A Comparative Analysis of the Effect of 24-Epibrassinolide on the Tolerance of Wheat Cultivars with Different Drought Adaptation Strategies Under Water Deficit Conditions
Abstract
1. Introduction
2. Results
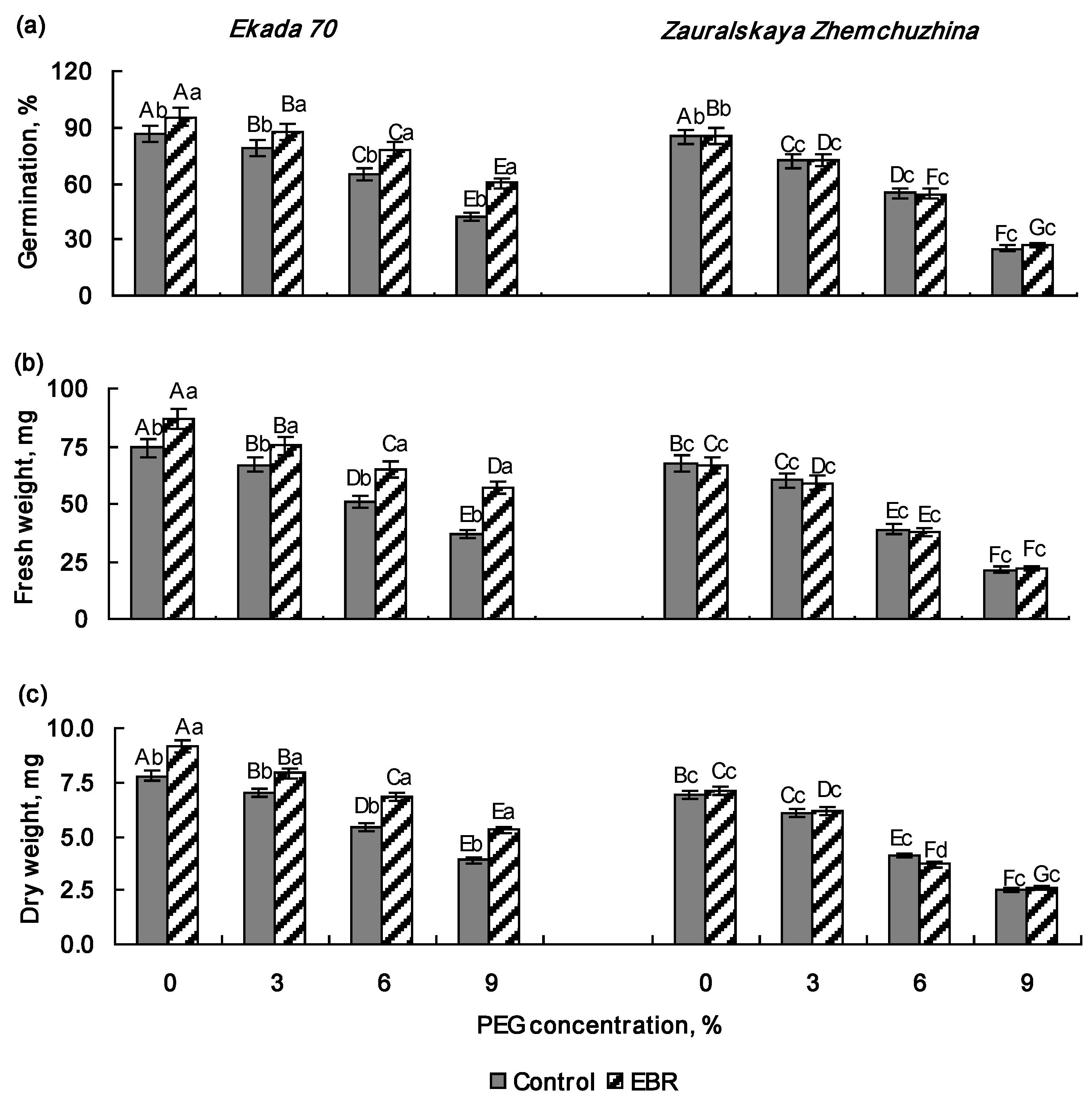
2.1. Effect of 24-Epibrassinolide Pretreatment on Seed Germination and Early Growth of Wheat Seedlings Under PEG Exposure
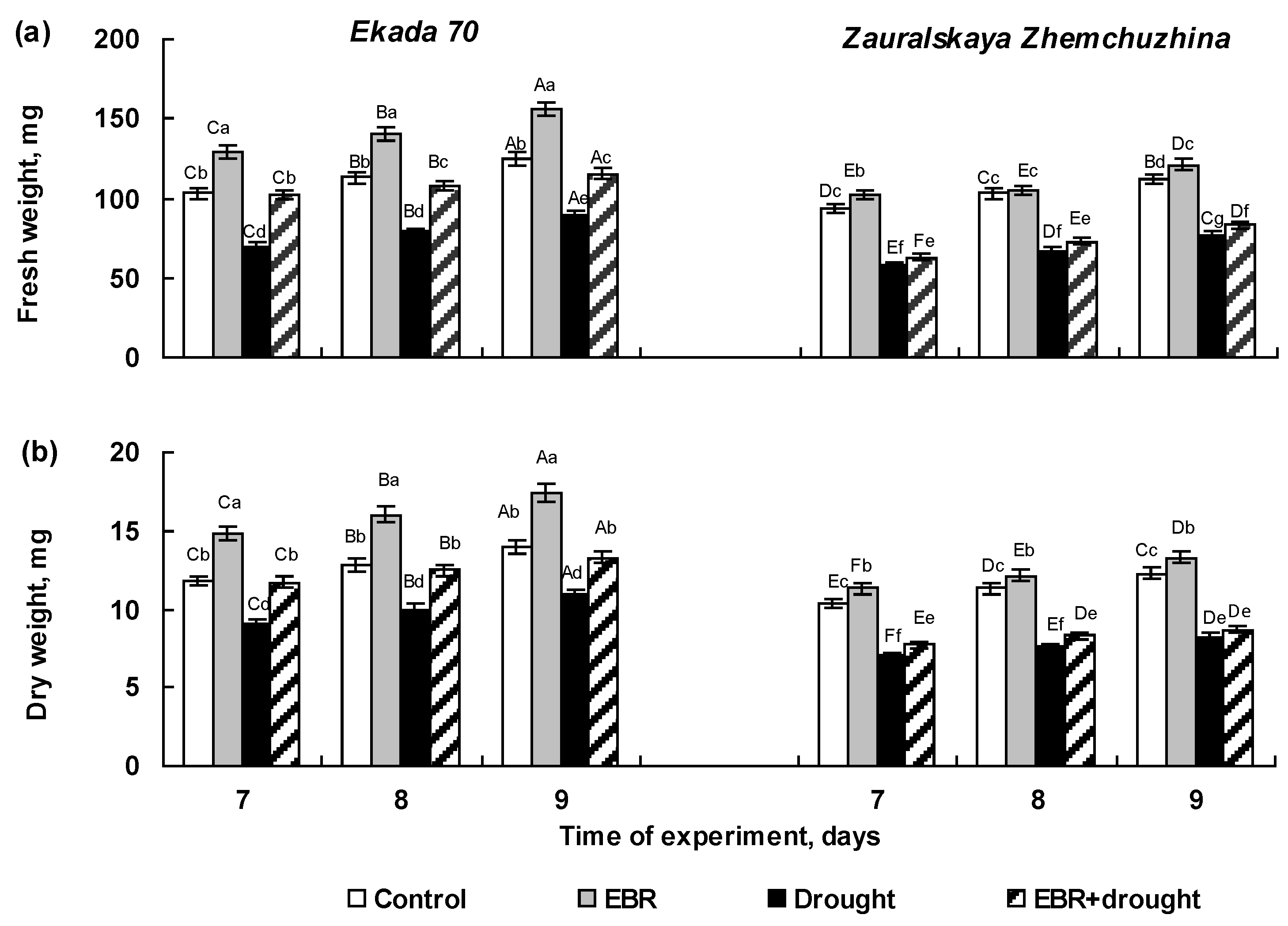
2.2. The Effect of 24-Epibrassinolide Seed Pretreatment on Growth of Wheat Seedlings Exposed to Soil Drought in Pot Experiments
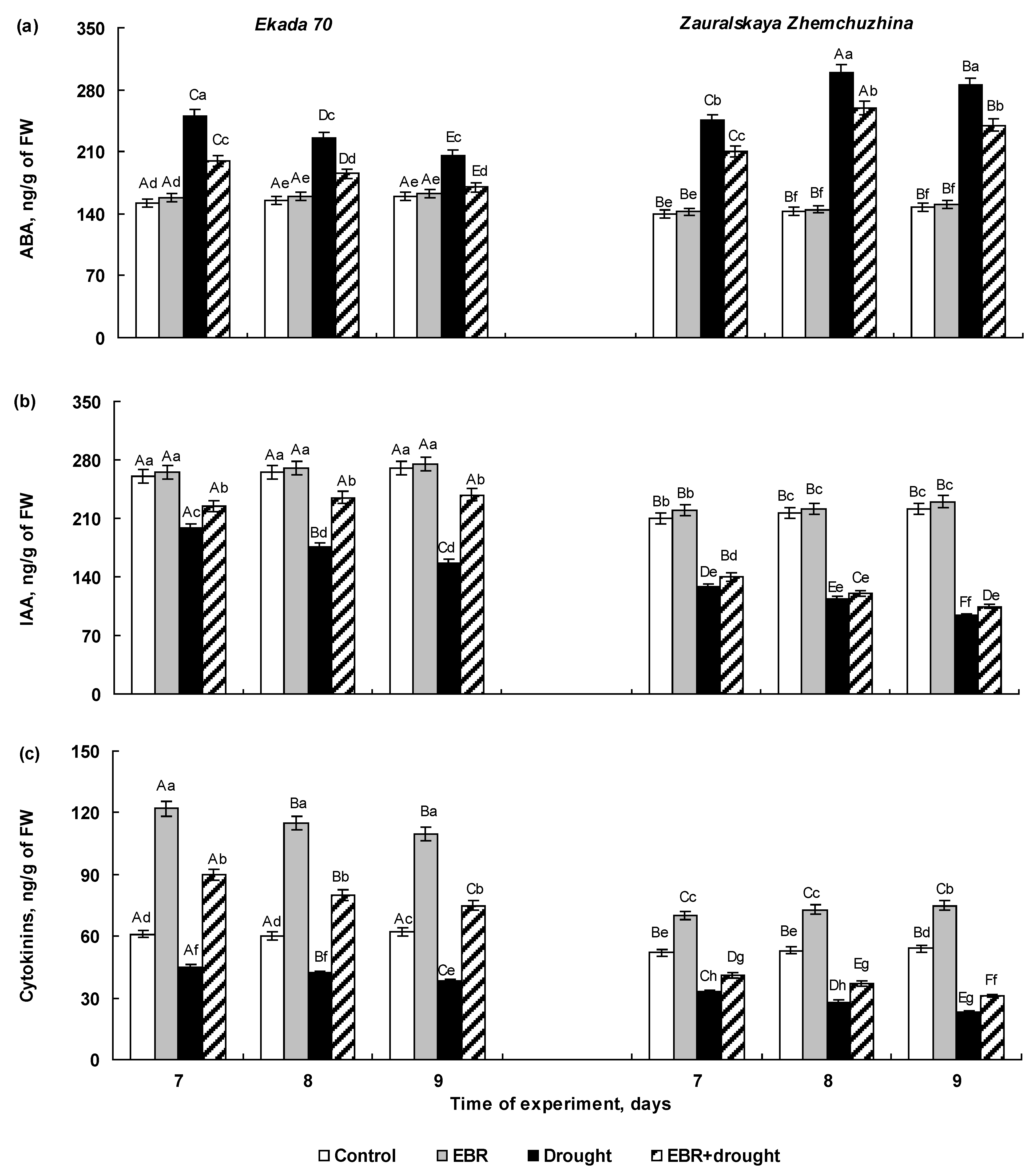
2.3. The Effect of 24-Epibrassinolide Seed Pretreatment on Hormonal Balance of Wheat Seedlings Subjected to Soil Drought
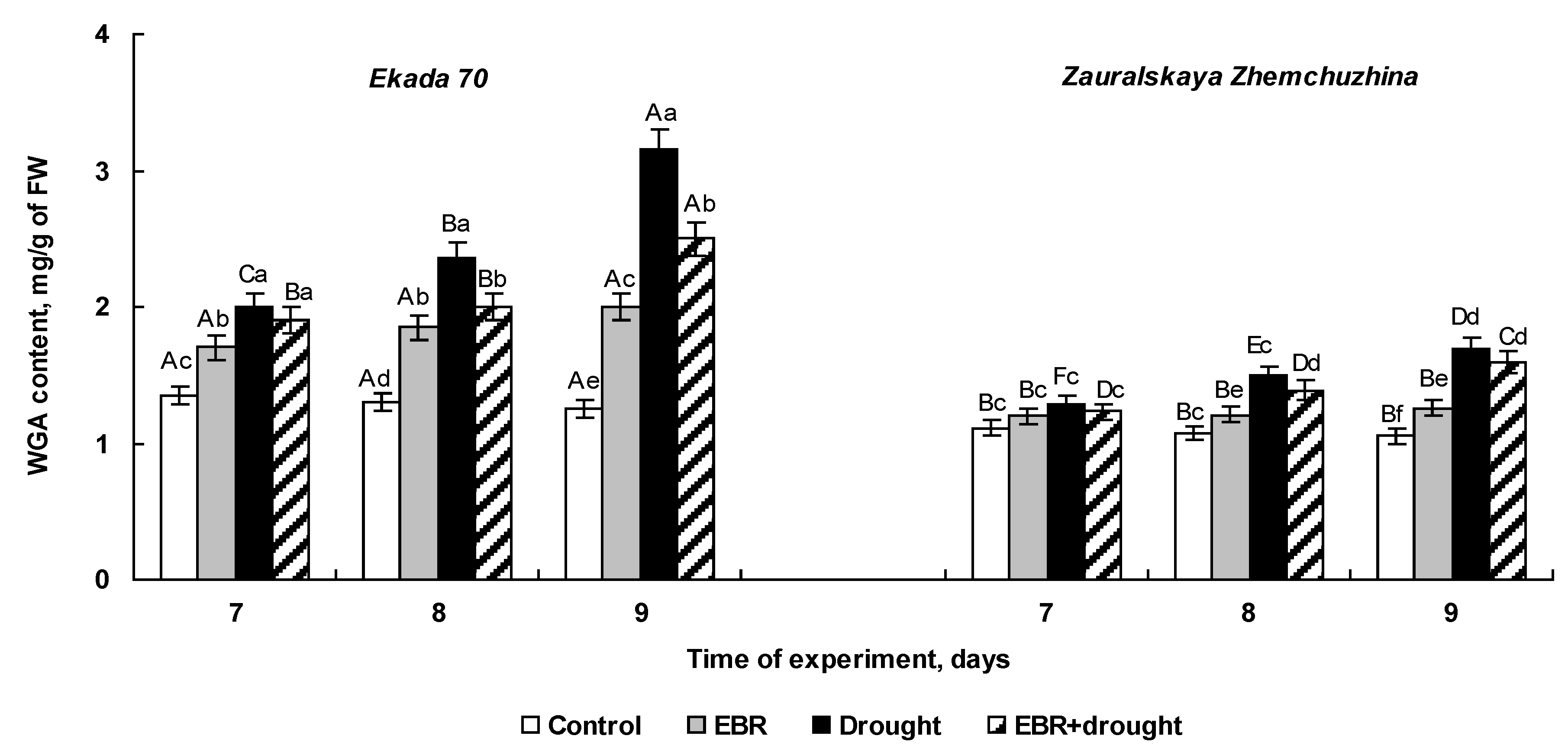
2.4. The Effect of 24-Epibrassinolide Seed Pretreatment on WGA Accumulation of Wheat Seedlings Subjected to Soil Drought
2.5. The Effect of 24-Epibrassinolide Seed Pretreatment on Drought-Induced MDA Accumulation and Elektrolyte Leackage in Wheat Seedlings
3. Discussion
4. Materials and Methods
4.1. Seed Material, Pre-Sowing EBR-Treatment
4.2. Assessment of Seed Germination and Early Seedling Growth Assays
4.3. Pot Experiment Design, Treatments, and Soil Drought Conditions
4.4. Immunoassay of Phytohormones and WGA
4.5. Measurements of MDA and Electrolyte Leakage
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dietz, K.J.; Zörb, C.; Geilfus, C.M. Drought and Crop Yield. Plant Biol. 2021, 23, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Stallmann, J.; Schweiger, R.; Müller, C. Effects of continuous versus pulsed drought stress on physiology and growth of wheat. Plant Biol. 2018, 20, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Aliyeva, D.R.; Gurbanova, U.A.; Rzayev, F.H.; Gasimov, E.K.; Huseynova, I.M. Biochemical and Ultrastructural Changes in Wheat Plants During Drought Stress. Biochemistry 2023, 88, 1944–1955. [Google Scholar] [CrossRef] [PubMed]
