Cultivated St. John’s Wort Flower Heads Accumulate Tocotrienols over Tocopherols, Regardless of the Year of the Plant
Abstract
1. Introduction
2. Results and Discussion
2.1. Tocopherols and Tocotrienols in Four Aerial Parts of Cultivated H. perforatum
2.2. Distribution of Tocochromanols in Cultivated H. perforatum: Flower Heads in the Spotlight
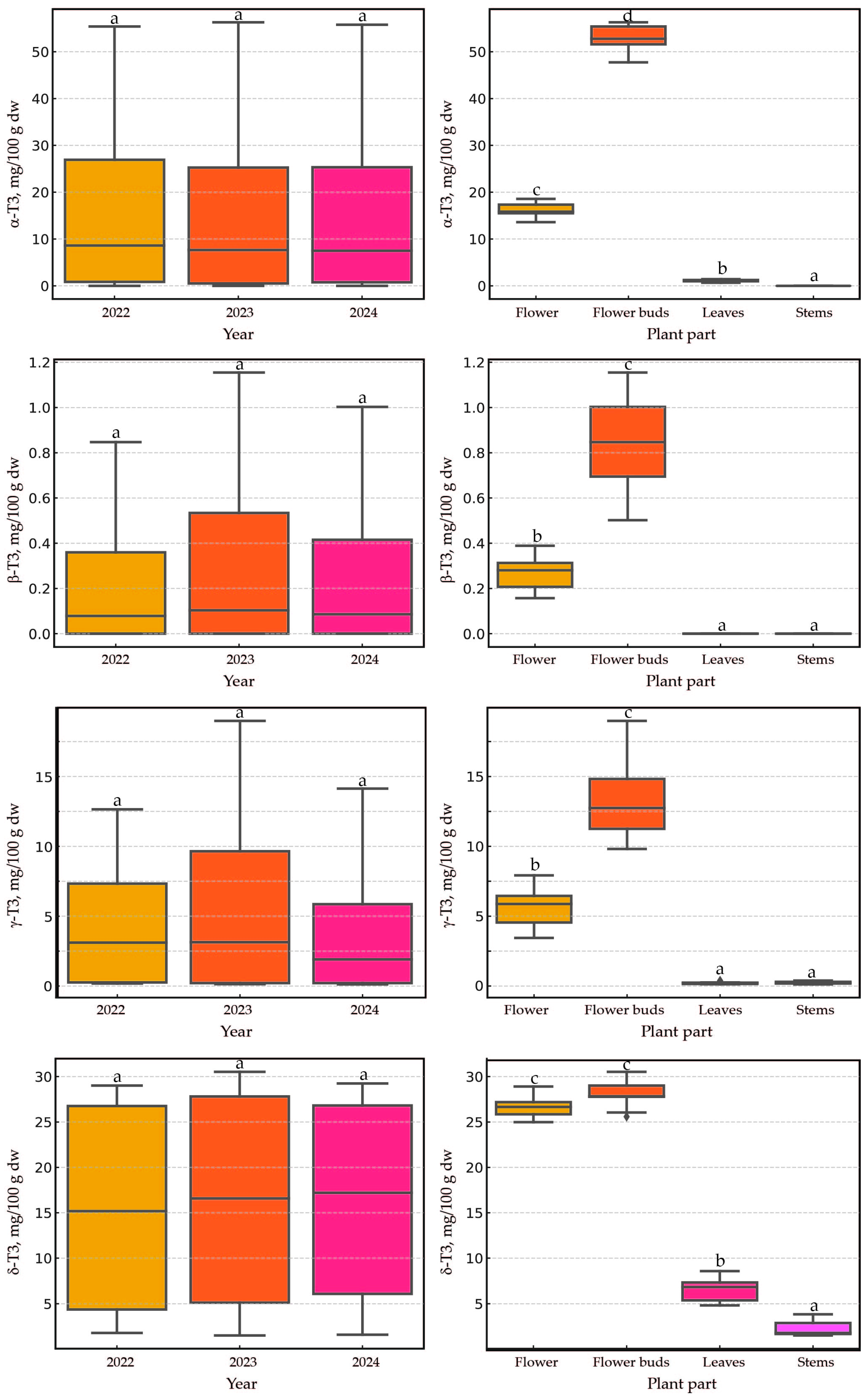
2.3. Impact of Harvest Year and Plant Part on Tocopherols and Tocotrienols Content in Cultivated H. perforatum
3. Materials and Methods
3.1. Reagents
3.2. Plant Material
3.3. H. perforatum Sample Preparation for Tocochormanols’ Determination (Saponification Protocol)
3.4. Tocochromanols’ (Tocopherols and Tocotrienols) Determination by RP-HPLC-FLD
3.5. RPLC-APCI-HRMS Analysis
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Angiosperm Phylogeny Group. An ordinal classification for the families of flowering plants. Ann. Mo. Bot. Gard. 1998, 85, 531–553. [Google Scholar] [CrossRef]
- The Angiosperm Phylogeny Group. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Bot. J. Linn. Soc. 2003, 141, 399–436. [Google Scholar] [CrossRef]
- Mišina, I.; Lazdiņa, D.; Górnaś, P. Tocochromanols in the leaves of plants in the Hypericum and Clusia genera. Molecules 2025, 30, 709. [Google Scholar] [CrossRef]
- Lazdiņa, D.; Mišina, I.; Górnaś, P. Tocotrienols in eleven species of Hypericum genus leaves. Molecules 2025, 30, 662. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Alegre, L. The function of tocopherols and tocotrienols in plants. Crit. Rev. Plant Sci. 2002, 21, 31–57. [Google Scholar] [CrossRef]
- Napoli, E.; Siracusa, L.; Ruberto, G.; Carrubba, A.; Lazzara, S.; Speciale, A.; Cimino, F.; Saija, A.; Cristani, M. Phytochemical profiles, phototoxic and antioxidant properties of eleven Hypericum species–A comparative study. Phytochemistry 2018, 152, 162–173. [Google Scholar] [CrossRef]
- Górnaś, P.; Mišina, I.; Lazdiņa, D. Annatto (Bixa orellana) seeds, palm (Elaeis guineensis) oils, and St. John’s wort (Hypericum perforatum) aerial parts as sources of rare prenyllipids. Eur. Food Res. Technol. 2025. [Google Scholar] [CrossRef]
- Zainal, Z.; Khaza’ai, H.; Radhakrishnan, A.K.; Chang, S.K. Therapeutic potential of palm oil vitamin E-derived tocotrienols in inflammation and chronic diseases: Evidence from preclinical and clinical studies. Food Res. Int. 2022, 156, 111175. [Google Scholar] [CrossRef]
