Abstract
Biogenic volatile organic compounds (BVOCs) significantly impact air quality and climate. Mechanical injury is a common stressor affecting plants in both natural and urban environments, and it has potentially large influences on BVOC emissions. However, the interspecific variability in wounding-induced BVOC emissions remains poorly understood, particularly for subtropical trees and shrubs. In this study, we investigated the effects of controlled mechanical injury on isoprenoid and aromatic compound emissions in a taxonomically diverse set of 45 subtropical broad-leaved woody species, 26 species without and in 19 species with BVOC storage structures (oil glands, resin ducts and glandular trichomes for volatile compound storage). Emissions of light-weight non-stored isoprene and monoterpenes and aromatic compounds in non-storage species showed moderate and variable emission increases after mechanical injury, likely reflecting the wounding impacts on leaf physiology. In storage species, mechanical injury triggered a substantial release of monoterpenes and aromatic compounds due to the rupture of storage structures. Across species, the proportion of monoterpenes in total emissions increased from 40.9% to 85.4% after mechanical injury, with 32.2% of this increase attributed to newly released compounds not detected in emissions from intact leaves. Sesquiterpene emissions, in contrast, were generally low and decreased after mechanical injury. Furthermore, wounding responses varied among plant functional groups, with evergreen species and those adapted to high temperatures and shade exhibiting stronger damage-induced BVOC emissions than deciduous species and those adapted to dry or cold environments. These findings suggest that mechanical disturbances such as pruning can significantly enhance BVOC emissions in subtropical urban forests and should be considered when modeling BVOC fluxes in both natural and managed ecosystems. Further research is needed to elucidate the relationship between storage structure characteristics and BVOC emissions, as well as their broader ecological and atmospheric implications.
1. Introduction
Plant-produced biogenic volatile organic compounds (BVOCs) play crucial roles in plant growth, reproduction, defense and stress responses [1,2,3]. They also significantly influence atmospheric chemistry and climate by affecting ozone and particulate matter formation [4,5,6]. Predicting the source strength of vegetation BVOC release and impacts of vegetation on atmospheric processes requires detailed information of the capacity of individual species to produce BVOC, as well as the stress responses of BVOC emissions. The East-Asian subtropics, with its high species diversity, is predicted to be a major source of global BVOC emissions [7,8,9]. However, the information on BVOC emissions and their stress responses in this region remains limited [10].
Based on the temporal variation of emissions and stress responses of emissions, BVOC emissions can be divided into constitutive and induced emissions. Constitutive BVOC production occurs in both stressed and unstressed conditions, whereas only certain species are strong constitutive emitters [2,11,12]. Induced BVOC emissions are elicited by stress, including biological stresses like pathogen infection and herbivory and abiotic stresses such as heat or mechanical injury [11,13,14]. Volatile isoprenoids, including isoprene, monoterpenes and sesquiterpenes, are the most abundant BVOCs emitted by plants [2,15,16]. Isoprene, a C5 hemiterpene, is constitutively emitted at high rates from some widespread temperate and subtropical and tropical woody species and from a few herbaceous species [10,17,18,19]. Monoterpenes (C10) are mainly emitted constitutively in evergreen conifers, as well as in several widespread evergreen and deciduous broad-leaved species [20,21]. Compared with monoterpenes, the constitutive emissions of sesquiterpenes (C15) are generally lower across emitting species and mainly reflect stress-induced emissions; they are typically emitted as part of a complex blend of stress-induced volatiles [11,22]. Volatile aromatics, including many benzenoids, form another major class of emitted BVOCs that play key roles in reproduction and physiological stress responses [11,23].
Upon production, volatiles can be immediately released to the atmosphere or stored in specialized storage structures [11,24,25]. Isoprene, due to its chemical properties, cannot be stored within leaves, whereas larger isoprenoids and aromatics may either be released immediately or stored, depending on the species, site of compound synthesis, and environmental stimuli [23,26]. Only species with specialized storage structures such as idioblasts, oil glands, glandular trichomes and resin ducts can store volatile isoprenoids and benzenoids in large quantities [21]. BVOC emissions vary in dependence on short- and long-term alterations in environmental conditions and in response to biotic and abiotic stresses [1,26]. In non-storage species, stress effects are modulated by changes in substrate availability for compound synthesis and gene expression levels that control the induction of new compounds [27,28]. In storage species with intact storage structures, the volatiles can leak out from the storage at a low rate, but the emissions can increase massively when these structures are mechanically damaged by herbivores or mechanical stress generated, e.g., by wind [29,30,31].
Mechanical injury, a common stressor in both natural and managed ecosystems (especially through pruning in urban areas), triggers both immediate and longer-term plant responses [31]. Immediate responses include the release of stored volatiles due to storage structure breakage, along with production of methanol and green leaf volatiles (GLVs) at the sites of damage [31,32,33]. These emissions can expel herbivores and attract their natural enemies [32] and possess antimicrobial properties [34]. Wounding can also reduce photosynthesis and thus affect the release of non-stored volatiles like isoprene [31]. Longer-term responses involve changes in substrate availability and gene expression related to BVOC synthesis, leading to altered emission rates and changed emission blends [35,36,37].
Although the importance of wounding on BVOC emissions is recognized, comparative studies across species, particularly in the East-Asian subtropics, are limited [31,33,38]. We hypothesize that (1) mechanical-injury-induced changes in BVOC emission rates and composition will vary significantly among species; (2) these variations will be related to the presence and characteristics of storage structures; and (3) species’ ecological adaptations (e.g., resistance to drought) will influence their wounding responses. Specifically, we predict that species adapted to moist, low-light conditions will have well-developed storage structures and show a rapid burst of diverse emissions upon mechanical injury, while species adapted to dry, high-light conditions will have reduced storage capacity and a less pronounced emission response. To test these hypotheses, we investigated the effects of mechanical injury on BVOC emissions from a set of 45 diverse broad-leaved tree and shrub species commonly found in the northern subtropical region. The species were grouped according to presence of storage structures (with and without storage structures), leaf longevity (evergreen and deciduous), and ecological tolerance category. In this study, we focused on isoprenoid (especially monoterpenes and sesquiterpenes) and aromatic compound emissions and report GLV emissions in a separate paper. Our previous work [11,39,40] has shown a correlation between constitutive BVOC emissions and species’ adaptations to light availability and drought. This study extends that work by examining the impact of wounding, a key stressor in both natural and urban environments.
2. Results
2.1. Impacts of Mechanical Injury on BVOC Emission Rates as Affected by the Presence of Storage Structures
2.1.1. Isoprene Emissions
Mechanical injury differently altered the emission rate depending on compound class and species (Figure 1, Table 1 and Table 2, Appendix A). Isoprene was emitted in nine tree species, and its emission rate was moderately affected by mechanical injury with some species showing increases and others decreases. A negative impact of mechanical injury on isoprene emission was observed in Sophora japonica (on average ± SE reduction of 58.3 ± 7.5%, p < 0.05) and Cinnamomum japonicum (75.3 ± 13.3% reduction, p < 0.05; Figure 1A), while in Platanus orientalis there was a significant increase (Figure 1A, p < 0.05). Given the contrasting species and overall moderate responses, the mechanical injury impact for all species pooled was not statistically significant (p > 0.05 for paired samples t-test, Figure 1B).
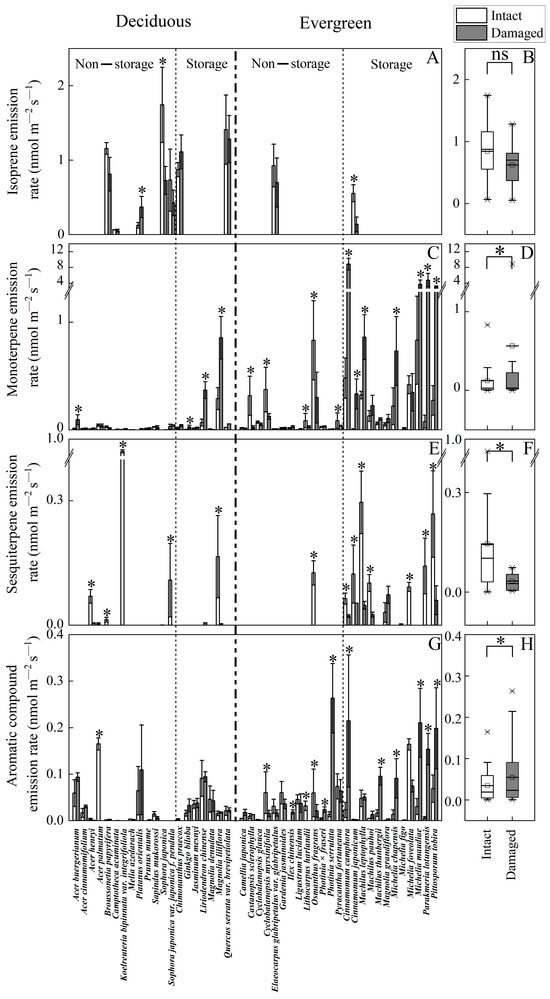
Figure 1.
Emission rates of isoprene (A,B), monoterpenes (C,D), sesquiterpenes (E,F) and aromatic compounds (G,H) from intact and mechanically injured leaves of 45 subtropical tree species (Table 1) classified among deciduous and evergreen species and species having or lacking specialized volatile-storing structures (resin ducts, glandular trichomes, oil cells and oil glands). (A,C,E,G) are the species actual average (±SE) emission rates, and (B,D,F,H) are the mean emission rates for intact and damaged leaves. Three replicate trees were sampled for each species, and from each tree, two leaves were measured. The values for two replicate leaves per plant were averaged and then the average for three replicate plants was calculated. * denotes significant differences between intact and injured leaf blade emission rates according to paired-samples t-tests (p < 0.05). “ns” denotes no significant difference between the emission rates of intact and injured leaf blade.

Table 1.
Information on leaf storage type, plant functional type (evergreen vs. deciduous) and species ecological characteristics for 45 subtropical woody broad-leaved species. “No” indicates that no storage compartment has been found in mature leaves. Species ecological characteristics are HT—high temperature resistant (thermophilus); CT—cold tolerant; LT—high-light resistant; ST—shade tolerant.

Table 2.
Changes in emissions before and after mechanical injury in 45 East-Asian subtropical woody species. Depending on the change in compound emission rate, the compounds are classified among four categories: increased emission after injury; reduced emission after injury; compound disappearance (emission below detection limit) after injury; newly emitted compound after injury.
