Effects of Different Temperatures on the Physiological Characteristics of Sweet Potato (Ipomoea batatas L. Lam) Storage Roots and Growth of Seedlings During the Sprouting and Seedling Period
Abstract
1. Introduction
2. Results
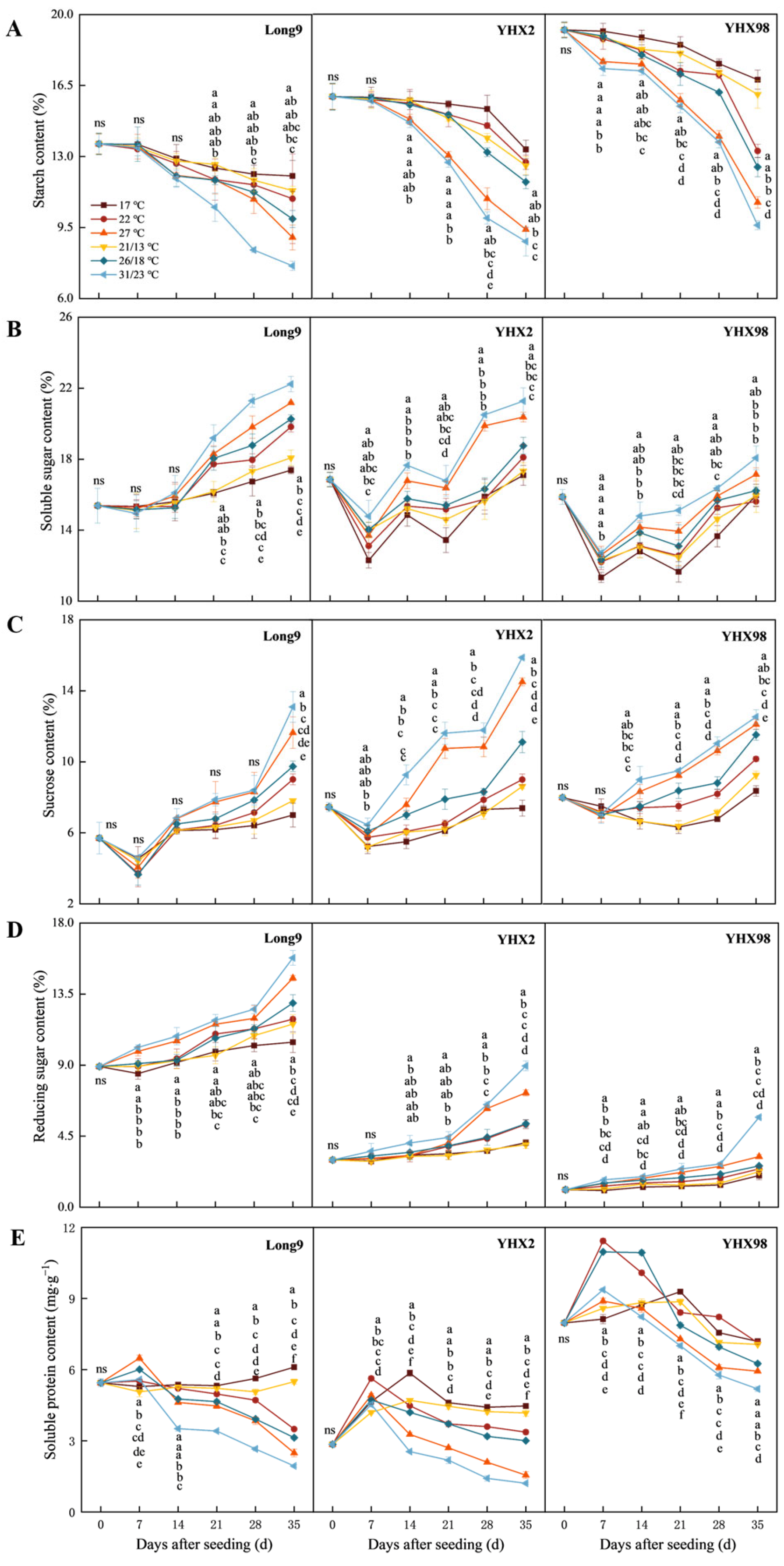
2.1. Changes in Nutrients of Sweet Potato Storage Roots During Sprouting and Seedling Period
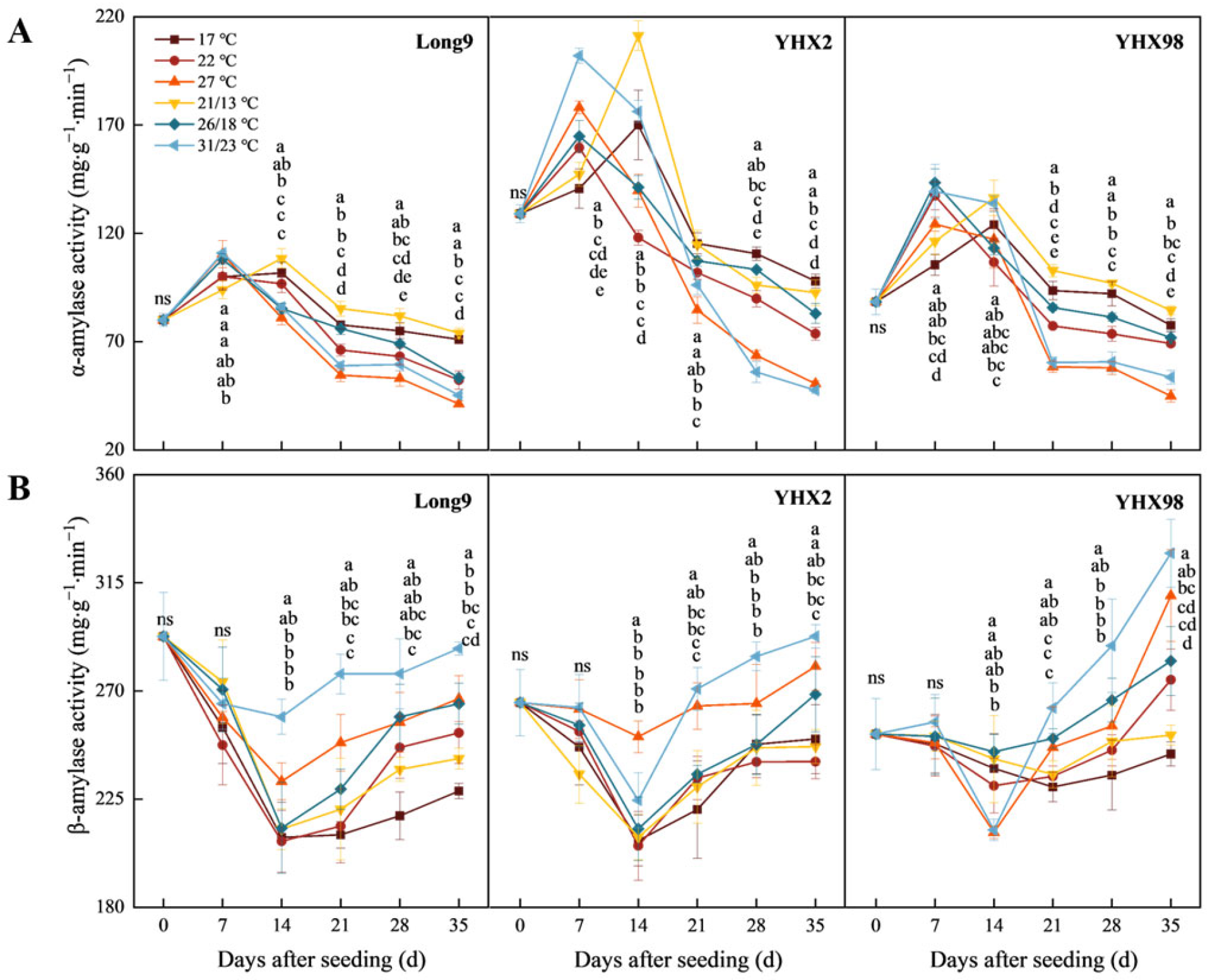
2.2. Changes in Amylase Activity of Sweet Potato Storage Roots During Sprouting and Seedling Period
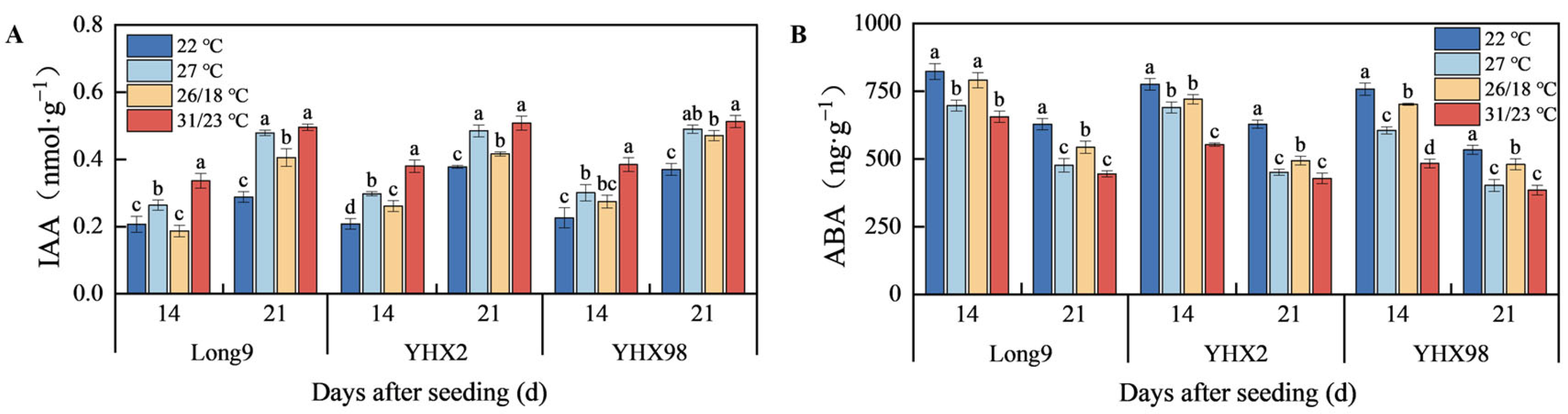
2.3. Changes in Endogenous Hormones of Sweet Potato Storage Roots During Sprouting and Seedling Period
