Biochar Amendment Increases Peanut Production Through Improvement of the Extracellular Enzyme Activities and Microbial Community Composition in Replanted Field
Abstract
1. Introduction
2. Results
2.1. Peanut Yield and Kernel Quality
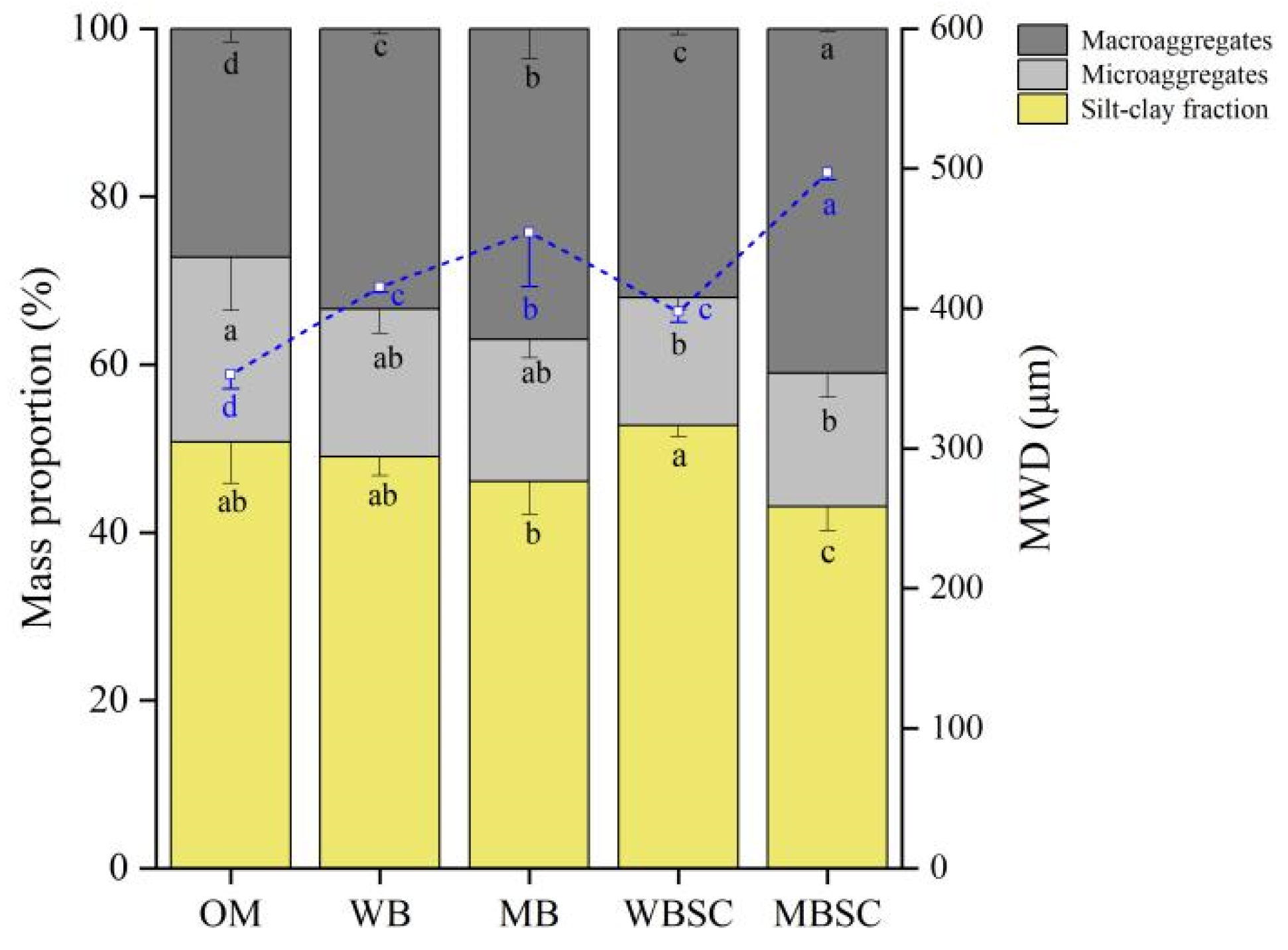
2.2. Soil Properties
2.3. Soil Extracellular Enzyme Activities and Function
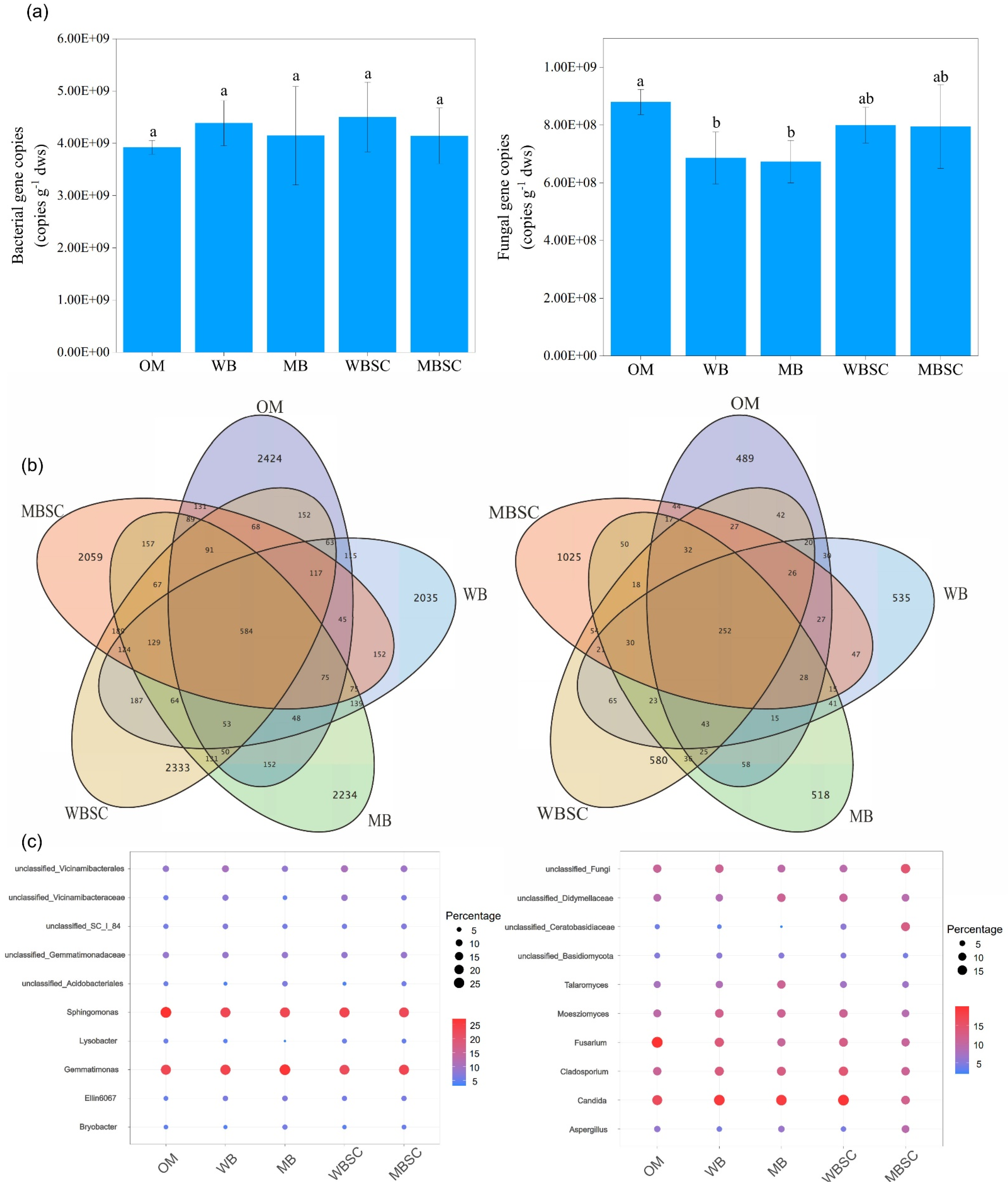
2.4. Gene Abundance and Community Composition of Rhizosphere Microbiome
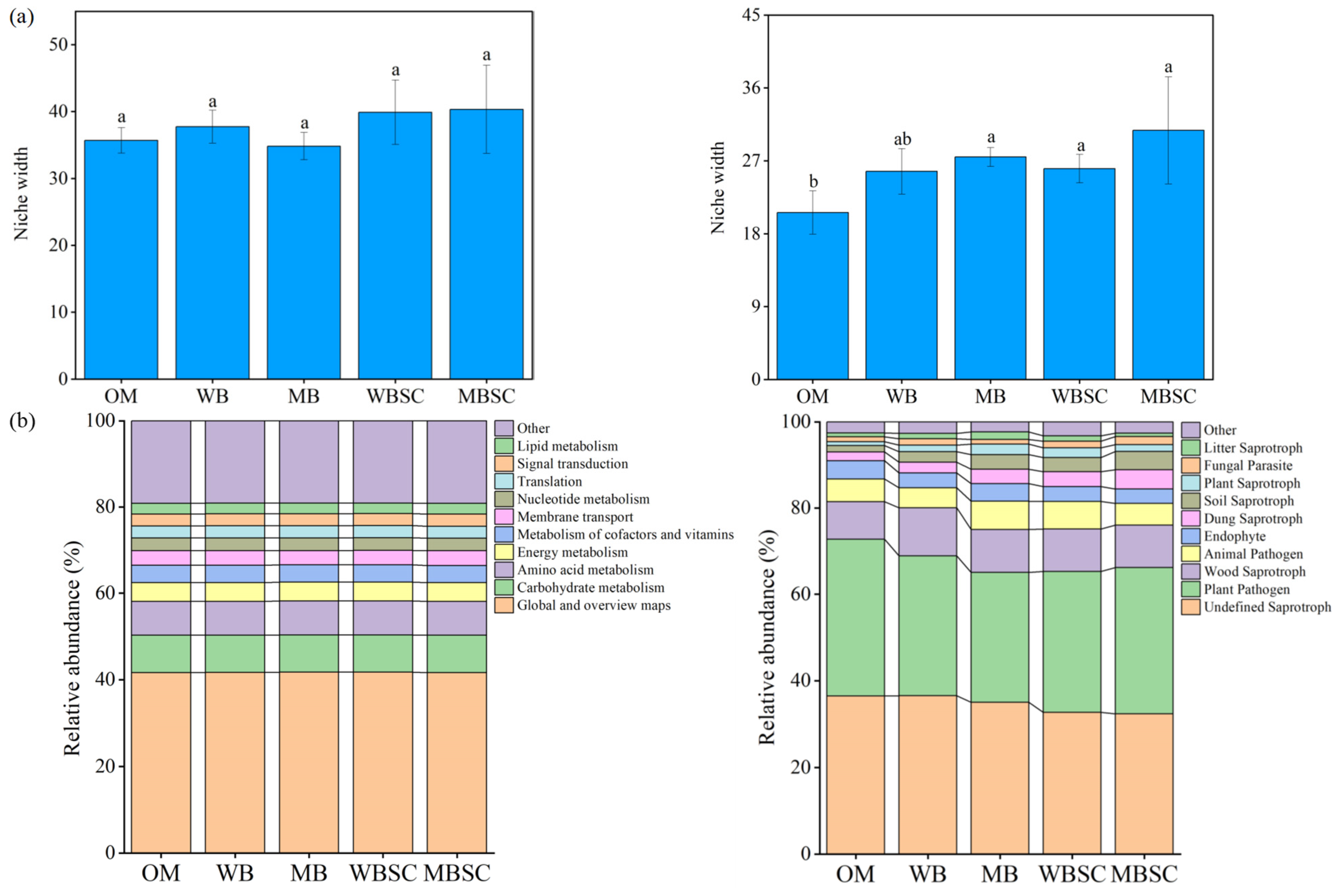
2.5. Diversity and Community Composition of Rhizosphere Microbiome
3. Discussion
3.1. Biochar–Compost Synergistically Improves Peanut Production and Soil Quality
3.2. Microbial Manipulation and Enzyme Activity Shifted with Biochar–Compost
4. Materials and Methods
4.1. Experimental Site and Soil Condition
4.2. Experimental Design
4.3. Plant Sampling and Analysis
4.4. Soil Sampling and Analysis
4.5. DNA Extraction and Real-Time qPCR Analysis
4.6. Illumina Hiseq Sequencing and Bioinformatics Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lehmann, J.; Bossio, D.A.; Kögel-Knabner, I.; Rillig, M.C. The concept and future prospects of soil health. Nat. Rev. Earth Environ. 2020, 1, 544–553. [Google Scholar] [CrossRef]
- IPBES. Summary for Policymakers of the Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; IPBES Secretariat: Bonn, Germany, 2019; 56p. [Google Scholar] [CrossRef]
- Ma, Z.; Guan, Z.; Liu, Q.; Hu, Y.; Liu, L.; Wang, B.; Huang, L.; Li, H.; Yang, Y.; Han, M.; et al. Obstacles in continuous cropping: Mechanisms and control measures. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2023; pp. 205–256. [Google Scholar]
- Huang, L.; Song, L.; Xia, X.; Mao, W.; Shi, K.; Zhou, Y.; Yu, J. Plant-Soil Feedbacks and Soil Sickness: From Mechanisms to Application in Agriculture. J. Chem. Ecol. 2013, 39, 232–242. [Google Scholar] [CrossRef]
- Li, H.; Li, C.; Song, X.; Liu, Y.; Gao, Q.; Zheng, R.; Li, J.; Zhang, P.; Liu, X. Impacts of continuous and rotational cropping practices on soil chemical properties and microbial communities during peanut cultivation. Sci. Rep. 2022, 12, 2758. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ding, C.; Hua, K.; Zhang, T.; Zhang, Y.; Zhao, L.; Yang, Y.; Liu, J.; Wang, X. Soil sickness of peanuts is attributable to modifications in soil microbes induced by peanut root exudates rather than to direct allelopathy. Soil Biol. Biochem. 2014, 78, 149–159. [Google Scholar] [CrossRef]