- Spicher, L.; Almeida, J.; Gutbrod, K.; Pipitone, R.; Dörmann, P.; Glauser, G.; Rossi, M.; Kessler, F. Essential role for phytol kinase and tocopherol in tolerance to combined light and temperature stress in tomato. J. Exp. Bot. 2017, 68, 5845–5856. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.; Garcia, Q.S.; Siqueira-Silva, A.I.; Silva, M.C.; Munné-Bosch, S. Tocotrienols in Vellozia gigantea leaves: Occurrence and modulation by seasonal and plant size effects. Planta 2014, 240, 437–446. [Google Scholar] [CrossRef]
- Matringe, M.; Ksas, B.; Rey, P.; Havaux, M. Tocotrienols, the unsaturated forms of vitamin E, can function as antioxidants and lipid protectors in tobacco leaves. Plant Physiol. 2008, 147, 764–778. [Google Scholar] [CrossRef]
- Lucht, J.M. Public acceptance of plant biotechnology and GM crops. Viruses 2015, 7, 4254–4281. [Google Scholar] [CrossRef]
- Lazzara, S.; Carrubba, A.; Napoli, E. Cultivating for the industry: Cropping experiences with Hypericum perforatum L. in a Mediterranean environment. Agriculture 2021, 11, 446. [Google Scholar] [CrossRef]
- Büter, B.; Orlacchio, C.; Soldati, A.; Berger, K. Significance of genetic and environmental aspects in the field cultivation of Hypericum perforatum. Planta Med. 1998, 64, 431–437. [Google Scholar] [CrossRef]
- de Abreu, I.N.; Mazzafera, P. Effect of water and temperature stress on the content of active constituents of Hypericum brasiliense Choisy. Plant Physiol. Biochem. 2005, 43, 241–248. [Google Scholar] [CrossRef]
- Górnaś, P.; Siger, A. Rare prenyllipids in wild St. John’s wort during three harvest seasons. Molecules 2025, 30, 901. [Google Scholar] [CrossRef] [PubMed]
- Górnaś, P.; Mišina, I.; Perkons, I.; Segliņa, D.; Czlapka-Matyasik, M. Characterization of tocochromanols in wild Hypericum perforatum populations in Latvia. Horticulturae 2025, 11, 205. [Google Scholar] [CrossRef]
- Mesa, T.; Munné-Bosch, S. α-Tocopherol in chloroplasts: Nothing more than an antioxidant? Curr. Opin. Plant Biol. 2023, 74, 102400. [Google Scholar] [CrossRef]
- Trela, A.; Szymańska, R. Less widespread plant oils as a good source of vitamin E. Food Chem. 2019, 296, 160–166. [Google Scholar] [CrossRef]
- Roriz, C.L.; Xavier, V.; Heleno, S.A.; Pinela, J.; Dias, M.I.; Calhelha, R.C.; Morales, P.; Ferreira, I.C.F.R.; Barros, L. Chemical and bioactive features of Amaranthus caudatus L. flowers and optimized ultrasound-assisted extraction of betalains. Foods 2021, 10, 779. [Google Scholar] [CrossRef]