2.4. Changes in Sprouting Characteristics of Sweet Potato During Sprouting and Seedling Period
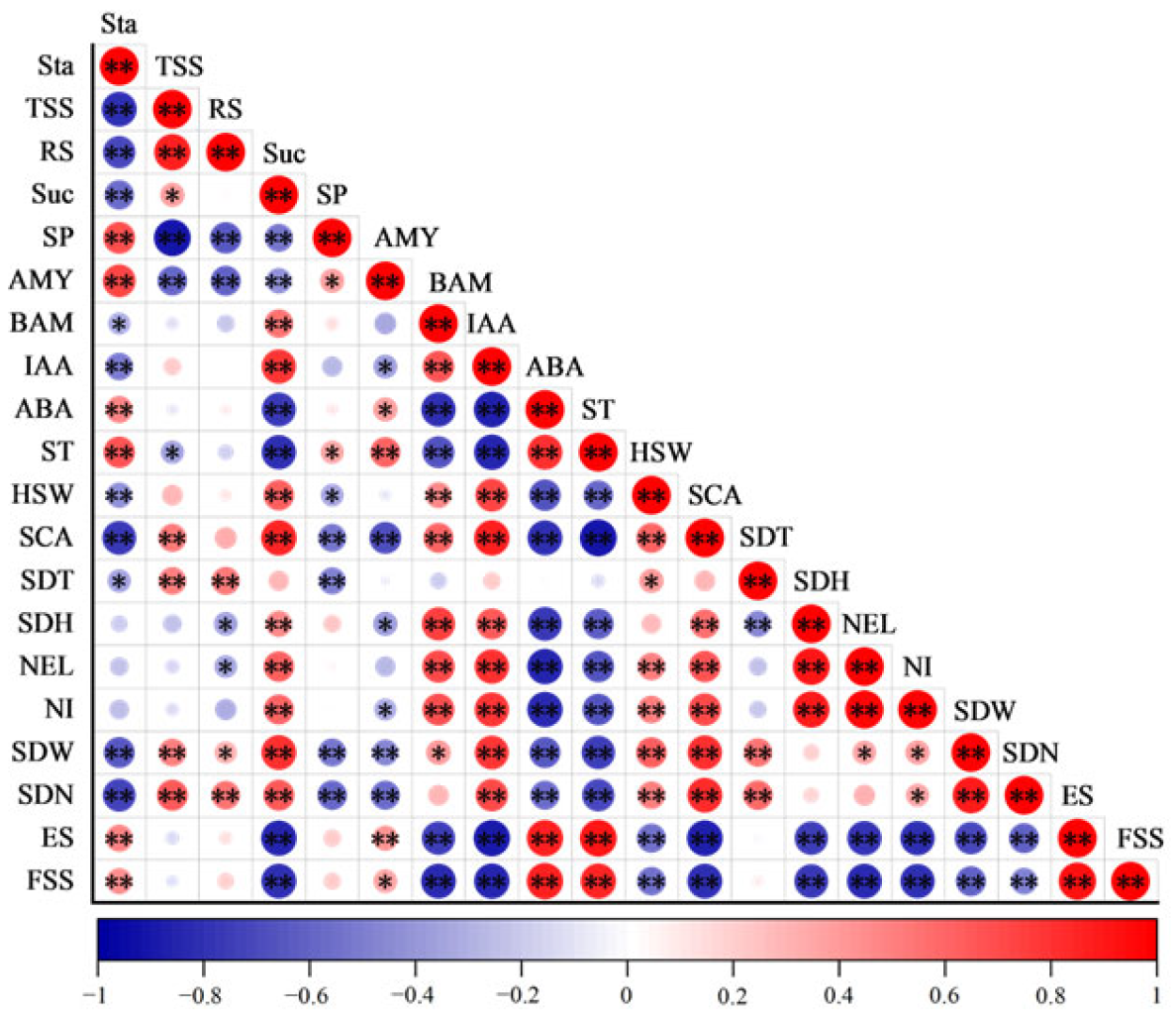
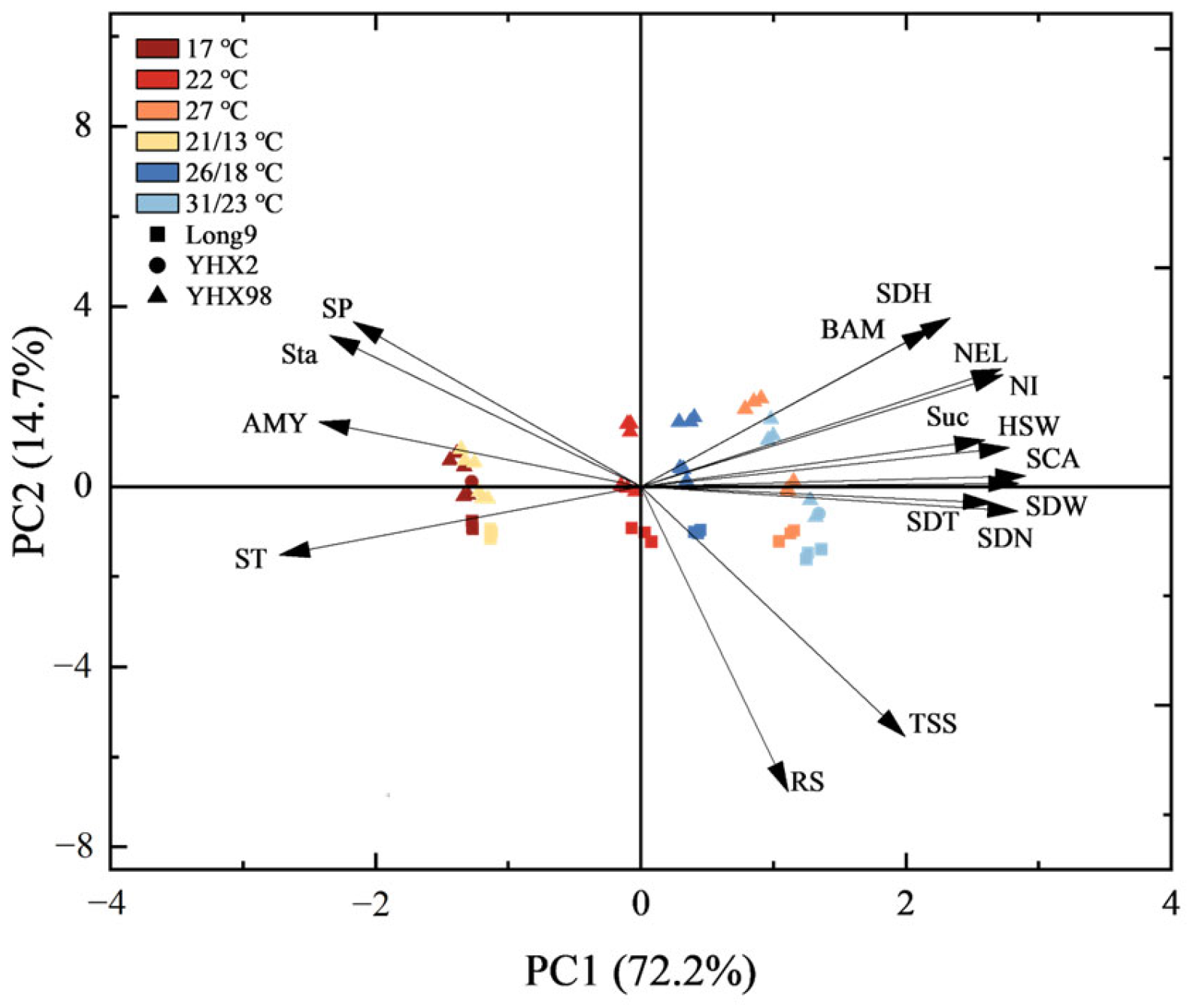
2.5. The Relationship Between the Physiological Characteristics and Sprouting Characteristics During Sprouting and Seedling Period
3. Discussion
3.1. Effect of Temperature on Starch Metabolism in Sweet Potato Storage Roots
3.2. Effect of Temperature on the Hormones of Sweet Potato Storage Roots
3.3. Effect of Temperature on the Sprouting Characteristics of Sweet Potato Storage Roots
4. Materials and Methods
4.1. Materials