- González, E.M. Drought Stress Tolerance in Plants. Int. J. Mol. Sci. 2023, 24, 6562. [Google Scholar] [CrossRef]
- Kruglova, N.N.; Zinatullina, A.E. Some methodological approaches to the identification of heat-resistant genotypes of crop plants (by the example of cereals). Biol. Bull. Rev. 2023, 13, 371–381. [Google Scholar] [CrossRef]
- Munns, R.; Millar, A.H. Seven Plant Capacities to Adapt to Abiotic Stress. J. Exp. Bot. 2023, 74, 4308–4323. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, W.; Ma, J.; Cheng, Z.; Zhang, X.; Liu, X.; Wu, X.; Zhang, J. An Integrated Framework for Drought Stress in Plants. Int. J. Mol. Sci. 2024, 25, 9347. [Google Scholar] [CrossRef]
- Volis, S.; Ormanbekova, D.; Yermekbayev, K.; Song, M.; Shulgina, I. Multi-approaches analysis reveals local adaptation in the emmer wheat (Triticum dicoccoides) at macro- but not microgeographical scale. PLoS ONE 2015, 10, e0125258. [Google Scholar] [CrossRef]
- Lastochkina, O.V.; Yuldashev, R.A.; Pusenkova, L.I. The effect of bacteria Bacillus subtilis 26D on drought resistance of soft spring wheat cultivars of the West Siberian forest-steppe and Volga steppe ecotypes in the early stages of ontogenesis. Proc. RAS Ufa Sci. Cent. 2017, 3, 99–102. [Google Scholar]
- Zhukovsky, P.M. Wheat in the USSR; State Publishing House of Agricultural Literature: Moscow/Leningrad, Russia, 1957; p. 632. [Google Scholar]
- Cigankov, V.I. Development of spring wheat cultivars adaptive to drought steppe regions of Kazakhstan. Proc. Orenbg. State Agric. Univ. 2012, 4, 112–115. [Google Scholar]