- Li, X.; Ding, C.; Zhang, T.; Wang, X. Fungal pathogen accumulation at the expense of plant-beneficial fungi as a consequence of consecutive peanut monoculturing. Soil Biol. Biochem. 2014, 72, 11–18. [Google Scholar] [CrossRef]
- Barrow, C.J. Biochar: Potential for countering land degradation and for improving agriculture. Appl. Geogr. 2012, 34, 21–28. [Google Scholar] [CrossRef]
- Bolan, N.; Hoang, S.A.; Beiyuan, J.; Gupta, S.; Hou, D.; Karakoti, A.; Joseph, S.; Jung, S.; Kim, K.-H.; Kirkham, M.B.; et al. Multifunctional applications of biochar beyond carbon storage. Int. Mater. Rev. 2022, 67, 150–200. [Google Scholar] [CrossRef]
- Wang, Y.; Villamil, M.B.; Davidson, P.C.; Akdeniz, N. A quantitative understanding of the role of co-composted biochar in plant growth using meta-analysis. Sci. Total Environ. 2019, 685, 741–752. [Google Scholar] [CrossRef]
- Jien, S.H.; Wang, C.C.; Lee, C.H.; Lee, T.Y. Stabilization of organic matter by biochar application in compost-amended soils with contrasting pH values and textures. Sustainability 2015, 7, 13317–13333. [Google Scholar] [CrossRef]
- Sanchez-Monedero, M.A.; Cayuela, M.L.; Roig, A.; Jindo, K.; Mondini, C.; Bolan, N. Role of biochar as an additive in organic waste composting. Bioresour. Technol. 2018, 247, 1155–1164. [Google Scholar] [CrossRef]
- Antonangelo, J.A.; Sun, X.; Zhang, H. The roles of co-composted biochar (COMBI) in improving soil quality, crop productivity, and toxic metal amelioration. J. Environ. Manag. 2021, 277, 111443. [Google Scholar] [CrossRef]
- Kammann, C.I.; Schmidt, H.-P.; Messerschmidt, N.; Linsel, S.; Steffens, D.; Müller, C.; Koyro, H.-W.; Conte, P.; Joseph, S. Erratum: Plant growth improvement mediated by nitrate capture in co-composted biochar. Sci. Rep. 2015, 5, 12378. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; He, H.; Inthapanya, X.; Yang, C.; Lu, L.; Zeng, G.; Han, Z. Role of biochar on composting of organic wastes and remediation of contaminated soils—A review. Environ. Sci. Pollut. Res. 2017, 24, 16560–16577. [Google Scholar] [CrossRef]
- Chen, M.; Li, X.; Yang, Q.; Chi, X.; Pan, L.; Chen, N.; Yang, Z.; Wang, T.; Wang, M.; Yu, S. Dynamic succession of soil bacterial community during continuous cropping of peanut (Arachis hypogaea L.). PLoS ONE 2014, 9, e101355. [Google Scholar] [CrossRef]