4.2. Experimental Design
4.3. Measurement Indicators and Methods
4.3.1. Determination of Starch, Total Soluble Sugar, Sucrose, Reducing Sugar and Soluble Protein in Storage Roots
4.3.2. Determination of Amylase Activity in Storage Roots
4.3.3. Endogenous Hormone Measurement
4.3.4. Measurement of Sprouting and Seedling Characteristics
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| TSS | Total soluble sugar |
| RS | Reducing sugar |
| SP | Soluble protein |
| Suc | Sucrose |
| Sta | Starch |
| AMY | α-amylase |
| BAM | β-amylase |
| IAA | Growth hormone |
| ABA | Abscisic acid |
| ST | Sprouting time |
| ES | Emergence stage |
| FSS | Full stand of seedlings stage |
| HSW | 100-seedling weight |
| SCA | Seedling cutting amount |
| SDT | Seedling thick |
| SDH | Seedling height |
| NEL | Number of expanded leaves |
| NI | Number of internodes |
| SDW | Seedling weight |
| SDN | Seedling number |
References
- Wang, X.; Li, Q.; Cao, Q.H.; Ma, D.F. Current Status and Future Prospective of Sweetpotato Production and Seed Industry in China. Sci. Agric. Sin. 2021, 54, 483–492. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Production: Crops and Livestock Products. 2024. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 20 December 2024).
