The Diversity of Morphological Traits and Seed Metabolomic Composition in Buckwheat Genetic Resources
Abstract
1. Introduction
2. Results and Discussion
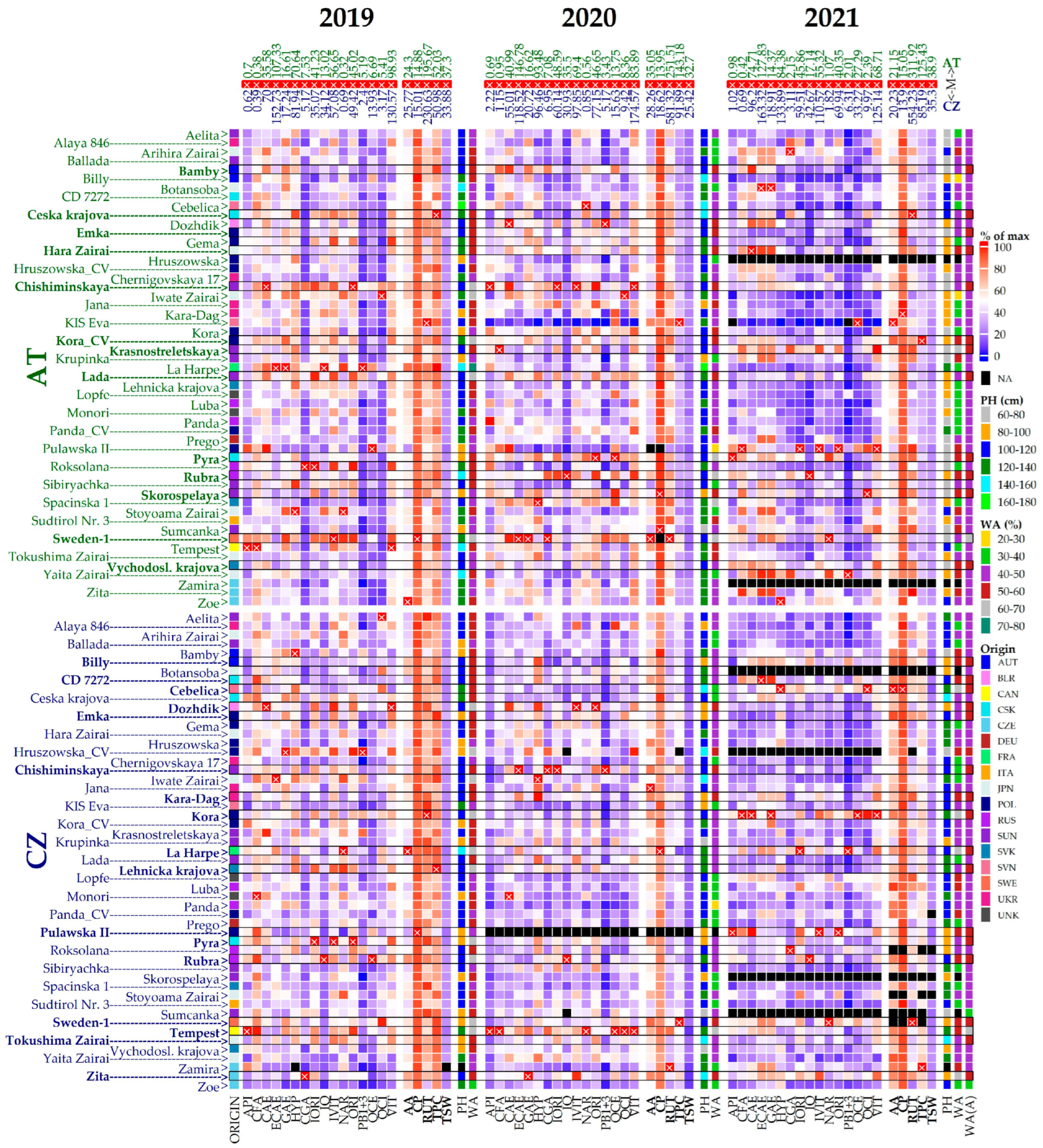
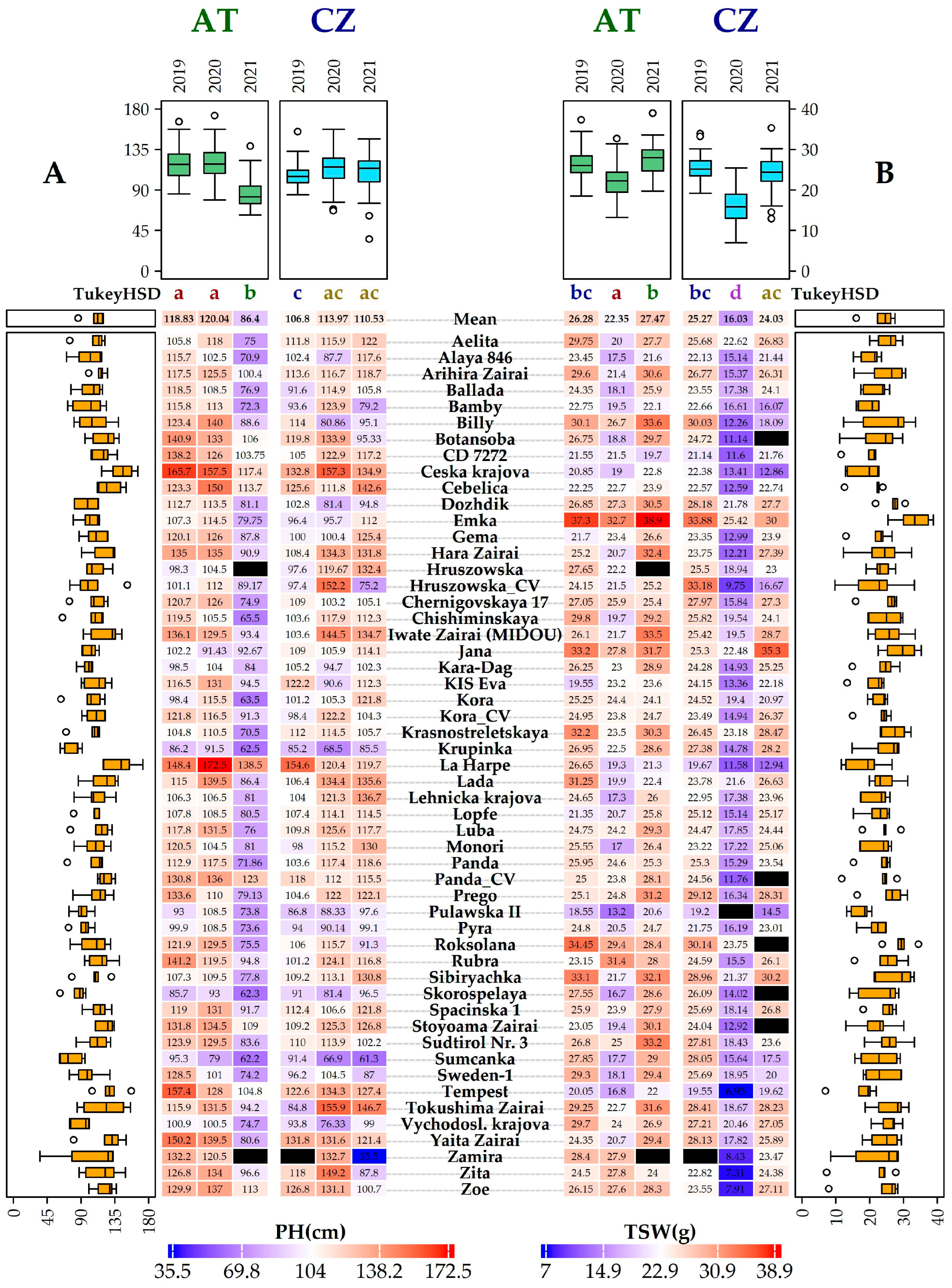
2.1. Morphological and Phenological Evaluation
2.2. Chemical Compounds Analysis
2.3. Metabolomic Profiling in Buckwheat Accessions
3. Materials and Methods
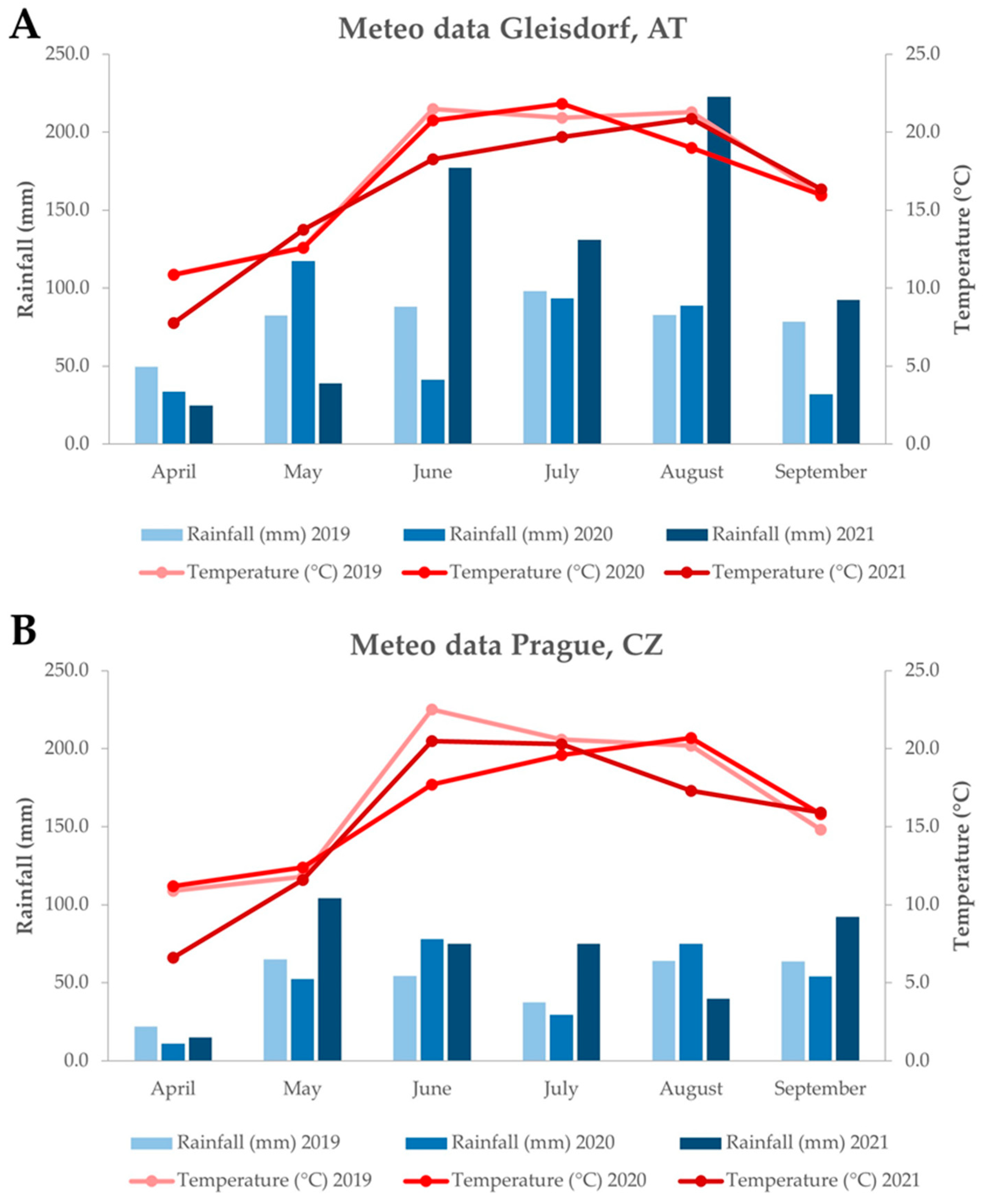
3.1. Plant Material and Weather Conditions
3.2. Chemicals
3.3. Standards Preparation and Sample Isolation
3.4. UHPLC-ESI-MS/MS Instrumentation
3.5. UHPLC-ESI-MS/MS Analysis
3.6. Determination of Phenolic Compound Concentration in Buckwheat Samples and Statistical Analysis
3.7. Chemical Analyses
3.8. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Jha, R.; Zhang, K.X.; He, Y.Q.; Mendler-Drienyovszki, N.; Magyar-Tábori, K.; Quinet, M.; Germ, M.; Kreft, I.; Meglic, V.; Ikeda, K.; et al. Global nutritional challenges and opportunities: Buckwheat, a potential bridge between nutrient deficiency and food security. Trends Food Sci. Technol. 2024, 145, 104365. [Google Scholar] [CrossRef]
- Raguindin, P.F.; Itodo, O.A.; Stoyanov, J.; Dejanovic, G.M.; Gamba, M.; Asllanaj, E.; Minder, B.; Bussler, W.; Metzger, B.; Muka, T.; et al. A systematic review of phytochemicals in oat and buckwheat. Food Chem. 2021, 338, 127982. [Google Scholar] [CrossRef]
- Chrungoo, N.K.; Chettry, U. Buckwheat: A critical approach towards assessment of its potential as a super crop. Indian J. Genet. Pl. Br. 2021, 81, 1–23. [Google Scholar] [CrossRef]
- Chettry, U.; Chrungoo, N.K. Beyond the Cereal Box: Breeding Buckwheat as a Strategic Crop for Human Nutrition. Plant Foods Hum. Nutr. 2021, 76, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Huda, M.N.; Lu, S.; Jahan, T.; Ding, M.Q.; Jha, R.; Zhang, K.X.; Zhang, W.; Georgiev, M.I.; Park, S.U.; Zhou, M.L. Treasure from garden: Bioactive compounds of buckwheat. Food Chem. 2021, 335, 127653. [Google Scholar] [CrossRef]