- Muhitov, L.A.; Samuilov, F.D. Drought tolerance of different ecotypes of wheat in Forest-Steppe of Orenburg Pre-Ural. Proc. Kazan State Agric. Univ. 2007, 1, 57–59. [Google Scholar]
- Hong, J.H.; Seah, S.W.; Xu, J. The root of ABA action in environmental stress response. Plant Cell Rep. 2013, 32, 971–983. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, F.; Yan, Q.; Duan, Z.; Wang, S.; Ao, B.; Han, Y.; Zhang, J. Genome-wide analysis of the Rab. gene family in Melilotus albus reveals their role in salt tolerance. Int. J. Mol. Sci. 2022, 24, 126. [Google Scholar] [CrossRef] [PubMed]
- Raikhel, N.V.; Lee, H.I.; Broekaert, W.F. Structure and Function of Chitin-Binding Proteins. Ann. Rev. Plant Biol. 1993, 44, 591–615. [Google Scholar] [CrossRef]
- Avalbaev, A.; Bezrukova, M.; Allagulova, C.; Lubyanova, A.; Kudoyarova, G.; Fedorova, K.; Maslennikova, D.; Yuldashev, R.; Shakirova, F. Wheat germ agglutinin is involved in the protective action of 24-epibrassinolide on the roots of wheat seedlings under drought conditions. Plant Physiol. Biochem. 2020, 146, 420–427. [Google Scholar] [CrossRef]
- Jiang, S.Y.; Ma, Z.; Ramachandran, S. Evolutionary history and stress regulation of the lectin superfamily in higher plants. BMC Evol. Biol. 2010, 10, 79–103. [Google Scholar] [CrossRef]
- Singh, P.; Bhaglal, P.; Bhullar, S. Wheat germ agglutinin (WGA) gene expression and ABA accumulation in the developing embryos of wheat (Triticum aestivum) in response to drought. Plant Growth Regul. 2000, 30, 145–150. [Google Scholar] [CrossRef]
- Shakirova, F.M.; Bezrukova, M.V.; Yuldashev, R.A.; Fatkhutdinova, R.A.; Murzabaev, A.R. Involvement of lectin in the salicylic acid-induced wheat tolerance to cadmium and the role of endogenous ABA in the regulation of its level. Dokl. Biol. Sci. 2013, 448, 1–3. [Google Scholar] [CrossRef]
