Diversity of Phytochemical Content, Antioxidant Activity, and Fruit Morphometry of Three Mallow, Malva Species (Malvaceae)
Abstract
1. Introduction
2. Results
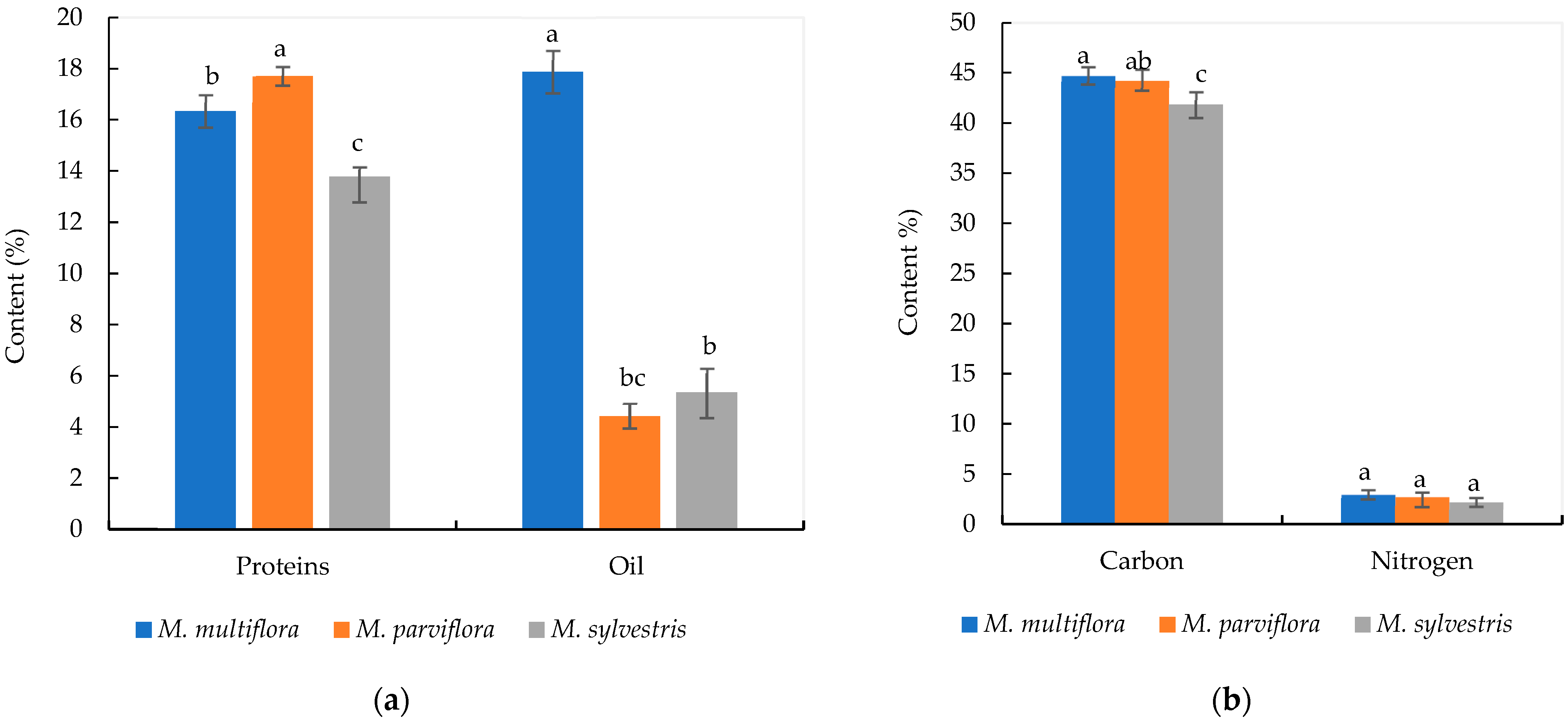
2.1. Phytochemical Characteristics
2.2. Antioxidant Activity
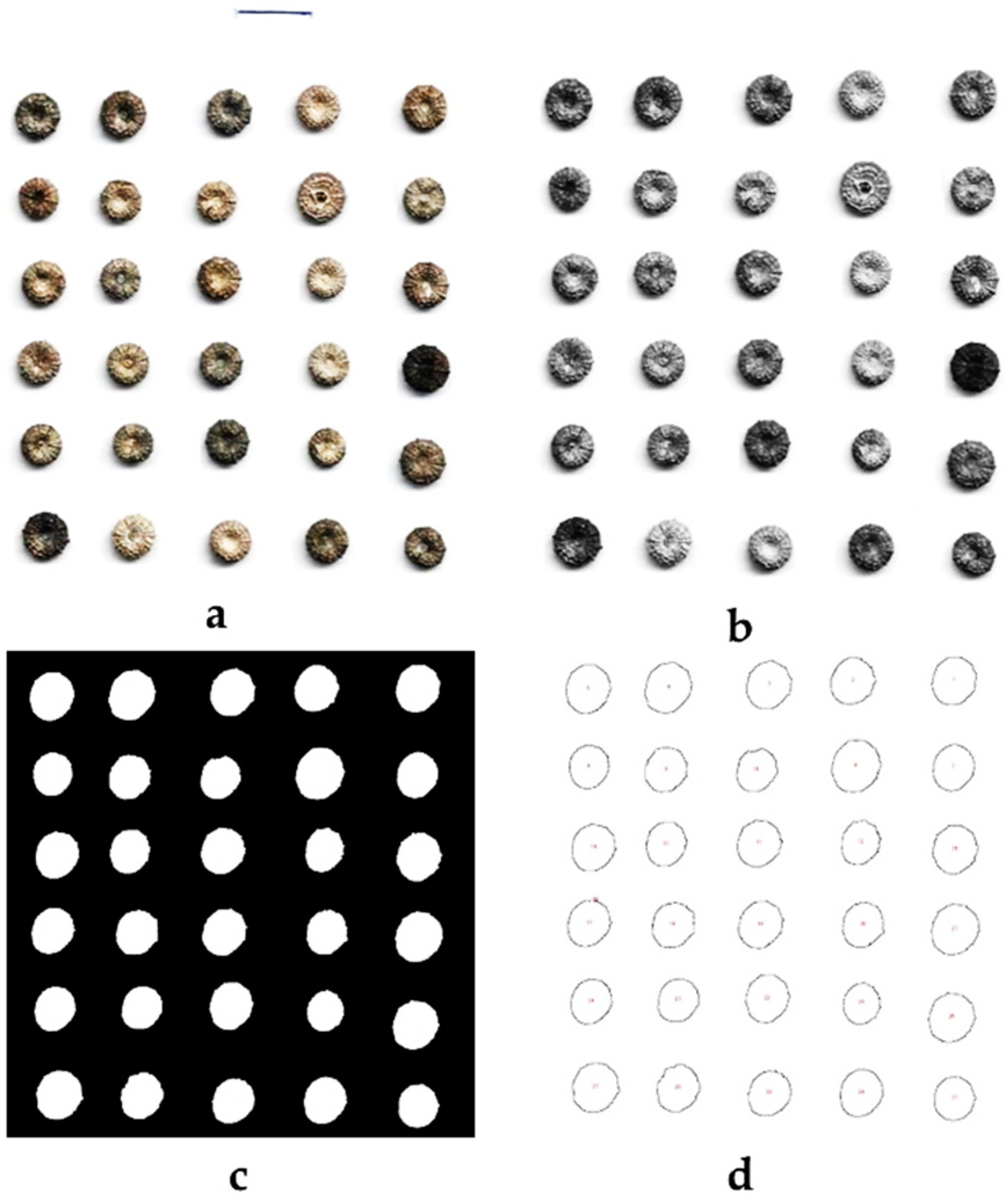
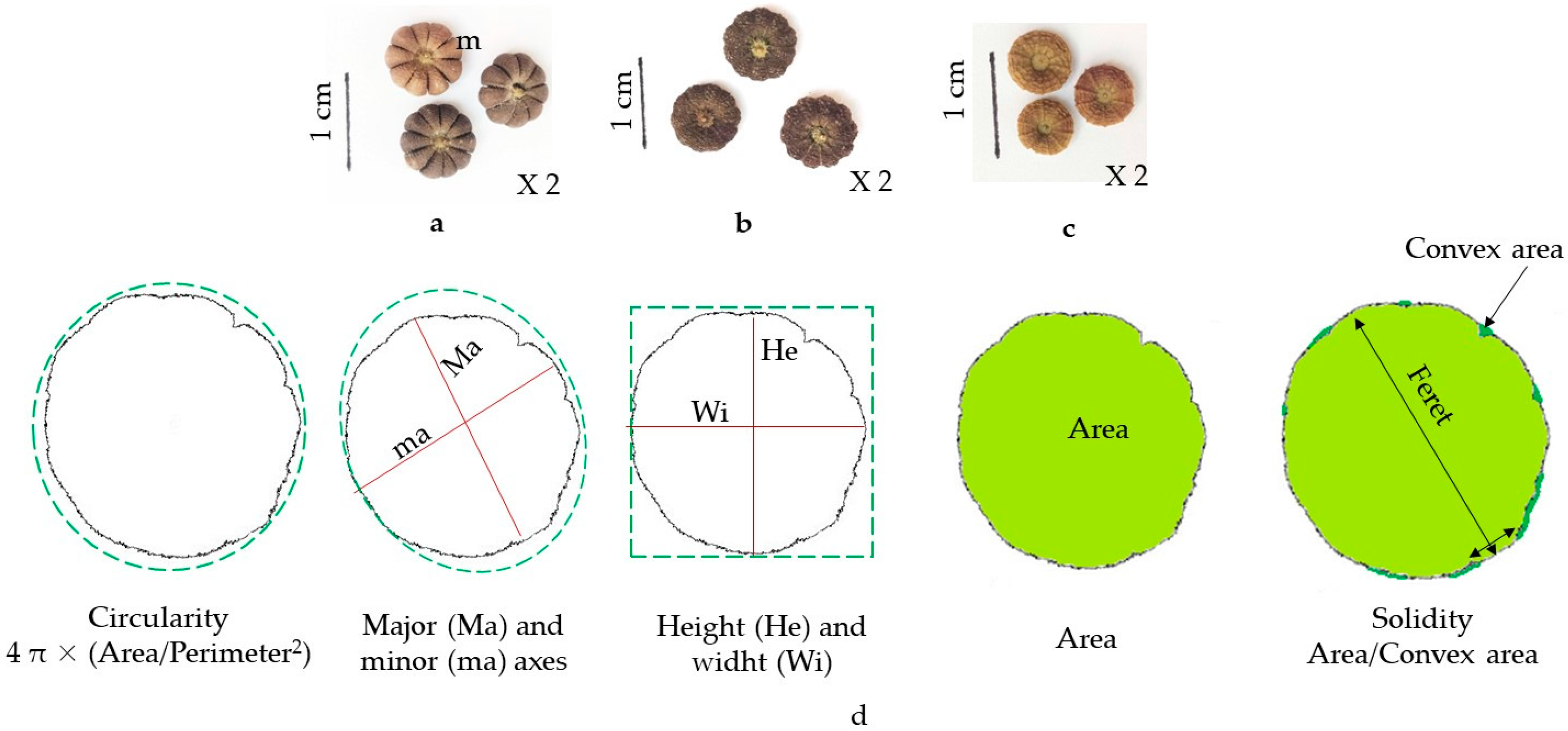
2.3. Morphometric and Texture Characters of Malva Fruits
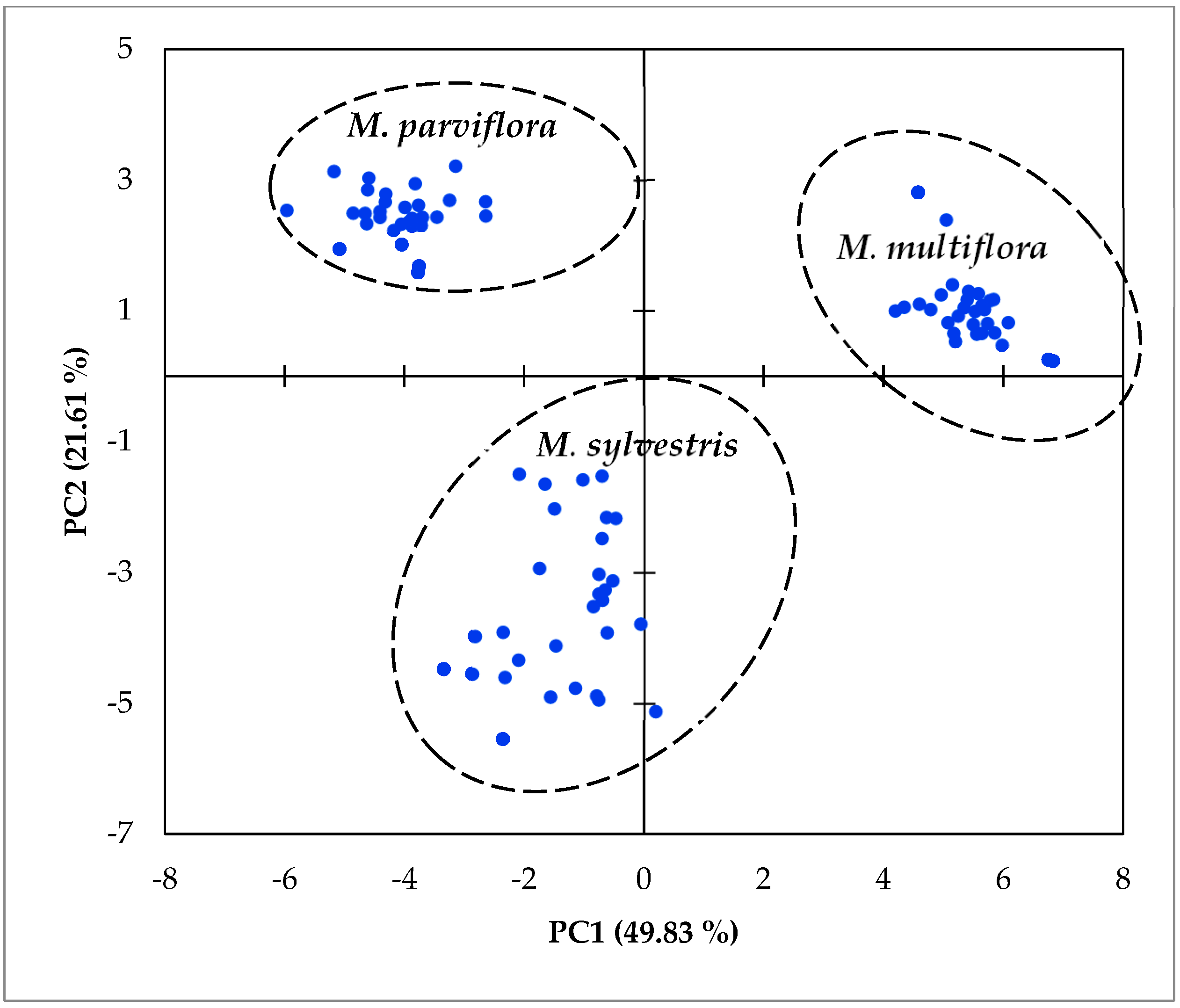
2.4. Multivariate Analysis
3. Discussion
3.1. Phytochemical Data
3.2. Morphometric and Texture
3.3. Multivariate Analysis
4. Materials and Methods
4.1. Malva Fruits Sampling
4.2. Preparation of the Fruit Extract
4.3. Determination of Total Polyphenols Content
4.4. Determination of Total Flavonoid Content
4.5. Determination of Protein Content by LECO Analyzer
4.6. Determination of Oil Content
4.7. Total Soluble Sugar and Starch Contents
4.8. Antioxidant Activity
4.8.1. Total Antioxidant Capacity
4.8.2. Radical Scavenging Ability
4.8.3. Ferric Reducing–Antioxidant Power (FRAP)
4.9. Morphometric and Texture Analysis
4.10. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Leaves, flowers, immature fruits and leafy flowered stems of Malva sylvestris: A comparative study of the nutraceutical potential and composition. Food Chem. Toxicol. 2010, 48, 1466–1472. [Google Scholar] [CrossRef] [PubMed]
- Zouari, N.; Fakhfakh, N.; Zouari, S.; Sellami, M.; Abid, M.; Ayadi, M.A.; Zaidi, S.; Neffati, M. Volatile and lipid analyses by gas chromatography/mass spectrometry and nutraceutical potential of edible wild Malva aegyptiaca L. (Malvaceae). Int. J. Food Sci. Nutr. 2011, 62, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Akbar, S.; Hanif, U.; Ali, J.; Ishtiaq, S. Pharmacognostic studies of stem, roots and leaves of Malva parviflora L. Asian Pac. J. Trop. Biomed. 2014, 4, 410–415. [Google Scholar] [CrossRef] [PubMed]
