Selenium Improves Yield and Quality in Prunella vulgaris by Regulating Antioxidant Defense, Photosynthesis, Growth, Secondary Metabolites, and Gene Expression Under Acid Stress
Abstract
1. Introduction
2. Results
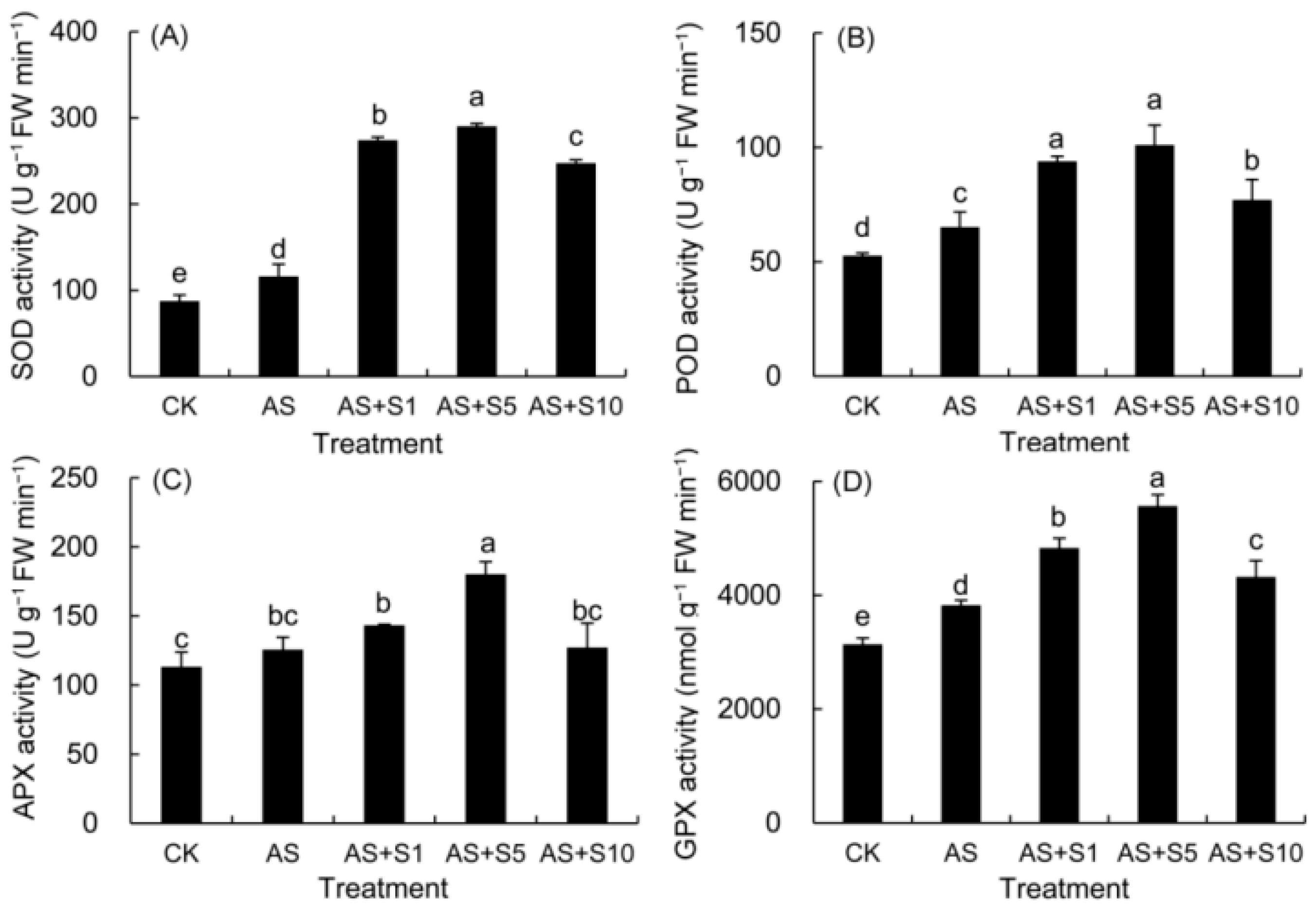
2.1. Antioxidant Enzyme Activity
2.2. Osmolytes Contents
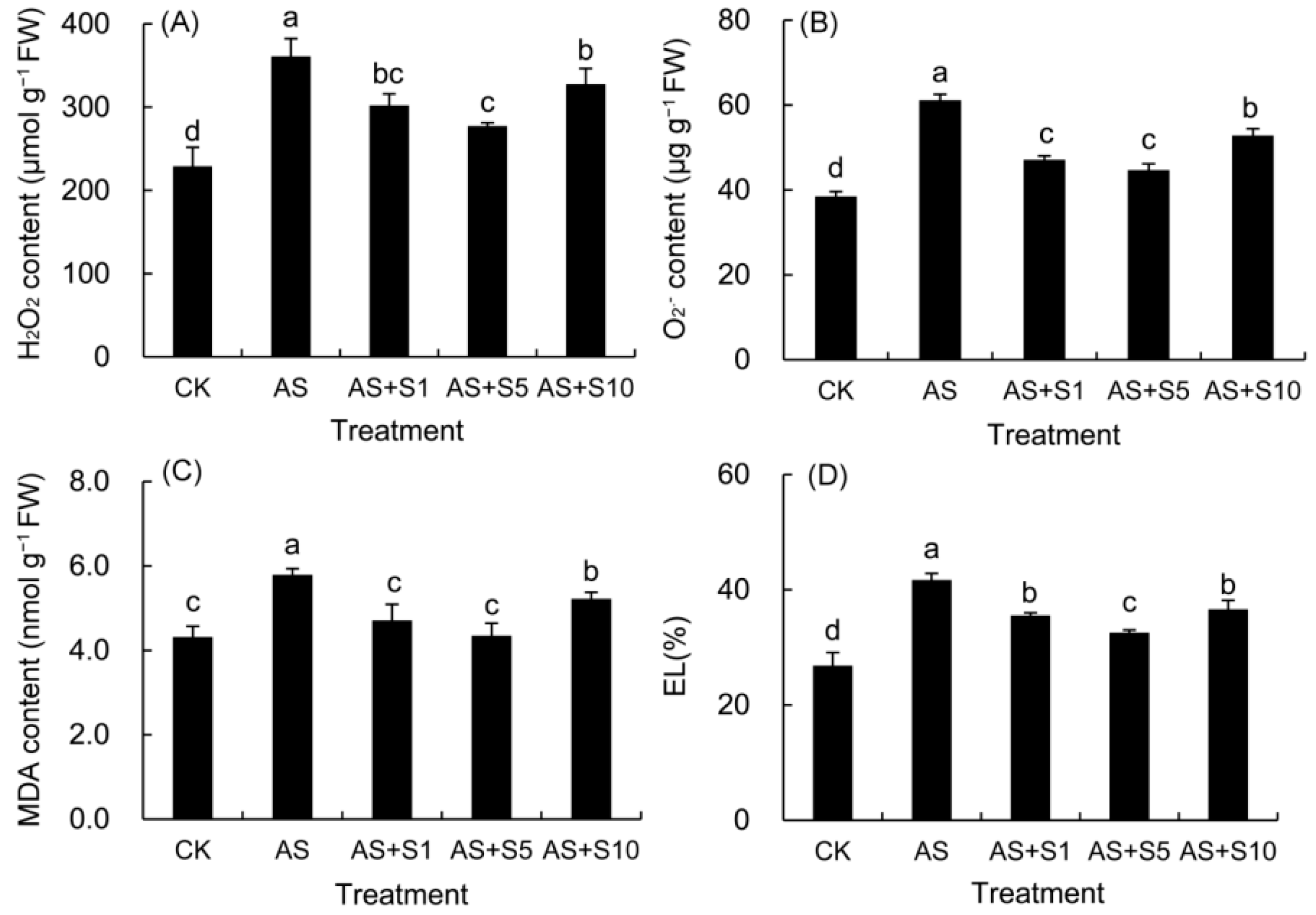
2.3. H2O2, O2− and MDA Content and Electrolyte Leakage
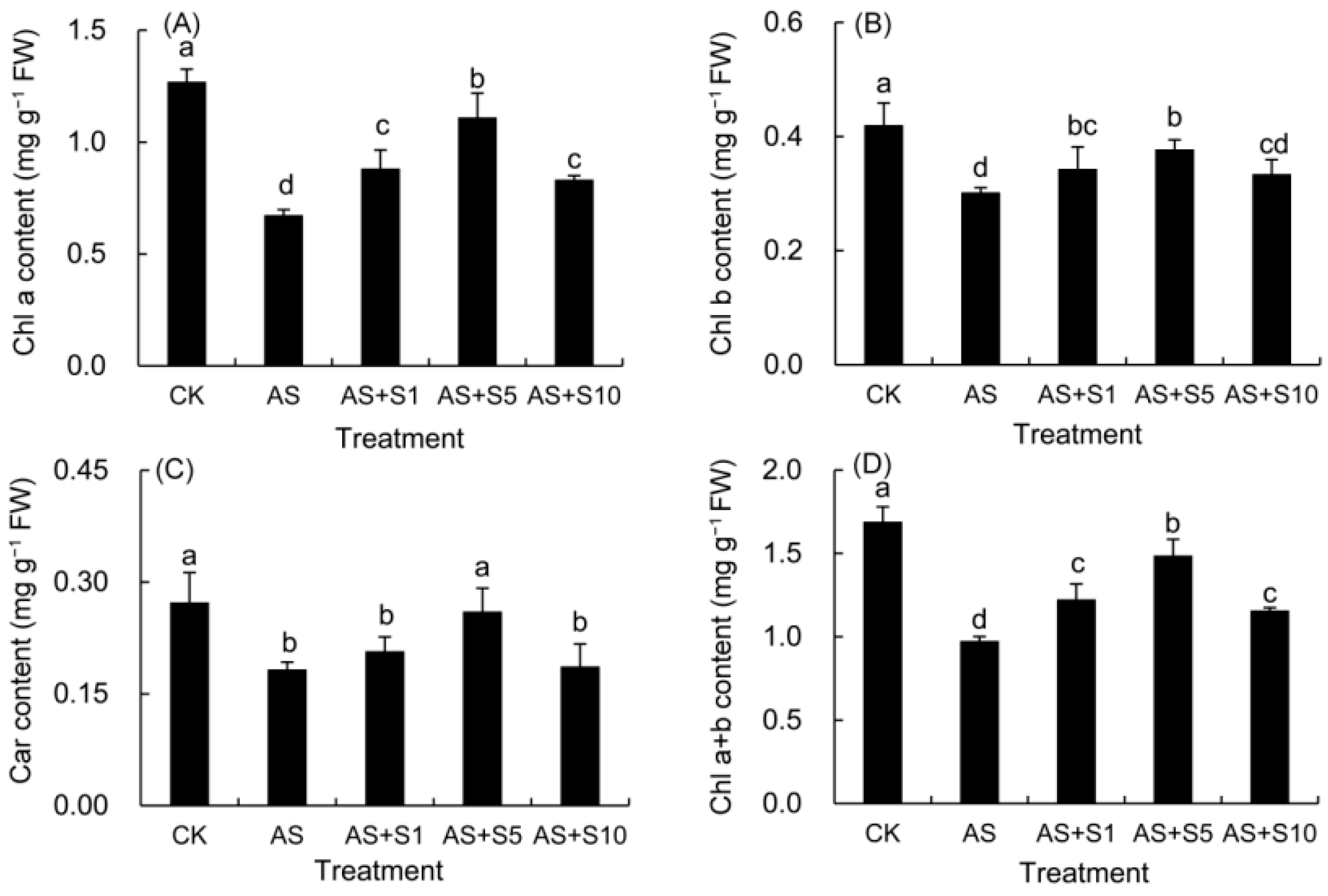
2.4. Photosynthetic Pigment
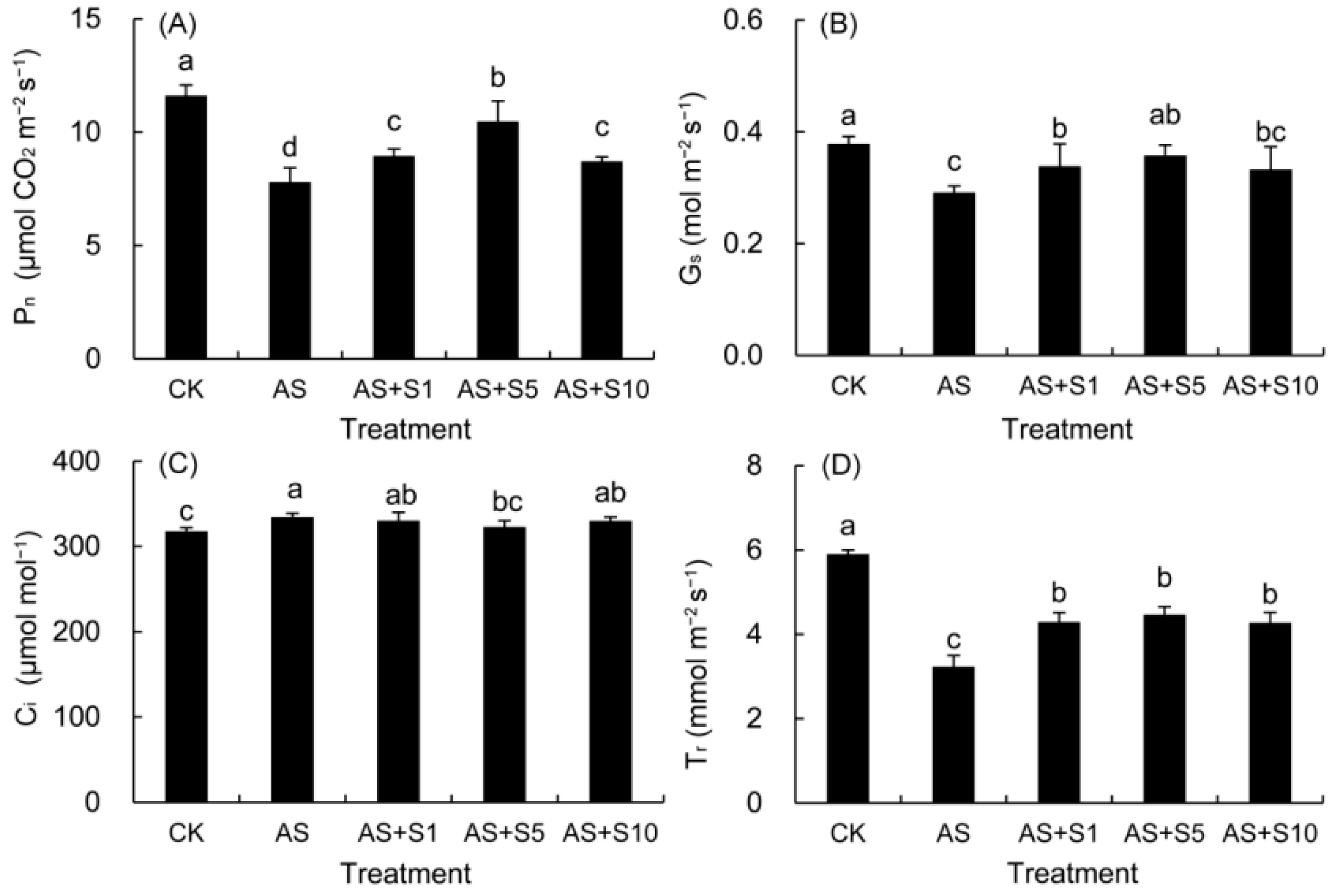
2.5. Gas Exchange Parameters
2.6. Root Architecture
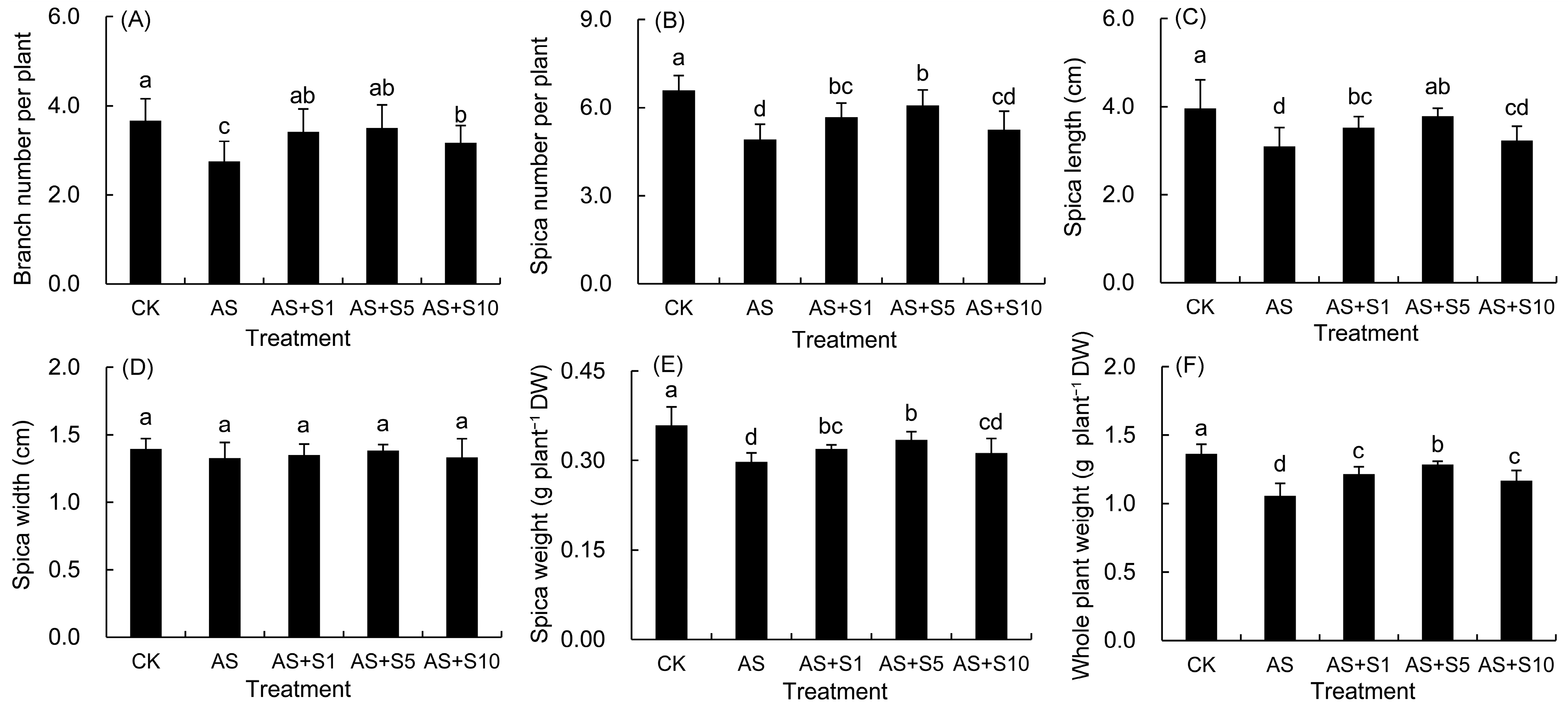
2.7. Morphology and Biomass
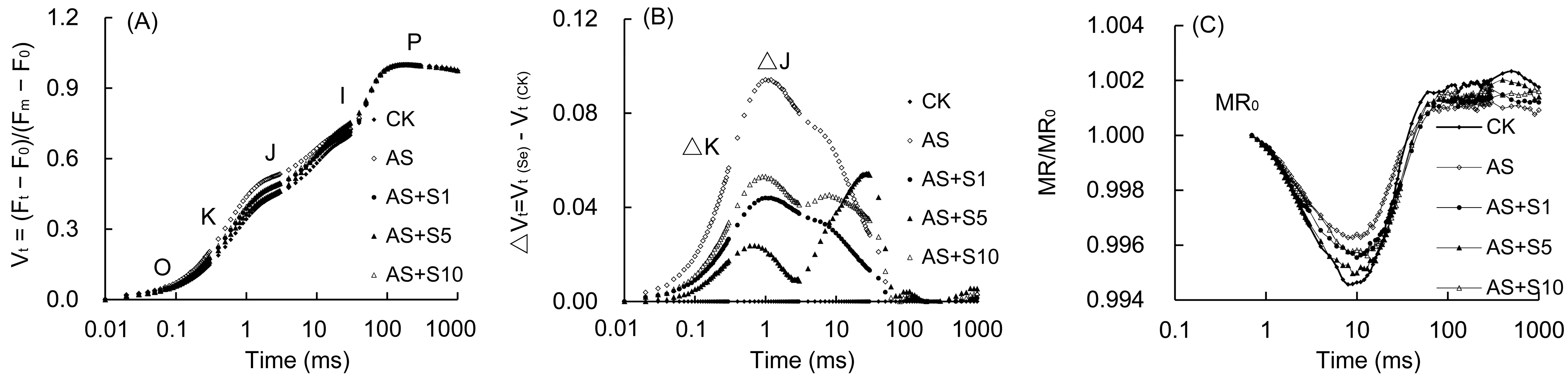
2.8. OJIP Curve and 820 nm Modulated Reflection
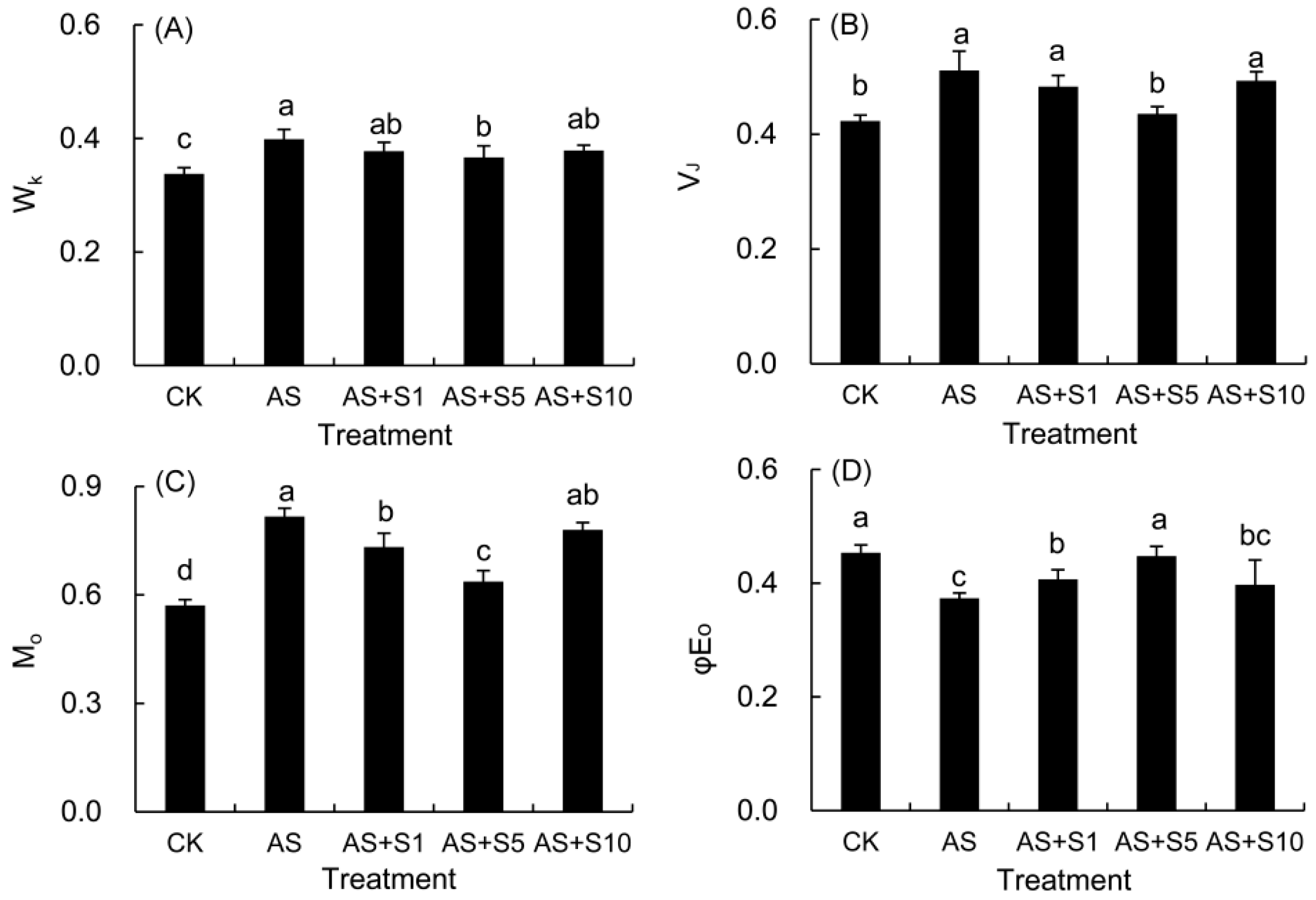
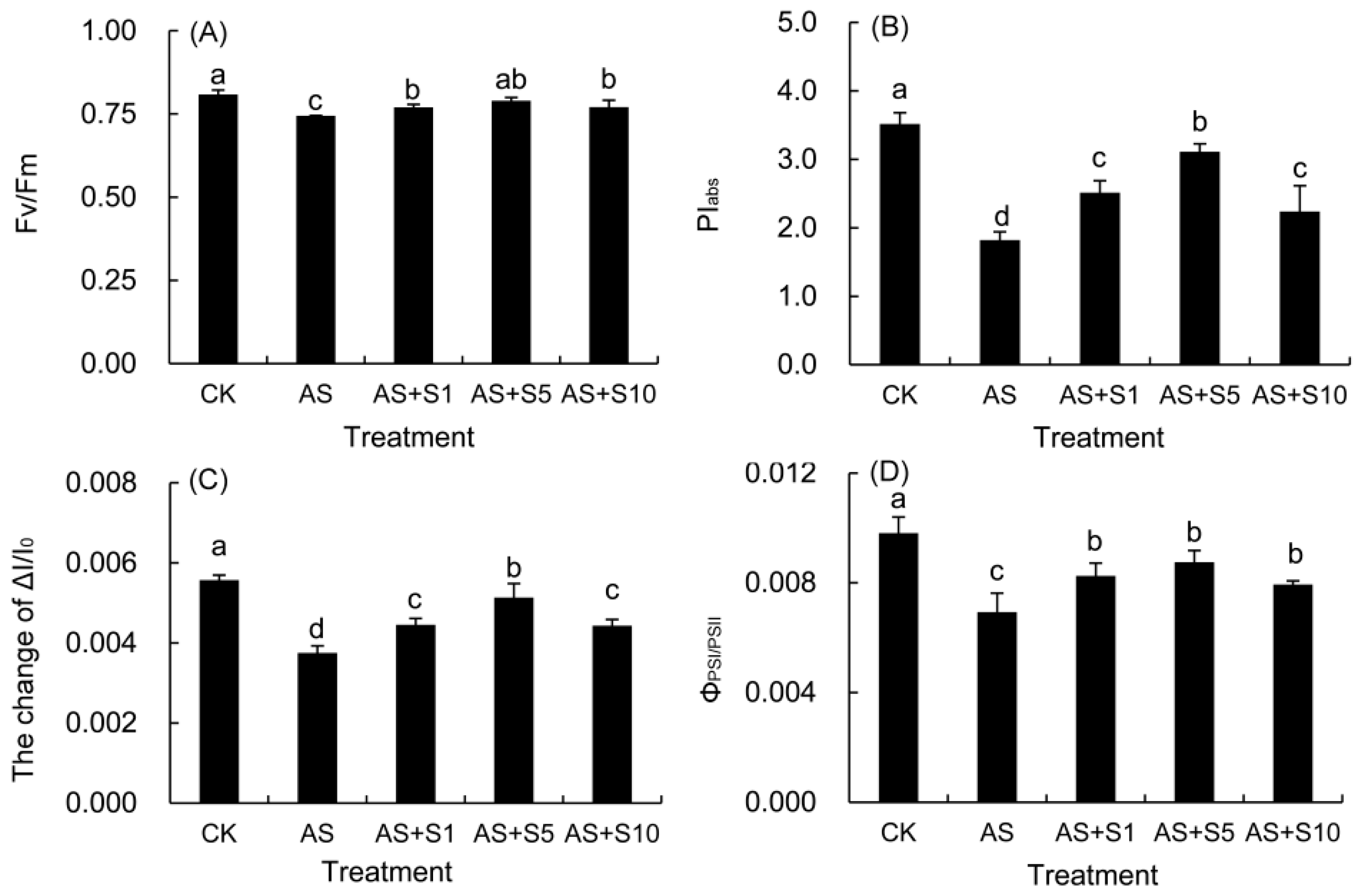
2.9. JIP Parameters
2.10. The Function and Coordination of PSII and PSI
2.11. The Contents of Secondary Metabolites
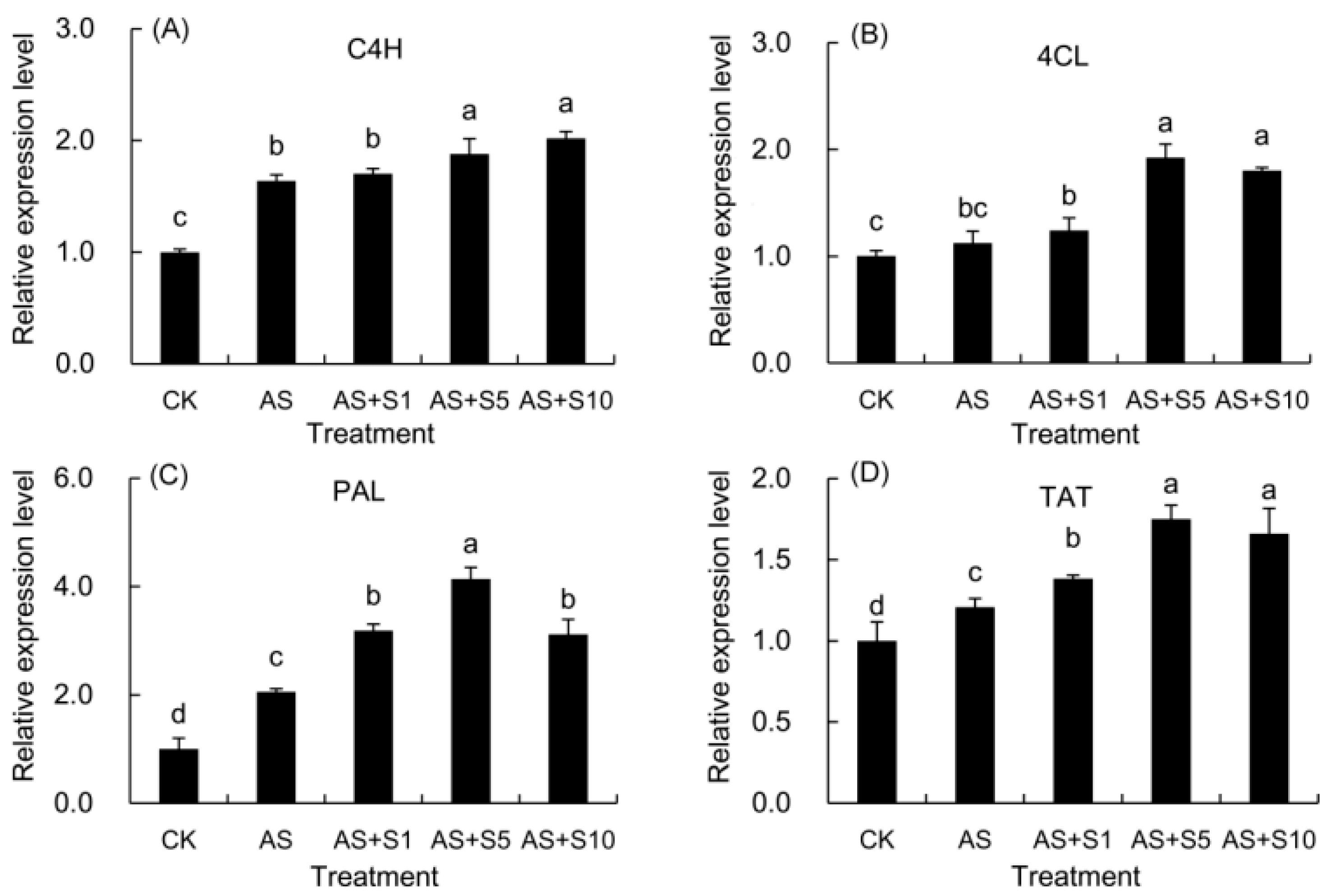
2.12. Expression Level of Key Genes
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Experimental Design
4.3. Determination of Antioxidant Enzyme Activity
4.4. Hydrogen Peroxide, Superoxide Anion, MDA Content, and Electrolyte Leakage
4.5. Determination of Pigment Contents and Gas Exchange Parameters
4.6. Determination of Osmolyte Content
4.7. Root Architecture Analysis
4.8. Determination of Morphological and Yield Traits
4.9. Fast Chlorophyll Fluorescence Induction Kinetic Curve (OJIP) and 820 nm Modulated Reflection
4.10. Determination of Secondary Metabolites Content
4.11. qRT-PCR Analysis
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bian, M.; Jin, X.; Broughton, S.; Zhang, X.Q.; Zhou, G.; Zhou, M.; Zhang, G.; Sun, D.; Li, C. A new allele of acid soil tolerance gene from a malting barley variety. BMC Genet. 2015, 16, 92. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Zhang, M.; Li, Y.; Zhang, Y.R.; Huang, X.C.; Yang, Y.H.; Zhu, H.Q.; Xiong, H.; Jiang, T.M. Influence of nitrogen fertilizer application on soil acidification characteristics of tea plantations in karst areas of southwest China. Agriculture 2023, 13, 849. [Google Scholar] [CrossRef]
- Wei, H.; Liu, Y.; Xiang, H.; Zhang, J.; Li, S.; Yang, J. Soil pH responses to simulated acid rain leaching in three agricultural soils. Sustainability 2020, 12, 280. [Google Scholar] [CrossRef]
- Von Uwxküll, H.; Mutert, E. Global extent, development and economic impact of acid soils. Plant Soil. 1995, 171, 1–15. [Google Scholar] [CrossRef]
- Shen, R.F.; Zhao, X.Q. The sustainable use of acid soils. J. Agric. 2019, 9, 16–20. [Google Scholar]
- Ahmad, G.; Khan, A.A.; Mohamed, H.I. Changes in Growth, Yield, Photosynthetic Pigments, Biochemical Substances, Oxidative Damage, and Antioxidant Activities Induced by Treatment with Different pH of Artificial acid rain in Pumpkin (Cucurbita Moschata). Gesunde Pflanz. 2021, 73, 623–637. [Google Scholar] [CrossRef]
- Sun, J.; Hu, H.; Li, Y.; Wang, L.; Zhou, Q.; Huang, X. Effects and mechanism of acid rain on plant chloroplast ATP synthase. Env. Sci. Pollut. Res. Int. 2016, 23, 18296–18306. [Google Scholar] [CrossRef]
- Zhang, L.X.; Hao, G.B.; Chang, Q.S.; Liu, W.; Xue, X.; Chen, S.D.; Chen, Y.M.; Zhang, S.W.; Yang, X.J. Antioxidant capacity and photosynthetic characteristics of Prunella vulgaris seedlings in response to simulated acid rain stress. Chin. J. Grassl. 2020, 42, 56–61. [Google Scholar]
- Li, B.; Wang, S.C.; Zhang, Y.; Qiu, D.W. Acid soil improvement enhances disease tolerance in citrus infected by Candidatus Liberibacter asiaticus. Int. J. Mol. Sci. 2020, 21, 3614. [Google Scholar] [CrossRef]
- Chen, Y.H.; Zhang, X.R.; Guo, Q.S.; Cao, L.P.; Qin, Q.; Li, C.; Zhao, M.; Wang, W.M. Plant morphology, physiological characteristics, accumulation of secondary metabolites and antioxidant activities of Prunella vulgaris L. under UV solar exclusion. Biol. Res. 2019, 52, 17. [Google Scholar] [CrossRef]