2.1.2. Monoterpene Emissions
Monoterpene emissions were detected in all 45 tree species, with the effects of mechanical injury varying based on storage structures and leaf longevity (Figure 1C, Table 2, Appendix A). Mechanical injury increased monoterpene emissions in several evergreen species with leaf storage structures: Parakmeria lotungensis (62-fold), Cinnamomum camphora (17-fold), Pittosporum tobira (10-fold), and Michelia maudiae (3.5-fold). Conversely, wounding decreased emissions in most evergreen species without storage tissues, such as Castanopsis sclerophylla (12.7-fold reduction), Cyclobalanopsis myrsinifolia (3.0-fold), Lithocarpus harlandii (31-fold), and Osmanthus fragrans (2.8-fold). In deciduous species, wounding impacts ranged from moderately positive to negligible, except for Magnolia liliflora and Liriodendron chinense, which have storage structures. Overall, monoterpene emissions significantly increased in species with storage structures, especially evergreens (Figure 1C).
2.1.3. Sesquiterpene Emissions
Sesquiterpene emissions were detected in 20 species, predominantly in evergreen species with specialized storage structures, though the emission rates were generally low (<0.3 nmol m−2 s−1 for most species; Figure 1E,F, Table 2, Appendix A). Mechanical injury reduced sesquiterpene emissions in 16 species by an average of 3.4-fold compared to intact leaves. Post-injury, only nine woody species continued to emit detectable levels of sesquiterpenes, while the emissions fell below the detection limit in the remaining seven species, e.g., in Koelreuteria bipinnata var. Integrifoliola from 0.86 ± 0.02 nmol m−2 s−1 and Sophora japonica var. japonica f. pendula from 0.108 ± 0.08 nmol m−2 s−1 to below the detection limit. Overall, sesquiterpene emissions were generally low and decreased after injury in most species.
2.1.4. Aromatic Compound Emissions
Emissions of aromatic compounds from intact leaves were observed in 38 woody species, and wounding strongly stimulated these emissions, in particular in evergreen species with storage structures (Figure 1G, Table 2). For instance, after the injury, the emissions of aromatics increased 25-fold in Parakmeria lotungensis, 18-fold in Photinia serrulata, 8-fold in Cinnamomum camphora, and 6-fold in Michelia maudiae compared to the emissions from intact leaves (Figure 1G). In contrast, the emissions were reduced in several evergreen species without storage structures, including Cyclobalanopsis myrsinifolia, Lithocarpus harlandii and Osmanthus fragrans. In most deciduous species, the emission rates were only moderately affected by wounding (p < 0.05). For all species pooled, aromatic compound emissions significantly increased in species with storage structures. (Figure 1H, Table 2, Appendix A).
2.2. Impacts of Mechanical Injury on the BVOC Composition and Abundance in Storage and Non-Storage Species
Wounding altered the proportion of different BVOC compound classes and individual compounds in the emissions of 45 tree species, whereas the changes in BVOC composition depended on the presence of storage structures (Figure 1 and Figure 2, Table 2). In non-storage species, the share of isoprene (from 54.7% in intact leaves to 64.0% in wounded leaves) and aromatics (8.3% in intact vs. 16.1% in wounded leaves) increased upon wounding (Figure 2C,D). This was associated with reduced monoterpene (23.2% in intact vs. 19.8% in wounded leaves) and sesquiterpene (13.6% in intact vs. 0.10% in wounded leaves) emissions (Figure 2C,D). In storage species, the share of isoprene (from 35.5% in intact to 8.8% in wounded leaves), aromatics (7.6% in intact vs. 4.7% in wounded leaves), and sesquiterpenes (16.1% in intact vs. 1.0% in wounded (Figure 2E,F) decreased after mechanical injury. The monoterpene share strongly increased upon wounding (40.9% in intact vs. 85.4% wounded leaves) (Figure 2E,F).
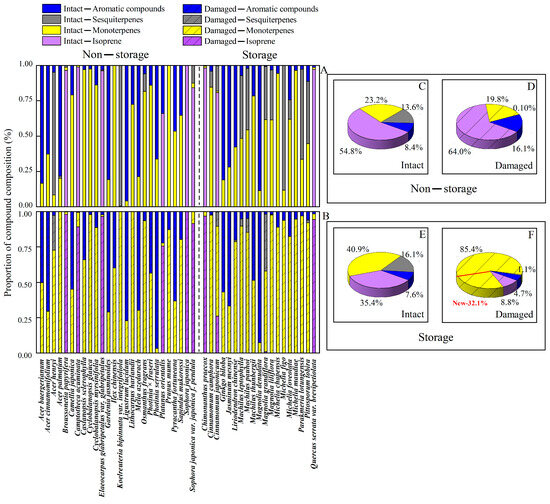
Figure 2.
The proportions of compounds (monoterpenes, sesquiterpenes and aromatic compounds) in intact (A) and damaged (B) leaves in species with and without volatile storage structures and group-average share of BVOC classes for intact (C) and damaged (D) leaves in species without storage structures and for intact (E) and damaged (F) leaves in storage species. “New” represents the share of compounds not observed in the emissions of intact leaves and emitted after the leaves were injured.
2.2.1. Injury-Dependent Changes in the Composition of Emitted Monoterpenes
Wounding altered the compound share within key volatile classes, especially for monoterpenes (Table 2, Appendix A). In species with storage structures, wounding enhanced common monoterpene emissions (e.g., α-pinene, α-terpinolene, limonene, camphor, cyclofeuchene, β-phellandrene, α-phellandrene, camphene, (E)-β-ocimene, γ-terpinene, pseudolimonene, α-terpinene, p-cymene, fenchene, α-thujene and eucalyptol, Appendix A). Furthermore, many new monoterpene compounds not emitted from intact leaves in the given species were induced after mechanical injury, including limonene in Cinnamomum japonicum and Michelia figo; camphor in Magnolia grandiflora, M. liliflora, Michelia foveolata and Parakmeria lotungensis; and cyclofeuchene in C. camphora, P. lotungensis and Pittosporum tobira (Table 2, Appendix A). Overall, 32.2% of wounding-dependent increase in the share of monoterpene emission was due to induction of new compounds. In storage species, a reduction or disappearance of emissions of certain monoterpenes was rare after wounding; such modifications were only observed in Machilus pauhoi (cyclofeuchene), Machilus thunbergii and P. tobira (p-cymene) and Michelia foveolata (α-pinene).
2.2.2. Changes in Sesquiterpene Composition in Response to Mechanical Injury
Sesquiterpene diversity was overall low, and the main compounds observed in emissions from intact leaves were β-longipinene, α-muurolene, β-maaliene, cis-muurola-4(14),5-diene, β-caryophyllene and α-bulnesene (Table 2, Appendix A). The elicitation of new compounds after wounding was rare, with cis-muurola-3,5-diene observed in wounded leaves of Liriodendron chinense, β-bourbonene in Magnolia denudata and β-copaene in M. grandiflora. Consistent with the overall reduction in emission rate, the emission rate of most individual monoterpenes was reduced, and multiple sesquiterpenes were no longer detected in the emission blends (Table 2, Appendix A).
2.2.3. Modifications in the Composition of Aromatics After Mechanical Injury
The change in composition of aromatic compounds after mechanical injury was different for storage and non-storage species (Table 2, Appendix A). 2, 5-dimethylstyrene was mainly emitted from evergreen species with storage structure belonging to the families Lauraceae and Magnoliaceae, and its emissions were significantly stimulated after wounding in these species. The compounds 2-phenylethyl formate and 3, 4-dimethylbenzyl alcohol were only observed in evergreens from Lauraceae and Magnoliaceae, e.g., in Michelia chapensis, M. maudiae and Parakmeria lotungensis.
In non-storage species, the responses of different aromatic compound emissions to wounding were variable. Emissions of 4-cyclopentyl ethylbenzoate, 2,5-dimethyl styrene, 2-phenylethyl benzoate and 2,4-butylethyl benzoate increased in Camellia japonica, Ligustrum lucidum and Photinia serrulata, while the emissions of these compounds decreased and reached even to levels below the detection limit in Cyclobalanopsis myrsinifolia, Elaeocarpus glabripetalus var. glabripetalus and Osmanthus fragrans (Table 2, Appendix A).
2.3. Response of BVOC Emission to Mechanical Injury in Relation to Leaf Longevity and Species Ecological Adaptations
2.3.1. Changes in BVOC Emission Due to Mechanical Injury Associated with Leaf Longevity
Mechanical injury altered BVOC composition and proportion among 45 woody species (Figure 1 and Figure 2), and the changes were related to leaf longevity and ecological tolerance (Figure 3 and Figure 4). Due to a limited number of isoprene- and sesquiterpene-emitting species, the impact of species ecology on wounding-dependent changes in emissions was analyzed for monoterpenes and aromatics. Storage species had strongly enhanced monoterpene emissions after mechanical injury, and non-storage species had unaffected emissions (Figure 3). Evergreen species generally had greater monoterpene emissions than deciduous woody species, and the wounding-dependent stimulation of monoterpene emission rate was greater in evergreen species (Figure 1C and Figure 3, Table 2, Appendix A). Aromatic compound emission was unaffected by wounding in deciduous species, and the emissions were enhanced in evergreens (Figure 3).
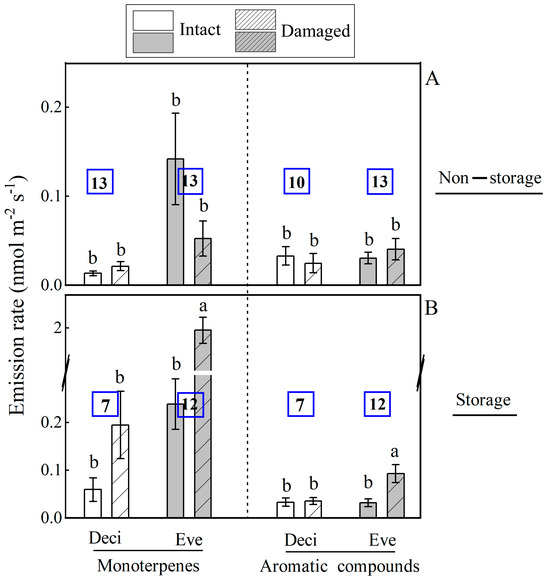
Figure 3.
Comparison of average ± SE monoterpene and aromatic compound emission rates among intact and mechanically injured evergreen and deciduous leaves with (19 species) and without (26 species) specialized storage for 45 subtropical woody species. The number of species for each group is shown within each bar. Different letters denote significant differences (p < 0.05) between intact and injured leaf blades among the groups according to ANOVA followed by Duncan tests. The comparisons for isoprene and sesquiterpenes were not informative due to too few species for presence of storage/leaf duration combinations (n = 19). The abbreviations are Deci—deciduous species; Eve—evergreen species.
