Supercritical CO2 Extraction of Bioactive Compounds from Corn Grains (Zea mays L., Hybrid Pri-15-7-16) with Metabolomic Profiling and Confocal Laser Microscopy
Abstract
1. Introduction
2. Results
2.1. Extraction of Bioactive Compounds
2.2. Identification of Bioactive Compounds
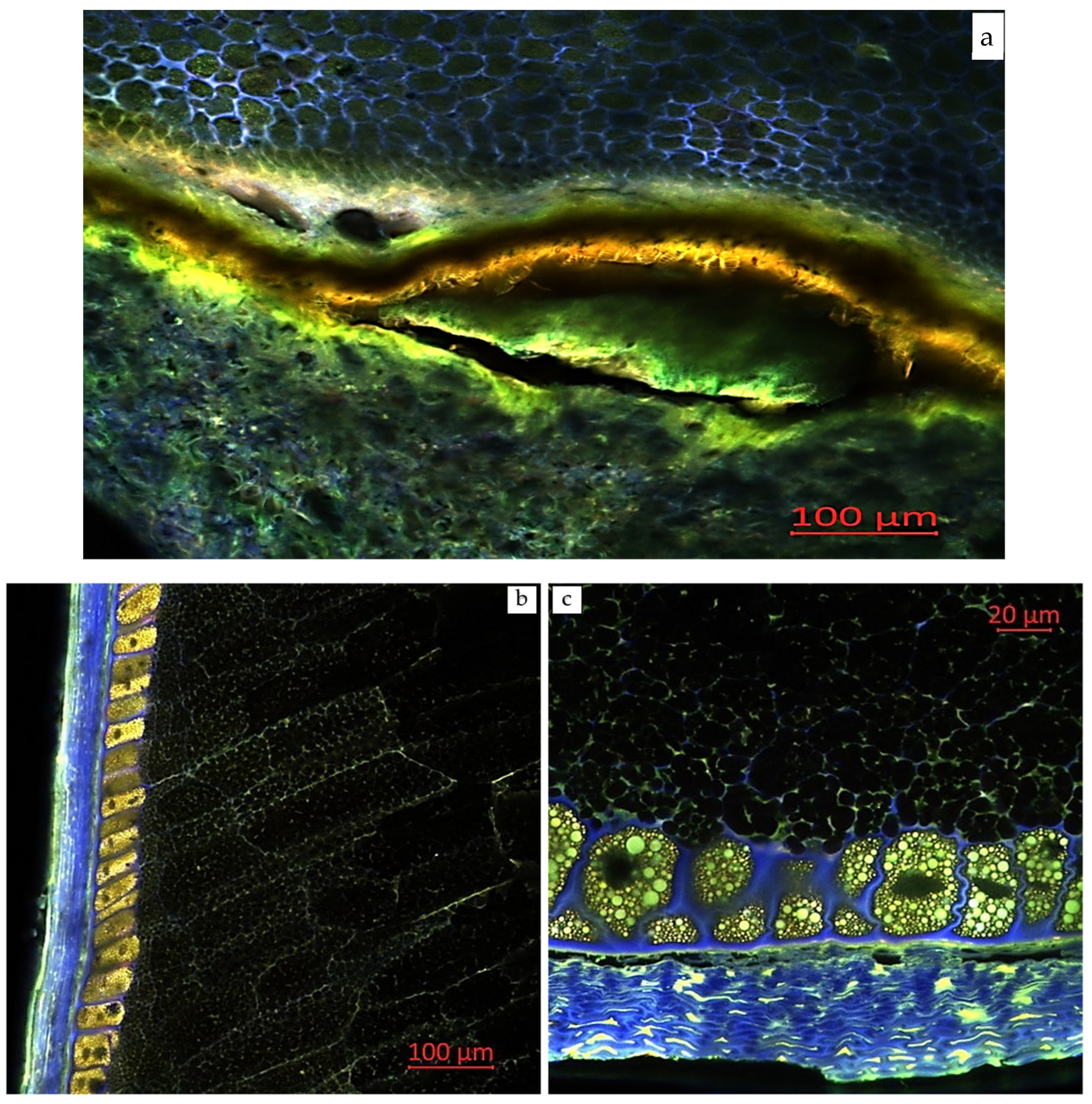
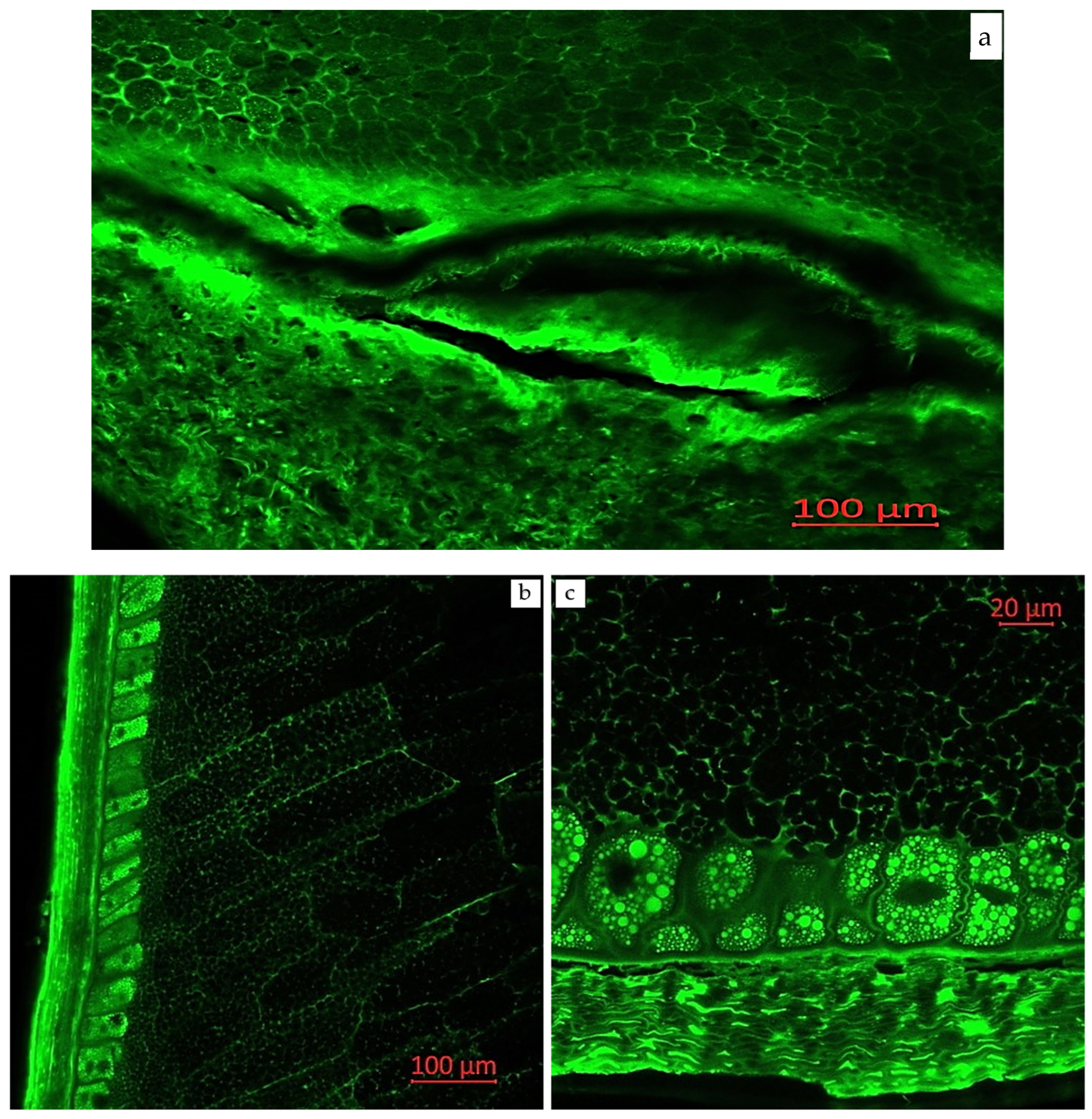
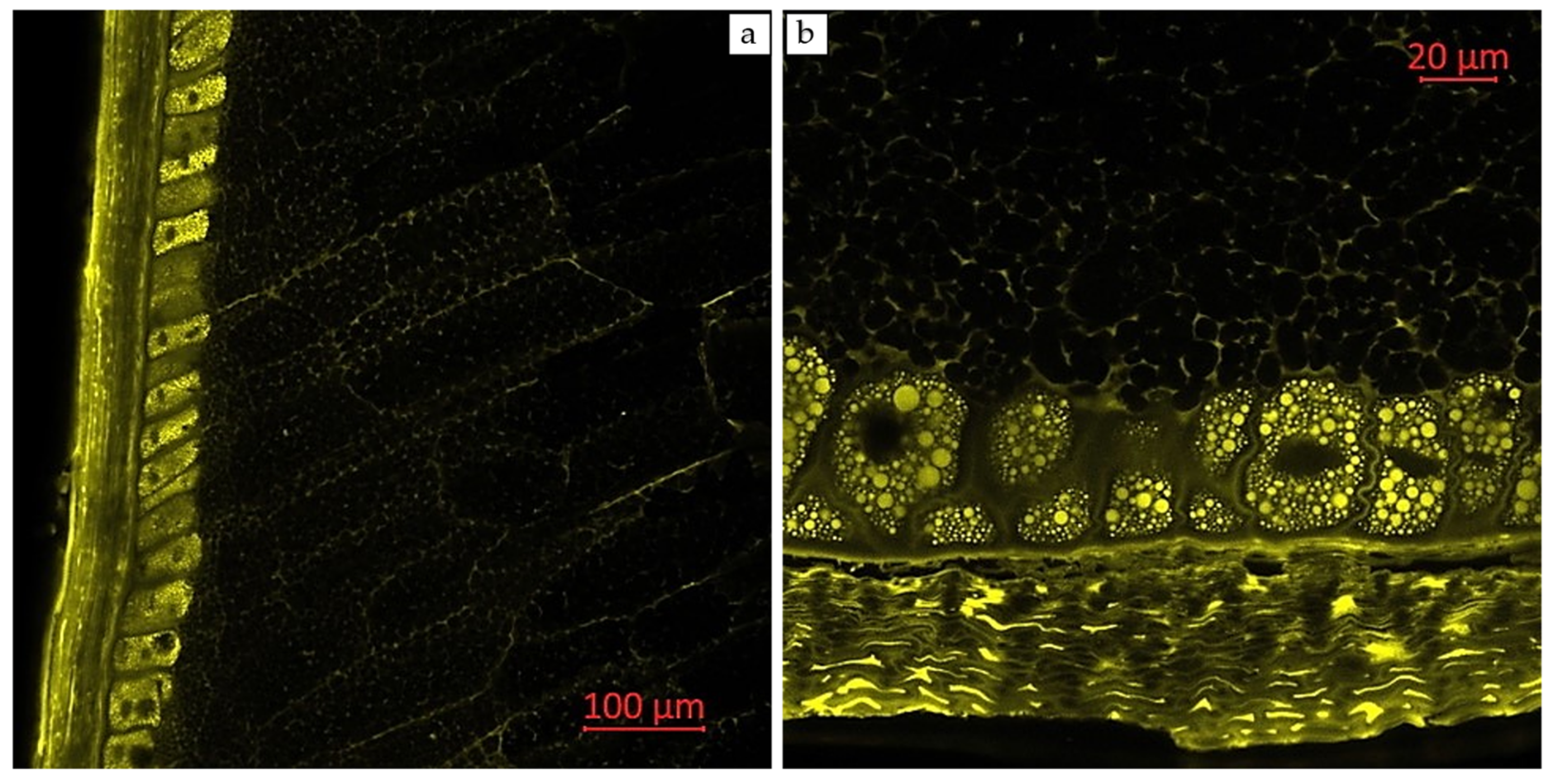
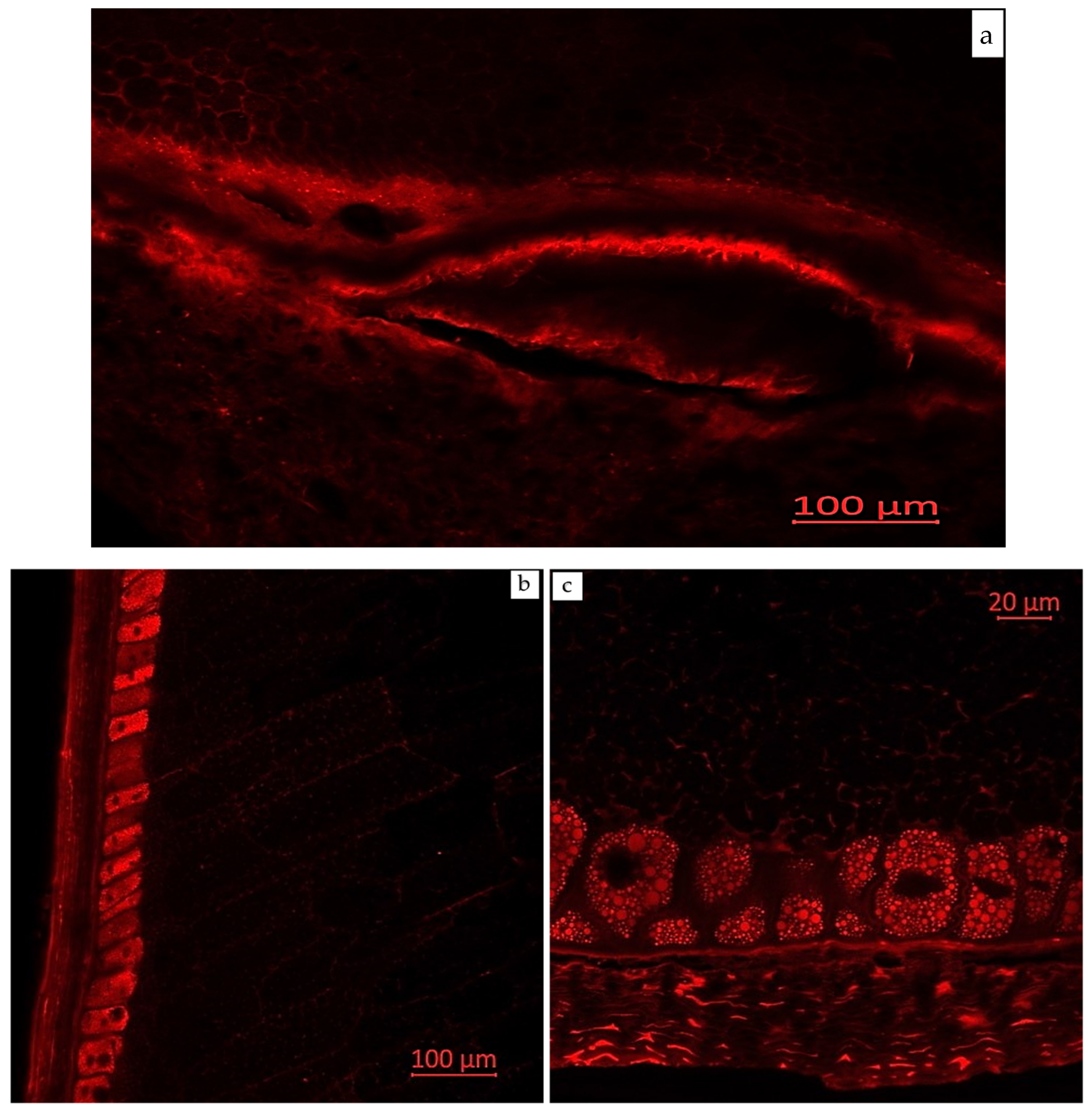
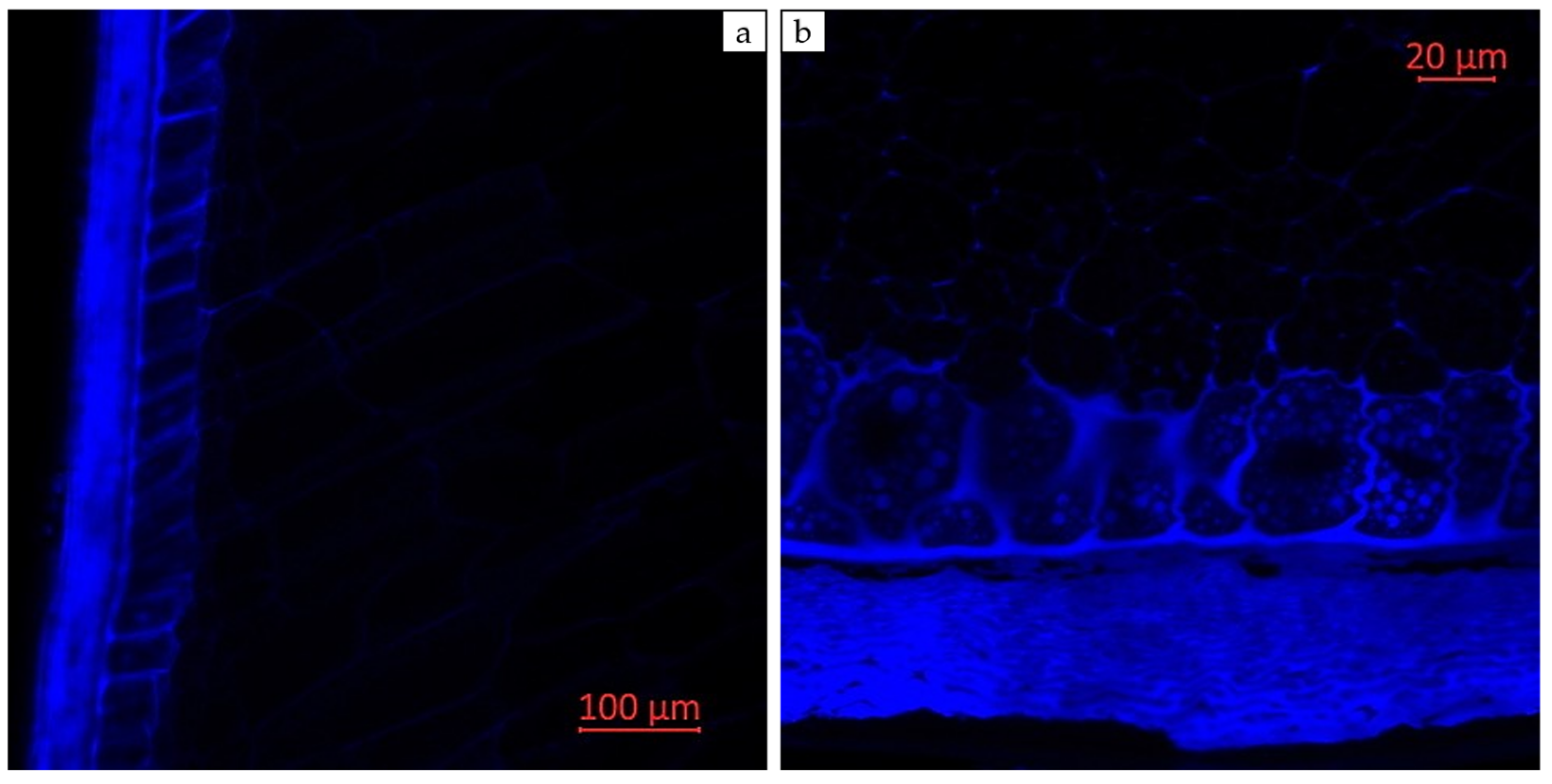
2.3. Visual Localization of Bioactive Compounds
3. Discussion
4. Materials and Methods
4.1. Fractional Maceration
4.2. Supercritical CO2 Extraction
4.3. Liquid Chromatography
4.4. Mass Spectrometry
4.5. Confocal Laser Scanning Microscopy
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Classes of Compounds | Identified Chemical Compounds | Chemical Formula | Molar Mass | Ion Adduct [M−H]− | Ion Adduct [M+H]+ | First-Order Fragmentation MS/MS | Second-Order Fragmentation MS/MS | Third-Order Fragmentation MS/MS | Sources | |
|---|---|---|---|---|---|---|---|---|---|---|
| Polyphenolic Compounds | ||||||||||
| 1 | Polyphenolic acid | Caffeic acid derivative | C16H18O9Na | 377.2985 | 377 | 341; 215 | 179; 113 | [63] | ||
| 2 | Polyphenolic acid | Caffeoylmalic acid | C13H12O8 | 296.2296 | 295 | 277; 171 | 233; 113 | [64,65] | ||
| 3 | Polyphenolic acid | 3,4-Diacetoxybenzoic acid | C10H11O6 | 238.1935 | 237 | 119 | [64,66] | |||
| 4 | Polyphenolic acid | Hydroxy methoxy dimethylbenzoic acid | C10H12O4 | 196.1999 | 197 | 177; 153 | 125 | [67] | ||
| 5 | Polyphenolic acid | Hydroxyferulic acid | C10H10O5 | 210.1834 | 211 | 193; 125 | [68] | |||
| 6 | Polyphenolic acid | Caffeic acid [(2E)-3-(3,4-Dihydroxyphenyl) acrylic acid] | C9H8O4 | 180.1574 | 181 | 135 | 119 | [69,70,71] | ||
| 7 | Stilbene | Resveratrol [trans-Resveratrol; 3,4’,5-Trihydroxystilbene; Stilbentriol] | C14H12O3 | 228.2433 | 229 | 209 | 163 | 146 | [67,72] | |
| 8 | Flavan-3-ol | Epiafzelechin [(epi)Afzelechin] | C15H14O5 | 274.2687 | 275 | 245; 176 | 175 | [67,73,74] | ||
| 9 | Flavonol | Kaempferol [3,5,7-Trihydroxy-2-(4-hydro- xyphenyl)-4H-chromen-4-one] | C15H10O6 | 286.2363 | 285 | 185; 117; 257 | 117 | [64,68,75,76,77] | ||
| 10 | Flavan-3-ol | Catechin [D-Catechol] | C15H14O6 | 290.2681 | 291 | 261; 189 | 173; 242 | 191; 143 | [67,72,78,79,80] | |
| 11 | Flavan-3-ol | (epi)catechin | C15H14O6 | 290.2681 | 291 | 261; 173 | 243; 173 | [67,72,80] | ||
| 12 | Flavonol | Quercetin | C15H10O7 | 302.2357 | 303 | 275; 245; 203; 175 | 175 | [63,64,75,78] | ||
| 13 | Flavan-3-ol | Gallocatechin [+ (-) Gallocatechin] | C15H14O7 | 306.2675 | 307 | 277; 207 | 207; 159 | [67,78,79] | ||
| 14 | Flavonol | Myricetin | C15H10O8 | 318.2351 | 319 | 291; 219; 174 | 259; 191 | 243; 161 | [71,72,77,81,82] | |
| 15 | Flavone | Cirsiliol | C17H14O7 | 330.2889 | 329 | 229; 171; 293 | 211; 155 | 183 | [83] | |
| 16 | Flavone | 5,7-Dimetoxy-3,3’,4’-trihydroxyflavone | C17H14O7 | 330.2889 | 331 | 315; 270 | 313 | 285; 257 | [84] | |
| 17 | Flavone | Luteolin 7,3’-disulphate | C15H10O12S2 | 446.3627 | 447 | 287 | 152 | [85] | ||
| 18 | Flavone | Apigenin 7-sulfate | C15H10O8S | 350.3001 | 351 | 337; 308 | 308; 291 | [67,86] | ||
| 19 | Lignan | Matairesinol [(-)-Matairesinol; Artigenin Congener] | C20H22O6 | 358.3851 | 359 | 324; 289; 127 | 144 | 127 | [87] | |
| 20 | Flavan-3-ol derivative | Epiafzelechin 3-O-gallate | C22H18O9 | 426.3729 | 427 | 301; 171; 382 | 171 | [80] | ||
| 21 | Flavone | Apigenin-C-hexoside | C21H20O10 | 432.3775 | 433 | 418; 314; 265; 219; 155 | 257; 169 | [88] | ||
| 22 | Anthocyanidin | Pelargonidin-3-O-glucoside (callistephin) | C21H21O10 | 433.3854 | 433 | 271; 185 | 253; 121 | 235 | [89,90] | |
| 23 | Anthocyanidin | Cyanidin-3-O-glucoside [Cyanidin 3-O-beta-D-Glucoside; Kuromarin] | C21H21O11+ | 449.3848 | 447 | 285 | 199 | [90,91,92] | ||
| 24 | Flavone | Luteolin-7-O-beta-glucuronide | C21H18O12 | 462.3604 | 463 | 447; 395; 359; 285; 199; 149 | 287; 199 | [93,94] | ||
| 25 | Flavonol | Kaempferol-3-O-glucuronide | C21H18O12 | 462.3604 | 463 | 287; 198 | 269; 198 | [65,67,75] | ||
| 26 | Anthocyanidin | Delphinidin malonyl hexoside | C24H23O15 | 551.4304 | 551 | 465; 287; 185 | 287; 115 | [67] | ||
| 27 | Flavone | Chrysoeriol C-hexoside-C-pentoside | C27H30O15 | 594.5181 | 595 | 578; 536; 509; 425 | 294 | [66] | ||