- Ahsan, T. Effects of microbial agent and microbial fertilizer input on soil microbial community structure and diversity in a peanut continuous cropping system. J. Adv. Res. 2024, 64, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Tian, J.; Chen, L.; He, Q.; Liu, X.; Bian, R.; Zheng, J.; Cheng, K.; Xia, S.; Zhang, X.; et al. Biochar boosted high oleic peanut production with enhanced root development and biological N fixation by diazotrophs in a sand-loamy Primisol. Sci. Total Environ. 2024, 932, 173061. [Google Scholar] [CrossRef]
- Xu, C.; Bai, S.H.; Hao, Y.; Rachaputi, R.C.N.; Xu, Z.; Wallace, H.M. Peanut shell biochar improves soil properties and peanut kernel quality on a red Ferrosol. J. Soils Sediments 2015, 15, 2220–2231. [Google Scholar] [CrossRef]
- Sohail, M.I.; Rehman, M.Z.U.; Rizwan, M.; Yousaf, B.; Ali, S.; ul Haq, M.A.; Anayat, A.; Waris, A.A. Efficiency of various silicon rich amendments on growth and cadmium accumulation in field grown cereals and health risk assessment. Chemosphere 2020, 244, 125481. [Google Scholar] [CrossRef]
- Reza, M.T.; Lynam, J.G.; Vasquez, V.R.; Coronella, C.J. Pelletization of biochar from hydrothermally carbonized wood. Environ. Prog. Sustain. Energy 2012, 31, 225–234. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X. Changes in physical, chemical, and microbiological properties during the two-stage co-composting of green waste with spent mushroom compost and biochar. Bioresour. Technol. 2014, 171, 274–284. [Google Scholar] [CrossRef]
- Agegnehu, G.; Srivastava, A.K.; Bird, M.I. The role of biochar and biochar-compost in improving soil quality and crop performance: A review. Appl. Soil. Ecol. 2017, 119, 156–170. [Google Scholar] [CrossRef]
- Pandit, N.R.; Schmidt, H.P.; Mulder, J.; Hale, S.E.; Husson, O.; Cornelissen, G. Nutrient effect of various composting methods with and without biochar on soil fertility and maize growth. Arch. Agron. Soil. Sci. 2020, 66, 250–265. [Google Scholar] [CrossRef]
- Vandecasteele, B.; Sinicco, T.; D’Hose, T.; Vanden Nest, T.; Mondini, C. Biochar amendment before or after composting affects compost quality and N losses, but not P plant uptake. J. Environ. Manag. 2016, 168, 200–209. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, G.; Sun, H.; Zhou, S.; Zou, G. Straw biochar hastens organic matter degradation and produces nutrient-rich compost. Bioresour. Technol. 2016, 200, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, X.; Yang, Z.; Siddikee, M.A.; Kong, M.; Lu, L.; Shen, J.; Dai, C. Physiological mechanisms behind endophytic fungus Phomopsis liquidambari-mediated symbiosis enhancement of peanut in a monocropping system. Plant Soil 2017, 416, 325–342. [Google Scholar] [CrossRef]