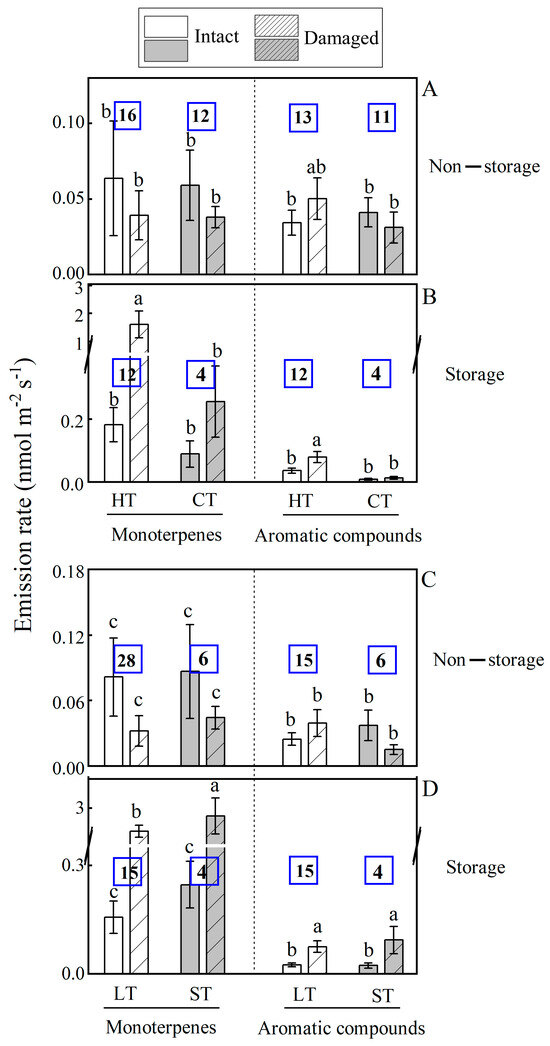
Figure 4.
Comparison of average ± SE monoterpene and aromatic compound emission rates among intact and mechanically injured leaves of species with varying stress resistance, tolerant to high and low temperatures (A,B) and to high light and shade (C,D) among the 45 subtropical woody species studied. The number of species for each group is shown within each bar. Different letters denote significant differences (p < 0.05) between intact and injured leaves among the groups according to ANOVA followed by Duncan tests. Too few combinations of stress tolerance/presence of storage structure were available for isoprene and sesquiterpenes. Species ecological potentials are HT—high temperature resistant (thermophilus, n = 28); CT—cold tolerant (n = 16); LT—high-light resistant (n = 33); ST—shade tolerant (n = 11).
2.3.2. Changes in BVOC Emission to Mechanical Injury in Species with Different Ecological Tolerance
The response of BVOC emission to mechanical injury was linked to the ecological tolerance of plant species, but differences in wounding responses among species were mainly due to variations in the proportion of storage species within each group (Figure 4). Wounding enhanced monoterpene emissions in storage species for all ecological groups analyzed (thermophilus species, HT; cold tolerant species, CT; high-light tolerant species, LT; shade tolerant species, ST), but wounding did not alter emissions in non-storage species for any of the ecological group. For aromatics, wounding increased the emissions in HT (Figure 4A,B), LT and ST (Figure 4C,D) species with storage structure and in none of the species groups without storage (Figure 4).
3. Discussion
Plants produce different volatile secondary metabolites (BVOC) as a defense strategy against different stresses. The volatiles can be immediately released after formation or stored in specialized storage structures and released upon the breakage of the storage structures. In this study, wounding responses of BVOC emissions from 45 widespread East-Asian subtropical woody species of contrasting volatile storage capacity and ecological requirements were investigated. Our study demonstrates major differences in the wounding responses among storage and non-storage species and among different volatile groups, isoprene, mono- and sesquiterpenes and aromatics, as discussed below.
3.1. Changes in Isoprene Emission in Response to Mechanical Injury
Isoprene, a major constitutively synthesized volatile organic compound, plays crucial role in enhancing plant protection against abiotic stress. Specifically, it improves thermotolerance [18,54,55] by maintaining thylakoid membrane integrity [56,57,58] and scavenging reactive oxygen species (ROS) [1,29,59,60]. Due to its high volatility, isoprene cannot be significantly stored within the leaves and is immediately released upon synthesis. Isoprene is synthesized in the chloroplasts from glyceraldehyde 3-phosphate and pyruvate via 2-C-methyl-D-erythritol-4-phosphate/ 1-deoxy-D-xylulose 5-phosphate (MEP/DOXP) pathway. Isoprene emission is closely associated with photosynthesis [61,62,63], which provides the necessary carbon substrates, ATP and NADPH [64,65,66,67]. However, differently from photosynthesis, moderately severe abiotic stresses typically stimulate isoprene emission [68,69]. The reduction in leaf stomatal conductance is typically one of the earliest responses to stress, limiting CO2 entry and thereby decreasing photosynthesis. The response of isoprene emission to CO2 concentration is a curve with an optimum; isoprene emissions decrease both at CO2 concentrations below and above the optimum. Therefore, a moderate decrease in stomatal conductance can initially stimulate isoprene emission [20,70]. However, with stress progression, decreases in isoprene emissions have been observed in many studies [63,69] due to reductions in the immediate isoprene substrate, dimethylallyl diphosphate (DMADP) concentration [71].
When plant leaves are wounded, their water conducting pathways are disrupted, reducing stomatal conductance. Consequently, this decreases photosynthesis and might alter carbon availability for DMADP formation [31,72]. The degree of reduction in conductance at the given level of wounding varies among species [31], suggesting that the response of isoprene emission rate to mechanical injury can also vary among species. In Populus tremula × P. tremuloides, moderate wounding did not affect isoprene emission, whereas in Eucalyptus globulus, isoprene emission was slightly stimulated [31]. Such contrasting responses were confirmed in our study, where significant negative effects of wounding were observed in two species and a positive effect of wounding in one species, and no significant wounding effects were observed in the remaining six species (Figure 1, Table 2, Appendix A). This evidence collectively indicates that in the short-term (minutes to a few hours), wounding effects on isoprene emission are expected to be only moderate, except for some species with stronger physiological responses to the given degree of wounding. However, mechanical injury can elicit enhanced expression of the isoprene synthase gene, thereby resulting in increased isoprene emissions in several hours to days after wounding [35]. Our study did not assess such longer-term wounding effects and so far, species differences in long-term wounding responses of isoprene emission have not been studied. Further research simultaneously assessing injury impacts on leaf photosynthetic characteristics, isoprene emission and DMADP pool size right after injury through recovery are needed to gain an insight into species differences in wounding responses.
3.2. Responses of Monoterpene Emissions to Mechanical Injury
Like isoprene, monoterpenes are synthesized via the MEP/DOXP pathway in plant plastids [73,74]. However, only a limited number of species are strong constitutive emitters of both isoprene and monoterpenes [75]. In our study, such a species was Cinnamomum japonicum (Figure 1). As mentioned in the Introduction, leaf monoterpene emissions can result from de novo synthesis in leaf mesophyll and immediate release (de novo emissions) or from release of monoterpenes synthesized and stored in special secretory structures such as idioblasts, oil glands, resin ducts and glandular trichomes (storage emissions). It is anticipated that de novo monoterpene emissions will respond to mechanical wounding similarly to isoprene emission, i.e., wounding is expected to lead to moderate positive or negative changes or no changes in emission rate depending on how wounding alters chloroplastic substrate availability for monoterpene synthesis. Indeed, among non-storage species in our study, mechanical injury enhanced de novo monoterpene emissions in two species (Acer buergerianum and Camptotheca acuminata), reduced in six species and did not significantly affect the emission rate in 18 species (Figure 1C,D). Both Camptotheca acuminate and Platanus orientalis are typically isoprene emitters, and the mechanical injury induced only a limited number of new monoterpene compounds in these species (Table 2, Appendix A). Furthermore, deciduous woody species without storage structures had generally a very low monoterpene emission rate either in intact or wounded leaves, whereas evergreen species without storage leaf structures had a higher monoterpene emission rate (Figure 3).
Terpene-storing species accumulate substantial amounts of terpenes in specialized storage structures, and breakage of these structures by herbivore feeding or mechanical damage leads to the release of stored compounds at high rates [30,31,76]. The release of stored compounds from wounded leaves plays crucial roles in direct and indirect defense, deterring herbivores and inhibiting the entry and growth of pathogenic microorganisms [77]. In our study, monoterpene emissions strongly increased after mechanical injury in 14 of 19 storage species, though the magnitude of responses differed among individual species (Figure 1C,D and Figure 3, Table 2, Appendix A). Particularly strong enhancement of emissions by wounding, 10- to more than 60-fold increase was observed in the evergreen tree species Cinnamomum camphora, Parakmeria lotungensis and Pittosporum tobira (Figure 1C, Table 2). In contrast, monoterpene emissions from the other five storage species (Jasminum mesnyi, Magnolia denudate, Machilus pauhoi, Magnolia grandiflora and Michelia foveolata) did not significantly respond to wounding. We propose that the interspecific variation in monoterpene baseline emissions and emission responses to wounding, reflects both genetic factors intrinsic to the species, including the number and regulation of expression of monoterpene synthase genes, overall activity of MEP/DOXP pathway and of course, the type, size and density of specialized storage structures. Further studies are warranted to elucidate the relationships of injury responses of monoterpene emissions with storage types, as well as with the number of storage structures per unit leaf area.
In this study, the emissions of α-pinene, limonene, α-terpinolene and camphor were most frequent, and the emissions of all these volatiles typically increased after leaf injury (Table 2, Appendix A). However, mechanical injury also altered the composition of emitted monoterpenes, and many new monoterpenes not observed in intact leaves were released in several woody species (Figure 1, Table 2, Appendix A). For instance, new oxygenated and non-oxygenated monoterpenes, like 3-carene, α-terpineol, endo-borneol, (E)-4-thujanol and fenchol, were detected in the emissions of Cinnamomum camphora, Michelia maudiae and Parakmeria lotungensis and several other woody species (Table 2). In Pittosporum tobira, even six new monoterpene compounds (camphor, α-phellandrene, (E)-β-ocimene, pseudolimonene, α-terpinene and α-fenchene) were detected after injury (Table 2). The increase in the proportion of oxygenated compounds may indicate oxidation processes occurring once these compounds are exposed to atmospheric conditions [78,79], although, the share of oxygenated terpenes can also increase in the case of de novo synthesized terpenes [80]. Furthermore, the storage species can have different types of oil glands and glandular trichomes with different BVOC composition [22,81,82,83]. Provided the possible differences in the abundance of different types of oil glands or glandular trichomes [81] and/or terpene permeability of cells lining the outer surface of storage structures, not all storage compartments might have contributed to the emitted terpene blend of non-injured leaves at a level exceeding the threshold for detection. Once injured, however, the emissions from these structures might have significantly contributed to the emission blend. Pittosporum tobira is a special case with both capitate glandular trichomes and oil canals, and it is plausible that the particularly strong enhancement of emissions and spectacular change of the emission blend after damage reflects additional sharing from a storage compartment that did not contribute to emissions in non-damaged leaves.
