021; p. 56. [Google Scholar]
- Zelnik, I.; Germ, M. Diversity of Inland Wetlands: Important Roles in Mitigation of Human Impacts. Diversity 2023, 15, 1050. [Google Scholar] [CrossRef]
- Troia, A. Macrophytes in Inland Waters: From Knowledge to Management. Plants 2023, 12, 582. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.; Efremov, A.; Davidson, T.A.; Molina-Navarro, E.; Fidanza, K.; Betiol, T.C.C.; Chambers, P.; Grimaldo, J.T.; Martins, S.V.; Springuel, I. World Distribution, Diversity and Endemism of Aquatic Macrophytes. Aquat. Bot. 2019, 158, 103127. [Google Scholar] [CrossRef]
- Lesiv, M.S.; Polishchuk, A.I.; Antonyak, H.L. Aquatic Macrophytes: Ecological Features and Functions. Stud. Biol. 2020, 14, 79–94. [Google Scholar] [CrossRef]
- Thomaz, S.M. Ecosystem Services Provided by Freshwater Macrophytes. Hydrobiologia 2023, 850, 2757–2777. [Google Scholar] [CrossRef]
- Srivastava, J.; Kalra, S.J.S.; Naraian, R. Environmental Perspectives of Phragmites australis (Cav.) Trin. Ex. Steudel. Appl. Water Sci. 2014, 4, 193–202. [Google Scholar] [CrossRef]
- Eller, F.; Skálová, H.; Caplan, J.S.; Bhattarai, G.P.; Burger, M.K.; Cronin, J.T.; Guo, W.-Y.; Guo, X.; Hazelton, E.L.; Kettenring, K.M. Cosmopolitan Species as Models for Ecophysiological Responses to Global Change: The Common Reed Phragmites australis. Front. Plant Sci. 2017, 8, 1833. [Google Scholar] [CrossRef]
- Nikolajevskij, V.G. Research into the Biology of the Common Reed (Phragmites communis Trin.) in the U.S.S.R. Folia Geobot. Phytotax. 1971, 6, 221–230. [Google Scholar] [CrossRef]
- Clevering, O.A.; Brix, H.; Lukavská, J. Geographic Variation in Growth Responses in Phragmites australis. Aquat. Bot. 2001, 69, 89–108. [Google Scholar] [CrossRef]
- Ludwig, D.F.; Iannuzzi, T.J.; Esposito, A.N. Phragmites and Environmental Management: A Question of Values. Estuaries 2003, 26, 624–630. [Google Scholar] [CrossRef]
- Köbbing, J.F.; Beckmann, V.; Thevs, N.; Peng, H.; Zerbe, S. Investigation of a Traditional Reed Economy (Phragmites australis) under Threat: Pulp and Paper Market, Values and Netchain at Wuliangsuhai Lake, Inner Mongolia, China. Wetl. Ecol. Manag. 2016, 24, 357–371. [Google Scholar] [CrossRef]
- Meyerson, L.A.; Cronin, J.T.; Pyšek, P. Phragmites Australis as a Model Organism for Studying Plant Invasions. Biol. Invasions 2016, 18, 2421–2431. [Google Scholar] [CrossRef]
- Ailstock, M.S.; Norman, C.M.; Bushmann, P.J. Common Reed Phragmites australis: Control and Effects Upon Biodiversity in Freshwater Nontidal Wetlands. Restor. Ecol. 2001, 9, 49–59. [Google Scholar] [CrossRef]
- Hazelton, E.L.; Mozdzer, T.J.; Burdick, D.M.; Kettenring, K.M.; Whigham, D.F. Phragmites australis Management in the United States: 40 Years of Methods and Outcomes. AoB Plants 2014, 6, plu001. [Google Scholar] [CrossRef] [PubMed]
- Kiviat, E. Ecosystem Services of Phragmites in North America with Emphasis on Habitat Functions. AoB Plants 2013, 5, plt008. [Google Scholar] [CrossRef]
- Wichtmann, W.; Couwenberg, J. Reed as a Renewable Resource and Other Aspects of Paludiculture. Mires Peat 2013, 13, 1–2. [Google Scholar]
- Brix, H.; Ye, S.; Laws, E.A.; Sun, D.; Li, G.; Ding, X.; Yuan, H.; Zhao, G.; Wang, J.; Pei, S. Large-Scale Management of Common Reed, Phragmites australis, for Paper Production: A Case Study from the Liaohe Delta, China. Ecol. Eng. 2014, 73, 760–769. [Google Scholar] [CrossRef]
- Joosten, H.; Gaudig, G.; Tanneberger, F.; Wichmann, S.; Wichtmann, W. Paludiculture: Sustainable Productive Use of Wet and Rewetted Peatlands; Cambridge University Press: Cambridge, UK, 2016; Volume 10. [Google Scholar]