- Keable, S.M.; Vertemara, J.; Zadvornyy, O.A.; Eilers, B.J.; Danyal, K.; Rasmussen, A.J.; De Gioia, L.; Zampella, G.; Seefeldt, L.C.; Peters, J.W. Structural characterization of the nitrogenase molybdenumiron protein with the substrate acetylene trapped near the active site. J. Inorg. Biochem. 2017, 180, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Dong, Y.; Zhang, X.; Wang, Q.; Xu, L.; Liu, S.; Hou, J.; Fan, Z. Effects of exogenous salicylic acid on physiological characteristics of peanut seedlings under iron-deficiency stress. J. Plant Nutr. 2015, 38, 127–144. [Google Scholar] [CrossRef]
- Qu, S. Effect of Medium and Microelement Fertilizer Combination on Growth, Development and Yield and Quality of Peanut. Master’s Thesis, Shenyang Agricultural University, Shenyang, China, 2019. [Google Scholar]
- Yuan, Y.; Kong, Q.; Zheng, Y.; Zheng, H.; Liu, Y.; Cheng, Y.; Zhang, X.; Li, Z.; You, X.; Li, Y. Co-application of biochar and pyroligneous acid improved peanut production and nutritional quality in a coastal soil. Environ. Technol. Innov. 2022, 28, 102886. [Google Scholar] [CrossRef]
- Glisczynski von, F.; Sandhage-Hofmanna, A.; Amelung, W.; Pude, R. Biochar-compost substrates do not promote growth and fruit quality of a replanted German apple orchard with fertile Haplic Luvicsol soils. Sci. Hortic. 2016, 213, 110–114. [Google Scholar] [CrossRef]
- Fu, H.; Zhang, G.; Zhang, F.; Sun, Z.; Geng, G.; Li, T. Effects of continuous tomato monoculture on soil microbial properties and enzyme activities in a solar greenhouse. Sustainability 2017, 9, 317. [Google Scholar] [CrossRef]
- Chen, W.; Teng, Y.; Li, Z.; Liu, W.; Ren, W.; Luo, Y.; Christie, P. Mechanisms by which organic fertilizer and effective microbes mitigate peanut continuous cropping yield constraints in a red soil of south China. Appl. Soil. Ecol. 2018, 128, 23–34. [Google Scholar] [CrossRef]
- Nishioka, T.; Elsharkawy, M.M.; Suga, H.; Kageyama, K.; Hyakumachi, M.; Shimizu, M. Development of culture medium for the isolation of Flavobacterium and Chryseobacterium from rhizosphere soil. Microbes Environ. 2016, 31, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xia, R.; Tang, M.; Chen, X.; Zhong, B.; Liu, X.; Bian, R.; Yang, L.; Zheng, J.; Cheng, K.; et al. Improved ginseng production under continuous cropping through soil health reinforcement and rhizosphere microbial manipulation with biochar: A field study of Panax ginseng from Northeast China. Hortic. Res. 2022, 9, uhac108. [Google Scholar] [CrossRef]