- Kreft, M. Buckwheat phenolic metabolites in health and disease. Nutr. Res. Rev. 2016, 29, 30–39. [Google Scholar] [CrossRef]
- Rana, J.C.; Singh, M.; Chauban, R.S.; Chahota, R.K.; Sharma, T.R.; Yadav, R.; Achak, S. Genetic resources of buckwheat in India. In Molecular Breeding and Nutritional Aspects of Buckwheat; Zhou, M., Kreft, I., Woo, S.H., Chrungoo, N., Wieslander, G., Eds.; Acadenic Press: Cambridge, MA, USA; Elsevier Inc.: London, UK, 2016; pp. 109–135. [Google Scholar]
- Rauf, M.; Yoon, H.; Lee, S.; Hyun, D.Y.; Lee, M.C.; Oh, S.; Choi, Y.M. Evaluation of Fagopyrum esculentum Moench germplasm based on agro-morphological traits and the rutin and quercetin content of seeds under spring cultivation. Genet. Resour. Crop Evol. 2020, 67, 1385–1403. [Google Scholar] [CrossRef]
- Zhou, M.; Tang, Y.; Den, X.; Ruan, C.; Kreft, I.; Tang, Y.; Wu, T. Overview of buckwheat resources in the world. In Buckwheat Germplasm in the World; Zhou, M., Kreft, I., Suvorova, G., Tang, Y., Woo, S.H., Eds.; Academic Press: Cambridge, MA, USA; Elsevier Inc.: London, UK, 2018; p. 355. [Google Scholar]
- Pipan, B.; Sinkovic, L.; Neji, M.; Janovská, D.; Zhou, M.L.; Meglic, V. Agro-Morphological and Molecular Characterization Reveal Deep Insights in Promising Genetic Diversity and Marker-Trait Associations in Fagopyrum esculentum and Fagopyrum tataricum. Plants 2023, 12, 3321. [Google Scholar] [CrossRef]
- Grahic, J.; Okic, A.; Simon, S.; Djikic, M.; Gadzo, D.; Pejic, I.; Gasi, F. Genetic Relationships and Diversity of Common Buckwheat Accessions in Bosnia and Herzegovina. Agronomy 2022, 12, 2676. [Google Scholar] [CrossRef]
- Park, J.E.; Kang, Y.; Han, G.D.; Yildiz, M.; Kim, S.H.; Kim, C.; Chung, Y.S. Diversity study of common buckwheat germplasm in the Republic of Korea using GBS. Plant Biotechnol. Rep. 2022, 16, 799–803. [Google Scholar] [CrossRef]