- Bezrukova, M.V.; Fatkhutdinova, R.A.; Shakirova, F.M. Protective effect of wheat germ agglutinin on the course of mitosis in the roots of Triticum aestivum seedlings exposed to cadmium. Russ. J. Plant Physiol. 2016, 63, 238–244. [Google Scholar] [CrossRef]
- Bezrukova, M.V.; Fatkhutdinova, R.A.; Lubyanova, A.R.; Murzabaev, A.R.; Fedyaev, V.V.; Shakirova, F.M. Lectin involvement in the development of wheat tolerance to cadmium toxicity. Russ. J. Plant Physiol. 2011, 58, 1048–1054. [Google Scholar] [CrossRef]
- Bogoeva, V.P.; Radeva, M.A.; Atanasova, L.Y.; Stoitsova, S.R.; Boteva, R.N. Fluorescence analysis of hormone binding activities of wheat germ agglutinin. Biochim. Biophys. Acta 2004, 1698, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Shakirova, F.M.; Kildibekova, A.R.; Bezrukova, M.V.; Avalbaev, A.M. Wheat germ agglutinin regulates cell division in wheat seedling roots. Plant Growth Regul. 2004, 42, 175–180. [Google Scholar] [CrossRef]
- Shakirova, F.M.; Bezrukova, M.V.; Avalbaev, A.M.; Gimalov, F.R. Stimulation of wheat germ agglutinin gene expression in root seedlings by 24-epibrassinolide. Russ. J. Plant Physiol. 2002, 49, 225–228. [Google Scholar] [CrossRef]
- Kutschera, U.; Wang, Z.Y. Brassinosteroid action in flowering plants: A Darwinian perspective. J. Exp. Bot. 2012, 63, 3511–3522. [Google Scholar] [CrossRef]
- Wei, Z.; Li, J. Brassinosteroids regulate root growth, development, and symbiosis. Mol. Plant 2016, 9, 86–100. [Google Scholar] [CrossRef]
- Khan, T.A.; Kappachery, S.; Karumannil, S.; AlHosani, M.; Almansoori, N.; Almansoori, H.; Yusuf, M.; Tran, L.P.; Gururani, M.A. Brassinosteroid signaling pathways: Insights into plant responses under abiotic stress. Int. J. Mol. Sci. 2023, 24, 17246. [Google Scholar] [CrossRef]