- Liu, C.; Xia, R.; Tang, M.; Liu, X.; Bian, R.; Yang, L.; Zheng, J.; Cheng, K.; Zhang, X.; Drosos, M.; et al. More microbial manipulation and plant defense than soil fertility for biochar in food production: A field experiment of replanted ginseng with different biochars. Front. Microbiol. 2022, 13, 1065313. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.Y.; Li, H.; Hao, M.M.; Ren, Y.N.; Zhang, M.K.; Liu, R.Y.; Zhang, Y.; Li, G.; Chen, J.S.; Ning, T.Y.; et al. Nitrogen fixation and crop productivity enhancements co-driven by intercrop root exudates and key rhizosphere bacteria. J. Appl. Ecol. 2021, 58, 2243–2255. [Google Scholar] [CrossRef]
- Zhou, G.; Fan, K.; Li, G.; Gao, S.; Chang, D.; Liang, T.; Li, S.; Liang, H.; Zhang, J.; Che, Z.; et al. Synergistic effects of diazotrophs and arbuscular mycorrhizal fungi on soil biological nitrogen fixation after three decades of fertilization. iMeta 2023, 2, e81. [Google Scholar] [CrossRef]
- Inderbitzin, P.; Ward, J.; Barbella, A.; Solares, N.; Izyumin, D.; Burman, P.; Chellemi, D.O.; Subbarao, K.V. Soil microbiomes associated with verticillium wilt suppressive broccoli and chitin amendments are enriched with potential biocontrol agents. Phytopathology 2018, 108, 31–43. [Google Scholar] [CrossRef]
- Long, N.; Liu, J.; Liu, J.; Li, Y.; Hou, Y.; Liao, X.; Zhou, L.; Shi, L.; Kong, W. Single molecule Real-time sequencing to explore the mycobiome diversity in malt. Microbiol. Spectr. 2022, 10, e00511–e00522. [Google Scholar] [CrossRef]
- Zhang, L.; Xiang, Y.; Jing, Y.; Zhang, R. Biochar amendment effects on the activities of soil carbon, nitrogen, and phosphorus hydrolytic enzymes: A meta-analysis. Environ. Sci. Pollut. Res. 2019, 26, 22990–23001. [Google Scholar] [CrossRef]
- Oldfield, T.L.; Sikirica, N.; Mondini, C.; Lopez, G.; Kuikman, P.J.; Holden, N.M. Biochar, compost and biochar-compost blend as options to recover nutrients and sequester carbon. J. Environ. Manag. 2018, 218, 465–476. [Google Scholar] [CrossRef]
- Dai, Z.; Xiong, X.; Zhu, H.; Xu, H.; Leng, P.; Li, J.; Tang, C.; Xu, J. Association of biochar properties with changes in soil bacterial, fungal and fauna communities and nutrient cycling processes. Biochar 2021, 3, 239–254. [Google Scholar] [CrossRef]
- Ouyang, L.; Tang, Q.; Yu, L.; Zhang, R. Effects of amendment of different biochars on soil enzyme activities related to carbon mineralisation. Soil Res. 2014, 52, 706–716. [Google Scholar] [CrossRef]