| 28 | Flavonol | Quercetin 3,4’-di-O-beta-glucopyranoside [Quercetin diglucoside] | C27H30O17 | 626.5169 | 627 | 465 | 447; 405; 303 | [64,77,92] | ||
| 29 | Flavone | Tricin trimethyl ether 7-O-hexosyl-hexoside | C30H36O17 | 668.5966 | 669 | 345; 387; 283 | [95] | |||
| 30 | Flavan-3-ol | (Epi)fisetinidol-(epi)catechin-A-(epi)fisetinidol | C45H36O16 | 832.7577 | 831 | 721; 693; 609; 575; 537; 506 | [96] | |||
| Compounds of Other Chemical Groups | ||||||||||
| 31 | Amino acid | L-Lysine | C6H14N2O2 | 146.1876 | 147 | 119 | [76] | |||
| 32 | Amino acid | L-threanine | C7H14N2O3 | 174.1977 | 175 | 159 | [80] | |||
| 33 | Amino acid | L-Tryptophan [Tryptophan; (S)-Tryptophan] | C11H12N2O2 | 204.2252 | 205 | 161; 159 | 143 | [80,97] | ||
| 34 | Omega-5 fatty acid | Myristoleic acid [Cis-9-Tetradecanoic acid] | C14H26O2 | 226.3550 | 227 | 209; 168 | 127 | [67] | ||
| 35 | Medium-chain fatty acid | Hydroxy dodecanoic acid | C12H22O5 | 246.3001 | 247 | 238 | 203 | 174 | [67] | |
| 36 | Omega-3 fatty acid | Stearidonic acid [6,9,12,15-Octadecatetraenoic acid; Moroctic acid] | C18H28O2 | 276.4137 | 277 | 259; 177 | 177 | [67,75,98] | ||
| 37 | Omega-3 fatty acid | Linolenic acid (Alpha-Linolenic acid; Linolenate) | C18H30O2 | 278.4296 | 279 | 243; 173 | 173 | 131 | [98,99] | |
| 38 | Diterpenoid | Isocryptotanshinone II | C19H20O3 | 296.3603 | 297 | 279; 197 | 173 | [98] | ||
| 39 | Alpha-omega Dicarboxylic acid | Octadecanedioic acid [1,16-Hexadecanedicarboxylic acid] | C18H34O4 | 314.4602 | 313 | 295; 183 | 293; 179 | 275; 177 | [67] | |
| 40 | Unsaturated fatty acid | Oxo-eicosatetraenoic acid | C20H30O3 | 318.4504 | 319 | 301 | 186 | [67] | ||
| 41 | Oxylipin | 13-Trihydroxy-Octadecenoic acid [THODE] | C18H34O5 | 330.4596 | 329 | 171; 211; 293 | 153 | [100,101] | ||
| 42 | Oxylipin | 9,12,13-Trihydroxy-trans-10-octadecenoic acid | C18H34O5 | 330.4596 | 329 | 171; 229 | 127 | [64] | ||
| 43 | Carotenoid | (all-E)-lutein 3’-O-myristate | C40H54O | 550.8562 | 551 | 533; 505; 469;429; 373; 345 | 453; 410 | [102] | ||
| 44 | Unsaturated fatty acid | Eicosatetraenedioic acid | C20H30O4 | 334.4498 | 335 | 321; 124 | 291 | [67] | ||
References
- Mora-Gutierrez, A.; Núñez de González, M.T.; Woldesenbet, S.; Attaie, R.; Jung, Y. Influence of Deliverable Form of Dietary Vitamin D3 on the Immune Response in Late-Lactating Dairy Goats. Dairy 2024, 5, 308–315. [Google Scholar] [CrossRef]
- Zafalon, R.V.A.; Ruberti, B.; Rentas, M.F.; Amaral, A.R.; Vendramini, T.H.A.; Chacar, F.C.; Kogika, M.M.; Brunetto, M.A. The Role of Vitamin D in Small Animal Bone Metabolism. Metabolites 2020, 10, 496. [Google Scholar] [CrossRef]
- Szymczak, I.; Pawliczak, R. The Active Metabolite of Vitamin D3 as a Potential Immunomodulator. Scand. J. Immunol. 2016, 83, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Šimoliūnas, E.; Rinkūnaitė, I.; Bukelskienė, Ž.; Bukelskienė, V. Bioavailability of Different Vitamin D Oral Supplements in Laboratory Animal Model. Medicina 2019, 55, 265. [Google Scholar] [CrossRef]
- Jäpelt, R.B.; Jakobsen, J. Vitamin D in Plants: A Review of Occurrence, Analysis, and Biosynthesis. Front. Plant Sci. 2013, 4, 136. [Google Scholar] [CrossRef]
- Azevedo, M.A.; Cerqueira, M.A.; Gonçalves, C.; Amado, I.R.; Teixeira, J.A.; Pastrana, L. Encapsulation of Vitamin D3 Using Rhamnolipids-Based Nanostructured Lipid Carriers. Food Chem. 2023, 427, 136654. [Google Scholar] [CrossRef] [PubMed]
- Peñuñuri-Pacheco, N.; Moreno-García, Y.A.; González-Ríos, H.; Astiazarán-García, H.; López-Franco, Y.L.; Tortoledo-Ortiz, O.; Pérez-Báez, A.J.; Dávila-Ramírez, J.L.; Lizardi-Mendoza, J.; Valenzuela-Melendres, M. Optimization of the Encapsulation of Vitamin D3 in Oil in Water Nanoemulsions: Preliminary Application in a Functional Meat Model System. Foods 2024, 13, 2842. [Google Scholar] [CrossRef]
- Dahiya, D.; Terpou, A.; Dasenaki, M.; Nigam, P.S. Current Status and Future Prospects of Bioactive Molecules Delivered through Sustainable Encapsulation Techniques for Food Fortification. Sustain. Food Technol. 2023, 1, 500–510. [Google Scholar] [CrossRef]
- Aggeletopoulou, I.; Kalafateli, M.; Geramoutsos, G.; Triantos, C. Recent Advances in the Use of Vitamin D Organic Nanocarriers for Drug Delivery. Biomolecules 2024, 14, 1090. [Google Scholar] [CrossRef]