Apart from physical explanations, mechanical injury activates the plant defense system and leads to the elicitation of the expression of genes responsible for synthesis of monoterpenes, sesquiterpenes and green leaf volatiles [35,84,85]. For example, when Pinus massoniana was artificially damaged or attacked by Dendrolimus punctatus larvae, in addition to the increase in stored monoterpene emissions, emissions of many new compounds were induced [86]. Although full activation of gene expression is relatively time-consuming [87,88], enhanced expression of isoprenoid synthases might be observed already in minutes to a few hours after stress application [35]. After damage, several species started to emit (E)-β-ocimene, which is a classic stress-induced monoterpene [89], suggesting that induction of de novo terpene synthesis did occur. Furthermore, we also observed emissions of several new monoterpenes from injured leaves of non-storage species, suggesting that elicitation of gene-expression-level responses did contribute to the modification of the blend of monoterpenes emitted from the injured leaves.
The South-East Asian subtropics are usually characterized by high humidity and supraoptimal temperatures, often exceeding 35–40 °C. In this study, the selected 45 species are commonly found and grow well in Chinese subtropical to tropical regions, although not all of them originate from warm humid climates. It is well established that warm humid climates are typically conducive to the proliferation of pathogenic microorganisms. In this study, deciduous woody species without leaf storage structure were mainly sun-adapted species resistant to photoinhibition and occasional drought in highly exposed habitats, while the evergreen species without leaf storage structures were moderately shade-tolerant with leathery leaves that provide strong resistance to biotic stress and prevent microbial invasion. From an ecological perspective, these results may reflect the trade-off between synthesis of different protective chemicals in plants. Constitutive isoprene emission primarily serves to maintain high photosynthetic efficiency under stress, while constitutive monoterpene emissions not only protect photosynthesis from stress but also deter insect attacks.
3.3. Alterations in Sesquiterpene Emissions by Mechanical Injury
Sesquiterpenes are characteristic components of the volatile bouquets produced by many flowers, whereas the emission rate from non-stressed leaves is typically barely detectable [30]. This was confirmed in our study where the sesquiterpene emissions were generally low, except for Koelreuteria bipinnata var. integrifoliola, and the emissions above the detection limit were observed in only 20 species (Figure 1, Figure 2 and Figure 3, Table 1 and Table 2). Similarly to monoterpenes, sesquiterpenes can be stored in different storage structures [25,27,29], and thus, damage to these storage sites is expected to significantly enhance sesquiterpene emissions. Leaf injury by herbivores has been shown to strongly induce sesquiterpene emission [90]. In contrast, in this study, in addition to Liriodendron chinense, sesquiterpene emissions significantly decreased in other species, often to the level below the detection limits (Figure 1, Table 2). These results maybe reflect the difference in the nature of mechanical injury compared to herbivore-induced damage.
Furthermore, these puzzling differences between mono- and sesquiterpene wounding responses might reflect different physico-chemical characteristics in these compound groups. Sesquiterpenes are generally more reactive in the ambient atmosphere than monoterpenes and volatile aromatics; most reactive sesquiterpenes, although not all, have atmospheric lifetimes on the order of a few minute [91,92]. There also can be spatial separation of mono- and sesquiterpene synthesis, e.g., in different types of glandular trichomes [93,94]. Given that application of wounding took 0.5–1 h, part of the sesquiterpenes released after the breakage of storage structures might have become oxygenated, sealing the sites of damage and reducing subsequent emissions of sesquiterpenes. Previously, a reduction in emissions after terpene oxidation has been observed in wounded needles of Pinus pinea [78]. In addition, analyses of the essential oil composition of broad-leaved woody species indicate that stored sesquiterpenes generally comprise a relatively small fraction of total stored terpenes [79,95,96,97]. Thus, we suggest that sesquiterpene oxidation in combination with a much lower source strength of sesquiterpenes explains the reduction in sesquiterpene emissions after wounding in our study (Figure 1E,F, Table 2, Appendix B). Similarly to our study, wounding reduced the content of sesquiterpenes in the broad-leaved evergreen Melaleuca alternifolia [79,98].
3.4. Aromatic Compound Responses to Mechanical Injury
In plants, the chloroplastic shikimic acid pathway provides aromatic amino acids and precursors for a variety of secondary metabolites [99]. This pathway also facilitates the synthesis of multiple volatile compounds that can be either immediately emitted (de novo emissions) or stored prior to emission, akin to terpenes [31,61,100,101]. Although the terpene and phenolic compound storage structures can be spatially separated [25,102,103], we observed that mechanical injury responses of emissions of aromatic compounds broadly reflected the responses of monoterpene emissions to wounding. In the case of de novo emissions, aromatics emissions were moderately reduced in four species and enhanced in three species, whereas in storage species, the emissions were strongly enhanced in six species (Figure 1G,H, Figure 3). Interestingly, the aromatic compound 2, 5-dimethyl styrene was significantly stimulated after wounding only in those species with storage structures, including Cinnamomum camphora, Magnolia grandiflora, Michelia chapensis, Michelia maudiae, Parakmeria lotungensis and Pittosporum tobira (Table 2, Appendix A). This result indicates that the leakage of aromatics from leaf storage compartments was that low in intact leaves that certain aromatics were non-detectable until the storage tissues were broken.
Additionally, we identified several novel aromatic compounds that were released only after wounding, e.g., 2-phenylethyl formate and 3, 4-dimethylbenzyl alcohol in species with storage structures and 4-cyclopentyl ethylbenzoate in some species without storage structures (Table 2, Appendix A). As discussed above, stresses can alter BVOC emissions by changing gene expression patterns, as has also been observed for volatile phenolics [26,61,100,104], and the change in the emission composition can reflect plant systemic response to mechanical injury [37]. Although the time between wounding and the measurements was relatively short, it is plausible that in our study, the composition change was partly driven by the initial phase of the gene expression level response.
Emissions of aromatics and monoterpenes from intact and wounded leaves were positively correlated for storage species (Table 3), indicating that the release of these compounds after wounding primarily reflects the breakage of storage structures. In non-storage species, there was also evidence of a moderately strong positive correlation between monoterpene and benzenoid release after damage (Table 3). As the synthesis of aromatics and monoterpenes occurs in plastids, this correlation indicates that both the MEP/DOXP and shikimate acid pathway responded similarly to the wounding stress in our study. However, in other studies, stress-dependent emissions of de novo released monoterpenes and aromatics were not strongly correlated [105] or were even negatively correlated [33,106]. Our study demonstrates that wounding of storage species does significantly contribute to the enhancement of BVOC emissions in vegetation and suggests that more work is needed to gain an insight into the cooperativity of different BVOC synthesis pathways under stress in non-storage species.

Table 3.
Correlations between emission rates of isoprene and monoterpenes, sesquiterpenes and aromatics for intact and mechanically injured evergreen and deciduous leaves with (19 species) and without (26 species) specialized storage. Due to insufficient numbers of evergreen species emitting isoprene and damaged deciduous species emitting sesquiterpenes (n < 9), only those species that were sufficient are shown in the following table. “n” represents 3 repeated data for each tree species.
3.5. Effects of Plant Functional Types and Different Ecological Requirements on Species Emission After Mechanical Injury
Urban woody floras are comprised of species that belong to diverse plant functional types and exhibit varying ecological tolerances. In this study, average monoterpene emissions were enhanced by wounding both in deciduous and evergreen plant functional types, but the enhancement was greater for evergreens, especially for those with storage structures (Figure 1 and Figure 4). This reflected the more widespread occurrence of storage structures in evergreen than in deciduous species, and these differences are consistent with the so-called leaf economics spectrum that embraces the life strategy of deciduous and evergreen species [107]. The deciduous species typically have higher investment in photosynthetic machinery and lower investments in structural and chemical defenses, whereas longer-living leaves in evergreens have lower photosynthetic capacity and greater investments in more robust leaf structure and chemical defenses to protect the leaves from abiotic and biotic stresses [107].
Regarding species ecological requirements, high-temperature and high-light resistance, shade tolerance and drought tolerance were associated with a stronger wounding response in monoterpene and aromatics emissions (Figure 4). This is consistent with the predictions from the leaf economics spectrum that plants in more stressful environments should have higher defense investments to enhance leaf longevity [15,107,108]. As noted above, plants are exposed to particularly strong pressure of pathogenic microorganisms in warm humid subtropical and tropical climates. The thirteen deciduous woody species without storage structure primarily emitted isoprene (Appendix A); these species are primarily high-light-adapted species that also moderately tolerate drought. Thirteen evergreen species without storage structures were constitutive emitters of monoterpenes; these species were moderately shade-tolerant (Figure 1 and Figure 3). Notably, almost all of these evergreen species without storage structures originated from the subtropical regions of China, indicating these species have adapted to high-temperature and high-humidity climates. In such conditions their robust leaf structure provides strong resistance to biotic stress and prevents microbial invasion, and we argue that the emissions of monoterpenes contribute to the pathogen resistance in these species.
Nineteen species had specialized storage structures and with the exception for Chimonanthus praecox and Jasminum mesnyi, 5 deciduous species and 12 evergreen species originated from subtropics, and most of these species were shade-tolerance. These species exhibited significant emissions of monoterpenes and aromatic compounds, especially following mechanical damage. This suggests that the release of monoterpenes and aromatics plays a crucial role in maintaining leaf resistance to environments rich in pathogenic microorganisms.
From an adaptation perspective, the interspecific variations in emissions of isoprenoids and aromatics from intact leaves and after wounding may reflect the trade-offs in how plants invest energy and carbon to adapt to the variable environments. We hypothesize that constitutive isoprene emissions primarily serves to maintain high photosynthetic efficiency, in contrast, constitutive light-dependent monoterpene emissions not only support photosynthesis but also deter insect attacks. However, the stored monoterpenes and aromatics serve dual functions: they protect against herbivores in intact leaves and facilitate wound repair and healing upon mechanical injury. These distinct emission roles are closely associated with the presence or absence of leaf storage structures that reflect convergent evolution to enhance resistance to biotic stresses but also play an important role in plant survival in different environments (environmental tolerance; Figure 5).