- Gajanayake, B.; Reddy, K.R.; Shankle, M.W.; Arancibia, R.A.; Villordon, A.O. Quantifying Storage Root Initiation, Growth, and Developmental Responses of Sweetpotato to Early Season Temperature. Agron. J. 2014, 106, 1795–1804. [Google Scholar] [CrossRef]
- Dong, S.; Xie, B.; Hou, F.; Li, A.; Wang, Q.; Zhang, L. Effect of Temperature during Root Germination on Quality Traits of Sweet Potato. Shandong Agric. Sci. 2018, 50, 82–87. [Google Scholar] [CrossRef]
- Yang, H.L. Analysis of sweetpotato tuber sprouting habit and factors affecting seedling establishment. Mod. Agric. Sci. Technol. 2019, 12, 25–26. [Google Scholar]
- Wijewardana, C.; Reddy, K.R.; Shankle, M.W.; Meyers, S.; Gao, W. Low and high-temperature effects on sweetpotato storage root initiation and early transplant establishment. Sci. Hortic. 2018, 240, 38–48. [Google Scholar] [CrossRef]
- Sheikh, F.R.; Jose-Santhi, J.; Kalia, D.; Singh, K.; Singh, R.K. Sugars as the regulators of dormancy and sprouting in geophytes. Ind. Crops Prod. 2022, 189, 115817. [Google Scholar] [CrossRef]
- Di, X.; Wang, Q.; Zhang, F.; Feng, H.; Wang, X.; Cai, C. Advances in the Modulation of Potato Tuber Dormancy and Sprouting. Int. J. Mol. Sci. 2024, 25, 5078. [Google Scholar] [CrossRef]
- Ranwala, A.P.; Miller, W.B. Gibberellin-mediated changes in carbohydrate metabolism during flower stalk elongation in tulips. Plant Growth Regul. 2008, 55, 241–248. [Google Scholar] [CrossRef]
- Wiltshire, J.J.J.; Cobb, A.H. A review of the physiology of potato tuber dormancy. Ann. Appl. Biol. 1996, 129, 553–569. [Google Scholar] [CrossRef]
- Sonnewald, S.; Sonnewald, U. Regulation of potato tuber sprouting. Planta 2014, 239, 27–38. [Google Scholar] [CrossRef]
- Wang, R.J.; Li, W.H.; Zhao, Q.Q.; Xie, Y.Q.; Li, Y.; Wu, X.W. Effects of Different Temperature Treatments on Dormancy Regulation of Konjac Bulbil. Acta Bot. Boreal. Occident. Sin. 2023, 43, 2088–2099. [Google Scholar] [CrossRef]
- Sun, Z.T.; Henson, C.A. A quantitative assessment of the importance of barley seed α-amylase, β-amylase, debranching enzyme, and α-glucosidase in starch degradation. Arch. Biochem. Biophys. 1991, 284, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Irving, D.E.; Shingleton, G.J.; Hurst, P.L. Starch degradation in buttercup squash (Cucurbita maxima). J. Am. Soc. Hortic. Sci. 1999, 124, 587–590. [Google Scholar] [CrossRef]
- Fadón, E.; Rodrigo, J.; Herrero, M. Is there a specific stage to rest? Morphological changes in flower primordia in relation to endodormancy in sweet cherry (Prunus avium L.). Trees 2018, 32, 1583–1594. [Google Scholar] [CrossRef]
- Kaufmann, H.; Blanke, M. Changes in carbohydrate levels and relative water content (RWC) to distinguish dormancy phases in sweet cherry. J. Plant Physiol. 2017, 218, 1–5. [Google Scholar] [CrossRef]
- Gholizadeh, J.; Sadeghipour, H.R.; Abdolzadeh, A.; Hemmati, K.; Hassani, D.; Vahdati, K. Redox rather than carbohydrate metabolism differentiates endodormant lateral buds in walnut cultivars with contrasting chilling requirements. Sci. Hortic. 2017, 225, 29–37. [Google Scholar] [CrossRef]
- Dong, S.; Beckles, D.M. Dynamic changes in the starch-sugar interconversion within plant source and sink tissues promote a better abiotic stress response. J. Plant Physiol. 2019, 234–235, 80–93. [Google Scholar] [CrossRef]
- Li, H.; Yue, Y.S.; Teng, K.; Zhang, Q.G.; Teng, W.J.; Zhang, H.; Wen, H.F.; Wu, J.Y.; Fan, X.F. Physiological Characteristics of Seed Germination of Carex rigescens at different Temperatures. Acta Agrestia Sin. 2023, 31, 426–432. [Google Scholar] [CrossRef]
- Qiu, T.Y. Variation of Main Components in Sweet Potato Storage Rootsduring Germination and Its Relationship with Germination Property. Master’s Thesis, Zhejiang A&F University, Hangzhou, China, 2019. [Google Scholar]
- Arnau, J.A.; Tadeo, F.R.; Guerri, J.; Primo-Millo, E. Cytokinins in peach: Endogenous levels during early fruit development. Plant Physiol. Biochem. 1999, 37, 741–750. [Google Scholar] [CrossRef]
- Wang, F.; Cheng, F.M.; Liu, Y.; Zhong, L.J.; Zhang, G.P. Dynamic Changes of Plant Hormones in Developing Grains at Rice Filling Stage under Different Temperatures. Acta Agron. Sin. 2006, 32, 25–29. [Google Scholar] [CrossRef]