- Bai, Y.B.; Xia, B.H.; Xie, W.J.; Zhou, Y.M.; Xie, J.C.; Li, H.Q.; Liao, D.F.; Lin, L.M.; Li, C. Phytochemistry and pharmacological activities of the genus Prunella. Food Chem. 2016, 204, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Xia, F.; Pan, J.Y.; Chen, Y.P.; Peng, H.M.; Chen, S.H. Discuss on present situation and countermeasures for acid rain prevention and control in China. J. Env. Sci. Manag. 2011, 36, 30–35. [Google Scholar]
- Jia, H.; Song, Z.; Wu, F.; Ma, M.; Li, Y.; Han, D.; Yang, Y.; Zhang, S.; Cui, H. Low selenium increases the auxin concentration and enhances tolerance to low phosphorous stress in tobacco. Env. Exp. Bot. 2018, 153, 127–134. [Google Scholar] [CrossRef]
- Sun, M.F.; Hui, X.R.; Tong, C.L.; Yuan, L.Y.; Zhang, D.J. The physiological and molecular responses of exogenous selenium to selenium content and fruit quality in walnut. Phyton-Int. J. Exp. Bot. 2023, 92, 851–860. [Google Scholar] [CrossRef]
- Khalofah, A.; Migdadi, H.; El-Harty, E. Antioxidant enzymatic activities and growth response of quinoa (Chenopodium quinoa Willd) to exogenous selenium application. Plants 2021, 10, 719. [Google Scholar] [CrossRef]
- Puccinelli, M.; Pezzarossa, B.; Rosellini, I.; Malorgio, F. Selenium enrichment enhances the quality and shelf life of basil leaves. Plants 2020, 9, 801. [Google Scholar] [CrossRef]
- Feng, R.; Wei, C.; Tu, S. The roles of selenium in protecting plants against abiotic stresses. Env. Exp. Bot. 2013, 87, 58–68. [Google Scholar] [CrossRef]
- Handa, N.; Kohli, S.K.; Sharma, A.; Thukral, A.K.; Bhardwaj, R.; Abd Allah, E.F.; Alqarawi, A.A.; Ahmad, P. Selenium modulates dynamics of antioxidative defence expression, photosynthetic attributes and secondary metabolites to mitigate chromium toxicity in Brassica juncea L. plants. Env. Exp. Bot. 2019, 161, 180–192. [Google Scholar] [CrossRef]
- Ghasemian, S.; Masoudian, N.; Saeid Nematpour, F.; Safipour Afshar, A. Selenium nanoparticles stimulate growth, physiology, and gene expression to alleviate salt stress in Melissa officinalis. Biológia 2021, 76, 2879–2888. [Google Scholar] [CrossRef]
- Ghanbari, F.; Bag-Nazari, M.; Azizi, A. Exogenous application of selenium and nano-selenium alleviates salt stress and improves secondary metabolites in lemon verbena under salinity stress. Sci. Rep. 2023, 13, 5352. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; García-Caparrós, P.; Parvin, K.; Zulfiqar, F.; Ahmed, N.; Fujita, M. Selenium supplementation and crop plant tolerance to metal/metalloid toxicity. Front. Plant Sci. 2022, 12, 792770. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Wu, S.; Meng, X.; Xue, J.; Duan, Y. Selenium Regulates Antioxidant Capacities and Diterpenoid Biosynthesis in the Medicinal Plant Isodon rubescens. Phyton 2024, 93, 1705–1716. [Google Scholar] [CrossRef]
- Liu, H.D.; Xiao, C.M.; Qiu, T.C.; Deng, J.; Cheng, H.; Cong, X.; Cheng, S.Y.; Rao, S.; Zhang, Y. Selenium regulates antioxidant, photosynthesis, and cell permeability in plants under various abiotic stresses: A review. Plants 2022, 12, 44. [Google Scholar] [CrossRef]
- Moloi, M.J.; Khoza, B.M. The effect of selenium foliar application on the physiological responses of edamame under different water treatments. Agronomy 2022, 12, 2400. [Google Scholar] [CrossRef]
- Wang, M.; Ali, F.; Qi, M.; Peng, Q.; Wang, M.; Bañuelos, G.S.; Miao, S.; Li, Z.; Dinh, Q.T.; Liang, D. Insights into uptake, accumulation, and subcellular distribution of selenium among eight wheat (Triticum aestivum L.) cultivars supplied with selenite and selenate. Ecotox. Env. Safe 2021, 207, 111544. [Google Scholar] [CrossRef]
- Liu, H.; Blankenship, R.E. On the interface of light-harvesting antenna complexes and reaction centers in oxygenic photosynthesis. BBA-Bioenerg. 2019, 1860, 148079. [Google Scholar] [CrossRef]