- Milke, J.; Gałczyńska, M.; Wróbel, J. The Importance of Biological and Ecological Properties of Phragmites australis (Cav.) Trin. Ex Steud., in Phytoremendiation of Aquatic Ecosystems—The Review. Water 2020, 12, 1770. [Google Scholar] [CrossRef]
- Kuprina, K.; Seeber, E.; Rudyk, A.; Wichmann, S.; Schnittler, M.; Bog, M. Changes in Genotype Composition and Morphology at an Experimental Site of Common Reed (Phragmites australis) Over a Quarter of a Century. Wetlands 2023, 43, 90. [Google Scholar] [CrossRef]
- Köbbing, J.-F.; Thevs, N.; Zerbe, S. The Utilisation of Reed (Phragmites australis): A Review. Mires Peat 2013, 13, 1–14. [Google Scholar]
- Thevs, N.; Beckmann, V.; Akimalieva, A.; Köbbing, J.F.; Nurtazin, S.; Hirschelmann, S.; Piechottka, T.; Salmurzauli, R.; Baibagysov, A. Assessment of Ecosystem Services of the Wetlands in the Ili River Delta, Kazakhstan. Env. Earth Sci. 2017, 76, 30. [Google Scholar] [CrossRef]
- Baranowski, E.; Thevs, N.; Khalil, A.; Baibagyssov, A.; Iklassov, M.; Salmurzauli, R.; Nurtazin, S.; Beckmann, V. Pastoral Farming in the Ili Delta, Kazakhstan, under Decreasing Water Inflow: An Economic Assessment. Agriculture 2020, 10, 281. [Google Scholar] [CrossRef]
- Baibagyssov, A.; Thevs, N.; Nurtazin, S.; Waldhardt, R.; Beckmann, V.; Salmurzauly, R. Biomass Resources of Phragmites australis in Kazakhstan: Historical Developments, Utilization, and Prospects. Resources 2020, 9, 74. [Google Scholar] [CrossRef]
- Thevs, N. Forest Landscape Restoration and Sustainable Biomass Utilization in Central Asia. In Ustainable Life on Land; MDPI: Basel, Switzerland, 2022; Volume 153. [Google Scholar]
- Carus, M.; Dammer, L.; Raschka, A.; Skoczinski, P. Renewable Carbon: Key to a Sustainable and Future-oriented Chemical and Plastic Industry: Definition, Strategy, Measures and Potential. Greenh. Gases 2020, 10, 488–505. [Google Scholar] [CrossRef]
- McCartney, M.P.; Rebelo, L.-M.; Sellamuttu, S.S. Wetlands, Livelihoods and Human Health. In Wetlands and Human Health; Finlayson, C.M., Horwitz, P., Weinstein, P., Eds.; Wetlands: Ecology, Conservation and Management; Springer: Dordrecht, The Netherlands, 2015; pp. 123–148. ISBN 978-94-017-9609-5. [Google Scholar]
- Hirschelmann, S. The Use of Reed in the Ili-Delta, Kazakhstan—A Social-Ecological Investigation in the Village Region of Kuigan. Master’s Thesis, The University of Greifswald, Greifswald, Germany, 2014. [Google Scholar]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Eid, E.M.; Shaltout, K.H.; Al-Sodany, Y.M.; Soetaert, K.; Jensen, K. Modeling Growth, Carbon Allocation and Nutrient Budgets of Phragmites australis in Lake Burullus, Egypt. Wetlands 2010, 30, 240–251. [Google Scholar] [CrossRef]
- Thevs, N.; Zerbe, S.; Gahlert, F.; Mijit, M.; Succow, M. Productivity of Reed (Phragmites australis Trin. Ex Steud.) in Continental-Arid NW China in Relation to Soil, Groundwater, and Land-Use. J. Appl. Bot. Food Qual. 2007, 81, 62–68. [Google Scholar]
- Engloner, A.I. Annual Growth Dynamics and Morphological Differences of Reed (Phragmites australis [Cav.] Trin. Ex Steudel) in Relation to Water Supply. Flora-Morphol. Distrib. Funct. Ecol. Plants 2004, 199, 256–262. [Google Scholar] [CrossRef]
- Engloner: Structure, Growth Dynamics and Biomass of Reed—Google Scholar. Available online: https://scholar.google.com/scholar_lookup?title=Structure%2C%20growth%20dynamics%20and%20biomass%20of%20reed%20%20%20A%20review&publication_year=2009&author=A.I.%20Engloner (accessed on 28 February 2025).