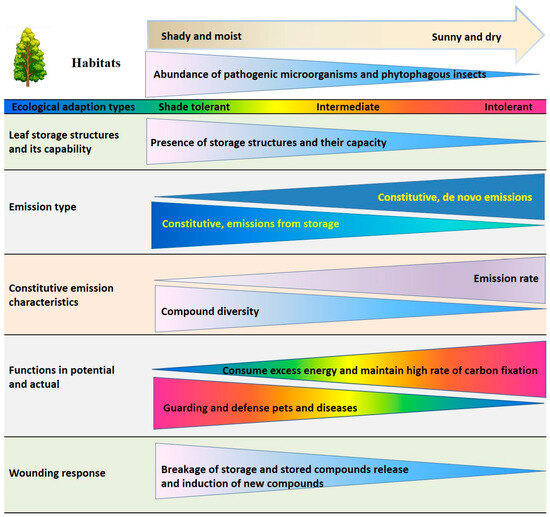
Figure 5.
Schematic overview of emission characteristics of volatile isoprenoids and aromatics, the wounding responses of emissions, and volatile functions as associated with presence of leaf storage structures and ecological adaptations. The direction from the base to the vertex of the isosceles triangles indicates the change in the expression of the given trait.
4. Materials and Methods
4.1. Plant Selection and Sampling
The study was conducted in the campus of the Zhejiang Agriculture and Forestry University (30°15′23″ N, 119°43′29″ E, elevation 100–110 m). Firstly, 45 common ornamental woody species (25 evergreen and 20 deciduous species) were selected for the analysis. Based on species ecological characteristics, the selected species were classified as: high-temperature resistant (thermophilus species, MT, n = 28), cold tolerant (CT, n = 16), drought tolerant (DT, n = 13), waterlogging tolerant (WT, n = 5), high-light resistant (LT, n = 33) and shade tolerant (ST, n = 11) (Table 1) [10].
Sampling and BVOC emission measurements were conducted in summer (June–July) 2017, as described in detail in Yuan et al. (2020) [10]. For each species, three healthy adult individuals were selected. From each individual, two terminal branches (100–110 cm length) with mature leaves exposed to full sunlight were excised, immediately recut under water to avoid cavitation and transported to the laboratory for measurements.
4.2. Experimental Treatments
Two sets of BVOC measurements were conducted, one with intact branches and the other with mechanically damaged branches, as described in the next section. After the measurement of intact leaves, the leaves were subject to mechanical injury using a custom-made square (40 mm × 40 mm) hole punch with sharp micro-needles (88 needles uniformly distributed over the square surface). In each branch, all the leaves used for measurements of BVOC emissions were pierced, avoiding the leaf midrib. Once the mechanical injury was completed, typically in 30–40 min, BVOC release was immediately measured. Both for the control and damage treatment, 6 samples from 3 different plants (3 different plants × 2 branches) were measured for each species. During BVOC sampling, the chamber was illuminated with cold white LEDs, resulting in a light intensity at the leaf surface of 300 μmol m−2 s−1. This intensity corresponds to a mid-canopy light intensity in sunny days or above-canopy light intensity on cloudy days. It is typically not saturating for light-dependent BVOC emissions, but it was selected to avoid an excessive rise in temperature during sampling (leaf temperature during sampling was 27–29 °C). We note that the emission rates can be potentially scaled to standard conditions typically used to define the basal emission factor (incident light intensity of 1000 μmol m−2 s−1 and leaf temperature of 30 °C) [69]. The details are described in Yuan et al. (2020) [10].
4.3. Compound Sampling and Analysis
To increase the detection limit for low-level emitter and enhance the accuracy of detection of less dominant volatile compounds, a semi-closed dynamic sampling system was used, allowing the achievement of moderately high concentrations of volatiles during sampling. For BVOC sampling, the branches were enclosed in custom-made cylindrical glass (95% quartz glass) containers (either 15 cm × 25 cm or 10 cm × 15 cm, depending on the branch size). Extended glass tubes (0.8 cm diameter) were attached to the containers for BVOC sampling and ambient air entry. The effect of diffusion of BVOC-enriched air out of the container through the air inlet was estimated to affect the calculated BVOC emission rates <1% [10].
BVOC were collected with an air sampling pump (Beijing Municipal Institute of Labour Protection, Beijing, China) onto the adsorption tubes filled with Tenax TA, Carbograph TD and Carboxen 1003 (Markes, Bridgend, UK) at 120 mL·min−1 for 40 min. Further details of sampling are provided in Yuan et al. (2020). Volatile analysis was conducted with a gas chromatograph (GC; 7890B, Agilent, Santa Clara, CA, USA) equipped with a mass spectrometric (MS) detector (5977B, Agilent, Santa Clara, CA, USA) and thermal desorption (TD) system (TD-100, Markes, Bridgend, UK). A DB-624 (length 60 m, inner diameter 0.25 mm, film thickness 0.25 μm; Agilent, Santa Clara, CA, USA) capillary column was used. The MS was operated in electron impact (EI) ionization (70 eV) mode using the mass scan range of m/z 45–400. The ion source and transfer line temperature was 230 °C, the quadrupole temperature was 150 °C, and the flow rate of the carrier gas (He) was 1 mL min−1 for desorption and 1.2 mL min−1 for GC-MS analysis. The GC oven program used was as in [10].
Authentic GC-grade standards (Sigma, St. Louis, MO, USA) and MassHunter software (version B.07.00, Agilent, Santa Clara, CA, USA) with the NIST 14 spectral library were used to identify the compounds. To calibrate the system, a standard gas (Ionicon GmbH, Innsbruck, Austria) containing 15 different volatiles (each individual volatile at 1 ppm) was used and quantitatively diluted with high purity nitrogen (99.999%). The diluted calibration samples were collected onto the adsorption tubes and analyzed according to the same protocol as plant BVOC samples [10,98].
4.4. Determination of Retention Index and Compound Identification
To accurately identify biovolatile organic compounds (BVOCs), the retention index (RI) method combined with mass spectrometry data was employed to confirm the identity of the compounds. A series of known alkane standard substances (with carbon chain lengths ranging from C5 to C20, Sigma) were utilized to estimate the retention index of the test compound. By analyzing these standard substances, we obtained their respective retention times on the chromatographic column (tRn, tRn+1). Subsequently, BVOC samples were analyzed under identical chromatographic conditions as those used for the alkane standards, allowing us to record the retention time (tRx) for each target compound. The retention index (RI) was calculated using the following formula:
n: number of carbon atoms in the alkane with a shorter retention time than that of the test compound; tRx: retention time of the test compound; tRn and tRn+1: retention times of two alkanes with slightly shorter and longer retention times than that of the test compound, respectively.
The retention index of each BVOC calculated by the above method was then compared with the retention index data in the NIST 14 mass spectrometry database to quickly confirm the identity of the test compound, and the mass spectrometry data of each target compound were further confirmed. The detailed retention index data of each compound measured in this study are listed in Appendix B.
4.5. Classification and Identification of Leaf BVOC Storage Structures
As highlighted in the Introduction, the presence of BVOC storage structures can importantly alter the magnitude of terpene and aromatics emissions. Thus, the studied species were classified according to the presence of storage structures in leaves as non-storage and storage species (Table 1). The storage species were further classified according to the type of storage structures as species with oil cells, glandular trichomes or secretion (resin) ducts (Table 1). Out of 45 studied species, 15 species contained oil cells, including all Lauraceae species (Cinnamomum camphora, Cinnamomum japonicum, Machilus leptophylla, Machilus pauhoi and Machilus thunbergii) [45,46], Magnoliaceae species (Magnolia denudata, Magnolia grandiflora, Magnolia liliflora, Michelia chapensis, Michelia figo, Michelia foveolata, Michelia maudiae, Parakmeria lotungensis and Liriodendron chinense) [43,51] and a Calycanthaceae species (Chimonanthus praecox) [43]. Among the studied species, only Ginkgo biloba has resin ducts in leaves [47], and Pittosporum tobira has oil ducts [52].
Although the taxonomic signature for the presence of oil cells was strong, the association of glandular trichomes to taxonomy was weaker. Among the studied species, 4 species (Cinnamomum camphora from Lauraceae, Jasminum mesnyi from Oleaceae, Pittosporum tobira from Pittosporaceae and Quercus serrata var. brevipetiolata from Fagaceae) had glandular trichomes on the leaf surface [45,46,48,53]. In contrast, another species from Fagaceae, Lithocarpus harlandii, does not have glandular trichomes on the leaf surface [50]. On the other hand, Koelreuteria bipinnata var. integrifoliola has non-glandular trichomes and no glandular trichomes, so trichomes on the surface of this species do not play a role in the storage of volatiles [49]. In Camptotheca acuminata (Nyssaceae), glandular trichomes are found on the stem and on the surface of young leaves but they are not present on the surface of mature leaves [42]. Likewise, Melia azedarach (Meliaceae) has glandular trichomes on the surface of young but not on old leaves [109]. Therefore, these species were classified as non-storage species. Analogously, in most Rosaceae and Oleaceae species, the glandular trichomes are found on the flowers not on the leaves [41]. In Fabaceae and Aceraceae species, storage structures (oil cells) or glandular trichomes have not been found [41]. Altogether 19 species were confirmed to have at least one type of storage. As for some storage structure types, only one or a few species were available; the effect of storage structure type was assessed in the analyses of the impact of presence of storage structures on BVOC emissions (Table 1).
4.6. Statistical Analyses
All reported values are the averages of three independent replicates per species. First, BVOC emission rates were averaged for each plant individual (two replicate samples taken from each individual), and then the species average (three replicate plants) was calculated. The differences between the emission rates of intact and mechanically injured leaves were analyzed by paired sample t-tests. Differences in average emission rates before and after mechanical injury among species groups with and without storage structures, among deciduous and evergreen species and among ecological tolerance groups were analyzed by ANOVA followed by Duncan tests. Correlations between individual isoprenoid classes (isoprene, monoterpenes and sesquiterpenes) and aromatic compounds were explored by linear and nonlinear regression analyses. All statistical tests were considered significant at p < 0.05. The statistical analyses were performed with SPSS 24.0 for Windows (Statistical Products and Service Solutions, IBM, Armonk, NY, USA), and figures were drawn with Origin 8.0 software (Origin Lab, Northampton, MA, USA).