- Kim, E.; Yokono, M.; Tsugane, K.; Ishii, A.; Noda, C.; Minagawa, J. Formation of a stable PSI-PSII megacomplex in rice that conducts energy spillover. Plant Cell Physiol. 2023, 64, 858–865. [Google Scholar] [CrossRef]
- Chang, Q.S.; Zhang, L.X.; Chen, S.C.; Gong, M.G.; Liu, L.C.; Hou, X.G.; Mi, Y.F.; Wang, X.H.; Wang, J.Z.; Zhang, Y.; et al. Exogenous melatonin enhances the yield and secondary metabolite contents of Prunella vulgaris by modulating antioxidant system, root architecture and photosynthetic capacity. Plants 2023, 12, 1129. [Google Scholar] [CrossRef]
- Yokono, M.; Takabayashi, A.; Akimoto, S.; Tanaka, A. A megacomplex composed of both photosystem reaction centres in higher plants. Nat. Commun. 2015, 6, 6675. [Google Scholar] [CrossRef]
- Souza, A.F.C.; Martins, J.P.R.; Gontijo, A.B.P.L.; Falqueto, A.R. Selenium improves the transport dynamics and energy conservation of the photosynthetic apparatus of in vitro grown Billbergia zebrina (Bromeliaceae). Photosynthetica 2019, 57, 931–941. [Google Scholar] [CrossRef]
- Martins, J.P.R.; Moreira, S.W.; Braga, P.C.S.; Conde, L.T.; Cipriano, R.; Falqueto, A.R.; Gontijo, A.B.P.L. Photosynthetic apparatus performance and anatomical modulations of Alcantarea imperialis (Bromeliaceae) exposed to selenium during in vitro growth. Photosynthetica 2021, 59, 529–537. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Ma, J.; Wu, F.; Wang, L.; Sun, L.; Zhang, P.; Wang, W.; Xu, J. Comparative physiological and soil microbial community structural analysis revealed that selenium alleviates cadmium stress in Perilla frutescens. Front. Plant Sci. 2022, 13, 1022935. [Google Scholar] [CrossRef] [PubMed]
- Azimi, F.; Oraei, M.; Gohari, G.; Panahirad, S.; Farmarzi, A. Chitosan-selenium nanoparticles (Cs–Se NPs) modulate the photosynthesis parameters, antioxidant enzymes activities and essential oils in Dracocephalum moldavica L. under cadmium toxicity stress. Plant Physiol. Biochem. 2021, 167, 257–268. [Google Scholar] [CrossRef]
- Skrypnik, L.; Feduraev, P.; Golovin, A.; Maslennikov, P.; Styran, T.; Antipina, M.; Riabova, A.; Katserov, D. The integral boosting effect of selenium on the secondary metabolism of higher plants. Plants 2022, 11, 3432. [Google Scholar] [CrossRef]
- Sayed, T.E.; Ahmed, E.S. Improving artemisinin and essential oil production from Artemisia plant through in vivo elicitation with gamma irradiation nano-selenium and chitosan coupled with bio-organic fertilizers. Front. Energy Res. 2022, 10, 996253. [Google Scholar] [CrossRef]
- Wang, G.; Wu, L.Y.; Zhang, H.; Wu, W.J.; Zhang, M.M.; Li, X.F.; Wu, H. Regulation of the phenylpropanoid pathway: A mechanism of selenium tolerance in peanut (Arachis hypogaea L.) seedlings. J. Agr. Food Chem. 2016, 64, 3626–3635. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Zhang, B.; Han, Z.; Wang, W.; Chi, Q.; Zhou, J.; Nie, L.; Xu, S.; Liu, D.; et al. Concentration and distribution of selenium in soils of mainland China, and implications for human health. J. Geochem. Explor. 2021, 220, 106654. [Google Scholar] [CrossRef]
- Wang, R.; Yu, T.; Zeng, Q.; Yang, Z. Distribution characteristics, origin and influencing factors of soil selenium concentration of main farming areas in China. Curr. Biotechnol. 2017, 7, 359–366. [Google Scholar]
- Xie, B.T.; He, L.; Jiang, G.J.; Sun, B.B.; Zeng, D.M. Regulation and evaluation of selenium availability in Se-rich soils in southern China. Rock. Miner. Anal. 2017, 36, 273–281. [Google Scholar]
- Poggi, V.; Arcioni, A.; Filippini, P.; Pifferi, P.G. Foliar application of selenite and selenate to potato (Solanum tuberosum): Effect of a ligand agent on selenium content of tubers. J. Agric. Food Chem. 2000, 48, 4749–4751. [Google Scholar] [CrossRef]