- Razgonova, M.; Zinchenko, Y.; Pikula, K.; Tekutyeva, L.; Son, O.; Zakharenko, A.; Kalenik, T.; Golokhvast, K. Spatial Distribution of Polyphenolic Compounds in Corn Grains (Zea mays L. var. Pioneer) Studied by Laser Confocal Microscopy and High-Resolution Mass Spectrometry. Plants 2022, 11, 630. [Google Scholar] [CrossRef]
- Razgonova, M.P.; Navaz, M.A.; Sabitov, A.S.; Zinchenko, Y.N.; Rusakova, E.A.; Petrusha, E.N.; Golokhvast, K.S.; Tikhonova, N.G. The Global Metabolome Profiles of Four Varieties of Lonicera caerulea, Established via Tandem Mass Spectrometry. Horticulturae 2023, 9, 1188. [Google Scholar] [CrossRef]
- Waqas, M.; Salman, M.; Sharif, M.S. Application of Polyphenolic Compounds in Animal Nutrition and Their Promising Effects. J. Anim. Feed Sci. 2023, 32, 233–256. [Google Scholar] [CrossRef]
- Godlewska, K.; Pacyga, P.; Szumny, A.; Szymczycha-Madeja, A.; Wełna, M.; Michalak, I. Methods for Rapid Screening of Biologically Active Compounds Present in Plant-Based Extracts. Molecules 2022, 27, 7094. [Google Scholar] [CrossRef] [PubMed]
- Tyśkiewicz, K.; Konkol, M.; Rój, E. The Application of Supercritical Fluid Extraction in Phenolic Compounds Isolation from Natural Plant Materials. Molecules 2018, 23, 2625. [Google Scholar] [CrossRef]
- Serra, V.; Salvatori, G.; Pastorelli, G. Dietary Polyphenol Supplementation in Food Producing Animals: Effects on the Quality of Derived Products. Animals 2021, 11, 401. [Google Scholar] [CrossRef]
- Salinas-Moreno, Y.; García-Salinas, C.; Ramírez-Díaz, J.L.; Alemán-de la Torre, I. Phenolic Compounds in Maize Grains and Its Nixtamalized Products. In Phenolic Compounds-Natural Sources, Importance and Applications; InTech: Hong Kong, China, 2017. [Google Scholar]
- Siyuan, S.; Tong, L.; Liu, R. Corn Phytochemicals and Their Health Benefits. Food Sci. Hum. Wellness 2018, 7, 185–195. [Google Scholar] [CrossRef]
- Kim, J.-T.; Chung, I.-M.; Kim, M.-J.; Lee, J.-S.; Son, B.-Y.; Bae, H.-H.; Go, Y.S.; Kim, S.-L.; Baek, S.-B.; Kim, S.-H.; et al. Comparison of Antioxidant Activity Assays in Fresh Purple Waxy Corn (Zea mays L.) during Grain Filling. Appl. Biol. Chem. 2022, 65, 1. [Google Scholar] [CrossRef]
- Chen, L.; Guo, Y.; Li, X.; Gong, K.; Liu, K. Phenolics and Related in Vitro Functional Activities of Different Varieties of Fresh Waxy Corn: A Whole Grain. BMC Chem. 2021, 15, 14. [Google Scholar] [CrossRef]
- Hu, Q.; Xu, J. Profiles of Carotenoids, Anthocyanins, Phenolics, and Antioxidant Activity of Selected Color Waxy Corn Grains during Maturation. J. Agric. Food Chem. 2011, 59, 2026–2033. [Google Scholar] [CrossRef]
- Piazza, S.; Bani, C.; Colombo, F.; Mercogliano, F.; Pozzoli, C.; Martinelli, G.; Petroni, K.; Roberto Pilu, S.; Sonzogni, E.; Fumagalli, M.; et al. Pigmented Corn as a Gluten-Free Source of Polyphenols with Anti-Inflammatory and Antioxidant Properties in CaCo-2 Cells. Food Res. Int. 2024, 191, 114640. [Google Scholar] [CrossRef]
- Feregrino-Pérez, A.A.; Mercado-Luna, A.; Murillo-Cárdenas, C.A.; González-Santos, R.; Chávez-Servín, J.L.; Vargas-Madriz, A.F.; Luna-Sánchez, E. Polyphenolic Compounds and Antioxidant Capacity in Native Maize of the Sierra Gorda of Querétaro. Agronomy 2024, 14, 142. [Google Scholar] [CrossRef]
- Chen, Z.; Świsłocka, R.; Choińska, R.; Marszałek, K.; Dąbrowska, A.; Lewandowski, W.; Lewandowska, H. Exploring the Correlation Between the Molecular Structure and Biological Activities of Metal–Phenolic Compound Complexes: Research and Description of the Role of Metal Ions in Improving the Antioxidant Activities of Phenolic Compounds. Int. J. Mol. Sci. 2024, 25, 11775. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-Antioxidant Activity Relationship of Methoxy, Phenolic Hydroxyl, and Carboxylic Acid Groups of Phenolic Acids. Sci. Rep. 2020, 10, 2611. [Google Scholar] [CrossRef]
- Kuś, P.M.; Okińczyc, P.; Jakovljević, M.; Jokić, S.; Jerković, I. Development of Supercritical CO2 Extraction of Bioactive Phytochemicals from Black Poplar (Populus nigra L.) Buds Followed by GC–MS and UHPLC-DAD-QqTOF-MS. J. Pharm. Biomed. Anal. 2018, 158, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Razgonova, M.P.; Nawaz, M.A.; Ivanova, E.P.; Cherevach, E.I.; Golokhvast, K.S. Supercritical CO2-Based Extraction and Detection of Phenolic Compounds and Saponins from the Leaves of Three Medicago varia Mart. Varieties by Tandem Mass Spectrometry. Processes 2024, 12, 1041. [Google Scholar] [CrossRef]