- Han, G.D.; Mansoor, S.; Kim, J.; Park, J.; Heo, S.; Yu, J.K.; Kim, S.H.; Chung, Y.S. A study of the morphological and geographical diversity of Korean indigenous buckwheat landraces for breeding. J. King Saud Univ. Sci. 2024, 36, 103387. [Google Scholar]
- Naik, S.; Mahajan, R.; Sofi, P.A.; Abidi, I.; Ali, G.; Nehvi, F.A.; Khan, I.; Bhat, S.A.; Bhat, M.A.; Bhat, B.A. Characterisation of buckwheat (Fagopyrum spp.) diversity of the norhtwestern Himalyas. Crop Pasture Sci. 2023, 74, 1069–1079. [Google Scholar] [CrossRef]
- Swarup, S.; Cargill, E.J.; Crosby, K.; Flagel, L.; Kniskern, J.; Glenn, K.C. Genetic diversity is indispensable for plant breeding to improve crops. Crop Sci. 2021, 61, 839–852. [Google Scholar] [CrossRef]
- Vieites-Alvarez, Y.; Reigosa, M.J.; Sánchez-Moreiras, A.M. A decade of advances in the study of buckwheat for organic farming and agroecology (2013–2023). Front. Plant Sci. 2024, 15, 1354672. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Lv, Q.Y.; Liu, A.; Wang, J.R.; Sun, X.Q.; Deng, J.; Chen, Q.F.; Wu, Q. Comparative metabolomics study of Tartary (Fagopyrum tataricum (L.) Gaertn) and common (Fagopyrum esculentum Moench) buckwheat seeds. Food Chem. 2022, 371, 131125. [Google Scholar] [CrossRef]
- Deng, J.; Dong, F.; Wu, C.X.; Zhao, J.L.; Li, H.Y.; Huang, J.; Shi, T.X.; Meng, Z.Y.; Cai, F.; Chen, Q.F.; et al. Comparative Metabolomics Analysis between Red- and White-Flowered Common Buckwheat Cultivars. Phyton-Int. J. Exp. Bot. 2021, 90, 859–870. [Google Scholar] [CrossRef]
- Janovska, D.; Jagr, M.; Svoboda, P.; Dvoracek, V.; Meglic, V.; Cepkova, P.H. Breeding Buckwheat for Nutritional Quality in the Czech Republic. Plants 2021, 10, 1262. [Google Scholar] [CrossRef]