5. Conclusions
The presence and activity of different secondary metabolic pathways, including those responsible for BVOC production, are primarily governed by the intrinsic phylogenetic relationships and genetic backgrounds of the species. Nevertheless, even plant species with closely related genetic profiles have evolved distinct biological traits that align with their ecological niches. Wounding is a recurrent stress for plants, especially in urban environments where the vegetation is regularly pruned, but the information of interspecific variability in modifications in BVOC emission rates after wounding is scare. Our study emphasizes that the presence of storage structures is the key factor controlling the degree of wounding-dependent change in BVOC emissions to the ambient atmosphere. In particular, emissions of monoterpenes and aromatics in species with storage structures respond much more strongly to wounding than in species lacking storage structures. In the case of isoprene and de novo synthesized monoterpenes and aromatics in species without storage structures, the responses are moderate and their magnitude and direction likely depend on the impact of wounding on foliage photosynthetic characteristics that ultimately control the substrate availability for BVOC synthesis. Our study uncovered large differences in the wounding response among evergreen and deciduous species and among species with different tolerance of ecological factors. These differences were primarily driven by greater abundance of BVOC-storing species among evergreens and among species with higher tolerance of different abiotic stress factors. Evergreen and presence of storage structures reflect long-term plant adaptation to warm high-humidity environments, and furthermore, many evergreens can grow under low light conditions and have high shade tolerance. We argue that wounding-dependent stimulation of the emission of monoterpenes and aromatics need to be considered in emission inventories for urban forests. Our results can be applied in regional natural and urban emission inventories in the subtropical biome. Furthermore, the impact of wounding as an important factor in the broad-leaved evergreen forest biome has to be incorporated in global models. To better understand the modulation of BVOC emissions in response to wounding, further studies should explore the phylogenetic relationships in the presence of BVOC storage and ecological adaption traits and plant capacity to emit BVOC.
Author Contributions
Z.S. designed the research, participated in the measurements, data analysis and manuscript writing. Y.Y. and Y.M. performed the measurements and participated in data analysis and manuscript writing. Ü.N. participated in data analysis and manuscript writing. H.Y., M.G. and G.Z. contributed to manuscript writing and revised submission version. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Grant No. 32071731), the Science and Technology Commonweal Project Fund of Zhejiang province (Grant No. 2016C32018), the Key Research and Development Program of Zhejiang Province (Grant No. 2021C02005), and the Personnel Startup Project of the Scientific Research and Development Foundation of Liaocheng University (Grant No. 318042402), and Estonian Science Foundation (Grant No. PRG2207).
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon request.
Acknowledgments
We thank Chunyang Li for his great help in the experimental design and for facilitation of the measurements.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
List of the biogenic volatile organic compounds (BVOC) observed and wounding-dependent changes for monoterpenes, sesquiterpenes and aromatic compounds emitted from leaves of 45 subtropical woody species: compound name, molecular formula, emission rate ± SE.
Table A1.
List of the biogenic volatile organic compounds (BVOC) observed and wounding-dependent changes for monoterpenes, sesquiterpenes and aromatic compounds emitted from leaves of 45 subtropical woody species: compound name, molecular formula, emission rate ± SE.
| Species | Isoprene | Monoterpenes | Sesquiterpenes | Aromatic Compounds | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rate (nmol m−2 s−1) | Compound | Rate (nmol m−2 s−1) | Compound | Rate (nmol m−2 s−1) | Compound | Rate (nmol m−2 s−1) | |||||
| Intact | Injured | Intact | Injured | Intact | Injured | Intact | Injured | ||||
| Acer buergerianum | C10H16O Camphor | 0.012 ± 0.0021 | 0.014 ± 0.002 | C10H12 2,5-dimethylstyrene | 0.059 ± 0.014 | 0.092 ± 0.009 | |||||
| C10H16 α-pinene | 0.069 ± 0.0012 | C10H12O benzenebutanal | 0.0015 ± 0.00021 | ||||||||
| C10H16 Limonene | 0.024 ± 0.0010 | ||||||||||
| C10H16 α-phellandrene | 0.012 ± 0.0042 | ||||||||||
| C10H16 Pseudolimonene | 0.011 ± 0.0011 | ||||||||||
| C10H16 α-terpinolene | 0.0011 ± 0.00043 | ||||||||||
| Acer cinnamomifolium | C10H16 γ-terpinene | 0.0017 ± 0.00023 | 0.0041 ± 0.00062 | C10H12 2,5-dimethylstyrene | 0.021 ± 0.0056 | 0.031 ± 0.0020 | |||||
| C10H16 α-phellandrene | 0.00072 ± 0.00004 | * 0.0029 ± 0.0004 | |||||||||
| C10H16O Camphor | 0.0037 ± 0.0007 | 0.0059 ± 0.00081 | |||||||||
| C10H16 (E)-β-ocimene | 0.0020 ± 0.00033 | ||||||||||
| Acer henryi | C10H16 (E)-β-ocimene | 0.0031 ± 0.000010 | 0.0032 ± 0.0013 | C15H24 bicyclogermacrene | 0.028 ± 0.003 | C10H12 2,5-dimethylstyrene | * 0.0038 ± 0.0009 | 0.00061 ± 0.00025 | |||
| C10H16 α-pinene | 0.0018 ± 0.00020 | 0.0030 ± 0.00033 | C15H24 δ-guaiene | 0.024 ± 0.00034 | |||||||
| C10H16 Camphor | 0.011 ± 0.0018 | C15H24 aciphyllene | 0.015 ± 0.0013 | ||||||||
| C10H16 Limonene | 0.0022 ± 0.00049 | C15H24 α-muurolene | 0.018 ± 0.0053 | ||||||||
| C15H24 δ-selinene | 0.011 ± 0.00022 | ||||||||||
| Acer palmatum | C10H16 α-pinene | 0.012 ± 0.00083 | 0.015 ± 0.0028 | C15H24 cis-muurola-4(14),5-diene | 0.0032 ± 0.0012 | C14H18O2 4-cyclopentyl ethylbenzoate | 0.17 ± 0.018 | ||||
| C10H16O Camphor | 0.030 ± 0.0055 | 0.029 ± 0.0035 | |||||||||
| Broussonetia papyrifera | 1.2 ± 0.051 | 0.81 ± 0.33 | C10H16 α-pinene | 0.0080 ± 0.0011 | 0.0054 ± 0.00096 | C15H24 α-muurolene | 0.011 ± 0.00001 | ||||
| C10H16O Camphor | 0.019 ± 0.0051 | 0.0085 ± 0.0016 | C15H24 bicyclogermacrene | 0.0078 ± 0.00072 | |||||||
| Camellia japonica | C10H16 Limonene | 0.0034 ± 0.00018 | C14H18O2 4-cyclopentyl ethylbenzoate | 0.0032 ± 0.00063 | * 0.017 ± 0.00053 | ||||||
| C10H16 α-pinene | 0.0032 ± 0.00032 | 0.0025 ± 0.00033 | C7H6O benzaldehyde | * 0.0023 ± 0.0004 | 0.00071 ± 0.00038 | ||||||
| C10H16O Camphor | 0.011 ± 0.0013 | 0.011 ± 0.0010 | |||||||||
| Camptotheca acuminata | 0.064 ± 0.0062 | 0.051 ± 0.016 | C10H16 α-pinene | 0.00044 ± 0.00001 | 0.0031 ± 0.00023 | C13H18O2 2-methyl-3-phenylpropyl benzoate | 0.00043 ± 0.0000 | ||||
| C10H16O Camphor | 0.0014 ± 0.00013 | ||||||||||
| C10H16 Limonene | 0.0016 ± 0.00001 | ||||||||||
| Castanopsis sclerophylla | C10H16 α-pinene | * 0.097 ± 0.034 | 0.020 ± 0.0024 | C10H12 2,5-dimethylstyrene | 0.012 ± 0.0060 | ||||||