- Pop, A.; Fizeșan, I.; Vlase, L.; Rusu, M.E.; Cherfan, J.; Babota, M.; Gheldiu, A.-M.; Tomuta, I.; Popa, D.-S. Enhanced recovery of phenolic and tocopherolic compounds from walnut (Juglans regia L.) male flowers based on process optimization of ultrasonic assisted-extraction: Phytochemical profile and biological activities. Antioxidants 2021, 10, 607. [Google Scholar] [CrossRef]
- López-Cervantes, J.; Sánchez-Machado, D.I.; Cruz-Flores, P.; Mariscal-Domínguez, M.F.; de la Mora-López, G.S.; Campas-Baypoli, O.N. Antioxidant capacity, proximate composition, and lipid constituents of Aloe vera flowers. J. Appl. Res. Med. Aromat. Plants 2018, 10, 93–98. [Google Scholar] [CrossRef]
- Fernandes, L.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E.; Casal, S. Phytochemical characterization of Borago officinalis L. and Centaurea cyanus L. during flower development. Food Res. Int. 2019, 123, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.; Ramalhosa, E.; Pereira, J.A.; Saraiva, J.A.; Casal, S. Borage, camellia, centaurea and pansies: Nutritional, fatty acids, free sugars, vitamin E, carotenoids and organic acids characterization. Food Res. Int. 2020, 132, 109070. [Google Scholar] [CrossRef] [PubMed]
- Tlili, N.; Nasri, N.; Saadaoui, E.; Khaldi, A.; Triki, S. Carotenoid and tocopherol composition of leaves, buds, and flowers of Capparis spinosa grown wild in Tunisia. J. Agric. Food Chem. 2009, 57, 5381–5385. [Google Scholar] [CrossRef]
- Lockowandt, L.; Pinela, J.; Roriz, C.L.; Pereira, C.; Abreu, R.M.; Calhelha, R.C.; Alves, M.J.; Barros, L.; Bredol, M.; Ferreira, I.C.F.R. Chemical features and bioactivities of cornflower (Centaurea cyanus L.) capitula: The blue flowers and the unexplored non-edible part. Ind. Crop. Prod. 2019, 128, 496–503. [Google Scholar] [CrossRef]
- Arrom, L.; Munné-Bosch, S. Tocopherol composition in flower organs of Lilium and its variations during natural and artificial senescence. Plant Sci. 2010, 179, 289–295. [Google Scholar] [CrossRef]
- Muñoz, P.; Briones, M.; Munné-Bosch, S. Photoinhibition and photoprotection during flower opening in lilies. Plant Sci. 2018, 272, 220–229. [Google Scholar] [CrossRef]
- Fernandes, Â.; Bancessi, A.; Pinela, J.; Dias, M.I.; Liberal, Â.; Calhelha, R.C.; Ćirić, A.; Soković, M.; Catarino, L.; Ferreira, I.C.F.R. Nutritional and phytochemical profiles and biological activities of Moringa oleifera Lam. edible parts from Guinea-Bissau (West Africa). Food Chem. 2021, 341, 128229. [Google Scholar] [CrossRef]