- Singh, M.; Malhotra, N.; Sharma, K. Buckwheat (Fagopyrum sp.) genetic resources: What can they contribute towards nutritional security of changing world? Genet. Resour. Crop Evol. 2020, 67, 1639–1658. [Google Scholar] [CrossRef]
- Morishita, T.; Hara, T.; Hara, T. Important agronomic characteristics of yielding ability in common buckwheat; ecotype and ecological differentiation, preharvest sprouting resistance, shattering resistance, and lodging resistance. Breed. Sci. 2020, 70, 39–47. [Google Scholar] [CrossRef]
- Ghiselli, L.; Tallarico, R.; Mariotti, M.; Romagnoli, S.; Baglio, A.P.; Donnarumma, P.; Benedettelli, S. Agronomic and nutritional characteristics of three buckwheat cultivars under organic farming in three environments of the Garfagnana mountain district. Ital. J. Agron. 2016, 11, 188–194. [Google Scholar] [CrossRef]
- Domingos, I.F.N.; Bilsborrow, P.E. The effect of variety and sowing date on the growth, development, yield and quality of common buckwheat (Fagopyrum esculentum Moench). Eur. J. Agron. 2021, 126, 126264. [Google Scholar] [CrossRef]
- Nandan, A.; Koirala, P.; Tripathi, A.D.; Vikranta, U.; Shah, K.R.; Gupta, A.J.; Agarwal, A.; Nirmal, N. Nutritional and functional perspectives of pseudocereals. Food Chem. 2024, 448, 139072. [Google Scholar] [CrossRef] [PubMed]
- Wisniewska, M.; Mankowski, D.R.; Fras, A. Variations in chemical composition of common buckwheat (Fagopyrum esculentum Moench) as a result of different environmental conditions. J. Sci. Food Agric. 2024, 104, 286–294. [Google Scholar] [CrossRef]
- Gavrić, T.; Čadro, S.; Gadžo, D.; Mirha, D.; Bezdrob, M.; Jovovic, Z.; Jurkovic, J.; Hamidovic, S. Influence of meteorological parameters on the yield and chemical composition of common buckwheat (Fagopyrum esculentum Moench). J. Agric. For. 2018, 64, 113–120. [Google Scholar] [CrossRef]