- Kuś, P.; Jerković, I.; Jakovljević, M.; Jokić, S. Extraction of Bioactive Phenolics from Black Poplar (Populus nigra L.) Buds by Supercritical CO2 and Its Optimization by Response Surface Methodology. J. Pharm. Biomed. Anal. 2018, 152, 128–136. [Google Scholar] [CrossRef]
- Razgonova, M.; Zakharenko, A.; Shin, T.-S.; Chung, G.; Golokhvast, K. Supercritical CO2 Extraction and Identification of Ginsenosides in Russian and North Korean Ginseng by HPLC with Tandem Mass Spectrometry. Molecules 2020, 25, 1407. [Google Scholar] [CrossRef]
- Razgonova, M.P.; Cherevach, E.I.; Tekutyeva, L.A.; Fedoreyev, S.A.; Mishchenko, N.P.; Tarbeeva, D.V.; Demidova, E.N.; Kirilenko, N.S.; Golokhvast, K. Maackia Amurensis Rupr. et Maxim.: Supercritical CO2 Extraction and Mass Spectrometric Characterization of Chemical Constituents. Molecules 2023, 28, 2026. [Google Scholar] [CrossRef]
- Zakharenko, A.M.; Razgonova, M.P.; Pikula, K.S.; Golokhvast, K.S. Simultaneous Determination of 78 Compounds of Rhodiola rosea Extract by Supercritical CO2-Extraction and HPLC-ESI-MS/MS Spectrometry. Biochem. Res. Int. 2021, 2021, 9957490. [Google Scholar] [CrossRef]
- Essien, S.O.; Young, B.; Baroutian, S. Recent Advances in Subcritical Water and Supercritical Carbon Dioxide Extraction of Bioactive Compounds from Plant Materials. Trends Food Sci. Technol. 2020, 97, 156–169. [Google Scholar] [CrossRef]
- Pimentel-Moral, S.; Borrás-Linares, I.; Lozano-Sánchez, J.; Arráez-Román, D.; Martínez-Férez, A.; Segura-Carretero, A. Supercritical CO2 Extraction of Bioactive Compounds from Hibiscus Sabdariffa. J. Supercrit. Fluids 2019, 147, 213–221. [Google Scholar] [CrossRef]
- Molino, A.; Larocca, V.; Di Sanzo, G.; Martino, M.; Casella, P.; Marino, T.; Karatza, D.; Musmarra, D. Extraction of Bioactive Compounds Using Supercritical Carbon Dioxide. Molecules 2019, 24, 782. [Google Scholar] [CrossRef]
- Villacís-Chiriboga, J.; Voorspoels, S.; Uyttebroek, M.; Ruales, J.; Van Camp, J.; Vera, E.; Elst, K. Supercritical CO2 Extraction of Bioactive Compounds from Mango (Mangifera indica L.) Peel and Pulp. Foods 2021, 10, 2201. [Google Scholar] [CrossRef]
- Razgonova, M.; Zakharenko, A.; Ercisli, S.; Grudev, V.; Golokhvast, K. Comparative Analysis of Far East Sikhotinsky Rhododendron (Rh. sichotense) and East Siberian Rhododendron (Rh. adamsii) Using Supercritical CO2-Extraction and HPLC-ESI-MS/MS Spectrometry. Molecules 2020, 25, 3774. [Google Scholar] [CrossRef] [PubMed]
- Razgonova, M.P.; Tekutyeva, L.A.; Podvolotskaya, A.B.; Stepochkina, V.D.; Zakharenko, A.M.; Golokhvast, K. Zostera marina L.: Supercritical CO2-Extraction and Mass Spectrometric Characterization of Chemical Constituents Recovered from Seagrass. Separations 2022, 9, 182. [Google Scholar] [CrossRef]
- Marinho, C.M.; Lemos, C.O.T.; Arvelos, S.; de Souza Barrozo, M.A.; Hori, C.E.; Watanabe, É.O. Extraction of Corn Germ Oil with Supercritical CO2 and Cosolvents. J. Food Sci. Technol. 2019, 56, 4448–4456. [Google Scholar] [CrossRef]
- Monroy, Y.M.; Rodrigues, R.A.F.; Sartoratto, A.; Cabral, F.A. Optimization of the Extraction of Phenolic Compounds from Purple Corn Cob (Zea mays L.) by Sequential Extraction Using Supercritical Carbon Dioxide, Ethanol and Water as Solvents. J. Supercrit. Fluids 2016, 116, 10–19. [Google Scholar] [CrossRef]
- Razgonova, M.P.; Zakharenko, A.M.; Golokhvast, K.S. Investigation of the Supercritical CO2 Extracts of Wild Ledum palustre L. (Rhododendron tomentosum Harmaja) and Identification of Its Metabolites by Tandem Mass Spectrometry. Russ. J. Bioorganic Chem. 2023, 49, 1645–1657. [Google Scholar] [CrossRef]
- Berger, M.; Devaux, M.-F.; Legland, D.; Barron, C.; Delord, B.; Guillon, F. Darkfield and Fluorescence Macrovision of a Series of Large Images to Assess Anatomical and Chemical Tissue Variability in Whole Cross-Sections of Maize Stems. Front. Plant Sci. 2021, 12, 792981. [Google Scholar] [CrossRef]
- Corcel, M.; Devaux, M.-F.; Guillon, F.; Barron, C. Comparison of UV and Visible Autofluorescence of Wheat Grain Tissues in Macroscopic Images of Cross-Sections and Particles. Comput. Electron. Agric. 2016, 127, 281–288. [Google Scholar] [CrossRef]
- Razgonova, M.P.; Zinchenko, Y.N.; Kozak, D.K.; Kuznetsova, V.A.; Zakharenko, A.M.; Ercisli, S.; Golokhvast, K.S. Autofluorescence-Based Investigation of Spatial Distribution of Phenolic Compounds in Soybeans Using Confocal Laser Microscopy and a High-Resolution Mass Spectrometric Approach. Molecules 2022, 27, 8228. [Google Scholar] [CrossRef] [PubMed]