- Golovina, E.A.; Van As, H.; Hoekstra, F.A. Membrane chemical stability and seed longevity. Eur. Biophys. J. Biophys. Lett. 2010, 39, 657–668. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, X.B.; Liu, Y.; Zhao, M.S.; Tu, S.P. Embryo Development and Dormancy Releasing of Acer yangjuechi, the Extremely Endangered Plant. Sci. Silvae Sin. 2017, 53, 65–73. [Google Scholar] [CrossRef]
- Yang, R.C.; Zhang, H.J.; Wang, Q.; Guo, Y.D. Regulatory Mechanism of Plant Hormones on Seed Dormancy and Germination (Review). Acta Agrestia Sin. 2012, 20, 1–9. [Google Scholar]
- Khan, A.A. Primary, preventive and permissive roles of hormones in plant systems. Bot. Rev. 1975, 41, 391–420. [Google Scholar] [CrossRef]
- Wu, J.H.; Xie, C.P.; Tian, C.G.; Zhou, Y.P. Molecular Mechanism of Abscisic Acid Regulation During Seed Dormancy and Germination. Chin. Bull. Bot. 2018, 53, 542–555. [Google Scholar] [CrossRef]
- Saini, S.; Sharma, I.; Kaur, N.; Pati, P.K. Auxin: A master regulator in plant root development. Plant Cell Rep. 2013, 32, 741–757. [Google Scholar] [CrossRef]
- Davies, P.J. Plant Hormones: Biosynthesis, Signal Transduction, Action! 3rd ed.; Springer: New York, NY, USA, 2010; pp. 485–486. ISBN 978-1-4020-2684-3. [Google Scholar]
- López-González, C.; Juárez-Colunga, S.; Morales-Elías, N.C.; Tiessen, A. Exploring regulatory networks in plants: Transcription factors of starch metabolism. PeerJ 2019, 7, e6841. [Google Scholar] [CrossRef]
- Liu, Y.X.; Shi, K.M.; Lin, J.; Cui, L.J. Response of endogenous hormone to temperatures changes during the seed germination of Phoebe zhennan. J. Cent. South Univ. For. Technol. 2020, 40, 89–95. [Google Scholar] [CrossRef]
- Kebreab, E.; Murdoch, A.J. A Model of the Effects of a Wide Range of Constant and Alternating Temperatures on Seed Germination of Four Orobanche Species. Ann. Bot. 1999, 84, 549–557. [Google Scholar] [CrossRef]
- Liu, K.; Baskin, J.M.; Baskin, C.C.; Bu, H.; Du, G.; Ma, M. Effect of Diurnal Fluctuating versus Constant Temperatures on Germination of 445 Species from the Eastern Tibet Plateau. PLoS ONE 2013, 8, e69364. [Google Scholar] [CrossRef]
- Wang, Y.W.; Fu, H.J.; Shi, P.J.; Lin, S.Y. Effects of variable temperatures on seed germination and seedling growth of Phyllostachys edulis. J. Nanjing For. Univ. Nat. Sci. Ed. 2021, 45, 97–106. [Google Scholar] [CrossRef]
- Zhang, Q.; Shan, C.; Song, W.; Cai, W.; Zhou, F.; Ning, M.; Tang, F. Transcriptome analysis of starch and sucrose metabolism change in Gold Queen Hami melons under different storage temperatures. Postharvest Biol. Technol. 2021, 174, 111445. [Google Scholar] [CrossRef]
- Collins, W.W.; Walter, W.M.; Torgersenbelding, S. Carbohydrate Changes in Sprouting Sweetpotato Roots. HortScience 1990, 25, 979. [Google Scholar] [CrossRef]
- Wang, Q.M.; Zhang, L.M.; Wang, J.J.; Wang, D.Z. Influence of Main Nutrients in Storage Rooton Budding of Sweetpotato. J. Shandong Agric. Sci. 1998, 7–9. [Google Scholar] [CrossRef]
- Miele, N.A.; Volpe, S.; Torrieri, E.; Cavella, S. Improving physical properties of sodium caseinate based coating with the optimal formulation: Effect on strawberries’ respiration and transpiration rates. J. Food Eng. 2022, 331, 111123. [Google Scholar] [CrossRef]
- Gao, J.F. Experimental Guidance for Plant Physiology, 1st ed.; Higher Education Press: Beijing, China, 2006; pp. 144–148. ISBN 7-04-019170-9. [Google Scholar]
- Zhang, C. Regulation of Starch-sugar Metabolism via Repression of AcidInvertase in Potato (Solanum tuberosum L.) Tubers. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2007. [Google Scholar]
- Li, H.S. Principle and Technology of Plant Physiological and Biochemical Experiment, 1st ed.; Higher Education Press: Beijing, China, 2000; pp. 182–185. ISBN 7-04-008076-1. [Google Scholar]
- Abedi, E.; Sayadi, M.; Pourmohammadi, K. Effect of freezing-thawing pre-treatment on enzymatic modification of corn and potato starch treated with activated α-amylase: Investigation of functional properties. Food Hydrocoll. 2022, 129, 107676. [Google Scholar] [CrossRef]
- He, Z.P. The Experimental Guide for Crops Chemical Control, 1st ed.; China Agricultural University Press: Beijing, China, 1993; pp. 60–68. ISBN 7-81002-421-3. [Google Scholar]
- NY/T 2939-2016; Standard Description of Sweet Potato Germplasm Resources. Ministry of Agriculture and Rural Affairs: Beijing, China, 2016.