- Zebosi, B.; Vollbrecht, E.; Best, N.B. Brassinosteroid biosynthesis and signaling: Conserved and diversified functions of core genes across multiple plant species. Plant Comm. 2024, 5, 100982. [Google Scholar] [CrossRef]
- Shakirova, F.M.; Bezrukova, M.V. Effect of 24-epibrassinolide and salinity on the level of ABA and lectin in wheat seedling roots. Russ. J. Plant Physiol. 1998, 45, 388–391. [Google Scholar]
- Razi, K.; Muneer, S. Drought stress-induced physiological mechanisms, signaling pathways and molecular response of chloroplasts in common vegetable crops. Crit. Rev. Biotechnol. 2021, 41, 669–691. [Google Scholar] [CrossRef]
- Hura, T.; Hura, K.; Ostrowska, A. Drought-Stress Induced Physiological and Molecular Changes in Plants. Int. J. Mol. Sci. 2022, 23, 4698. [Google Scholar] [CrossRef]
- Kong, H.; Meng, X.; Akram, N.A.; Zhu, F.; Hu, J.; Zhang, Z. Seed priming with fullerol improves seed germina-tion, seedling growth and antioxidant enzyme system of two winter wheat cultivars under drought stress. Plants 2023, 12, 1417. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hu, X.; Jiang, J.; Wang, X. Functions and Mechanisms of Brassinosteroids in Regulating Crop Agronomic Traits. Plant Cell Physiol. 2024, 65, 1568–1580. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S. BRacing for Water Stress: Brassinosteroid Signaling Promotes Drought Survival in Wheat. Plant Physiol. 2019, 180, 18–19. [Google Scholar] [CrossRef] [PubMed]
- Perez-Borroto, L.S.; Guzzo, M.C.; Posada, G.; Peña Malavera, A.N.; Castagnaro, A.P.; Gonzalez-Olmedo, J.L.; Coll-García, Y.; Pardo, E.M. A brassinosteroid functional analogue increases soybean drought resilience. Sci. Rep. 2022, 12, 11294. [Google Scholar] [CrossRef]