- El Khoury, M.; Haykal, T.; Hodroj, M.H.; Abou Najem, S.; Sarkis, R.; Taleb, R.I.; Rizk, S. Malva pseudolavatera Leaf Extract Promotes ROS Induction Leading to Apoptosis in Acute Myeloid Leukemia Cells In Vitro. Cancers 2020, 12, 435. [Google Scholar] [CrossRef]
- Jabri, M.-A.; Wannes, D.; Hajji, N.; Sakly, M.; Marzouki, L.; Sebai, H. Role of laxative and antioxidant properties of Malva sylvestris leaves in constipation treatment. Biomed. Pharmacother. 2017, 89, 29–35. [Google Scholar] [CrossRef]
- Romitelli, I.; Martins, M.B.G. Comparison of leaf morphology and anatomy among Malva sylvestris (“Gerânio-aromático”), Pelargonium graveolens (“Falsa-malva”) and Pelargonium odoratissimum (“Gerânio-de-cheiro”). Rev. Bras. Plantas Med. 2013, 15, 91–97. [Google Scholar] [CrossRef]
- Sarmiento-Tomalá, G.; Santos-Ordóñez, E.; Miranda-Martínez, M.; Pacheco-Coello, R.; Scull-Lizama, R.; Gutiérrez-Gaitén, Y.; Delgado Hernández, R.R. Molecular barcode and morphology analysis of Malva pseudolavatera Webb & Berthel and Malva sylvestris L. from Ecuador. Biodivers. J. Biol. Divers. 2022, 21, 3554–3561. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Kang, H. Random Amplified Polymorphic DNA profiling in detecting genetic variation in Malva L. species: Edible and medicinal plants. Caryologia 2022, 75, 77–87. [Google Scholar] [CrossRef]
- Blunden, G.; Patel, A.V.; Armstrong, N.J.; Gorham, J. Betaine distribution in the Malvaceae. Phytochemistry 2001, 58, 451–454. [Google Scholar] [CrossRef]
- Baum, D.A.; DeWitt Smith, S.; Yen, A.; Alverson, W.S.; Nyffeler, R.; Whitlock, B.A.; Oldham, R.L. Phylogenetic relationships of Malvatheca (Bombacoideae and Malvoideae; Malvaceae sensu lato) as inferred from plastid DNA sequences. Am. J. Bot. 2004, 91, 1863–1871. [Google Scholar] [CrossRef]