- Baranauskienė, R.; Venskutonis, P.R. Supercritical CO2 extraction of Narcissus poeticus L. flowers for the isolation of volatile fragrance compounds. Molecules 2022, 27, 353. [Google Scholar] [CrossRef]
- Saini, R.K.; Ahn, H.-Y.; Park, G.-W.; Shin, J.-W.; Lee, J.-H.; Yu, J.-W.; Song, M.-H.; Keum, Y.-S.; Lee, J.-H. Quantitative profiling of carotenoids, tocopherols, phytosterols, and fatty acids in the flower petals of ten marigold (Tagetes spp. L.) cultivars. Foods 2023, 12, 3549. [Google Scholar] [CrossRef]
- Montoya-Arroyo, A.; Toro-González, C.; Sus, N.; Warner, J.; Esquivel, P.; Jiménez, V.M.; Frank, J. Vitamin E and carotenoid profiles in leaves, stems, petioles and flowers of stinging nettle (Urtica leptophylla Kunth) from Costa Rica. J. Sci. Food Agric. 2022, 102, 6340–6348. [Google Scholar] [CrossRef] [PubMed]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Ferreira, I.C.F.R. Nutritional and chemical characterization of edible petals and corresponding infusions: Valorization as new food ingredients. Food Chem. 2017, 220, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Górnaś, P.; Segliņa, D.; Lācis, G.; Pugajeva, I. Dessert and crab apple seeds as a promising and rich source of all four homologues of tocopherol (α, β, γ and δ). LWT-Food Sci. Technol. 2014, 59, 211–214. [Google Scholar] [CrossRef]
- Mišina, I.; Perkons, I.; Siger, A.; Soliven, A.; Górnaś, P. Residues of St. John’s wort (Hypericum perforatum) tea infusions/water extracts as a valuable source of tocotrienols: An extraction study. Appl. Sci. 2025, 15, 2047. [Google Scholar] [CrossRef]
- Górnaś, P.; Mišina, I.; Waśkiewicz, A.; Perkons, I.; Pugajeva, I.; Segliņa, D. Simultaneous extraction of tocochromanols and flavan-3-ols from the grape seeds: Analytical and industrial aspects. Food Chem. 2025, 462, 140913. [Google Scholar] [CrossRef]



| Plant Part | Tocochromanols, mg/100 g dw | Ratio Ts/T3s | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| α-T * | β-T | γ-T | δ-T | α-T3 | β-T3 | γ-T3 | δ-T3 | Total Ts | Total T3s | Total Ts + T3s | ||
| Stems | ||||||||||||
| Minimal | 2.1 | nd | 0.2 | nd | nd | nd | 0.1 | 1.5 | 2.4 | 1.7 | 4.1 | 0.9 |
| Maximal | 5.0 | tr | 0.6 | tr | tr | tr | 0.4 | 3.8 | 5.5 | 4.2 | 8.2 | 2.9 |
| Average | 3.6 | – | 0.4 | – | – | – | 0.2 | 2.2 | 3.9 | 2.5 | 6.4 | 1.7 |
| STDEV | 0.9 | – | 0.1 | – | – | – | 0.1 | 0.8 | 1.0 | 0.9 | 1.5 | 0.6 |
| Coefficient of variation | 24.5 | – | 39.0 | – | – | – | 40.6 | 37.1 | 24.6 | 37.0 | 23.4 | 35.4 |
| Leaves | ||||||||||||