- Raza, M.A.S.; Ibrahim, M.A.; Ditta, A.; Iqbal, R.; Aslam, M.U.; Muhammad, F.; Ali, S.; Çiğ, F.; Ali, B.; Muhammad Ikram, R.; et al. Exploring the recuperative potential of brassinosteroids and nano-biochar on growth, physiology, and yield of wheat under drought stress. Sci. Rep. 2023, 13, 15015. [Google Scholar] [CrossRef]
- Fontanet-Manzaneque, J.B.; Laibach, N.; Herrero-García, I.; Coleto-Alcudia, V.; Blasco-Escámez, D.; Zhang, C.; Orduña, L.; Alseekh, S.; Miller, S.; Bjarnholt, N.; et al. Untargeted mutagenesis of brassinosteroid receptor SbBRI1 confers drought tolerance by altering phenylpropanoid metabolism in Sorghum bicolor. Plant Biotechnol. J. 2024, 22, 3406–3423. [Google Scholar] [CrossRef]
- Llanes, A.; Andrade, A.; Alemano, S.; Luna, V. Alterations of endogenous hormonal levels in plants under drought and salinity. American J. Plant Sci. 2016, 7, 1357–1371. [Google Scholar] [CrossRef]
- Matkowski, H.; Daszkowska-Golec, A. Wisdom comes after facts—An update on plants priming using phytohormones. J. Plant Physiol. 2024, 305, 154414. [Google Scholar] [CrossRef]
- Jardim-Messeder, D.; de Souza-Vieira, Y.; Sachetto-Martins, G. Dressed Up to the Nines: The Interplay of Phytohormones Signaling and Redox Metabolism During Plant Response to Drought. Plants 2025, 14, 208. [Google Scholar] [CrossRef]
- Zhang, X.; Goatley, M.; Wu, W.; Ervin, E.; Shang, C. Drought-induced Injury is Associated with Hormonal Alteration in Kentucky bluegrass. Plant Signal Behav. 2019, 14, e1651607. [Google Scholar] [CrossRef]
- Ji, X.; Dong, B.; Shiran, B.; Talbot, M.J.; Edlington, J.E.; Hughes, T.; White, R.G.; Gubler, F.; Dolferus, R. Control of Abscisic Acid Catabolism and Abscisic Acid Homeostasis is Important for Reproductive Stage Stress tolerance in Cereals. Plant Physiol. 2011, 156, 647–662. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ervin, E.H. Cytokinin-containing Seaweed and Humic Acid Extracts Associated with Creeping Bentgrass Leaf Cytokinins and Drought Resistance. Crop Sci. 2004, 44, 1737–1745. [Google Scholar] [CrossRef]