| Cultivars | Temperature | Sprouting Time | Emergence Stage | Full Stand of Seedling Stage |
|---|---|---|---|---|
| (d) | (d) | (d) | ||
| Long9 | 17 °C | 21.33 ± 1.21 a | n.d. | n.d. |
| 22 °C | 7.33 ± 0.82 c | 22.50 ± 1.22 a | 27.33 ± 1.21 a | |
| 27 °C | 4.50 ± 0.55 d | 13.33 ± 1.03 c | 20.83 ± 1.33 c | |
| 21/13 °C | 19.83 ± 1.17 b | n.d. | n.d. | |
| 26/18 °C | 6.50 ± 0.84 c | 20.17 ± 1.60 b | 25.33 ± 1.21 b | |
| 31/23 °C | 4.00 ± 0.63 d | 11.33 ± 0.52 d | 18.50 ± 0.84 d | |
| YHX2 | 17 °C | 18.33 ± 0.82 a | n.d. | n.d. |
| 22 °C | 6.67 ± 0.82 c | 18.00 ± 1.10 a | 25.00 ± 0.89 a | |
| 27 °C | 4.50 ± 0.55 d | 10.50 ± 0.84 c | 15.67 ± 1.03 c | |
| 21/13 °C | 16.50 ± 1.52 b | n.d. | n.d. | |
| 26/18 °C | 6.33 ± 1.03 c | 16.50 ± 0.84 b | 21.33 ± 1.21 b | |
| 31/23 °C | 3.83 ± 0.75 d | 8.67 ± 0.82 d | 13.00 ± 0.89 d | |
| YHX98 | 17 °C | 16.33 ± 0.82 a | n.d. | n.d. |
| 22 °C | 6.50 ± 0.55 c | 16.33 ± 1.21 a | 23.50 ± 1.52 a | |
| 27 °C | 4.17 ± 0.75 d | 9.67 ± 0.52 c | 14.17 ± 0.75 c | |
| 21/13 °C | 14.17 ± 1.17 b | n.d. | n.d. | |
| 26/18 °C | 6.00 ± 1.10 c | 15.00 ± 0.63 b | 20.50 ± 1.64 b | |
| 31/23 °C | 3.67 ± 0.52 d | 8.50 ± 0.55 d | 11.50 ± 1.05 d | |
| Significance | ||||
| Cultivar | ** | ** | ** | |
| Temperature | ** | ** | ** | |
| Cutivar × Temperature | ** | * | * | |
| Cultivars | Temperature | Days After Seeding | |||||
|---|---|---|---|---|---|---|---|
| 21 d | 28 d | 35 d | |||||
| A 100-Seedling Weight | Seedling Cutting Amount | A 100-Seedling Weight | Seedling Cutting Amount | A 100-Seedling Weight | Seedling Cutting Amount | ||
| (g·100-Plants−1) | (Plant) | (g·100-Plants−1) | (Plant) | (g·100-Plants−1) | (Plant) | ||
| Long9 | 17 °C | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 22 °C | n.d. | n.d. | n.d. | n.d. | 752.33 ± 23.46 c | 2.43 ± 0.09 d | |
| 27 °C | 159.67 ± 11.24 b | 0.77 ± 0.20 b | 591.90 ± 3.86 b | 3.50 ± 0.17 b | 1177.65 ± 174.54 a | 6.35 ± 0.14 b | |
| 21/13 °C | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| 26/18 °C | n.d. | n.d. | 223.27 ± 7.82 c | 0.90 ± 0.09 c | 1049.98 ± 155.87 b | 3.30 ± 0.04 c | |
| 31/23 °C | 250.58 ± 10.93 a | 1.3 ± 0.05 a | 626.33 ± 27.43 a | 4.11 ± 0.51 a | 1228.86 ± 184.79 a | 6.73 ± 0.12 a | |
| YHX2 | 17 °C | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 22 °C | n.d. | n.d. | n.d. | n.d. | 762.22 ± 42.34 c | 2.47 ± 0.21 c | |
| 27 °C | 503.70 ± 1.79 b | 1.18 ± 0.17 a | 597.13 ± 11.80 b | 4.50 ± 0.17 b | 1174.23 ± 41.51 b | 6.50 ± 0.17 a | |
| 21/13 °C | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| 26/18 °C | n.d. | n.d. | 281.31 ± 5.69 c | 1.32 ± 0.21 c | 1301.62 ± 49.71 a | 3.55 ± 0.19 b | |
| 31/23 °C | 568.08 ± 4.63 a | 1.33 ± 0.33 a | 690.63 ± 21.85 a | 5.50 ± 0.17 a | 1268.35 ± 35.65 a | 6.51 ± 0.17 a | |
| YHX98 | 17 °C | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 22 °C | n.d. | n.d. | 320.5 ± 14.91 d | 1.43 ± 0.17 d | 737.88 ± 57.82 c | 2.52 ± 0.17 d | |
| 27 °C | 525.42 ± 28.22 b | 1.15 ± 0.16 b | 832.32 ± 9.14 b | 4.66 ± 0.34 b | 987.27 ± 97.11 b | 5.55 ± 0.09 b | |
| 21/13 °C | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| 26/18 °C | n.d. | n.d. | 452.50 ± 5.68 c | 1.77 ± 0.20 c | 1327.88 ± 20.27 a | 3.72 ± 0.26 c | |
| 31/23 °C | 572.72 ± 10.33 a | 1.77 ± 0.20 a | 999.42 ± 13.49 a | 5.50 ± 0.17 a | 1264.82 ± 62.69 a | 6.19 ± 0.17 a | |
| Significance | |||||||
| Cultivar | ** | * | ** | ** | ns | * | |
| Temperature | ** | ** | ** | ** | ** | ** | |
| Cultivar × Temperature | ns | ns | ** | * | * | ** | |
| Cultivars | Temperature | Seedling Thickness | Seedling Height | Number of Expanded Leaves | Number of Internodes | Seedling Weight | Seedling Number |