- Razgonova, M.P.; Burlyaeva, M.O.; Zinchenko, Y.N.; Krylova, E.A.; Chunikhina, O.A.; Ivanova, N.M.; Zakharenko, A.M.; Golokhvast, K.S. Identification and Spatial Distribution of Bioactive Compounds in Seeds Vigna unguiculata (L.) Walp. by Laser Microscopy and Tandem Mass Spectrometry. Plants 2022, 11, 2147. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, L.; Radotić, K.; Kalauzi, A.; Djikanović, D.; Jeremić, M. Quantification of Compression Wood Severity in Tracheids of Pinus Radiata D. Don Using Confocal Fluorescence Imaging and Spectral Deconvolution. J. Struct. Biol. 2010, 169, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, L. Softwood and Hardwood Lignin Fluorescence Spectra of Wood Cell Walls in Different Mounting Media. IAWA J. 2013, 34, 3–19. [Google Scholar] [CrossRef]
- Donaldson, L. Autofluorescence in Plants. Molecules 2020, 25, 2393. [Google Scholar] [CrossRef]
- Donaldson, L.; Williams, N. Imaging and Spectroscopy of Natural Fluorophores in Pine Needles. Plants 2018, 7, 10. [Google Scholar] [CrossRef]
- Roshchina, V.V.; Kuchin, A.V.; Yashin, V.A. Application of Autofluorescence for Analysis of Medicinal Plants. Int. J. Spectrosc. 2017, 2017, 7159609. [Google Scholar] [CrossRef]
- Sudo, E.; Teranishi, M.; Hidema, J.; Taniuchi, T. Visualization of Flavonol Distribution in the Abaxial Epidermis of Onion Scales via Detection of Its Autofluorescence in the Absence of Chemical Processes. Biosci. Biotechnol. Biochem. 2009, 73, 2107–2109. [Google Scholar] [CrossRef]
- Monago-Maraña, O.; Durán-Merás, I.; Galeano-Díaz, T.; Muñoz de la Peña, A. Fluorescence Properties of Flavonoid Compounds. Quantification in Paprika Samples Using Spectrofluorimetry Coupled to Second Order Chemometric Tools. Food Chem. 2016, 196, 1058–1065. [Google Scholar] [CrossRef]
- Talamond, P.; Verdeil, J.-L.; Conéjéro, G. Secondary Metabolite Localization by Autofluorescence in Living Plant Cells. Molecules 2015, 20, 5024–5037. [Google Scholar] [CrossRef]
- Mackon, E.; Ma, Y.; Jeazet Dongho Epse Mackon, G.C.; Li, Q.; Zhou, Q.; Liu, P. Subcellular Localization and Vesicular Structures of Anthocyanin Pigmentation by Fluorescence Imaging of Black Rice (Oryza sativa L.) Stigma Protoplast. Plants 2021, 10, 685. [Google Scholar] [CrossRef] [PubMed]
- Collings, D.A. Anthocyanin in the Vacuole of Red Onion Epidermal Cells Quenches Other Fluorescent Molecules. Plants 2019, 8, 596. [Google Scholar] [CrossRef] [PubMed]
- Corcel, M.; Devaux, M.-F.; Guillon, F.; Barron, C. Identification of Tissular Origin of Particles Based on Autofluorescence Multispectral Image Analysis at the Macroscopic Scale. EPJ Web Conf. 2017, 140, 05012. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Schweiger, J. Cell Wall Bound Ferulic Acid, the Major Substance of the Blue-Green Fluorescence Emission of Plants. J. Plant Physiol. 1998, 152, 272–282. [Google Scholar] [CrossRef]
- Rudall, P. Investigation of the Presence of Phenolic Compounds in Monocotyledonous Cell Walls, Using UV Fluorescence Microscopy. Ann. Bot. 1994, 74, 483–491. [Google Scholar] [CrossRef]
- Antoine, C.; Peyron, S.; Mabille, F.; Lapierre, C.; Bouchet, B.; Abecassis, J.; Rouau, X. Individual Contribution of Grain Outer Layers and Their Cell Wall Structure to the Mechanical Properties of Wheat Bran. J. Agric. Food Chem. 2003, 51, 2026–2033. [Google Scholar] [CrossRef]
- Vanholme, R.; De Meester, B.; Ralph, J.; Boerjan, W. Lignin Biosynthesis and Its Integration into Metabolism. Curr. Opin. Biotechnol. 2019, 56, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Philippe, S.; Tranquet, O.; Utille, J.-P.; Saulnier, L.; Guillon, F. Investigation of Ferulate Deposition in Endosperm Cell Walls of Mature and Developing Wheat Grains by Using a Polyclonal Antibody. Planta 2007, 225, 1287–1299. [Google Scholar] [CrossRef]
- Das, A.K.; Singh, V. Antioxidative Free and Bound Phenolic Constituents in Pericarp, Germ and Endosperm of Indian Dent (Zea mays var. indentata) and Flint (Zea mays var. indurata) Maize. J. Funct. Foods 2015, 13, 363–374. [Google Scholar] [CrossRef]
- Hernanz, D.; Nuñez, V.; Sancho, A.I.; Faulds, C.B.; Williamson, G.; Bartolomé, B.; Gómez-Cordovés, C. Hydroxycinnamic Acids and Ferulic Acid Dehydrodimers in Barley and Processed Barley. J. Agric. Food Chem. 2001, 49, 4884–4888. [Google Scholar] [CrossRef]