- Luo, Y.; Zang, H.; Yu, Z.; Chen, Z.; Gunina, A.; Kuzyakov, Y.; Xu, J.; Zhang, K.; Brookes, P.C. Priming effects in biochar enriched soils using a three-source-partitioning approach: C-14 labelling and C-13 natural abundance. Soil Biol. Biochem. 2017, 106, 28–35. [Google Scholar] [CrossRef]
- Butler, J.L.; Williams, M.A.; Bottomley, P.J.; Myrold, D.D. Microbial community dynamics associated with rhizosphere carbon f low. Appl. Environ. Microbiol. 2003, 69, 6793–6800. [Google Scholar] [CrossRef]
- Lu, R. Soil Agro-Chemical Analysis. In Analysis Method of Soil Agricultural Chemistry; Liu, X.S., Chen, S.H., Eds.; China Agricultural Science Technology Press: Beijing, China, 2000. [Google Scholar]
- Smith, A.P.; Marín-Spiotta, E.; de Graaff, M.A.; Balser, T.C. Microbial community structure varies across soil organic matter aggregate pools during tropical land cover change. Soil Biol. Biochem. 2014, 77, 292–303. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- DeForest, J.L. The inf luence of time, storage temperature, and substrate age on potential soil enzyme activity in acidic forest soils using MUB-linked substrates and L-DOPA. Soil Biol. Biochem. 2009, 41, 1180–1186. [Google Scholar] [CrossRef]
- German, D.P.; Weintraub, M.N.; Grandy, A.S.; Lauber, C.L.; Rinkes, Z.L.; Allison, S.D. Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biol. Biochem. 2011, 43, 1387–1397. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]

| Treatment | Pod Yield (kg ha−1) | Plant Height (cm) | Pod Number (plant−1) | Pod Weight (g plant−1) | Kernel Weight (g 100 kerner−1) | Pod Weight (g 100 pod−1) | Kernel to Pod Ratio (%) | Survival Rate (%) |
|---|---|---|---|---|---|---|---|---|
| OM | 3039.03 ± 189.43 b | 59.61 ± 3.82 b | 44.33 ± 12.18 b | 51.01 ± 10.98 c | 68.20 ± 3.94 a | 104.67 ± 4.73 b | 65.13 ± 0.83 d | 89.44 ± 2.68 b |
| WB | 3715.24 ± 167.59 a | 63.50 ± 2.26 a | 54.00 ± 11.52 ab | 53.50 ± 22.17 bc | 78.71 ± 3.04 a | 111.01 ± 6.24 ab | 70.96 ± 1.99 ab | 95.83 ± 1.67 a |
| MB | 3469.64 ± 257.09 ab | 63.21 ± 5.67 a | 52.17 ± 8.30 ab | 53.83 ± 15.28 bc | 81.90 ± 8.40 a | 120.67 ± 12.66 a | 67.89 ± 0.38 c | 95.28 ± 1.73 a |
| WBSC | 3736.45 ± 380.94 a | 64.82 ± 2.37 a | 64.67 ± 12.79 a | 71.40 ± 10.97 ab | 79.22 ± 4.53 a | 110.33 ± 5.03 ab | 71.76 ± 1.15 a | 95.83 ± 2.20 a |
| MBSC | 3578.16 ± 346.83 a | 63.88 ± 2.94 a | 59.83 ± 7.08 a | 68.25 ± 10.47 a | 83.47 ± 6.05 a | 119.67 ± 6.51 ab | 69.70 ± 1.36 abc | 95.56 ± 2.41 a |