| C10H16 Limonene | * 0.022 ± 0.010 | 0.0034 ± 0.00076 | C13H18O2 2,4-butylethylbenzoate | 0.013 ± 0.00023 | |||||||
| C10H16 Cyclofeuchene | * 0.047 ± 0.015 | 0.0086 ± 0.00016 | |||||||||
| C10H16 γ-terpinene | * 0.046 ± 0.023 | 0.0079 ± 0.0020 | |||||||||
| C10H16 α-terpinene | 0.076 ± 0.017 | ||||||||||
| C10H16 camphene | 0.041 ± 0.023 | ||||||||||
| C10H16 Pseudolimonene | 0.021 ± 0.010 | ||||||||||
| C10H16 α-terpinolene | 0.013 ± 0.0049 | ||||||||||
| C10H16 (E)-β-ocimene | 0.011 ± 0.0056 | ||||||||||
| C10H16 α-phellandrene | 0.0086 ± 0.0027 | ||||||||||
| C10H16 α-fenchene | 0.0060 ± 0.0028 | ||||||||||
| C10H14 p-cymene | 0.069 ± 0.035 | ||||||||||
| Chimonanthus praecox | * 0.87 ± 0.077 | 0.14 ± 0.44 | C10H16 α-phellandrene | 0.0064 ± 0.00023 | C14H18O2 2,4-butylethylbenzoate | 0.0027 ± 0.00033 | |||||
| C10H16 α-pinene | 0.0023 ± 0.00063 | 0.0042 ± 0.00043 | C8H10 p-xylene | 0.0015 ± 0.0004 | |||||||
| C10H16 γ-terpinene | 0.0078 ± 0.00003 | * 0.024 ± 0.0033 | |||||||||
| C10H16 Pseudolimonene | 0.0037 ± 0.00058 | 0.0075 ± 0.00068 | |||||||||
| C10H16 β-phellandrene | 0.0025 ± 0.00022 | 0.0037 ± 0.00063 | |||||||||
| C10H16 Limonene | 0.0012 ± 0.000042 | * 0.0029 ± 0.0003 | |||||||||
| C10H16 α-terpinolene | 0.00061 ± 0.00001 | * 0.0015 ± 0.0002 | |||||||||
| C10H16O Camphor | 0.0022 ± 0.00050 | * 0.0058 ± 0.0011 | |||||||||
| C10H14 p-cymene | 0.0021 ± 0.00059 | * 0.010 ± 0.0034 | |||||||||
| C10H16 (E)-β-ocimene | 0.0071 ± 0.0013 | ||||||||||
| Cinnamomum camphora | C10H16 (E)-β-ocimene | 0.20 ± 0.085 | 0.37 ± 0.0093 | C15H24 γ-humulene | 0.039 ± 0.0057 | C10H12 2,5-dimethylstyrene | 0.013 ± 0.0063 | * 0.21 ± 0.083 | |||
| C10H16 α-pinene | 0.041 ± 0.015 | * 0.57 ± 0.040 | C15H24 bicyclogermacrene | 0.015 ± 0.0041 | C14H18O2 2,4-butylethylbenzoate | * 0.030 ± 0.011 | 0.012 ± 0.00023 | ||||
| C10H16 γ-terpinene | 0.024 ± 0.0097 | * 0.27 ± 0.0056 | C15H24 β-longipinene | 0.0089 ± 0.0032 | C10H12O2 dicyclopentadiene diepoxide | 0.012 ± 0.00010 | |||||
| C10H16 α-terpinene | 0.018 ± 0.0070 | * 0.30 ± 0.14 | C15H24 cis-muurola-4(14),5-diene | 0.0050 ± 0.0000 | |||||||
| C10H16 Limonene | 0.011 ± 0.0037 | * 0.56 ± 0.040 | C15H24 δ-selinene | 0.0053 ± 0.00001 | * 0.022 ± 0.0016 | ||||||
| C10H16 α-phellandrene | 0.0048 ± 0.00073 | 0.17 ± 0.066 | |||||||||
| C10H16 Pseudolimonene | 0.011 ± 0.0028 | * 0.18 ± 0.055 | |||||||||
| C10H16 α-terpinolene | 0.0069 ± 0.0027 | * 0.21 ± 0.085 | |||||||||
| C10H16 α-fenchene | 0.0010 ± 0.0001 | * 0.026 ± 0.0046 | |||||||||
| C10H16O Camphor | 0.30 ± 0.000012 | * 2.6 ± 0.79 | |||||||||
| C10H18O eucalyptol | 0.16 ± 0.095 | * 1.1 ± 0.39 | |||||||||
| C10H14 p-cymene | 0.058 ± 0.029 | * 0.49 ± 0.13 | |||||||||
| C10H16 camphene | 0.52 ± 0.068 | ||||||||||
| C10H16 3-carene | 0.27 ± 0.046 | ||||||||||
| C10H16 β-phellandrene | 0.25 ± 0.090 | ||||||||||
| C10H18O α-terpineol | 0.45 ± 0.13 | ||||||||||
| C10H18O trans-4-thujanol | 0.23 ± 0.074 | ||||||||||
| C10H18O endo-borneol | 0.099 ± 0.0081 | ||||||||||
| Cinnamomum japonicum | * 0.55 ± 0.071 | 0.16 ± 0.099 | C10H16 α-pinene | 0.0055 ± 0.0014 | * 0.075 ± 0.028 | C15H24 cis-muurola-4(14),5-diene | 0.0039 ± 0.0010 | C10H12 2,5-dimethylstyrene | 0.0020 ± 0.0011 | 0.0017 ± 0.00066 | |
| C10H16 camphene | 0.00090 ± 0.00009 | * 0.030 ± 0.012 | C15H24 γ-gurjunene | 0.026 ± 0.012 | 0.018 ± 0.00019 | ||||||
| C10H14 p-cymene | 0.00053 ± 0.00005 | * 0.0065 ± 0.0030 | C15H24 β-copaene | 0.11 ± 0.0069 | |||||||
| C10H16 Limonene | 0.035 ± 0.010 | C15H24 caryophyllene | 0.059 ± 0.000013 | ||||||||
| C10H16 Pseudolimonene | 0.027 ± 0.0097 | C15H24 bicyclogermacrene | 0.055 ± 0.0013 | ||||||||
| C10H16 β-phellandrene | 0.011 ± 0.0055 | C15H24 δ-guaiene | 0.033 ± 0.014 | ||||||||
| C10H16 α-phellandrene | 0.011 ± 0.0039 | C15H24 γ-humulene | 0.017 ± 0.00012 | ||||||||
| C10H16 (E)-β-ocimene | 0.0056 ± 0.0010 | C15H24 β-maaliene | 0.014 ± 0.0044 | ||||||||
| C10H16 γ-terpinene | 0.0027 ± 0.00025 | C15H24 δ-selinene | 0.0052 ± 0.0017 | 0.0072 ± 0.00052 | |||||||
| C10H16O Camphor | 0.24 ± 0.073 | ||||||||||
| C10H18O endo-Borneol | 0.010 ± 0.00064 | ||||||||||
| Cyclobalanopsis glauca | C10H16 α-pinene | 0.047 ± 0.0053 | 0.035 ± 0.016 | C10H12 3,4-dimethylstyrene | 0.00093 ± 0.0002 | 0.00070 ± 0.00002 | |||||
| C10H16 Pseudolimonene | 0.0050 ± 0.0013 | 0.0088 ± 0.00007 | C10H12O benzenebutanal | 0.0012 ± 0.00009 | |||||||
| C10H16 camphene | 0.0026 ± 0.00022 | 0.0039 ± 0.00019 | C13H18O2 2,4-butylethylbenzoate | 0.0018 ± 0.00021 | |||||||
| C10H16 Limonene | 0.0025 ± 0.00023 | 0.0032 ± 0.00086 | C8H10 p-xylene | 0.00062 ± 0.000061 | |||||||
| C10H16 α-terpinolene | 0.00051 ± 0.00003 | * 0.0013 ± 0.0001 | C15H14O2 2-phenylethyl benzoate | 0.00086 ± 0.00002 | |||||||
| C10H16O Camphor | 0.013 ± 0.0015 | 0.016 ± 0.0024 | |||||||||
| C10H14 p-cymene | 0.00073 ± 0.00022 | 0.00077 ± 0.0002 | |||||||||
| Cyclobalanopsis myrsinifolia | C10H16 α-pinene | 0.11 ± 0.0098 | 0.052 ± 0.023 | C10H12 3,4-dimethylstyrene | 0.010 ± 0.00021 | ||||||
| C10H16 γ-terpinene | * 0.050 ± 0.026 | 0.015 ± 0.0069 | C14H18O2 4-cyclopentyl ethylbenzoate | 0.065 ± 0.025 | 0.016 ± 0.0040 | ||||||
| C10H16 Cyclofeuchene | 0.055 ± 0.016 | ||||||||||
| C10H16 α-terpinene | 0.032 ± 0.16 | 0.016 ± 0.0061 | |||||||||
| C10H16 Pseudolimonene | 0.027 ± 0.0066 | 0.016 ± 0.0039 | |||||||||
| C10H16 Limonene | 0.015 ± 0.0040 | 0.011 ± 0.0038 | |||||||||
| C10H16 camphene | * 0.014 ± 0.0040 | 0.0040 ± 0.0013 | |||||||||
| C10H16 α-terpinolene | * 0.011 ± 0.0047 | 0.0043 ± 0.0010 | |||||||||
| C10H16 β-phellandrene | * 0.010 ± 0.0042 | 0.0036 ± 0.00091 | |||||||||
| C10H16 α-phellandrene | 0.0065 ± 0.0028 | 0.0036 ± 0.00090 | |||||||||
| C10H16 α-fenchene | 0.0023 ± 0.00046 | ||||||||||
| C10H16O Camphor | 0.0032 ± 0.00063 | 0.0024 ± 0.00017 | |||||||||
| C10H14 p-cymene | * 0.069 ± 0.032 | 0.025 ± 0.012 | |||||||||
| Elaeocarpus glabripetalus var. glabripetalus | 0.93 ± 0.18 | 0.70 ± 0.37 | C10H16 α-pinene | 0.0016 ± 0.00031 | 0.0025 ± 0.00022 | C13H18O2 2,4-butylethylbenzoate | * 0.031 ± 0.0089 | 0.013 ± 0.0040 | |||
| C10H16O Camphor | 0.0045 ± 0.0010 | 0.0067 ± 0.0010 | C15H14O2 2-phenylethyl benzoate | 0.00048 ± 0.000073 | |||||||
| C16H16O2 2-phenylethyl benzoate | 0.00086 ± 0.00009 | ||||||||||
| Gardenia jasminoides | C10H16O camphor | 0.013 ± 0.0035 | 0.014 ± 0.0037 | C14H18O2 4-cyclopentyl ethylbenzoate | 0.060 ± 0.014 | 0.036 ± 0.0069 | |||||
| C10H16 α-pinene | 0.0014 ± 0.00013 | ||||||||||
| Ginkgo biloba | C10H16 α-pinene | 0.0038 ± 0.0005 | * 0.014 ± 0.0043 | C14H18O2 4-cyclopentyl ethylbenzoate | 0.015 ± 0.0034 | * 0.040 ± 0.0084 | |||||
| C10H16O Camphor | 0.035 ± 0.00078 | ||||||||||
| Ilex chinensis | C10H16 α-pinene | 0.0015 ± 0.00045 | * 0.0081 ± 0.0016 | C11H14O2 2-phenylethyl benzoate | 0.0031 ± 0.00076 | ||||||
| C10H16O Camphor | 0.012 ± 0.0020 | 0.025 ± 0.011 | C14H18O2 4-cyclopentyl ethylbenzoate | 0.016 ± 0.0034 | |||||||
| Jasminum mesnyi | C10H16 α-pinene | 0.0035 ± 0.00051 | 0.0055 ± 0.00030 | C15H14O2 2-phenylethyl benzoate | 0.0014 ± 0.00001 | ||||||