- Abdul, S.; Cheema, M.A.; Sher, A.; Ijaz, M.; Ul Allah, S.; Nawaz, A.; Abbas, T.; Ali, Q. Physiological and biochemical attributes of bread wheat (Triticum aestivum L.) seedlings are infuenced by foliar application of silicon and selenium under water defcit. Acta Physiol. Plant. 2019, 41, 146. [Google Scholar]
- Kápolna, E.; Laursen, K.H.; Husted, S.; Larsen, E.H. Bio-fortification and isotopic labelling of Se metabolites in onions and carrots following foliar application of Se and 77Se. Food Chem. 2012, 133, 650–657. [Google Scholar] [CrossRef]
- Longchamp, M.; Castrec-Rouelle, M.; Biron, P.; Bariac, T. Variations in the accumulation, localization and rate of metabolization of selenium in mature Zea mays plants supplied with selenite or selenate. Food Chem. 2015, 182, 128–135. [Google Scholar] [CrossRef]
- Wang, M.; Ali, F.; Wang, M.; Dinh, Q.T.; Zhou, F.; Bañuelos, G.S.; Liang, D. Understanding boosting selenium accumulation in Wheat (Triticum aestivum L.) following foliar selenium application at different stages, forms, and doses. Environ. Sci. Pollut. Res. Int. 2020, 27, 717–728. [Google Scholar] [CrossRef]
- Xu, X.; Wang, J.; Wu, H.; Yuan, Q.; Wang, J.; Cui, J.; Lin, A. Effects of selenium fertilizer application and tomato varieties on tomato fruit quality: A meta-analysis. Sci. Hortic. 2022, 304, 111242. [Google Scholar] [CrossRef]
- Alsamadany, H.; Alharby, H.F.; Al-Zahrani, H.S.; Kuşvuran, A.; Kuşvuran, S.; Rady, M.M. Selenium fortification stimulates antioxidant- and enzyme gene expression-related defense mechanisms in response to saline stress in Cucurbita pepo. Sci. Hortic. 2023, 312, 111886. [Google Scholar] [CrossRef]
- Sardari, M.; Rezayian, M.; Niknam, V. Comparative study for the effect of selenium and nano-selenium on wheat plants grown under drought stress. Russ. J. Plant Physl. 2022, 69, 127. [Google Scholar] [CrossRef]
- Alves, L.R.; Prado, E.R.; de Oliveira, R.; Santos, E.F.; Lemos De Souza, I.; Dos Reis, A.R.; Azevedo, R.A.; Gratão, P.L. Mechanisms of cadmium-stress avoidance by selenium in tomato plants. Ecotoxicology 2020, 29, 594–606. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Zhang, X.; He, H.; Xiang, J.Q.; Yin, H.Q.; Hou, T. Selenium-containing proteins/peptides from plants: A review on the structures and functions. J. Agr. Food Chem. 2020, 68, 15061–15073. [Google Scholar] [CrossRef]
- Chauhan, R.; Awasthi, S.; Srivastava, S.; Dwivedi, S.; Pilon-Smits, E.A.H.; Dhankher, O.P.; Tripathi, R.D. Understanding selenium metabolism in plants and its role as a beneficial element. Crit. Rev. Env. Sci. Tec. 2019, 49, 1937–1958. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Prasad, P.V.V.; Seppanen, M. Selenium protects sorghum leaves from oxidative damage under high temperature stress by enhancing antioxidant defense system. Plant Physiol. Biochem. 2010, 48, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.Z.; Wang, Y.; Wang, S.H.; Yin, L.P.; Xu, G.J.; Zheng, C.; Lei, C.; Zhang, M.Z. Selenium increases chlorogenic acid, chlorophyll and carotenoids of Lycium chinense leaves. J. Sci. Food Agric. 2013, 93, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Ying, Z.; Chen, J.; Yang, Y.; Zhang, J.; Yang, L.; Liu, M. Effect of Selenium Application on Growth, Antioxidative Capacity, and Nutritional Quality in Purple Lettuce Seedlings. Agronomy 2023, 13, 1664. [Google Scholar] [CrossRef]
- Qi, W.Y.; Li, Q.; Chen, H.; Liu, J.; Xing, S.F.; Xu, M.; Yan, Z.; Song, C.; Wang, S.G. Selenium nanoparticles ameliorate Brassica napus L. cadmium toxicity by inhibiting the respiratory burst and scavenging reactive oxygen species. J. Hazard. Mater. 2021, 417, 125900. [Google Scholar] [CrossRef]
- Hussain, S.; Ahmed, S.; Akram, W.; Li, G.H.; Yasin, N.A. Selenium seed priming enhanced the growth of salt-stressed Brassica rapa L. through improving plant nutrition and the antioxidant system. Front. Plant Sci. 2022, 13, 1050359. [Google Scholar] [CrossRef]