- Rhodes, D.I.; Sadek, M.; Stone, B.A. Hydroxycinnamic Acids in Walls of Wheat Aleurone Cells. J. Cereal Sci. 2002, 36, 67–81. [Google Scholar] [CrossRef]
- El-sayed, M.; Abbas, F.; Refaat, S.; El-Shafae, A.; Fikry, E. UPLC-ESI-MS/MS Profile of The Ethyl Acetate Fraction of Aerial Parts of Bougainvillea “Scarlett O’Hara” Cultivated in Egypt. Egypt. J. Chem. 2020, 64, 793–806. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, C.; Gómez-Caravaca, A.M.; Guerra-Hernández, E.; Cerretani, L.; García-Villanova, B.; Verardo, V. Comprehensive Metabolite Profiling of Solanum tuberosum L. (Potato) Leaves by HPLC-ESI-QTOF-MS. Food Res. Int. 2018, 112, 390–399. [Google Scholar] [CrossRef]
- Spínola, V.; Pinto, J.; Castilho, P.C. Identification and Quantification of Phenolic Compounds of Selected Fruits from Madeira Island by HPLC-DAD–ESI-MSn and Screening for Their Antioxidant Activity. Food Chem. 2015, 173, 14–30. [Google Scholar] [CrossRef]
- Stallmann, J.; Schweiger, R.; Pons, C.A.A.; Müller, C. Wheat Growth, Applied Water Use Efficiency and Flag Leaf Metabolome under Continuous and Pulsed Deficit Irrigation. Sci. Rep. 2020, 10, 10112. [Google Scholar] [CrossRef] [PubMed]
- Hamed, A.R.; El-Hawary, S.S.; Ibrahim, R.M.; Abdelmohsen, U.R.; El-Halawany, A.M. Identification of Chemopreventive Components from Halophytes Belonging to Aizoaceae and Cactaceae Through LC/MS—Bioassay Guided Approach. J. Chromatogr. Sci. 2021, 59, 618–626. [Google Scholar] [CrossRef]
- Aita, S.; Capriotti, A.; Cavaliere, C.; Cerrato, A.; Giannelli Moneta, B.; Montone, C.; Piovesana, S.; Laganà, A. Andean Blueberry of the Genus Disterigma: A High-Resolution Mass Spectrometric Approach for the Comprehensive Characterization of Phenolic Compounds. Separations 2021, 8, 58. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Freire, C.S.R.; Domingues, M.R.M.; Silvestre, A.J.D.; Neto, C.P. Characterization of Phenolic Components in Polar Extracts of Eucalyptus Globulus Labill. Bark by High-Performance Liquid Chromatography–Mass Spectrometry. J. Agric. Food Chem. 2011, 59, 9386–9393. [Google Scholar] [CrossRef]
- Domingues, R.M.A.; Sousa, G.D.A.; Silva, C.M.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. High Value Triterpenic Compounds from the Outer Barks of Several Eucalyptus Species Cultivated in Brazil and in Portugal. Ind. Crops Prod. 2011, 33, 158–164. [Google Scholar] [CrossRef]
- Jiang, R.-W.; Lau, K.-M.; Hon, P.-M.; Mak, T.; Woo, K.-S.; Fung, K.-P. Chemistry and Biological Activities of Caffeic Acid Derivatives from Salvia Miltiorrhiza. Curr. Med. Chem. 2005, 12, 237–246. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, J.; Gao, X.; Amponsem, E.; Kang, L.; Hu, L.; Zhang, B.; Chang, Y. Simultaneous Determination of Stilbenes, Phenolic Acids, Flavonoids and Anthraquinones in Radix Polygoni Multiflori by LC–MS/MS. J. Pharm. Biomed. Anal. 2012, 62, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Marcía-Fuentes, J.; Santos-Aleman, R.; Borrás-Linares, I.; Sánchez, J.L. The Carao (Cassia grandis L.): Its Potential Usage in Pharmacological, Nutritional, and Medicinal Applications. In Innovations in Biotechnology for a Sustainable Future; Springer International Publishing: Cham, Switzerland, 2021; pp. 403–427. [Google Scholar]
- Sobeh, M.; Mahmoud, M.F.; Abdelfattah, M.A.O.; Cheng, H.; El-Shazly, A.M.; Wink, M. A Proanthocyanidin-Rich Extract from Cassia abbreviata Exhibits Antioxidant and Hepatoprotective Activities In Vivo. J. Ethnopharmacol. 2018, 213, 38–47. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Ali-Shtayeh, M.S.; Jamous, R.M.; Arráez-Román, D.; Segura-Carretero, A. HPLC–DAD–ESI-MS/MS Screening of Bioactive Components from Rhus coriaria L. (Sumac) Fruits. Food Chem. 2015, 166, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Wang, C.; Zou, L.; Liu, X.; Chen, J.; Tan, M.; Mei, Y.; Wei, L. Comparison of Multiple Bioactive Constituents in the Flower and the Caulis of Lonicera Japonica Based on UFLC-QTRAP-MS/MS Combined with Multivariate Statistical Analysis. Molecules 2019, 24, 1936. [Google Scholar] [CrossRef] [PubMed]
- Oertel, A.; Matros, A.; Hartmann, A.; Arapitsas, P.; Dehmer, K.J.; Martens, S.; Mock, H.-P. Metabolite Profiling of Red and Blue Potatoes Revealed Cultivar and Tissue Specific Patterns for Anthocyanins and Other Polyphenols. Planta 2017, 246, 281–297. [Google Scholar] [CrossRef]