| Minimal | 62.7 | 0.3 | 0.5 | nd | 0.7 | nd | 0.1 | 4.8 | 66.4 | 6.3 | 73.1 | 6.6 |
| Maximal | 85.8 | 0.7 | 3.0 | tr | 1.4 | tr | 0.4 | 8.6 | 87.3 | 10.1 | 95.6 | 11.9 |
| Average | 73.1 | 0.5 | 1.5 | – | 1.1 | – | 0.2 | 6.5 | 75.1 | 7.8 | 82.9 | 9.8 |
| STDEV | 7.5 | 0.1 | 0.8 | – | 0.3 | – | 0.1 | 1.3 | 7.2 | 1.2 | 7.4 | 1.5 |
| Coefficient of variation | 10.3 | 23.1 | 55.7 | – | 24.5 | – | 35.6 | 19.3 | 9.6 | 15.3 | 8.9 | 15.5 |
| Flower buds | ||||||||||||
| Minimal | 42.3 | 1.9 | 5.8 | 1.0 | 47.7 | 0.5 | 9.8 | 25.6 | 53.0 | 88.2 | 147.2 | 0.5 |
| Maximal | 63.3 | 4.5 | 8.3 | 2.1 | 56.3 | 1.7 | 19.0 | 30.5 | 76.0 | 104.7 | 173.9 | 0.8 |
| Average | 52.8 | 3.1 | 7.0 | 1.4 | 53.0 | 0.9 | 13.4 | 28.0 | 64.3 | 95.2 | 159.6 | 0.7 |
| STDEV | 7.0 | 0.8 | 0.7 | 0.3 | 2.8 | 0.2 | 3.0 | 1.5 | 7.4 | 5.9 | 8.4 | 0.1 |
| Coefficient of variation | 13.3 | 25.7 | 10.5 | 22.1 | 5.4 | 25.5 | 22.4 | 5.5 | 11.5 | 6.2 | 5.3 | 13.5 |
| Flowers | ||||||||||||
| Minimal | 39.4 | 2.5 | 4.9 | 0.9 | 13.6 | 0.2 | 3.4 | 25.0 | 48.9 | 43.1 | 97.1 | 1.0 |
| Maximal | 52.0 | 4.3 | 7.7 | 1.7 | 18.6 | 0.4 | 7.9 | 29.0 | 65.0 | 52.0 | 117.0 | 1.3 |
| Average | 45.6 | 3.5 | 6.4 | 1.2 | 16.1 | 0.3 | 5.7 | 26.8 | 56.8 | 48.8 | 105.6 | 1.2 |
| STDEV | 4.6 | 0.6 | 0.9 | 0.3 | 1.6 | 0.1 | 1.5 | 1.2 | 5.3 | 2.5 | 6.2 | 0.1 |
| Coefficient of variation | 10.0 | 16.2 | 14.7 | 21.8 | 10.0 | 29.8 | 26.6 | 4.4 | 9.3 | 5.1 | 5.8 | 9.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miķelsone, I.; Sipeniece, E.; Mišina, I.; Bondarenko, E.; Górnaś, P. Cultivated St. John’s Wort Flower Heads Accumulate Tocotrienols over Tocopherols, Regardless of the Year of the Plant. Plants 2025, 14, 852. https://doi.org/10.3390/plants14060852
Miķelsone I, Sipeniece E, Mišina I, Bondarenko E, Górnaś P. Cultivated St. John’s Wort Flower Heads Accumulate Tocotrienols over Tocopherols, Regardless of the Year of the Plant. Plants. 2025; 14(6):852. https://doi.org/10.3390/plants14060852
Chicago/Turabian StyleMiķelsone, Ieva, Elise Sipeniece, Inga Mišina, Elvita Bondarenko, and Paweł Górnaś. 2025. "Cultivated St. John’s Wort Flower Heads Accumulate Tocotrienols over Tocopherols, Regardless of the Year of the Plant" Plants 14, no. 6: 852. https://doi.org/10.3390/plants14060852
APA StyleMiķelsone, I., Sipeniece, E., Mišina, I., Bondarenko, E., & Górnaś, P. (2025). Cultivated St. John’s Wort Flower Heads Accumulate Tocotrienols over Tocopherols, Regardless of the Year of the Plant. Plants, 14(6), 852. https://doi.org/10.3390/plants14060852