- Khattab, H.I.; Emam, M.A.; Emam, M.M.; Helal, N.M.; Mohamed, M.R. Effect of selenium and silicon on transcription factors NAC5 and DREB2A involved in drought-responsive gene expression in rice. Biol. Plant. 2014, 58, 265–273. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, G.; Qin, H.; Guan, Y.; Wang, T.; Ni, W.; Xie, H.; Xing, Y.; Tian, G.; Lyu, M.; et al. Metabolomics combined with physiology and transcriptomics reveal key metabolic pathway responses in apple plants exposure to different selenium concentrations. J. Hazard. Mater. 2024, 464, 132953. [Google Scholar] [CrossRef]
- Petrov, V.; Hille, J.; Mueller-Rober, B.; Gechev, T.S. ROS-mediated abiotic stress-induced programmed cell death in plants. Front. Plant Sci. 2015, 6, 69. [Google Scholar] [CrossRef]
- Xu, H.; Xie, C.; Wei, Y.; Huang, Y.; Lin, X.; Xu, H.; Xie, C.; Wei, Y.K. Comparison of tolerance between two salvia plants to stress of simulated acid rain and study of the physiological mechanisms. Asian J. Ecotoxicol. 2017, 12, 206–214. (In Chinese) [Google Scholar]
- Dogru, A. Effects of heat stress on photosystem II activity and antioxidant enzymes in two maize cultivars. Planta 2021, 253, 85. [Google Scholar] [CrossRef] [PubMed]
- Dar, Z.; Malik, M.; Aziz, M.; Masood, A.; Dar, A.; Hussan, S.; Dar, Z. Role of selenium in regulation of plant antioxidants, chlorophyll retention and osmotic adjustment under drought conditions: A Review. Int. J. Plant Sci. 2021, 33, 52–60. [Google Scholar] [CrossRef]
- Balal, R.M.; Shahid, M.A.; Javaid, M.M.; Iqbal, Z.; Anjum, M.A.; Garcia-Sanchez, F.; Mattson, N.S. The role of selenium in amelioration of heat-induced oxidative damage in cucumber under high temperature stress. Acta Physiol. Plant. 2016, 38, 1–14. [Google Scholar] [CrossRef]
- Zhao, P.; Wu, Z.; Zheng, Y.; Shen, J.; Zhu, Y.; Chen, Q.; Wang, B.; Yang, F.; Ding, Y.; Liu, H.; et al. Selenite affected photosynthesis of Oryza sativa L. exposed to antimonite: Electron transfer, carbon fixation, pigment synthesis via a combined analysis of physiology and transcriptome. Plant Physiol. Biochem. 2023, 201, 107904. [Google Scholar] [CrossRef] [PubMed]
- Hawrylak-Nowak, B.; Rubinowska, K.; Molas, J.; Woch, W.; Matraszek-Gawron, R.; Szczurowska, A. Selenium-induced improvements in the ornamental value and salt stress resistance of Plectranthus scutellarioides (L.) R. Br. Folia Hortic. 2019, 31, 213–221. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, J.T.; Zhang, H.W.; Xu, Y.; An, Y.Y.; Wang, L.J. Effect of 5-Aminolevulinic acid (5-ALA) on leaf chlorophyll fast fluorescence characteristics and mineral element content of Buxus megistophylla grown along urban roadsides. Horticulturae 2021, 7, 95. [Google Scholar] [CrossRef]
- Velikova, V.; Tsonev, T.; Yordanov, I. Light and CO2 responses of photosynthesis and chlorophyll fluorescence characteristics in bean plants after simulated acid rain. Physiol. Plant. 1999, 107, 77–83. [Google Scholar] [CrossRef]
- Ju, S.; Wang, L.; Chen, J. Effects of silicon on the growth, photosynthesis and chloroplast ultrastructure of Oryza sativa L. seedlings under acid rain stress. Silicon 2020, 12, 655–664. [Google Scholar] [CrossRef]
- Jiang, C.; Zu, C.; Lu, D.; Zheng, Q.; Shen, J.; Wang, H.; Li, D. Effect of exogenous selenium supply on photosynthesis, Na+ accumulation and antioxidative capacity of maize (Zea mays L.) under salinity stress. Sci. Rep. 2017, 7, 42039. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.; Wassie, M.; Chen, L. Selenium supplementation alleviates cadmium-induced damages in tall fescue through modulating antioxidant system, photosynthesis efficiency, and gene expression. Env. Sci. Pollut. Res. Int. 2020, 27, 9490–9502. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, G.; Chen, J.; Wu, J.; Liu, S.; He, X. Effects of Foliar Spraying of Organic Selenium and Nano-Selenium Fertilizer on Pak Choi (Brassica chinensis var. pekinensis. cv. ‘Suzhouqing’) under Low Temperature Stress. Agriculture 2023, 13, 2140. [Google Scholar] [CrossRef]