- Jiang, X.; Weijun, L.; Huiyuan, G.; Shazhou, A. Studies on Morpha and Structure of Three Distinct Growth Forms Phragmites australis. Grassl. China 1995, 5, 49–52. [Google Scholar]
- Dykyjová, D.; Hradecká, D. Production Ecology of Phragmites communis 1. Relations of Two Ecotypes to the Microclimate and Nutrient Conditions of Habitat. Folia Geobot. Phytotaxon. 1976, 11, 23–61. [Google Scholar] [CrossRef]
- Kuprina, K. Genetic and Phenotypic Variation of Phragmites australis (Common Reed) under Environmental Stressors. Ph.D. Thesis, Universität Greifswald, Greifswald, Germany, 2024. [Google Scholar]
- Shatalov, V.G. Forestry, Forest Plantations, Protection and Protection of Forest; Voronezh University Publishing House: Voronezh, Russia, 1973; Volume 135. [Google Scholar]
- Chave, J.; Coomes, D.; Jansen, S.; Lewis, S.L.; Swenson, N.G.; Zanne, A.E. Towards a Worldwide Wood Economics Spectrum. Ecol. Lett. 2009, 12, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Thevs, N.; Fehrenz, S.; Aliev, K.; Emileva, B.; Fazylbekov, R.; Kentbaev, Y.; Qonunov, Y.; Qurbonbekova, Y.; Raissova, N.; Razhapbaev, M. Growth Rates of Poplar Cultivars across Central Asia. Forests 2021, 12, 373. [Google Scholar] [CrossRef]
- Kozlovskiy, V.B.; Pavlov, V.M. Growth of Main Forest Forming Species of the USSR; (In Russian). Forest Industry: Moscow, Russia, 1967; pp. 1–327. [Google Scholar]
- Beckmann, V. Transitioning to Sustainable Life on Land; MDPI-Multidisciplinary Digital Publishing Institute: Basel, Switzerland, 2021. [Google Scholar]
- Pandit, S.; Tsuyuki, S.; Dube, T. Estimating Above-Ground Biomass in Sub-Tropical Buffer Zone Community Forests, Nepal, Using Sentinel 2 Data. Remote Sens. 2018, 10, 601. [Google Scholar] [CrossRef]
- Su, H.; Shen, W.; Wang, J.; Ali, A.; Li, M. Machine Learning and Geostatistical Approaches for Estimating Aboveground Biomass in Chinese Subtropical Forests. For. Ecosyst. 2020, 7, 64. [Google Scholar] [CrossRef]
- Yang, Q.; Niu, C.; Liu, X.; Feng, Y.; Ma, Q.; Wang, X.; Tang, H.; Guo, Q. Mapping High-Resolution Forest Aboveground Biomass of China Using Multisource Remote Sensing Data. GIScience Remote Sens. 2023, 60, 2203303. [Google Scholar] [CrossRef]
- Pham, T.D.; Yokoya, N.; Xia, J.; Ha, N.T.; Le, N.N.; Nguyen, T.T.T.; Dao, T.H.; Vu, T.T.P.; Pham, T.D.; Takeuchi, W. Comparison of Machine Learning Methods for Estimating Mangrove Above-Ground Biomass Using Multiple Source Remote Sensing Data in the Red River Delta Biosphere Reserve, Vietnam. Remote Sens. 2020, 12, 1334. [Google Scholar] [CrossRef]
- Hu, T.; Zhang, Y.; Su, Y.; Zheng, Y.; Lin, G.; Guo, Q. Mapping the Global Mangrove Forest Aboveground Biomass Using Multisource Remote Sensing Data. Remote Sens. 2020, 12, 1690. [Google Scholar] [CrossRef]