- Qadir, S.A.; Noori Meerza, C.H.; Faraj, A.M.; Hamafaraj, K.K.; Abdalla Tobakari, S.R.; Hamarashid, S.H. Comparative study and genetic diversity in Malva using srap molecular markers. Caryologia 2022, 75, 101–108. [Google Scholar] [CrossRef]
- García, P.E.; Schönswetter, P.; Aguilar, J.F.; Feliner, G.N.; Schneeweiss, G.M. Five molecular markers reveal extensive morphological homoplasy and reticulate evolution in the Malva alliance (Malvaceae). Mol. Phylogenet. Evol. 2009, 50, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Bianco, M.L.; Grillo, O.; Garcia, P.E.; Mascia, F.; Venora, G.; Bacchetta, G. Morpho-colorimetric characterisation of Malva alliance taxa by seed image analysis. Plant Biol. 2016, 19, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Rovner, I.; Gyulai, F. Computer-assisted morphometry: A new method for assessing and distinguishing morphological variation in wild and domestic seed populations. Econ. Bot. 2007, 61, 154–172. [Google Scholar] [CrossRef]
- Hannachi, H.; Gómez Martín, J.J.; Saadaoui, E.; Cervantes, E. Stone diversity in wild and cultivated olive trees (Olea europaea L.). Dendrobiology 2017, 77, 19–32. [Google Scholar] [CrossRef]
- Ahmad, F.; Hameed, M.; Ahmad, M.S.A. Taxonomic significance of palynological studies for identification of two morphologically similar Malva species. Microsc. Res. Tech. 2022, 85, 2826–2834. [Google Scholar] [CrossRef]
- Bacchetta, G.; García, P.E.; Grillo, O.; Mascia, F.; Venora, G. Seed image analysis provides evidence of taxonomical differentiation within the Lavatera triloba aggregate (Malvaceae). Flora–Morphol. Distrib. Funct. Ecol. Plants 2011, 206, 468–472. [Google Scholar] [CrossRef]
- Parimalan, R.; Mahendranath, G.; Giridhar, P. Analysis of water soluble polysaccharides as a potential chemotaxonomic marker for landraces in Bixa orellana. Indian J. Biochem. Biophys. 2014, 51, 81–86. [Google Scholar]