- Novikova, L.Y.; Seferova, I.V.; Nekrasov, A.Y.; Perchuk, I.N.; Shelenga, T.V.; Samsonova, M.G.; Vishnyakova, M.A. Impact of weather and climate on seed protein and oil content of soybean in the North Caucasus. Vavilovskii Zh. Genet. Sel. 2018, 22, 708–715. [Google Scholar]
- Tomczak, A.; Zielinska-Dawidziak, M.; Piasecka-Kwiatkowska, D.; Lampart-Szczapa, E. Blue lupine seeds protein content and amino acids composition. PSE 2018, 64, 147–155. [Google Scholar] [CrossRef]
- Zielińska-Dawidziak, M.; Nawracała, J.; Piasecka-Kwiatkowska, D.; Król, E.; Staniek, H.; Krejpcio, Z. The impact of the year of soybean (Glycine max L. Merrill) harvest on the accumulation of iron from FeSO4 solutions. Fragm. Agron. 2012, 29, 183–193. [Google Scholar]
- Sinkovic, L.; Meglic, V.; Pipan, B. Grain characteristics of eleven buckwheat varieties (Fagopyrum spp.) grown under Central European climatic conditions. J. Elem. 2024, 29, 419–431. [Google Scholar] [CrossRef]
- Terpinc, P.; Cigic, B.; Polak, T.; Hribar, J.; Pozrl, T. LC-MS analysis of phenolic compounds and antioxidant activity of buckwheat at different stages of malting. Food Chem. 2016, 210, 9–17. [Google Scholar] [CrossRef]
- Giménez-Bastida, J.A.; Zielinski, H.; Piskula, M.; Zielinska, D.; Szawara-Nowak, D. Buckwheat bioactive compounds, their derived phenolic metabolites and their health benefits. Mol. Nutr. Food Res. 2017, 61, 1–10. [Google Scholar] [CrossRef]
- Marchev, A.S.; Vasileva, L.V.; Amirova, K.M.; Savova, M.S.; Balcheva-Sivenova, Z.P.; Georgiev, M.I. Metabolomics and health: From nutritional crops and plant-based pharmaceuticals to profling of human biofuids. CMLS 2021, 78, 6487–6503. [Google Scholar] [CrossRef] [PubMed]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef]
- Mashilo, J.; Odindo, A.O.; Shimelis, H.A.; Musenge, P.; Tesfay, S.Z.; Magwaza, L.S. Drought tolerance of selected bottle gourd Lagenaria siceraria (Molina) Standl. landraces assessed by leaf gas exchange and photosynthetic efficiency. Plant Physiol. Biochem. 2017, 120, 75–87. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar]