- Wang, Y.; Rao, C.; Huang, L.; Wu, J.; Sun, P.; Zhan, J.; Wu, J.; Liu, S.; Zhou, C.; Hu, L.; et al. Effects of Organic Selenium and Nanoselenium on Drought Stress of Pak Choi (Brassica chinensis var. pekinensis. cv. ‘Suzhouqing’) and Its Transcriptomic Analysis. Agronomy 2024, 14, 78. [Google Scholar] [CrossRef]
- Mir, A.R.; Siddiqui, H.; Alam, P.; Hayat, S. Melatonin modulates photosynthesis, redox status, and elemental composition to promote growth of Brassica juncea-a dose-dependent effect. Protoplasma 2020, 257, 1685–1700. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Zhang, K.R.; Zhang, Q.F.; Wang, W.B. Tolerant mechanism of Jatropha curcas L. roots to acid rain in soils with different acid-buffering capacities. Acta Physiol. Plant 2021, 43, 158. [Google Scholar] [CrossRef]
- Luo, Y.; Wei, Y.; Sun, S.; Wang, J.; Wang, W.; Han, D.; Shao, H.; Jia, H.; Fu, Y. Selenium Modulates the Level of Auxin to Alleviate the Toxicity of Cadmium in Tobacco. Int. J. Mol. Sci. 2019, 20, 3772. [Google Scholar] [CrossRef]
- Alyemeni, M.N.; Ahanger, M.A.; Wijaya, L.; Alam, P.; Bhardwaj, R.; Ahmad, P. Selenium mitigates cadmium-induced oxidative stress in tomato (Solanum lycopersicum L.) plants by modulating chlorophyll fluorescence, osmolyte accumulation, and antioxidant system. Protoplasma 2018, 255, 985–986. [Google Scholar] [CrossRef]
- Malik, J.A.; Kumar, S.; Thakur, P.; Sharma, S.; Kaur, N.; Kaur, R.; Pathania, D.; Bhandhari, K.; Kaushal, N.; Singh, K.; et al. Promotion of growth in mungbean (Phaseolus aureus Roxb.) by selenium is associated with stimulation of carbohydrate metabolism. Biol. Trace Elem. Res. 2011, 143, 530–539. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Liu, M.J.; Scheibe, R.; Selinski, J.; Zhang, L.T.; Yang, C.; Meng, X.L.; Gao, H.Y. Contribution of the alternative respiratory pathway to PSII photoprotection in C3 and C4 plants. Mol. Plant 2017, 10, 131–142. [Google Scholar] [CrossRef]
- Chalanika De Silva, H.C.; Asaeda, T. Effects of heat stress on growth, photosynthetic pigments, oxidative damage and competitive capacity of three submerged macrophytes. J. Plant Interact. 2017, 12, 228–236. [Google Scholar] [CrossRef]
- Mathur, S.; Jajoo, A.; Mehta, P.; Bharti, S. Analysis of elevated temperature-induced inhibition of photosystem II using chlorophyll a fluorescence induction kinetics in wheat leaves (Triticum aestivum). Plant Biol. 2011, 13, 1–6. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Qiang, S.; Goltsev, V. Simultaneous in vivo recording of prompt and delayed fluorescence and 820-nm reflection changes during drying and after rehydration of the resurrection plant Haberlea rhodopensis. BBA-Bioenerg. 2010, 1797, 1313–1326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.X.; Chang, Q.S.; Hou, X.G.; Wang, J.Z.; Chen, S.D.; Zhang, Q.M.; Wang, Z.; Yin, Y.; Liu, J.K. The effect of high temperature stress on the physiological indexes, chloroplast ultrastructure, photosystems of two herbaceous peony cultivars. J. Plant Growth Regul. 2023, 42, 1631–1646. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, J.; Hu, H.; Li, Y.; Xiang, M.; Wang, D. Integrated eco-physiological, biochemical, and molecular biological analyses of selenium fortification mechanism in alfalfa. Planta 2022, 256, 114. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, X.; Zhang, M.; Li, X.; Xie, J.; Liu, S.; Huang, Q.; Wang, J.; Guo, Q.; Wang, H. Hyperoside prevents Aβ42-induced neurotoxicity in PC12 cells and caenorhabditis elegans. Mol. Neurobiol. 2023, 60, 7136–7150. [Google Scholar] [CrossRef]
- Board of Pharmacopoeia of China. Pharmacopoeia of the People’s Republic of China; China Medico-Pharmaceutical Science & Technology Publishing House: Beijing, China, 2015. [Google Scholar]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Rao, M.V.; Paliyath, G.; Ormrod, D.P. Ultraviolet-B- and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol. 1996, 110, 125–136. [Google Scholar] [CrossRef]