- Hannachi, H.; Breton, C.; El Gazzah, M.; Msallem, M.; Ben El Hadj, S.; Bervillé, A. Differences between native and introduced olive cultivars as revealed by morphology of drupes, oil composition and SSR polymorphisms: A case study in Tunisia. Sci. Hortic. 2008, 116, 280–290. [Google Scholar] [CrossRef]
- Erarslan, Z.B.; Koçyiğit, M. The important taxonomic characteristics of the family Malvaceae and the herbarium specimens in ISTE. Turk. J. Biosci. Collect. 2019, 3, 1–7. [Google Scholar] [CrossRef]
- Da Silva, A.C.O.; De Oliveira, A.F.M.; Dos Santos, D.Y.A.C.; Da Silva, S.I. An approach to chemotaxonomy to the fatty acid content of some Malvaceae species. Biochem. Syst. Ecol. 2010, 38, 1035–1038. [Google Scholar] [CrossRef]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules 2019, 21, 1374. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, S.; Fernandez, M.; Cuervo, A.; Lasheras, C. Dietary intake of polyphenols and major food sources in an institutionalised elderly population. J. Hum. Nutr. Diet. 2014, 27, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Ghédira, K.; Goetz, P. Malva sylvestris L. (Malvaceae): Mauve. Phytotherapie 2016, 14, 68–72. [Google Scholar] [CrossRef]
- Mohammed, C.B.; Chahid, B.; Fatima, B.; Fatima-Zohra, S.; Meriem, B.; Farid, C. Antioxidant activity, phenolic and flavonoid content in leaves, flowers, stems and seeds of mallow (Malva sylvestris L.) from North Western of Algeria. Afr. J. Biotechnol. 2014, 13, 486–491. [Google Scholar] [CrossRef]
- Mustafa, A.; Ali, M. Comparative phyto and physico-chemical standardization of fresh and different market samples with the anti-inflammatory studies of fruit parts of Malva sylvestris L. Res. J. Pharmacol. Pharmacodyn. 2022, 14, 219–224. [Google Scholar] [CrossRef]