| Treatment | Fat | Protein | Oleic Acid | Linoleic Acid | Sugar | Cane Sugar | Soluble Sugar |
|---|---|---|---|---|---|---|---|
| OM | 47.58 ± 0.92 a | 27.36 ± 0.75 b | 51.07 ± 1.52 b | 29.73 ± 1.14 a | 14.96 ± 0.79 ab | 2.07 ± 0.11 ab | 3.01 ± 0.35 c |
| WB | 47.59 ± 0.64 a | 28.71 ± 0.51 ab | 51.72 ± 0.61 b | 30.22 ± 1.05 a | 15.15 ± 0.24 ab | 2.30 ± 0.35 a | 3.68 ± 0.11 b |
| MB | 46.26 ± 0.87 a | 30.44 ± 1.04 a | 51.54 ± 1.20 b | 30.44 ± 0.60 a | 16.18 ± 0.32 a | 2.51 ± 0.36 a | 4.33 ± 0.12 a |
| WBSC | 48.06 ± 1.77 a | 29.00 ± 0.31 ab | 53.77 ± 1.01 a | 27.03 ± 1.54 b | 13.98 ± 0.53 b | 1.95 ± 0.08 b | 3.16 ± 0.10 c |
| MBSC | 48.45 ± 0.69 a | 29.10 ± 0.83 ab | 53.21 ± 1.82 a | 27.58 ± 1.29 b | 14.8 ± 0.26 b | 1.96 ± 0.17 b | 3.41 ± 0.22 ab |
| Treatment | pH (H2O) | BD | SOC | Total N | Available P | Available K | CEC | MBC | MBN |
|---|---|---|---|---|---|---|---|---|---|
| (g cm−3) | (g kg−1) | (g kg−1) | (mg kg−1) | (mg kg−1) | (cmol kg−1) | (mg kg−1) | (mg kg−1) | ||
| OM | 6.49 ± 0.50 bc | 1.39 ± 0.14 a | 9.70 ± 1.00 c | 0.39 ± 0.03 b | 26.53 ± 3.17 b | 108.54 ± 7.69 b | 22.70 ± 2.41 a | 137.89 ± 11.25 b | 21.21 ± 2.04 c |
| WB | 7.37 ± 0.24 a | 1.30 ± 0.11 a | 16.26 ± 0.78 a | 0.38 ± 0.04 b | 28.71 ± 2.98 ab | 131.26 ± 6.05 a | 23.95 ± 0.97 a | 132.77 ± 12.5 b | 22.04 ± 3.92 bc |
| MB | 7.34 ± 0.19 a | 1.32 ± 0.15 a | 16.32 ± 1.32 a | 0.45 ± 0.04 ab | 31.84 ± 2.74 a | 148.39 ± 18.34 a | 23.38 ± 3.14 a | 162.80 ± 14.56 a | 24.02 ± 2.05 bc |
| WBSC | 6.82 ± 0.29 ab | 1.29 ± 0.10 a | 9.77 ± 0.86 c | 0.47 ± 0.02 a | 27.33 ± 2.44 ab | 96.80 ± 9.47 b | 24.28 ± 1.80 a | 177.90 ± 3.04 a | 27.29 ± 4.01 ab |
| MBSC | 6.70 ± 0.30 b | 1.22 ± 0.09 a | 11.33 ± 1.22 bc | 0.48 ± 0.04 a | 26.27 ± 2.61 b | 103.97 ± 7.14 b | 23.15 ± 3.08 a | 189.01 ± 14.96 a | 24.89 ± 3.88 abc |
| Treatment | α-Glucosidase (nmol g−1 h−1) | β-Glucosidase (nmol g−1 h−1) | β-Xylosidase (nmol g−1 h−1) | β-Cellobiohydrolase (nmol g−1 h−1) | N-Acetyl-Glucosaminidase (nmol g−1 h−1) | Acid Phosphatase (nmol g−1 h−1) | Sulfatase (nmol g−1 h−1) |
|---|---|---|---|---|---|---|---|
| OM | 11.95 ± 0.64 d | 104.86 ± 4.69 d | 11.93 ± 6.36 c | 22.54 ± 0.96 b | 52.69 ± 2.90 c | 449.15 ± 23.69 c | 4.98 ± 0.26 a |
| WB | 14.21 ± 0.77 c | 152.68 ± 8.49 b | 17.27 ± 0.84 b | 31.99 ± 1.57 a | 56.45 ± 2.85 b | 518.87 ± 34.26 ab | 6.58 ± 0.35 a |
| MB | 17.18 ± 0.45 b | 144.08 ± 4.59 c | 17.39 ± 0.58 b | 22.68 ± 1.06 b | 58.02 ± 2.08 b | 513.83 ± 16.42 b | 5.19 ± 0.17 a |