- Pinhero, R.G.U.O.; Rao, M.V.; Paliyath, G.; Murr, D.P.; Fletcher, R.A. Changes in activities of antioxidant enzymes and their relationship to genetic and paclobutrazol-induced chilling tolerance of maize seedlings. Plant Physiol. (Bethesda) 1997, 114, 695–704. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Elstner, E.F.; Heupel, A. Inhibition of nitrite formation from hydroxylammoniumchloride: A simple assay for superoxide dismutase. Anal. Biochem. 1976, 70, 616–620. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Analysis 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Gary, N.D.; Klausmeier, R.E. Colorimetric Determination of Ribose, Deoxyribose, and Nucleic Acid with Anthrone. Anal. Chem. 1954, 26, 1958–1960. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Li, Y.T.; Xu, W.W.; Ren, B.Z.; Zhao, B.; Zhang, J.; Liu, P.; Zhang, Z.S. High temperature reduces photosynthesis in maize leaves by damaging chloroplast ultrastructure and photosystem II. J. Agron. Crop Sci. 2020, 206, 548–564. [Google Scholar] [CrossRef]
- Zhang, L.X.; Chang, Q.S.; Hou, X.G.; Chen, S.D.; Zhang, Q.M.; Wang, J.Z.; Liu, S.S.; Li, S. Biochemical and photosystem characteristics of wild-type and Chl b-deficient mutant in tree peony (Paeonia suffruticosa). Photosynthetica 2021, 59, 256–265. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Jia, Y.J.; Gao, H.Y.; Zhang, L.T.; Li, H.D.; Meng, Q.W. Characterization of PSI recovery after chilling-induced photoinhibition in cucumber (Cucumis Sativus L.). Planta 2011, 234, 883–889. [Google Scholar] [CrossRef]
- Tang, H.; Hu, J.; Zhao, M.; Cao, L.; Chen, Y. Comparative study of the physiological responses, secondary metabolites, and gene expression of medicinal plant Prunella vulgaris L. treated with exogenous methyl jasmonate and salicylic acid. Acta Physiol. Plant. 2023, 45, 20. [Google Scholar] [CrossRef]

| Se Treatment | Root Length (cm) | Root Surface Area (cm2) | Root Volume (cm3) | The Number of Root Tip | Branch Number |
|---|---|---|---|---|---|
| CK | 760 ± 70 a | 112.41 ± 13.11 a | 1.41 ± 0.15 a | 1409 ± 137 b | 3133 ± 271 a |
| AS | 342 ± 34 e | 43.14 ± 4.42 d | 0.44 ± 0.08 c | 593 ± 135 d | 1159 ± 230 c |
| AS+S1 | 595 ± 23 c | 73.59 ± 5.58 bc | 0.73 ± 0.10 b | 1128 ± 105 c | 2525 ± 415 b |
| AS+S5 | 695 ± 49 b | 80.81 ± 10.29 b | 0.77 ± 0.15 b | 1743 ± 126 a | 2840 ± 443 ab |
| AS+S10 | 529 ± 30 d | 65.16 ± 6.95 c | 0.64 ± 0.12 b | 1143 ± 161 c | 2358 ± 226 c |
| Parameter | CK | AS | AS+S1 | AS+S5 | AS+S10 |
|---|---|---|---|---|---|
| ABS/RC | 1.43 ± 0.06 c | 1.72 ± 0.05 a | 1.58 ± 0.13 b | 1.56 ± 0.04 bc | 1.62 ± 0.05 ab |
| DI0/RC | 0.31 ± 0.03 c | 0.41 ± 0.02 a | 0.34 ± 0.02 bc | 0.32 ± 0.03 bc | 0.37 ± 0.04 ab |
| Tr0/RC | 1.12 ± 0.04 c | 1.31 ± 0.03 a | 1.24 ± 0.05 b | 1.22 ± 0.01 b | 1.25 ± 0.03 b |
| ET0/RC | 0.65 ± 0.03 a | 0.64 ± 0.09 a | 0.65 ± 0.07 a | 0.70 ± 0.02 a | 0.64 ± 0.10 a |
| φD0 | 0.19 ± 0.01 c | 0.26 ± 0.00 a | 0.23 ± 0.01 b | 0.21 ± 0.01 bc | 0.23 ± 0.02 b |
| ABS/CSm | 31,141.33 ± 845.22 a | 28,070.67 ± 714.81 b | 29,996.67 ± 1295.91 a | 30,211.00 ± 438.63 a | 30,518.00 ± 1253.21 a |
| DI0/CSm | 6332.00 ± 346.17 a | 6607.67 ± 527.45 a | 6734.00 ± 932.52 a | 6251.00 ± 685.96 a | 7011.00 ± 577.05 a |
| Tr0/CSm | 24,809.33 ± 569.70 a | 21,463.00 ± 730.87 b | 23,262.67 ± 2086.98 ab | 23,960.00 ± 789.86 a | 23,507.00 ± 1304.77 ab |
| ET0/CSm | 14,121.00 ± 531.10 a | 10,493.67 ± 784.19 b | 12,201.33 ± 1351.51 ab | 13,520.33 ± 251.29 a | 12,132.33 ± 1697.49 ab |
| RC/CSm | 18,159.41 ± 1290.96 a | 13,420.75 ± 655.54 d | 1556.20 ±231.12 bc | 16,415.79 ± 870.11 b | 14,845.98 ± 415.26 cd |
| Se Treatment | Total Flavonoids | Caffeic Acid | Ferulic Acid | Rosmarinic Acid | Hyperoside |