- Li, S.M.; Zheng, H.X.; Zhang, X.S.; Sui, N. Cytokinins as central regulators during plant growth and stress response. Plant Cell Rep. 2021, 40, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Mughal, N.; Shoaib, N.; Chen, J.; Li, Y.; He, Y.; Fu, M.; Li, X.; He, Y.; Guo, J.; Deng, J.; et al. Adaptive roles of cytokinins in enhancing plant resilience and yield against environmental stressors. Chemosphere 2024, 364, 143189. [Google Scholar] [CrossRef]
- Singh, P.; Chatterjee, S.; Ranjana, P.; Bhullar, S.S. Enhanced wheat germ agglutinin accumulation in the germinating embryos of wheat (Triticum aestivum L.) appears to be a general stress response. Curr. Sci. 1999, 76, 1140–1142. [Google Scholar]
- Demidchik, V. Mechanisms of oxidative stress in plants: From classical chemistry to cell biology. Environ. Exp. Bot. 2015, 109, 212–228. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Ma, C.; Kang, X.; Wang, J.; Zhang, T. 24-epibrassinolide enhanced cold tolerance of winter turnip rape (Brassica rapa L.). Biologia 2021, 76, 2859–2877. [Google Scholar] [CrossRef]
- Fan, J.; Tang, X.; Cai, J.; Tan, R.; Gao, X. Effects of exogenous EBR on the physiology of cold resistance and the expression of the VcCBF3 gene in blueberries during low-temperature stress. PLoS ONE 2025, 20, e0313194. [Google Scholar] [CrossRef]
- Kirova, E.; Pecheva, D.; Simova-Stoilova, L. Drought response in winter wheat: Protection from oxidative stress and mutagenesis effect. Acta Physiol. Plant. 2021, 43, 8. [Google Scholar] [CrossRef]
- Bezrukova, M.; Kildibekova, A.; Shakirova, F. WGA reduces the level of oxidative stress in wheat seedlings under salinity. Plant Growth Regul. 2008, 54, 195–201. [Google Scholar] [CrossRef]