- Aboukhalaf, A.; Tbatou, M.; Kalili, A.; Naciri, K.; Moujabbir, S.; Sahel, K.; Rocha, J.M.; Belahsen, R. Traditional knowledge and use of wild edible plants in Sidi Bennour region (Central Morocco). Ethnobot. Res. Appl. 2022, 23, 265–273. [Google Scholar] [CrossRef]
- Naser, E.H.; Mahdi, L.S.; Alasadi, R.T. Phytochemical constituents and pharmacological activity of Malva parviflora plant: A review. Sci. J. Med. Res. 2022, 23, 35–44. [Google Scholar] [CrossRef]
- Altyar, A.E.; Munir, A.; Ishtiaq, S.; Rizwan, M.; Abbas, K.; Kensara, O.; Elhady, S.S.; Rizg, W.Y.; Youssef, F.S.; Ashour, M.L. Malva parviflora leaves and fruits mucilage as natural sources of anti-inflammatory, antitussive and gastro-protective agents: A comparative study using rat models and Gas chromatography. Pharmaceuticals 2022, 15, 427. [Google Scholar] [CrossRef]
- Gautam, S.S.; Navneet, K.S. Screening of antibacterial and phytochemical constituents of Malva parviflora Linn. fruit extracts against respiratory tract pathogens. Res. Plant Biol. 2018, 8, 13–16. [Google Scholar] [CrossRef]
- Elfalleh, W.; Nasri, N.; Marzougui, N.; Thabti, I.; M’rabet, A.; Yahya, Y.; Lachiheb, B.; Guasmi, F.; Ferchichi, A. Physico-chemical properties and DPPH-ABTS scavenging activity of some local pomegranate (Punica granatum) ecotypes. Int. J. Food Sci. Nutr. 2009, 60, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Kayel, I.; Essghaier, B.; Benabderrahim, M.A.; Rodríguez-Arcos, R.; Jiménez-Araujo, A.; Guillén-Bejarano, R.; Hannachi, H. Three Mediterranean species from natural plant communities (Ceratonia siliqua, Pistacia lentiscus, and Olea europaea var. sylvestris): Phenolic acids, flavonoids, and biological activities. S. Afr. J. Bot. 2024, 175, 620–627. [Google Scholar] [CrossRef]
- Türker, M.; Dalar, A. In vitro antioxidant and enzyme inhibitory properties and phenolic composition of M. neglecta Wallr. (Malvaceae) fruit: A traditional medicinal fruit from Eastern Anatolia. Ind. Crop. Prod. 2013, 51, 376–380. [Google Scholar] [CrossRef]