- Magiera, A.; Feilhauer, H.; Waldhardt, R.; Wiesmair, M.; Otte, A. Modelling Biomass of Mountainous Grasslands by Including a Species Composition Map. Ecol. Indic. 2017, 78, 8–18. [Google Scholar] [CrossRef]
- Zeng, N.; Ren, X.; He, H.; Zhang, L.; Zhao, D.; Ge, R.; Li, P.; Niu, Z. Estimating Grassland Aboveground Biomass on the Tibetan Plateau Using a Random Forest Algorithm. Ecol. Indic. 2019, 102, 479–487. [Google Scholar] [CrossRef]
- Wang, S.; Tuya, H.; Zhang, S.; Zhao, X.; Liu, Z.; Li, R.; Lin, X. Random Forest Method for Analysis of Remote Sensing Inversion of Aboveground Biomass and Grazing Intensity of Grasslands in Inner Mongolia, China. Int. J. Remote Sens. 2023, 44, 2867–2884. [Google Scholar] [CrossRef]
- Mokarighahroodi, E. A Machine Learning Approach for Prediction of Rangeland Aboveground Production and Crop Water Use. Ph.D. Thesis, New Mexico State University, Las Cruces, NM, USA, 2021. [Google Scholar]
- Rapiya, M.; Ramoelo, A.; Truter, W. Seasonal Evaluation and Mapping of Aboveground Biomass in Natural Rangelands Using Sentinel-1 and Sentinel-2 Data. Environ. Monit Assess 2023, 195, 1544. [Google Scholar] [CrossRef]
- Carreiras, J.M.; Melo, J.B.; Vasconcelos, M.J. Estimating the Above-Ground Biomass in Miombo Savanna Woodlands (Mozambique, East Africa) Using L-Band Synthetic Aperture Radar Data. Remote Sens. 2013, 5, 1524–1548. [Google Scholar] [CrossRef]
- Karlson, M.; Ostwald, M.; Reese, H.; Sanou, J.; Tankoano, B.; Mattsson, E. Mapping Tree Canopy Cover and Aboveground Biomass in Sudano-Sahelian Woodlands Using Landsat 8 and Random Forest. Remote Sens. 2015, 7, 10017–10041. [Google Scholar] [CrossRef]
- Bispo, P.d.C.; Rodríguez-Veiga, P.; Zimbres, B.; do Couto de Miranda, S.; Henrique Giusti Cezare, C.; Fleming, S.; Baldacchino, F.; Louis, V.; Rains, D.; Garcia, M. Woody Aboveground Biomass Mapping of the Brazilian Savanna with a Multi-Sensor and Machine Learning Approach. Remote Sens. 2020, 12, 2685. [Google Scholar] [CrossRef]
- Forkuor, G.; Zoungrana, J.-B.B.; Dimobe, K.; Ouattara, B.; Vadrevu, K.P.; Tondoh, J.E. Above-Ground Biomass Mapping in West African Dryland Forest Using Sentinel-1 and 2 Datasets-A Case Study. Remote Sens. Environ. 2020, 236, 111496. [Google Scholar] [CrossRef]
- Mutanga, O.; Adam, E.; Cho, M.A. High Density Biomass Estimation for Wetland Vegetation Using WorldView-2 Imagery and Random Forest Regression Algorithm. Int. J. Appl. Earth Obs. Geoinf. 2012, 18, 399–406. [Google Scholar] [CrossRef]
- Wan, R.; Wang, P.; Wang, X.; Yao, X.; Dai, X. Mapping Aboveground Biomass of Four Typical Vegetation Types in the Poyang Lake Wetlands Based on Random Forest Modelling and Landsat Images. Front. Plant Sci. 2019, 10, 1281. [Google Scholar] [CrossRef]
- Hemati, M.; Mahdianpari, M.; Shiri, H.; Mohammadimanesh, F. Integrating SAR and Optical Data for Aboveground Biomass Estimation of Coastal Wetlands Using Machine Learning: Multi-Scale Approach. Remote Sens. 2024, 16, 831. [Google Scholar] [CrossRef]