- Chandrasekara, A.; Shahidi, F. Bioactivities and Antiradical Properties of Millet Grains and Hulls. J. Agric. Food Chem. 2011, 59, 9563–9571. [Google Scholar] [CrossRef]
- Sobeh, M.; Mahmoud, M.; Hasan, R.; Cheng, H.; El-Shazly, A.; Wink, M. Senna Singueana: Antioxidant, Hepatoprotective, Antiapoptotic Properties and Phytochemical Profiling of a Methanol Bark Extract. Molecules 2017, 22, 1502. [Google Scholar] [CrossRef]
- Qin, D.; Wang, Q.; Li, H.; Jiang, X.; Fang, K.; Wang, Q.; Li, B.; Pan, C.; Wu, H. Identification of Key Metabolites Based on Non-Targeted Metabolomics and Chemometrics Analyses Provides Insights into Bitterness in Kucha [Camellia kucha (Chang et Wang) Chang]. Food Res. Int. 2020, 138, 109789. [Google Scholar] [CrossRef]
- Zhou, X.-J.; Yan, L.-L.; Yin, P.-P.; Shi, L.-L.; Zhang, J.-H.; Liu, Y.-J.; Ma, C. Structural Characterisation and Antioxidant Activity Evaluation of Phenolic Compounds from Cold-Pressed Perilla frutescens var. arguta Seed Flour. Food Chem. 2014, 164, 150–157. [Google Scholar] [CrossRef]
- Paudel, L.; Wyzgoski, F.J.; Scheerens, J.C.; Chanon, A.M.; Reese, R.N.; Smiljanic, D.; Wesdemiotis, C.; Blakeslee, J.J.; Riedl, K.M.; Rinaldi, P.L. Nonanthocyanin Secondary Metabolites of Black Raspberry (Rubus occidentalis L.) Fruits: Identification by HPLC-DAD, NMR, HPLC-ESI-MS, and ESI-MS/MS Analyses. J. Agric. Food Chem. 2013, 61, 12032–12043. [Google Scholar] [CrossRef]
- Pandey, V.; Ansari, M.W.; Tula, S.; Sahoo, R.K.; Bains, G.; Kumar, J.; Tuteja, N.; Shukla, A. Ocimum Sanctum Leaf Extract Induces Drought Stress Tolerance in Rice. Plant Signal Behav. 2016, 11, e1150400. [Google Scholar] [CrossRef]
- Prasad Pandey, B.; Prakash Pradhan, S.; Adhikari, K. LC-ESI-QTOF-MS for the Profiling of the Metabolites and In Vitro Enzymes Inhibition Activity of Bryophyllum pinnatum and Oxalis corniculata Collected from Ramechhap District of Nepal. Chem. Biodivers. 2020, 17, e2000155. [Google Scholar] [CrossRef] [PubMed]
- Enerstvedt, K.H.; Jordheim, M.; Andersen, Ø.M. Isolation and Identification of Flavonoids Found in Zostera marina Collected in Norwegian Coastal Waters. Am. J. Plant Sci. 2016, 7, 1163–1172. [Google Scholar] [CrossRef]
- Teles, Y.; Souza, M.; Souza, M. Sulphated Flavonoids: Biosynthesis, Structures, and Biological Activities. Molecules 2018, 23, 480. [Google Scholar] [CrossRef] [PubMed]
- Smeds, A.I.; Eklund, P.C.; Sjöholm, R.E.; Willför, S.M.; Nishibe, S.; Deyama, T.; Holmbom, B.R. Quantification of a Broad Spectrum of Lignans in Cereals, Oilseeds, and Nuts. J. Agric. Food Chem. 2007, 55, 1337–1346. [Google Scholar] [CrossRef]
- Quifer-Rada, P.; Vallverdú-Queralt, A.; Martínez-Huélamo, M.; Chiva-Blanch, G.; Jáuregui, O.; Estruch, R.; Lamuela-Raventós, R. A Comprehensive Characterisation of Beer Polyphenols by High Resolution Mass Spectrometry (LC–ESI-LTQ-Orbitrap-MS). Food Chem. 2015, 169, 336–343. [Google Scholar] [CrossRef]
- Dinelli, G.; Segura-Carretero, A.; Di Silvestro, R.; Marotti, I.; Arráez-Román, D.; Benedettelli, S.; Ghiselli, L.; Fernadez-Gutierrez, A. Profiles of Phenolic Compounds in Modern and Old Common Wheat Varieties Determined by Liquid Chromatography Coupled with Time-of-Flight Mass Spectrometry. J. Chromatogr. A 2011, 1218, 7670–7681. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Chawla, M.; Chunduri, V.; Kumar, R.; Sharma, S.; Sharma, N.K.; Kaur, N.; Kumar, A.; Mundey, J.K.; Saini, M.K.; et al. Transfer of Grain Colors to Elite Wheat Cultivars and Their Characterization. J. Cereal Sci. 2016, 71, 138–144. [Google Scholar] [CrossRef]
- Vera de Rosso, V.; Hillebrand, S.; Cuevas Montilla, E.; Bobbio, F.O.; Winterhalter, P.; Mercadante, A.Z. Determination of Anthocyanins from Acerola (Malpighia emarginata DC.) and Açai (Euterpe oleracea Mart.) by HPLC–PDA–MS/MS. J. Food Compos. Anal. 2008, 21, 291–299. [Google Scholar] [CrossRef]
- Yin, N.-W.; Wang, S.-X.; Jia, L.-D.; Zhu, M.-C.; Yang, J.; Zhou, B.-J.; Yin, J.-M.; Lu, K.; Wang, R.; Li, J.-N.; et al. Identification and Characterization of Major Constituents in Different-Colored Rapeseed Petals by UPLC–HESI-MS/MS. J. Agric. Food Chem. 2019, 67, 11053–11065. [Google Scholar] [CrossRef]
- Xu, L.-L.; Xu, J.-J.; Zhong, K.-R.; Shang, Z.-P.; Wang, F.; Wang, R.-F.; Zhang, L.; Zhang, J.-Y.; Liu, B. Analysis of Non-Volatile Chemical Constituents of Menthae Haplocalycis Herba by Ultra-High Performance Liquid Chromatography-High Resolution Mass Spectrometry. Molecules 2017, 22, 1756. [Google Scholar] [CrossRef]