- Miller, H.; Rigelhof, F.; Marquart, L.; Prakash, A.; Kanter, M. Whole-grain products and antioxidants. Cereal Foods World 2000, 45, 5963. [Google Scholar]
- Chew, Y.-L.; Goh, J.-K.; Lim, Y.-Y. Assessment of in Vitro Antioxidant Capacity and Polyphenolic Composition of Selected Medicinal Herbs from Leguminosae Family in Peninsular Malaysia. Food Chem. 2009, 116, 13–18. [Google Scholar] [CrossRef]
- Cervantes, E.; Martín, J.J.; Saadaoui, E. Updated methods for seed shape analysis. Scientifica 2016, 2016, 5691825. [Google Scholar] [CrossRef]
- Dong, R.; Guo, Q.; Li, H.; Li, J.; Zuo, W.; Long, C. Estimation of morphological variation in seed traits of Sophora moorcroftiana using digital image analysis. Front. Plant Sci. 2023, 14, 1185393. [Google Scholar] [CrossRef]
- Vazquez, D.V.; Spetale, F.E.; Nankar, A.N.; Grozeva, S.; Rodríguez, G.R. Machine Learning-Based Tomato Fruit Shape Classification System. Plants 2024, 13, 2357. [Google Scholar] [CrossRef]
- Pottier-Alapetite, G. Flore de la Tunisie: Angiospermes-dicotylédones. Apétales-Dialypétales. Gamopétales; The Ministère de l’Enseignement Supérieur et de la Recherche Scientifique et le Ministère de l’Agriculture: Paris, France, 1981; pp. 665–1191. [Google Scholar]
- Bettaieb, I.; Benabderrahim, M.A.; Guillén-Bejarano, R.; Rodríguez-Arcos, R.; Jiménez-Araujo, A.; Bouaine, M.; Ghorbal, A.; Elfalleh, W. The effect of freeze-drying process and arabica coffee enrichment on bioactive content, aroma volatile, and sensory characteristics of date seed coffee. Food Biosci. 2024, 57, 103473. [Google Scholar] [CrossRef]