- Klemas, V. Remote Sensing of Coastal Wetland Biomass: An Overview. J. Coast. Res. 2013, 29, 1016–1028. [Google Scholar] [CrossRef]
- Lumbierres, M.; Méndez, P.F.; Bustamante, J.; Soriguer, R.; Santamaría, L. Modeling Biomass Production in Seasonal Wetlands Using MODIS NDVI Land Surface Phenology. Remote Sens. 2017, 9, 392. [Google Scholar] [CrossRef]
- O’Shea, C.J. Using Remote Sensing to Explore the Spectral and Spatial Characteristics of Wetland Vegetation; University of Stirling: Scotland, UK, 2005. [Google Scholar]
- Wang, C.; Pavelsky, T.M.; Kyzivat, E.D.; Garcia-Tigreros, F.; Podest, E.; Yao, F.; Yang, X.; Zhang, S.; Song, C.; Langhorst, T. Quantification of Wetland Vegetation Communities Features with Airborne AVIRIS-NG, UAVSAR, and UAV LiDAR Data in Peace-Athabasca Delta. Remote Sens. Environ. 2023, 294, 113646. [Google Scholar] [CrossRef]
- Samarkhanov, K.; Abuduwaili, J.; Samat, A.; Issanova, G. The Spatial and Temporal Land Cover Patterns of the Qazaly Irrigation Zone in 2003–2018: The Case of Syrdarya River’s Lower Reaches, Kazakhstan. Sustainability 2019, 11, 4035. [Google Scholar] [CrossRef]
- Wang, L.; Dronova, I.; Gong, P.; Yang, W.; Li, Y.; Liu, Q. A New Time Series Vegetation–Water Index of Phenological–Hydrological Trait across Species and Functional Types for Poyang Lake Wetland Ecosystem. Remote Sens. Environ. 2012, 125, 49–63. [Google Scholar] [CrossRef]
- Wang, E.; Huang, T.; Liu, Z.; Bao, L.; Guo, B.; Yu, Z.; Feng, Z.; Luo, H.; Ou, G. Improving Forest Above-Ground Biomass Estimation Accuracy Using Multi-Source Remote Sensing and Optimized Least Absolute Shrinkage and Selection Operator Variable Selection Method. Remote Sens. 2024, 16, 4497. [Google Scholar] [CrossRef]
- Rowan, G.S.; Kalacska, M. A Review of Remote Sensing of Submerged Aquatic Vegetation for Non-Specialists. Remote Sens. 2021, 13, 623. [Google Scholar] [CrossRef]
- Meyer, H.; Ludwig, M.; Milà, C.; Linnenbrink, J.; Schumacher, F. The CAST Package for Training and Assessment of Spatial Prediction Models in R 2024. arXiv 2024, arXiv:2404.06978. [Google Scholar]
- Niu, X.; Chen, B.; Sun, W.; Feng, T.; Yang, X.; Liu, Y.; Liu, W.; Fu, B. Estimation of Coastal Wetland Vegetation Aboveground Biomass by Integrating UAV and Satellite Remote Sensing Data. Remote Sens. 2024, 16, 2760. [Google Scholar] [CrossRef]
- Ge, C.; Zhang, C.; Zhang, Y.; Fan, Z.; Kong, M.; He, W. Synergy of UAV-LiDAR Data and Multispectral Remote Sensing Images for Allometric Estimation of Phragmites Australis Aboveground Biomass in Coastal Wetland. Remote Sens. 2024, 16, 3073. [Google Scholar] [CrossRef]
- Lesser Aral Sea and Delta of the Syrdarya River | Ramsar Sites Information Service. Available online: https://rsis.ramsar.org/ris/2083 (accessed on 20 June 2024).