- Borovaya, S.A.; Klykov, A.G. Some aspects of flavonoid biosynthesis and accumulation in buckwheat plants. Plant Biotechnol. Rep. 2020, 14, 213–225. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.F.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Kalinova, J.P.; Vrchotova, N.; Triska, J. Phenolics levels in different parts of common buckwheat (Fagopyrwn esculentum) achenes. J. Cereal Sci. 2019, 85, 243–248. [Google Scholar] [CrossRef]
- Kiprovski, B.; Mikulic-Petkovsek, M.; Slatnar, A.; Veberic, R.; Stampar, F.; Malencic, D.; Latkovic, D. Comparison of phenolic profiles and antioxidant properties of European Fagopyrum esculentum cultivars. Food Chem. 2015, 185, 41–47. [Google Scholar] [CrossRef]
- Podolska, G.; Gujska, E.; Klepacka, J.; Aleksandrowicz, E. Bioactive Compounds in Different Buckwheat Species. Plants 2021, 10, 961. [Google Scholar] [CrossRef]
- Qin, P.Y.; Wang, Q.; Shan, F.; Hou, Z.H.; Ren, G.X. Nutritional composition and flavonoids content of flour from different buckwheat cultivars. IJFST 2010, 45, 951–958. [Google Scholar] [CrossRef]
- Suzuki, T.; Noda, T.; Morishita, T.; Ishiguro, K.; Otsuka, S.; Brunori, A. Present status and future perspectives of breeding for buckwheat quality. Breed. Sci. 2020, 70, 48–66. [Google Scholar] [CrossRef] [PubMed]
- Sedej, I.; Sakac, M.; Mandic, A.; Misan, A.; Tumbas, V.; Canadanovic-Brunet, J. Buckwheat (Fagopyrum esculentum Moench) Grain and Fractions: Antioxidant Compounds and Activities. J. Food Sci. 2012, 77, C954–C959. [Google Scholar] [CrossRef] [PubMed]
- Kalinova, J.; Vrchotova, N. The influence of organic and conventional crop management, variety and year on the yield and flavonoid level in common buckwheat groats. Food Chem. 2011, 127, 602–608. [Google Scholar] [CrossRef]