| Treatment | MDA, mol g−1 Fresh Weight | EL, µS/g Fresh Weight | ||
|---|---|---|---|---|
| E70 | ZZh | E70 | ZZh | |
| Control | 41.3 ± 2.1 Cb | 49.9 ± 2.5 Ba | 20.1 ± 0.9 Cb | 23.3 ± 1.1 Ba |
| EBR | 43.8 ± 2.2 Cb | 51.7 ± 2.6 Ba | 21.2 ± 1.0 Cb | 24.4 ± 1.2 Ba |
| Drought | 70.3 ± 3.5 Ab | 98.3 ± 4.1 Aa | 35.1 ± 1.7 Ab | 47.8 ± 2.3 Aa |
| EBR + drought | 52.4 ± 2.6 Bb | 91.7 ± 3.8 Aa | 25.7 ± 1.2 Bb | 43.5 ± 2.1 Aa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avalbaev, A.; Yuldashev, R.; Plotnikov, A.; Allagulova, C. A Comparative Analysis of the Effect of 24-Epibrassinolide on the Tolerance of Wheat Cultivars with Different Drought Adaptation Strategies Under Water Deficit Conditions. Plants 2025, 14, 869. https://doi.org/10.3390/plants14060869
Avalbaev A, Yuldashev R, Plotnikov A, Allagulova C. A Comparative Analysis of the Effect of 24-Epibrassinolide on the Tolerance of Wheat Cultivars with Different Drought Adaptation Strategies Under Water Deficit Conditions. Plants. 2025; 14(6):869. https://doi.org/10.3390/plants14060869
Chicago/Turabian StyleAvalbaev, Azamat, Ruslan Yuldashev, Anton Plotnikov, and Chulpan Allagulova. 2025. "A Comparative Analysis of the Effect of 24-Epibrassinolide on the Tolerance of Wheat Cultivars with Different Drought Adaptation Strategies Under Water Deficit Conditions" Plants 14, no. 6: 869. https://doi.org/10.3390/plants14060869
APA StyleAvalbaev, A., Yuldashev, R., Plotnikov, A., & Allagulova, C. (2025). A Comparative Analysis of the Effect of 24-Epibrassinolide on the Tolerance of Wheat Cultivars with Different Drought Adaptation Strategies Under Water Deficit Conditions. Plants, 14(6), 869. https://doi.org/10.3390/plants14060869