- Soumaya, G.; Hédia, H.; Mouhiba, B.N.A. Morphological, physiological, and biochemical responses of Tunisian Urtica pilulifera L. under salt constraint. S. Afr. J. Bot. 2021, 142, 124–130. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Hammami, N.; Benabderrahim, M.A.; Hannachi, H. Modeling approach of allelopathy efect of Urospermum dalechampii and Andryala integrifolia on lettuce-seed germination and seedling growth. Euro-Mediterr. J. Environ. Integr. 2024. [Google Scholar] [CrossRef]
- Felix, F.C.; Kratz, D.; Ribeiro, R.; Nogueira, A.C. Characterization and differentiation of forest species by seed image analysis: A new methodological approach. Ciência Florest. 2023, 33, e73427. [Google Scholar] [CrossRef]

| TAA (mg AAE/g DM) | FRAP (% RP) | DPPH (IC50) (mg/mL) | |
|---|---|---|---|
| M. mulriflora | 22.01 ± 0.86 a | 58.81 ± 0.006 a | 0.20 ± 0.00 c |
| M. parviflora | 11.47 ± 2.02 b | 42.26 ± 0.022 c | 0.01 ± 0.00 a |
| M. sylvestris | 12.04 ± 0.94 b | 49.21 ± 0.047 b | 0.02 ± 0.00 b |
| M. multiflora | M. parviflora | M. sylvestris | |
|---|---|---|---|
| Morphometry parameters | |||
| Area | 60.46 ± 5.91 a | 30.00 ±3.37 c | 42.27 ± 5.17 b |
| Perimeter | 28.69 ± 1.48 a | 21.15 ± 1.16 c | 24.87 ± 1.47 b |
| Width | 8.74 ± 0.39 a | 6.14 ± 0.40 c | 7.28 ± 0.50 b |
| Height | 9.14 ± 0.34 a | 6.40 ±0.35 c | 7.55 ± 0.48 b |
| AR | 1.10 ± 0.03 a | 1.13 ± 0.02 a | 1.12 ± 0.08 a |
| Major | 9.26 ± 0.42 a | 6.54 ± 0.36 a | 7.72 ± 0.49 b |
| Minor | 8.38 ± 0.30 a | 5.81 ± 0.35 c | 6.93 ± 0.45 b |
| Angle | 61.31 ± 21.04 a | 62.00 ± 7.95 a | 59.67 ± 10.28 a |
| Circularity | 0.92 ± 0.02 a | 0.84 ± 0.04 c | 0.87 ± 0.04 b |
| Feret | 9.82 ± 0.43 a | 6.73 ± 0.39 c | 7.99 ± 0.50 b |
| FeretX coordinates | 119.40 ± 82.95 b | 329.90 ± 214.08 a | 400.50 ± 279.55 a |
| FeretY coordinates | 121.57 ± 63.05 b | 413.67 ± 216.76 a | 457.83 ± 233.90 a |
| Feret Angle | 58.20 ± 23.13 a | 59.78 ± 15.11 a | 57.50 ± 23.24 a |
| MinFeret | 8.65 ± 0.35 a | 5.94 ±0.33 c | 7.06 ± 0.43 b |