- Altikardes, E.; Güzel, N. Impact of germination pre-treatments on buckwheat and Quinoa: Mitigation of anti-nutrient content and enhancement of antioxidant properties. Food Chem.-X 2024, 21, 101182. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, M.; Liu, Z.; Huang, Y.; Zhang, L.; Yang, F.; Gong, J.Q.; Huo, D.A. Metabolomics and related genes analysis revealed the distinct mechanism of drought resistance in novel buckwheat and cultivated species. Plant Growth Regul. 2024, 104, 695–711. [Google Scholar] [CrossRef]
- Shomali, A.; Das, S.; Arif, N.; Sarraf, M.; Zahra, N.; Yadav, V.; Aliniaeifard, S.; Chauhan, D.K.; Hasanuzzaman, M. Diverse Physiological Roles of Flavonoids in Plant Environmental Stress Responses and Tolerance. Plants 2022, 11, 3158. [Google Scholar] [CrossRef]
- Drazic, S.; Glamoclija, D.; Ristic, M.; Dolijanovic, Z.; Drazic, M.; Pavlovic, S.; Jaramaz, M.; Jaramaz, D. Effect of environment of the rutin content in leaves of Fagopyrum esculentum Moench. PSE 2016, 62, 261–265. [Google Scholar]
- Guo, X.D.; Ma, Y.J.; Parry, J.; Gao, J.M.; Yu, L.L.; Wang, M. Phenolics Content and Antioxidant Activity of Tartary Buckwheat from Different Locations. Molecules 2011, 16, 9850–9867. [Google Scholar] [CrossRef]
- Siracusa, L.; Gresta, F.; Sperlinga, E.; Ruberto, G. Effect of sowing time and soil water content on grain yield and phenolic profile of four buckwheat (Fagopyrum esculentum Moench.) varieties in a Mediterranean environment. J. Food Compos. Anal. 2017, 62, 1–7. [Google Scholar] [CrossRef]
- Wang, K.Y.; Zhang, H.H.; Yuan, L.; Li, X.L.; Cai, Y.Q. Potential Implications of Hyperoside on Oxidative Stress-Induced Human Diseases: A Comprehensive Review. J. Inflamm. Res. 2023, 16, 4503–4526. [Google Scholar] [CrossRef]