- Thomford, N.; Dzobo, K.; Chopera, D.; Wonkam, A.; Maroyi, A.; Blackhurst, D.; Dandara, C. In Vitro Reversible and Time-Dependent CYP450 Inhibition Profiles of Medicinal Herbal Plant Extracts Newbouldia laevis and Cassia abbreviata: Implications for Herb-Drug Interactions. Molecules 2016, 21, 891. [Google Scholar] [CrossRef] [PubMed]
- Wojakowska, A.; Perkowski, J.; Góral, T.; Stobiecki, M. Structural Characterization of Flavonoid Glycosides from Leaves of Wheat (Triticum aestivum L.) Using LC/MS/MS Profiling of the Target Compounds. J. Mass Spectrom. 2013, 48, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Martín, M.L.; Fuguet, E.; Jiménez-Ardón, A.; Herrero-Uribe, L.; Tamayo-Castillo, G.; Torres, J.L. Identification of Polyphenols from Antiviral Chamaecrista nictitans Extract Using High-Resolution LC–ESI–MS/MS. Anal. Bioanal. Chem. 2014, 406, 5501–5506. [Google Scholar] [CrossRef]
- Perchuk, I.; Shelenga, T.; Gurkina, M.; Miroshnichenko, E.; Burlyaeva, M. Composition of Primary and Secondary Metabolite Compounds in Seeds and Pods of Asparagus Bean (Vigna unguiculata (L.) Walp.) from China. Molecules 2020, 25, 3778. [Google Scholar] [CrossRef]
- Zhang, G.; Tian, Y.; Zhang, J.; Shu, L.; Yang, S.; Wang, W.; Sheng, J.; Dong, Y.; Chen, W. Hybrid de Novo Genome Assembly of the Chinese Herbal Plant Danshen (Salvia miltiorrhiza Bunge). Gigascience 2015, 4, 62. [Google Scholar] [CrossRef] [PubMed]
- Ekeberg, D.; Flæte, P.-O.; Eikenes, M.; Fongen, M.; Naess-Andresen, C.F. Qualitative and Quantitative Determination of Extractives in Heartwood of Scots Pine (Pinus sylvestris L.) by Gas Chromatography. J. Chromatogr. A 2006, 1109, 267–272. [Google Scholar] [CrossRef]
- Llorent-Martínez, E.J.; Spínola, V.; Gouveia, S.; Castilho, P.C. HPLC-ESI-MSn Characterization of Phenolic Compounds, Terpenoid Saponins, and Other Minor Compounds in Bituminaria bituminosa. Ind. Crops Prod. 2015, 69, 80–90. [Google Scholar] [CrossRef]
- Park, S.K.; Ha, J.S.; Kim, J.M.; Kang, J.Y.; Lee, D.S.; Guo, T.J.; Lee, U.; Kim, D.-O.; Heo, H.J. Antiamnesic Effect of Broccoli (Brassica oleracea var. italica) Leaves on Amyloid Beta (Aβ)1–42-Induced Learning and Memory Impairment. J. Agric. Food Chem. 2016, 64, 3353–3361. [Google Scholar] [CrossRef]
- Rodrigues, D.B.; Mariutti, L.R.B.; Mercadante, A.Z. An in Vitro Digestion Method Adapted for Carotenoids and Carotenoid Esters: Moving Forward towards Standardization. Food Funct. 2016, 7, 4992–5001. [Google Scholar] [CrossRef]
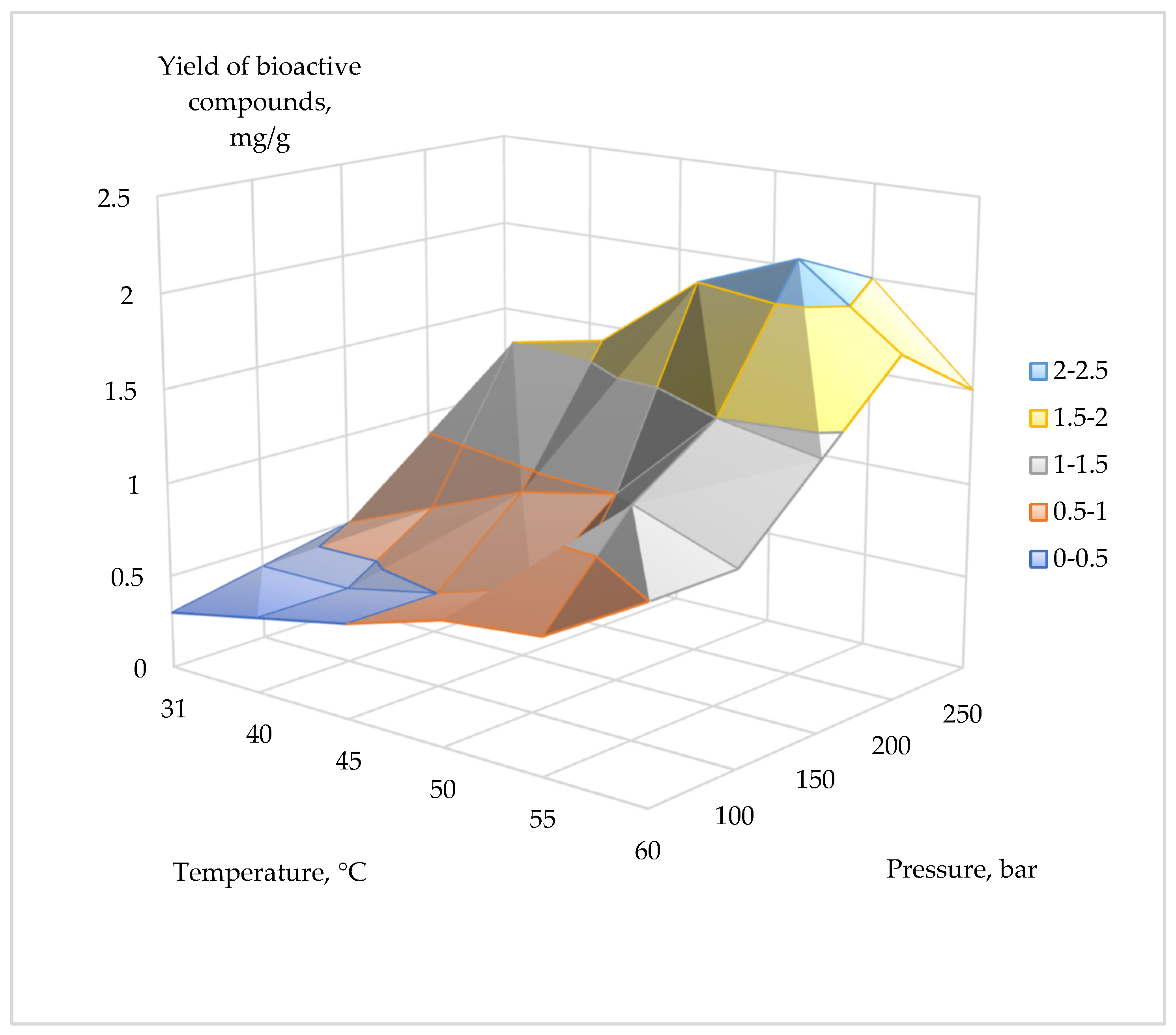
| Temperature, °C | 50 bar | 100 bar | 150 bar | 200 bar | 250 bar |
|---|---|---|---|---|---|
| 31 | 0.30 | 0.40 | 0.50 | 0.56 | 1.00 |
| 40 | 0.40 | 0.40 | 0.70 | 1.50 | 1.30 |
| 45 | 0.50 | 0.50 | 0.90 | 1.60 | 1.50 |
| 50 | 0.65 | 0.65 | 1.00 | 2.00 | 1.80 |
| 55 | 0.70 | 1.20 | 1.50 | 2.20 | 2.00 |
| 60 | 1.00 | 1.00 | 1.40 | 1.80 | 1.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razgonova, M.P.; Shinkaruk, P.A.; Maksimenko, A.A.; Podvolotskaya, A.B.; Tekutyeva, L.A. Supercritical CO2 Extraction of Bioactive Compounds from Corn Grains (Zea mays L., Hybrid Pri-15-7-16) with Metabolomic Profiling and Confocal Laser Microscopy. Plants 2025, 14, 913. https://doi.org/10.3390/plants14060913
Razgonova MP, Shinkaruk PA, Maksimenko AA, Podvolotskaya AB, Tekutyeva LA. Supercritical CO2 Extraction of Bioactive Compounds from Corn Grains (Zea mays L., Hybrid Pri-15-7-16) with Metabolomic Profiling and Confocal Laser Microscopy. Plants. 2025; 14(6):913. https://doi.org/10.3390/plants14060913
Chicago/Turabian StyleRazgonova, Mayya P., Pavel A. Shinkaruk, Anastasiia A. Maksimenko, Anna B. Podvolotskaya, and Liudmila A. Tekutyeva. 2025. "Supercritical CO2 Extraction of Bioactive Compounds from Corn Grains (Zea mays L., Hybrid Pri-15-7-16) with Metabolomic Profiling and Confocal Laser Microscopy" Plants 14, no. 6: 913. https://doi.org/10.3390/plants14060913
APA StyleRazgonova, M. P., Shinkaruk, P. A., Maksimenko, A. A., Podvolotskaya, A. B., & Tekutyeva, L. A. (2025). Supercritical CO2 Extraction of Bioactive Compounds from Corn Grains (Zea mays L., Hybrid Pri-15-7-16) with Metabolomic Profiling and Confocal Laser Microscopy. Plants, 14(6), 913. https://doi.org/10.3390/plants14060913