| Roundness index | 0.91 ± 0.03 a | 0.87 ± 0.07 b | 0.89 ± 0.06 ab |
| Solidity | 0.94 ± 0.01 b | 0.96 ± 0.01 a | 0.96 ± 0.03 a |
| Texture features | |||
| Mean of gray values | 87.17 ± 5.93 b | 89.34 ± 5.01 b | 115.38 ±19.15 a |
| Standard deviation | 29.02 ± 3.79 a | 24.42 ± 2.93 b | 24.68 ±7.92 b |
| Integrated Density | 5493.17 ± 559.15 a | 2949.85 ± 311.74 c | 4677.07 ± 722.61 b |
| Mode of gray value | 87.10 ± 9.27 b | 88.77 ± 8.73 b | 124.64 ± 7.37 a |
| Minimum of gray value | 30.47 ± 9.65 a | 20.08 ± 10.11 b | 25.42 ± 11.71 a |
| Maximum of gray value | 165.67 ± 0.71 b | 170.00 ± 0.00 a | 165.03 ± 0.19 c |
| Skewness | 0.67 ± 0.37 b | 1.17 ± 0.51 a | −0.73 ±0.82 c |
| Kurtosis | 0.39 ± 0.68 b | 1.62 ± 0.97 a | 2.33 ± 0.49 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rhimi, F.; Rejili, M.; Benabderrahim, M.A.; Hannachi, H. Diversity of Phytochemical Content, Antioxidant Activity, and Fruit Morphometry of Three Mallow, Malva Species (Malvaceae). Plants 2025, 14, 930. https://doi.org/10.3390/plants14060930
Rhimi F, Rejili M, Benabderrahim MA, Hannachi H. Diversity of Phytochemical Content, Antioxidant Activity, and Fruit Morphometry of Three Mallow, Malva Species (Malvaceae). Plants. 2025; 14(6):930. https://doi.org/10.3390/plants14060930
Chicago/Turabian StyleRhimi, Faten, Mokhtar Rejili, Mohamed Ali Benabderrahim, and Hédia Hannachi. 2025. "Diversity of Phytochemical Content, Antioxidant Activity, and Fruit Morphometry of Three Mallow, Malva Species (Malvaceae)" Plants 14, no. 6: 930. https://doi.org/10.3390/plants14060930
APA StyleRhimi, F., Rejili, M., Benabderrahim, M. A., & Hannachi, H. (2025). Diversity of Phytochemical Content, Antioxidant Activity, and Fruit Morphometry of Three Mallow, Malva Species (Malvaceae). Plants, 14(6), 930. https://doi.org/10.3390/plants14060930