- Zinabdin, N.; Akiyanova, F.; Yegemberdiyeva, K.; Temirbayeva, R.; Mazbayev, O. The Functional Zoning of the Syr Darya River’s Delta. Sustainability 2022, 14, 7153. [Google Scholar] [CrossRef]
- Jin, Q.; Wei, J.; Yang, Z.-L.; Lin, P. Irrigation-Induced Environmental Changes around the Aral Sea: An Integrated View from Multiple Satellite Observations. Remote Sens. 2017, 9, 900. [Google Scholar] [CrossRef]
- Bissenbayeva, S.B. Human Activities in the Syr Darya River Basin. J. Geogr. Environ. Manag. 2020, 58, 12–21. [Google Scholar] [CrossRef]
- Asarin, A.E.; Kravtsova, V.I.; Mikhailov, V.N. Amudarya and Syrdarya Rivers and Their Deltas. In The Aral Sea Environment; Kostianoy, A.G., Kosarev, A.N., Eds.; The Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2010; Volume 7, pp. 101–121. ISBN 978-3-540-88276-3. [Google Scholar]
- Degtyareva, O.; Muratova, N.; Salnikov, V.; Thiel, M. Monitoring of the Irrigated Rice Production Dynamic in the Kazalinsk Region, Kazakhstan, from 1984–2017. Cent. Asian J. Water Res. 2019, 5, 20–43. [Google Scholar] [CrossRef]
- Cai, X.; McKinney, D.C.; Rosegrant, M.W. Sustainability Analysis for Irrigation Water Management in the Aral Sea Region. Agric. Syst. 2003, 76, 1043–1066. [Google Scholar] [CrossRef]
- Kuderin, A.; Skorintseva, I.; Bassova, T.; Krylova, V.; Krasnoyarova, B. Landscape Planning of the Kazaly Irrigation Array of Southern Kazakhstan. Eur. J. Geogr. 2019, 10. [Google Scholar]
- Samakov, A. Livelihoods and Social-Environmental Change in the Syr Darya Delta: Adaptive Strategies and Practices. Ph.D. Thesis, Universität Bern, Bern, Switzerland, 2024. [Google Scholar]
- Micklin, P. The Future Aral Sea: Hope and Despair. Environ. Earth Sci. 2016, 75, 844. [Google Scholar] [CrossRef]
- Oskenbayev, A.; Xu, W.; Nyyssonen, V.; Neupane, R. Study on Environmental and Social Impact of Second Phase Syrdarya Control And Northern Aral Sea (SYNAS) Project. Int. J. Adv. Res. 2017, 5, 1659–1674. [Google Scholar] [CrossRef]
- White, K.; Micklin, P. Ecological Restoration and Economic Recovery in Kazakhstan’s Northern Aral Sea Region. Focus Geogr. 2021, 64, 162. [Google Scholar] [CrossRef]
- Kipshakbaev, N.; De Shoutter, J.; Dukhovny, V.; Malkovsky, I.; Ogar, N.; Haibullin, A.; Yaprintsev, V.; Tuchin, A.; Yakhiyaeva, K. Ecosystem Restoration in the Syrdarya Delta and Northern Part of the Aral Sea. Eur. J. Geogr. 2010, 10, 37–49. Available online: http://cawater-info.net/library/eng/north_aral_en.pdf (accessed on 12 March 2025).
- Mogano, K. The Utility of New Generation Multispectral Sensors in Assessing Aboveground Biomass of Phragmites Australis in Wetlands Areas in the City of Tshwane Metropolitan Municipality; South Africa. Ph.D. Thesis, University of KwaZulu-Natal, Durban, South Africa, 2017. [Google Scholar]
- Tiškus, E.; Vaičiūtė, D.; Bučas, M.; Gintauskas, J. Evaluation of Common Reed (Phragmites australis) Bed Changes in the Context of Management Using Earth Observation and Automatic Threshold. Eur. J. Remote Sens. 2023, 56, 2161070. [Google Scholar] [CrossRef]
- Rakhymberdina, M.Y.; Kulenova, N.A.; Shaimardanov, Z.K.; Assylkhanova, Z.A.; Toguzova, M.M.; Kassymov, D.K. Using Remote Sensing Data to Support Intelligent Agricultural GIS to Monitor the Condition of Arable Land and Crops. Chem. Eng. Trans. 2022, 94, 883–888. [Google Scholar]
- Siximbayeva, G.Т.; Nemec, J.; Khalitova, M.M.; Imangali, Z.G. Managing Corruption Risk in the Agricultural Sector of Kazakhstan and Its Assessment. Econ. Strategy Pract. 2022, 17, 199–211. [Google Scholar] [CrossRef]
- Cutler, D.R.; Edwards, T.C.; Beard, K.H.; Cutler, A.; Hess, K.T.; Gibson, J.; Lawler, J.J. Random Forests for Classification in Ecology. Ecology 2007, 88, 2783–2792. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Wang, J.; Zeng, W.; Song, J. Comparison and Evaluation of Three Methods for Estimating Forest above Ground Biomass Using TM and GLAS Data. Remote Sens. 2017, 9, 341. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Classification and Regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]