| WBSC | 17.18 ± 0.45 b | 152.59 ± 4.59 b | 21.51 ± 0.34 a | 23.88 ± 0.37 b | 63.61 ± 1.84 a | 515.46 ± 15.40 b | 5.39 ± 2.70 a |
| MBSC | 25.78 ± 0.62 a | 167.87 ± 3.52 a | 22.39 ± 0.47 a | 31.89 ± 1.56 a | 62.20 ± 1.23 a | 553.87 ± 10.47 a | 6.68 ± 0.13 a |
| Treatment | Polyphenol Oxidase (μmol g−1 h−1) | Peroxidase (μmol g−1 h−1) | H’ Index | Hydrolase/Non-Hydrolase | C/N Cycling | C/P Cycling | N/P Cycling |
| OM | 1.33 ± 0.74 a | 4.22 ± 0.89 c | 1.09 ± 0.03 c | 0.44 ± 0.02 d | 2.87 ± 0.08 c | 0.34 ± 0.01 d | 0.12 ± 0.00 a |
| WB | 1.81 ± 0.41 a | 5.65 ± 0.28 b | 1.15 ± 0.02 b | 0.51 ± 0.02 b | 3.84 ± 0.28 a | 0.42 ± 0.01 b | 0.11 ± 0.01 a |
| MB | 1.98 ± 0.34 a | 5.38 ± 0.48 b | 1.13 ± 0.01 b | 0.49 ± 0.00 c | 3.47 ± 0.03 b | 0.39 ± 0.00 c | 0.11 ± 0.00 a |
| WBSC | 1.83 ± 0.35 a | 5.28 ± 0.51 b | 1.16 ± 0.02 b | 0.53 ± 0.00 b | 3.37 ± 0.01 b | 0.42 ± 0.01 b | 0.12 ± 0.00 a |
| MBSC | 1.98 ± 0.21 a | 7.01 ± 0.74 a | 1.20 ± 0.01 a | 0.55 ± 0.00 a | 3.99 ± 0.11 a | 0.45 ± 0.00 a | 0.11 ± 0.00 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Shang, S.; Wang, C.; Tian, J.; Zhang, L.; Liu, X.; Bian, R.; He, Q.; Zhang, F.; Chen, L.; et al. Biochar Amendment Increases Peanut Production Through Improvement of the Extracellular Enzyme Activities and Microbial Community Composition in Replanted Field. Plants 2025, 14, 922. https://doi.org/10.3390/plants14060922
Liu C, Shang S, Wang C, Tian J, Zhang L, Liu X, Bian R, He Q, Zhang F, Chen L, et al. Biochar Amendment Increases Peanut Production Through Improvement of the Extracellular Enzyme Activities and Microbial Community Composition in Replanted Field. Plants. 2025; 14(6):922. https://doi.org/10.3390/plants14060922
Chicago/Turabian StyleLiu, Cheng, Shijie Shang, Chao Wang, Jing Tian, Liting Zhang, Xiaoyu Liu, Rongjun Bian, Qunling He, Fengye Zhang, Lei Chen, and et al. 2025. "Biochar Amendment Increases Peanut Production Through Improvement of the Extracellular Enzyme Activities and Microbial Community Composition in Replanted Field" Plants 14, no. 6: 922. https://doi.org/10.3390/plants14060922
APA StyleLiu, C., Shang, S., Wang, C., Tian, J., Zhang, L., Liu, X., Bian, R., He, Q., Zhang, F., Chen, L., Drosos, M., Azeem, M., Li, L., Shan, S., & Pan, G. (2025). Biochar Amendment Increases Peanut Production Through Improvement of the Extracellular Enzyme Activities and Microbial Community Composition in Replanted Field. Plants, 14(6), 922. https://doi.org/10.3390/plants14060922