|---|---|---|---|---|---|---|---|
| (cm) | (cm) | (Piece) | (Internode) | (g·kg−1) | (Plant·kg−1) | ||
| Long9 | 17 °C | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 22 °C | 0.29 ± 0.01 c | 19.54 ± 0.99 d | 4.66 ± 0.85 d | 6.69 ± 0.55 d | 197.18 ± 11.52 d | 16.7815 ± 0.47 d | |
| 27 °C | 0.38 ± 0.02 b | 55.97 ± 1.04 a | 10.19 ± 0.33 a | 15.23 ± 0.43 a | 422.38 ± 6.64 b | 50 ± 1.44 b | |
| 21/13 °C | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| 26/18 °C | 0.31 ± 0.01 c | 29.26 ± 1.6 c | 7.41 ± 0.47 c | 9.83 ± 0.76 c | 289.72 ± 3.13 c | 30.8524 ± 0.29 c | |
| 31/23 °C | 0.42 ± 0.09 a | 39.73 ± 3.33 b | 8.68 ± 0.12 b | 12.10 ± 0.14 b | 489.70 ± 3.43 a | 52.076 ± 1.23 a | |
| YHX2 | 17 °C | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 22 °C | 0.31 ± 0.02 c | 25.27 ± 0.73 d | 6.12 ± 0.29 c | 8.61 ± 0.38 d | 251.09 ± 2 d | 26.7118 ± 1.11 c | |
| 27 °C | 0.33 ± 0.01 b | 73.34 ± 1.02 a | 12.71 ± 0.44 a | 17.78 ± 0.16 a | 355.86 ± 2.27 b | 35.21 ± 0.88 b | |
| 21/13 °C | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| 26/18 °C | 0.32 ± 0.02 bc | 35.62 ± 2.7 c | 9.37 ± 0.23 b | 12.58 ± 0.53 c | 284.11 ± 4.29 c | 28.3169 ± 1.43 c | |
| 31/23 °C | 0.35 ± 0.03 a | 54.23 ± 0.63 b | 9.89 ± 0.62 b | 13.36 ± 0.07 b | 538.60 ± 11.85 a | 45.06 ± 2.20 a | |
| YHX98 | 17 °C | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 22 °C | 0.3 ± 0.01 b | 40.96 ± 2.41 d | 7.77 ± 0.8 d | 10.83 ± 0.52 d | 279.56 ± 3.94 d | 26.84 ± 1.20 c | |
| 27 °C | 0.19 ± 0.01 c | 131.18 ± 2.2 a | 14.64 ± 0.12 a | 20.01 ± 0.91 a | 308.87 ± 4.89 c | 31.03 ± 1.35 b | |
| 21/13 °C | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| 26/18 °C | 0.34 ± 0.01 a | 52.78 ± 2.73 c | 10.97 ± 0.27 c | 14.92 ± 0.72 c | 371.95 ± 10.43 a | 27.21 ± 0.15 c | |
| 31/23 °C | 0.29 ± 0.03 b | 95.04 ± 4.68 b | 12.24 ± 0.28 b | 16.71 ± 0.86 b | 353.11 ± 1.94 b | 33.66 ± 0.79 a | |
| Significance | |||||||
| Cultivar | ** | ** | ** | ** | ** | ** | |
| Temperature | * | ** | ** | ** | ** | ** | |
| Cultivar × Temperature | ** | ** | ns | ns | ** | ** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, G.; Gou, Y.; Zhang, L.; Tang, M.; Li, Y.; Song, Y.; Deng, S.; Du, K.; Lv, C.; Tang, D.; et al. Effects of Different Temperatures on the Physiological Characteristics of Sweet Potato (Ipomoea batatas L. Lam) Storage Roots and Growth of Seedlings During the Sprouting and Seedling Period. Plants 2025, 14, 868. https://doi.org/10.3390/plants14060868
Sun G, Gou Y, Zhang L, Tang M, Li Y, Song Y, Deng S, Du K, Lv C, Tang D, et al. Effects of Different Temperatures on the Physiological Characteristics of Sweet Potato (Ipomoea batatas L. Lam) Storage Roots and Growth of Seedlings During the Sprouting and Seedling Period. Plants. 2025; 14(6):868. https://doi.org/10.3390/plants14060868
Chicago/Turabian StyleSun, Guangyan, Yi Gou, Linxi Zhang, Mingjun Tang, Yucui Li, Yiming Song, Shuwen Deng, Kang Du, Changwen Lv, Daobin Tang, and et al. 2025. "Effects of Different Temperatures on the Physiological Characteristics of Sweet Potato (Ipomoea batatas L. Lam) Storage Roots and Growth of Seedlings During the Sprouting and Seedling Period" Plants 14, no. 6: 868. https://doi.org/10.3390/plants14060868
APA StyleSun, G., Gou, Y., Zhang, L., Tang, M., Li, Y., Song, Y., Deng, S., Du, K., Lv, C., Tang, D., & Wang, J. (2025). Effects of Different Temperatures on the Physiological Characteristics of Sweet Potato (Ipomoea batatas L. Lam) Storage Roots and Growth of Seedlings During the Sprouting and Seedling Period. Plants, 14(6), 868. https://doi.org/10.3390/plants14060868