|---|---|---|---|---|---|
| CK | 58.56 ± 2.22 d | 0.09 ± 0.00 b | 0.55 ± 0.01 d | 6.06 ± 0.04 c | 0.34 ± 0.01 c |
| AS | 62.89 ± 2.38 c | 0.09 ± 0.00 b | 0.58 ± 0.01 c | 6.43 ± 0.18 b | 0.36 ± 0.01 c |
| AS+S1 | 67.98 ± 2.05 b | 0.10 ± 0.01 b | 0.76 ± 0.01 b | 6.69 ± 0.19 b | 0.40 ± 0.02 b |
| AS+S5 | 82.40 ± 0.72 a | 0.11 ± 0.01 a | 0.82 ± 0.02 a | 7.40 ± 0.16 a | 0.44 ± 0.02 a |
| AS+S10 | 80.84 ± 0.88 a | 0.12 ± 0.00 a | 0.66 ± 0.01 c | 7.51 ± 0.16 a | 0.41 ± 0.03 ab |
| Fluorescence Parameters | Description |
|---|---|
| WK = (FK − F0)/(FJ − F0) | Normalized relative variable fluorescence |
| VJ = (FJ − F0)/(Fm − F0) | Relative variable fluorescence intensity at the J step |
| M0 = 4 (F300μs − F0)/(Fm − F0) | The initial slope of the relative variable fluorescence of the relative rate at which QA is reduced |
| φE0 = ET0/ABS = [1− (F0/Fm)]ψ0 | Quantum yield for electron transport |
| ABS/RC = M0 (1/VJ) (1/φP0) | Absorption flux per reaction center |
| TR0/RC = M0(1/VJ) | Trapped energy flux per reaction center (RC) |
| ET0/RC = M0 (1/VJ) ψE0 | Electron transport flux per RC |
| DI0/RC = (ABS/RC) − (TR0/RC) | Dissipated energy flux per RC |
| RC/CSm = φP0 (VJ/M0) (ABS/CSm) | Density of RCs per excited cross section (CS) |
| ABS/CSm | Absorbed energy flux per CS |
| TR0/CSm = φP0(ABS/CSm) | Trapped energy flux per CS |
| ET0/CSm = φE0(ABS/CSm) | Electron transport flux per CS |
| DI0/CSm = ABS/CSm-TR0/CSm | Dissipated energy flux per CS |
| Fv/Fm | The maximal quantum yield of PSII photochemistry |
| φD0 | Quantum yield of energy dissipation |
| PIABS = (RC/ABS) [φP0/(1 − φP0)][ψ0/(1 − ψ0)] | Performance index on absorption basis |
| Gene | Genbank Accession Number | Primer Name | Primer Sequence (5′ → 3′) | PCR Product (bp) |
|---|---|---|---|---|
| PvC4H | KJ010816 | PvC4H forward | ATCGTTGTCGCCGCCGTTGTGT | 136 |
| PvC4H reverse | CGTAGTCGGTGAGGTTTCGGTGGTTC | |||
| Pv4CL | KJ010817.1 | Pv4CL forward | CCACCATGGCCAATCCCTATT | 114 |
| Pv4CL reverse | CATAGTCCCGCACCTTGTCG | |||
| PvPAL | KJ010815.1 | PvPAL forward | TCCGTGCTTGTGTGTTTGTGCCTGTC | 203 |
| PvPAL reverse | GGCTTCCTGAACTCCTCCACCATCCT | |||
| PvTAT | KM053278 | PvTAT forward | CGTCTACTCGCATCAGCATCTCAGGA | 194 |
| PvTAT reverse | GCCAACCAGGGATCAACCACCTCTTC | |||
| β-actin | KJ010818 | β-actin forward | GCAGTTCTCTCCCTATACGCCAGTGG | 205 |
| β-actin reverse | GCTCGGCTGTGGTGGTGAATGAGTAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Chang, Q.; Zhao, X.; Guo, Q.; Chen, S.; Zhang, Q.; He, Y.; Chen, S.; Chen, K.; Ban, R.; et al. Selenium Improves Yield and Quality in Prunella vulgaris by Regulating Antioxidant Defense, Photosynthesis, Growth, Secondary Metabolites, and Gene Expression Under Acid Stress. Plants 2025, 14, 920. https://doi.org/10.3390/plants14060920
Zhang L, Chang Q, Zhao X, Guo Q, Chen S, Zhang Q, He Y, Chen S, Chen K, Ban R, et al. Selenium Improves Yield and Quality in Prunella vulgaris by Regulating Antioxidant Defense, Photosynthesis, Growth, Secondary Metabolites, and Gene Expression Under Acid Stress. Plants. 2025; 14(6):920. https://doi.org/10.3390/plants14060920
Chicago/Turabian StyleZhang, Lixia, Qingshan Chang, Xingli Zhao, Qi Guo, Shuangchen Chen, Qiaoming Zhang, Yinglong He, Sudan Chen, Ke Chen, Ruiguo Ban, and et al. 2025. "Selenium Improves Yield and Quality in Prunella vulgaris by Regulating Antioxidant Defense, Photosynthesis, Growth, Secondary Metabolites, and Gene Expression Under Acid Stress" Plants 14, no. 6: 920. https://doi.org/10.3390/plants14060920
APA StyleZhang, L., Chang, Q., Zhao, X., Guo, Q., Chen, S., Zhang, Q., He, Y., Chen, S., Chen, K., Ban, R., Hao, Y., & Hou, X. (2025). Selenium Improves Yield and Quality in Prunella vulgaris by Regulating Antioxidant Defense, Photosynthesis, Growth, Secondary Metabolites, and Gene Expression Under Acid Stress. Plants, 14(6), 920. https://doi.org/10.3390/plants14060920