- Dziedzic, K.; Gorecka, D.; Szwengiel, A.; Sulewska, H.; Kreft, I.; Gujska, E.; Walkowiak, J. The Content of Dietary Fibre and Polyphenols in Morphological Parts of Buckwheat (Fagopyrum tataricum). Plant Foods Hum Nutr. 2018, 73, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Proanthocyanidins in cereals and pseudocereals. Crit. Rev. Food Sci. Nutr. 2018, 59, 1–13. [Google Scholar] [CrossRef] [PubMed]
- IPGRI. Descriptors for Buckwheat (Fagopyrum spp.); International Plant Genetic Resources Institute (IPGRI): Rome, Italy, 1994. [Google Scholar]
- Holasova, M.; Fiedlerova, V.; Smrcinova, H.; Orsak, M.; Lachman, J.; Vavreinova, S. Buckwheat—The source of antioxidant activity in functional foods. Food Res. Int. 2002, 35, 207–211. [Google Scholar] [CrossRef]
- Sensoy, I.; Rosen, R.T.; Ho, C.T.; Karwe, M.V. Effect of processing on buckwheat phenolics and antioxidant activity. Food Chem. 2006, 99, 388–393. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hlásná Čepková, P.; Janovská, D.; Bernhart, M.; Svoboda, P.; Jágr, M.; Meglič, V. The Diversity of Morphological Traits and Seed Metabolomic Composition in Buckwheat Genetic Resources. Plants 2025, 14, 903. https://doi.org/10.3390/plants14060903
Hlásná Čepková P, Janovská D, Bernhart M, Svoboda P, Jágr M, Meglič V. The Diversity of Morphological Traits and Seed Metabolomic Composition in Buckwheat Genetic Resources. Plants. 2025; 14(6):903. https://doi.org/10.3390/plants14060903
Chicago/Turabian StyleHlásná Čepková, Petra, Dagmar Janovská, Maria Bernhart, Pavel Svoboda, Michal Jágr, and Vladimir Meglič. 2025. "The Diversity of Morphological Traits and Seed Metabolomic Composition in Buckwheat Genetic Resources" Plants 14, no. 6: 903. https://doi.org/10.3390/plants14060903
APA StyleHlásná Čepková, P., Janovská, D., Bernhart, M., Svoboda, P., Jágr, M., & Meglič, V. (2025). The Diversity of Morphological Traits and Seed Metabolomic Composition in Buckwheat Genetic Resources. Plants, 14(6), 903. https://doi.org/10.3390/plants14060903