| Land Cover Class | Number of Samples (n) | Standing Biomass (t ha−1) | |||
|---|---|---|---|---|---|
| Min | Max | Mean | Standard Deviation | ||
| Open reed and shrub vegetation | 26 | 1.14 | 8.58 | 4.36 | 2.46 |
| Non-submerged dense reed | 26 | 7.07 | 26.01 | 15.24 | 5.37 |
| Submerged dense deed | 26 | 6.57 | 51.33 | 28.21 | 11.71 |
| Land Cover Class | Productivity [t ha−1] | Area [ha] | Percentage | Standing Biomass [t] |
|---|---|---|---|---|
| Open reed and shrub vegetation | 0.0–3.5 | 425,432 | 75.4 | 744,506 |
| Non-submerged dense reed | 3.5–10.5 | 79,600 | 14.1 | 557,200 |
| 10.5–18.0 | 27,238 | 4.8 | 388,142 | |
| Submerged dense reed | 18.0–27.4 | 18,250 | 3.3 | 414,275 |
| 27.4–37.8 | 13,447 | 2.4 | 438,372 | |
| Total: 563,967 | Total: 2,542,495 | |||
| Land Cover Class (Strata) | Definition | Photograph |
|---|---|---|
| Open water | Open water bodies with <4% vegetation cover |  |
| Submerged dense reed | Reed (Phragmites australis-dominated) vegetation with a total vegetation cover of 70% or more and submerged soil during most of the year |  |
| Non-submerged dense reed | Reed (Phragmites australis-dominated) vegetation with a total vegetation cover of 70% or more non-submerged soil during most of the year |  |
| Open reed and shrub vegetation | Reed (Phragmites australis-dominated) vegetation, partly interspersed by shrubs with a total vegetation cover of <70%, but at least 20%, and non-submerged soil during most of the year |  |
| Bare land with open sands | Bare land with open sands with <4% vegetation cover |  |
| Investigation Areas | Land Cover and Land-Use Points | Reed Biomass Sampling Plots |
|---|---|---|
| (a) Wetland areas next to the Kok-Aral dam and dike complex | 60 | 12 |
| (b) Wetland areas around deltaic lakes, such as Aidarkol and Kotankol next to the Bekarystan Bi Village | 74 | 37 |
| (c) Wetland areas along the left branch of the Syr Darya River next to Tasaryk and Lakaly Villages and around the Maryamkol Lake next to the Kaukei Village | 71 | 29 |
| Total | 205 | 78 |
| Band | Definition | Wavelength (nm) | Spatial Resolution (m) |
|---|---|---|---|
| Band 1 | Coastal Aerosol | 443 | 60 |
| Band 2 | Blue | 490 | 10 |
| Band 3 | Green | 560 | 10 |
| Band 4 | Red | 665 | 10 |
| Band 5 | Red-edge 1 | 705 | 20 |
| Band 6 | Red-edge 2 | 740 | 20 |
| Band 7 | Red-edge 3 | 783 | 20 |
| Band 8 | Near-infrared (NIR) | 842 | 10 |
| Band 8a | Near-infrared (NIR) narrow | 865 | 20 |
| Band 9 | Water vapor | 945 | 60 |
| Band 10 | Short-wavelength infrared (SWIR-Cirrus) | 1375 | 60 |
| Band 11 | Short-wavelength infrared (SWIR-1) | 1610 | 20 |
| Band 12 | Short-wavelength infrared (SWIR-2) | 2190 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baibagyssov, A.; Magiera, A.; Thevs, N.; Waldhardt, R. Resource Characteristics of Common Reed (Phragmites australis) in the Syr Darya Delta, Kazakhstan, by Means of Remote Sensing and Random Forest. Plants 2025, 14, 933. https://doi.org/10.3390/plants14060933
Baibagyssov A, Magiera A, Thevs N, Waldhardt R. Resource Characteristics of Common Reed (Phragmites australis) in the Syr Darya Delta, Kazakhstan, by Means of Remote Sensing and Random Forest. Plants. 2025; 14(6):933. https://doi.org/10.3390/plants14060933
Chicago/Turabian StyleBaibagyssov, Azim, Anja Magiera, Niels Thevs, and Rainer Waldhardt. 2025. "Resource Characteristics of Common Reed (Phragmites australis) in the Syr Darya Delta, Kazakhstan, by Means of Remote Sensing and Random Forest" Plants 14, no. 6: 933. https://doi.org/10.3390/plants14060933
APA StyleBaibagyssov, A., Magiera, A., Thevs, N., & Waldhardt, R. (2025). Resource Characteristics of Common Reed (Phragmites australis) in the Syr Darya Delta, Kazakhstan, by Means of Remote Sensing and Random Forest. Plants, 14(6), 933. https://doi.org/10.3390/plants14060933