| C10H16O Camphor | 0.010 ± 0.0015 | 0.013 ± 0.0020 | C14H18O2 4-cyclopentyl ethylbenzoate | 0.034 ± 0.0059 | 0.038 ± 0.0057 | ||||||
| Koelreuteria bipinnata var. integrifoliola | C10H16 α-pinene | 0.0047 ± 0.00013 | 0.0046 ± 0.00052 | C15H24 β-longipinene | 0.36 ± 0.0019 | ||||||
| C15H24 valerena-4,7(11)-diene | 0.17 ± 0.010 | ||||||||||
| C15H24 γ-himachalene | 0.099 ± 0.00044 | ||||||||||
| C15H24 δ-guaiene | 0.059 ± 0.013 | ||||||||||
| C15H24 β-elemene | 0.056 ± 0.017 | ||||||||||
| C15H24 α-guaiene | 0.021 ± 0.00086 | ||||||||||
| C15H24 bicyclogermacrene | 0.017 ± 0.00063 | ||||||||||
| C15H24 β-copaene | 0.016 ± 0.00058 | ||||||||||
| C15H24 aromadendrene | 0.013 ± 0.000064 | ||||||||||
| Ligustrum lucidum | C10H16 α-pinene | 0.0020 ± 0.00045 | * 0.011 ± 0.00022 | C14H18O2 4-cyclopentyl ethylbenzoate | 0.046 ± 0.0069 | 0.051 ± 0.0077 | |||||
| C9H10O2 2-phenylethyl benzoate | 0.0036 ± 0.00025 | ||||||||||
| Liriodendron chinense | C10H16 Pseudolimonene | 0.027 ± 0.0073 | * 0.12 ± 0.025 | C15H24 cis-muurola-3,5-diene | 0.0054 ± 0.00027 | C13H18O2 2,4-butylethylbenzoate | 0.091 ± 0.022 | 0.093 ± 0.015 | |||
| C10H16 α-pinene | 0.024 ± 0.0060 | 0.049 ± 0.018 | C10H12 3,4-dimethylstyrene | 0.0041 ± 0.00063 | |||||||
| C10H16 Limonene | 0.013 ± 0.0044 | 0.016 ± 0.0040 | |||||||||
| C10H16 γ-terpinene | 0.0052 ± 0.0020 | * 0.055 ± 0.0057 | |||||||||
| C10H16 α-fenchene | 0.0022 ± 0.00064 | 0.0031 ± 0.00005 | |||||||||
| C10H16 (E)-β-ocimene | 0.082 ± 0.020 | ||||||||||
| C10H16 α-terpinene | 0.034 ± 0.0049 | ||||||||||
| C10H16 camphene | 0.025 ± 0.00083 | ||||||||||
| C10H16 α-phellandrene | 0.022 ± 0.011 | ||||||||||
| C10H16 3-carene | 0.0084 ± 0.00063 | ||||||||||
| C10H18O eucalyptol | 0.0095 ± 0.00044 | ||||||||||
| C10H14 p-cymene | 0.0099 ± 0.0026 | ||||||||||
| Lithocarpus harlandii | C10H16 (E)-β-ocimene | * 0.050 ± 0.021 | 0.016 ± 0.0039 | C15H24 cis-muurola-3,5-diene | 0.0030 ± 0.0000011 | C10H12 3,4-dimethylstyrene | 0.0010 ± 0.00001 | ||||
| C10H16 α-pinene | 0.012 ± 0.0051 | 0.0050 ± 0.00083 | C14H18O2 4-cyclopentyl ethylbenzoate | 0.041 ± 0.010 | |||||||
| C10H16O Camphor | 0.0080 ± 0.00031 | 0.0063 ± 0.00027 | |||||||||
| C10H16 α-terpinolene | 0.015 ± 0.0010 | ||||||||||
| C10H16 Pseudolimonene | 0.012 ± 0.00015 | ||||||||||
| C10H16 γ-terpinene | 0.010 ± 0.0039 | ||||||||||
| C10H16 Limonene | 0.0032 ± 0.000017 | ||||||||||
| C10H16 α-phellandrene | 0.0028 ± 0.000070 | ||||||||||
| C10H14 p-cymene | 0.0077 ± 0.0020 | ||||||||||
| Machilus leptophylla | C10H16 α-pinene | 0.11 ± 0.0028 | 0.21 ± 0.028 | C15H24 β-copaene | 0.094 ± 0.00087 | C10H12 3,4-dimethylstyrene | 0.019 ± 0.0059 | 0.022 ± 0.0014 | |||
| C10H16 camphene | 0.054 ± 0.019 | 0.072 ± 0.013 | C15H24 cis-muurola-4(14),5-diene | 0.040 ± 0.014 | C14H18O2 4-cyclopentyl ethylbenzoate | 0.058 ± 0.010 | 0.029 ± 0.0029 | ||||
| C10H16 Pseudolimonene | 0.036 ± 0.017 | * 0.071 ± 0.013 | C15H24 σ-selinene | 0.054 ± 0.0051 | |||||||
| C10H16 α-terpinene | 0.029 ± 0.0095 | 0.058 ± 0.017 | C15H24 β-maaliene | 0.040 ± 0.0043 | |||||||
| C10H16 Limonene | 0.029 ± 0.0088 | 0.059 ± 0.0095 | C15H24 bicyclogermacrene | 0.018 ± 0.0062 | |||||||
| C10H16 γ-terpinene | 0.028 ± 0.011 | 0.034 ± 0.0066 | C15H24 β-elemene | 0.049 ± 0.014 | |||||||
| C10H16 β-phellandrene | 0.0086 ± 0.0010 | * 0.040 ± 0.011 | C15H24 aromadendrene | 0.018 ± 0.0062 | |||||||
| C10H16 α-terpinolene | 0.0068 ± 0.0017 | 0.011 ± 0.0018 | C15H24 α-bulnesene | 0.012 ± 0.00073 | 0.0027 ± 0.00034 | ||||||
| C10H16 α-phellandrene | 0.0083 ± 0.0036 | * 0.16 ± 0.0049 | C15H24 α-ylangene | 0.0065 ± 0.0019 | |||||||
| C10H16 α-fenchene | 0.0058 ± 0.0022 | 0.0076 ± 0.0011 | C15H24 germacrene D | 0.0030 ± 0.0010 | |||||||
| C10H14 p-cymene | 0.050 ± 0.014 | 0.10 ± 0.016 | C15H24 β-longipinene | 0.0066 ± 0.0019 | |||||||
| C10H16 (E)-β-ocimene | 0.011 ± 0.0011 | C15H24 aromadendrene | 0.017 ± 0.0021 | ||||||||
| C10H16 β-fenchene | 0.0035 ± 0.00032 | C15H24 cis-muurola-3,5-diene | 0.041 ± 0.000011 | ||||||||
| C10H18O eucalyptol | 0.015 ± 0.0012 | C15H24 β-longipinene | 0.026 ± 0.0044 | ||||||||
| C10H16 Cyclofeuchene | 0.029 ± 0.00033 | 0.019 ± 0.0034 | C15H24 (-)-aristolene | 0.0038 ± 0.00081 | |||||||
| Machilus pauhoi | C10H16 α-pinene | 0.039 ± 0.022 | * 0.096 ± 0.021 | C15H24 σ-selinene | 0.055 ± 0.015 | C13H18O3 methanol, [4-(1,1-dimethylethyl)phenoxy]-, acetate | 0.0015 ± 0.00004 | ||||
| C10H16 γ-terpinene | 0.052 ± 0.0090 | 0.064 ± 0.012 | C15H24 (-)-aciphyllene | 0.018 ± 0.0065 | C10H12 o-cumenene | 0.0045 ± 0.0011 | 0.0068 ± 0.0015 | ||||
| C10H16 Pseudolimonene | 0.017 ± 0.0023 | 0.020 ± 0.0063 | C15H24 β-copaene | 0.018 ± 0.0078 | C16H16O2 2-phenylethyl benzoate | 0.019 ± 0.0071 | |||||
| C10H16 α-terpinolene | 0.0058 ± 0.00093 | 0.0066 ± 0.0016 | C15H24 α-muurolene | 0.0086 ± 0.0026 | |||||||
| C10H16 β-phellandrene | 0.0056 ± 0.000057 | 0.0063 ± 0.0017 | C15H24 aromadendrene | 0.0025 ± 0.0011 | |||||||
| C10H16 Limonene | 0.0056 ± 0.0024 | 0.012 ± 0.0028 | C15H24 β-patchoulene | 0.0055 ± 0.0011 | |||||||
| C10H16 camphene | 0.011 ± 0.0038 | 0.011 ± 0.00051 | C15H24 caryophyllene | 0.0030 ± 0.00060 | |||||||
| C10H16 α-phellandrene | 0.0070 ± 0.000052 | 0.0085 ± 0.0018 | |||||||||
| C10H16 (E)-β-ocimene | 0.0078 ± 0.0016 | ||||||||||
| C10H14 p-cymene | 0.038 ± 0.015 | 0.021 ± 0.0078 | |||||||||
| Machilus thunbergii | C10H16 α-terpinolene | 0.025 ± 0.0017 | 0.053 ± 0.0037 | C15H14O2 2-phenylethyl benzoate | 0.0012 ± 0.00026 | ||||||
| C10H16 γ-terpinene | 0.0039 ± 0.00023 | * 0.010 ± 0.0024 | C14H18O2 4-cyclopentyl ethylbenzoate | 0.017 ± 0.0047 | * 0.094 ± 0.045 | ||||||
| C10H16 α-terpinene | 0.0029 ± 0.000010 | * 0.013 ± 0.00005 | C9H10O2 2-phenylethyl formate | 0.0037 ± 0.00033 | |||||||
| C10H16 Limonene | 0.00063 ± 0.00024 | * 0.012 ± 0.0038 | |||||||||
| C10H16 Pseudolimonene | 0.058 ± 0.0011 | ||||||||||
| C10H16 camphene | 0.0024 ± 0.00013 | ||||||||||
| C10H14 p-cymene | 0.0041 ± 0.0010 | ||||||||||
| C10H16 α-thujene | 0.011 ± 0.00083 | 0.0093 ± 0.00044 | |||||||||
| C10H16O Camphor | 0.023 ± 0.0046 | 0.021 ± 0.0054 | |||||||||
| Magnolia denudata | C10H16 α-pinene | 0.0013 ± 0.00025 | 0.0013 ± 0.00007 | C14H18O2 4-cyclopentyl ethylbenzoate | 0.046 ± 0.018 | 0.038 ± 0.013 | |||||
| C10H16O Camphor | 0.0017 ± 0.00020 | 0.0020 ± 0.00023 | C15H14O2 2-phenylethyl benzoate | 0.0010 ± 0.000071 | |||||||
| C10H18O eucalyptol | 0.0092 ± 0.00034 | ||||||||||
| Magnolia grandiflora | C10H16 camphene | 0.0028 ± 0.000051 | 0.0071 ± 0.0014 | C15H24 β-elemene | 0.0078 ± 0.0044 | * 0.081 ± 0.0076 | C10H12 3,4-dimethylstyrene | 0.00066 ± 0.0002 | * 0.0023 ± 0.00090 | ||
| C10H16 Pseudolimonene | 0.0026 ± 0.00053 | * 0.011 ± 0.0041 | C15H24 β-copaene | 0.0040 ± 0.0007 | C12H16O 1-(4-methylphenyl)-1-Pentanone | 0.0033 ± 0.0012 | |||||
| C10H16 Limonene | 0.0014 ± 0.0003 | * 0.0064 ± 0.0016 | C15H24 cis-muurola-4(14),5-diene | 0.028 ± 0.0079 | |||||||
| C10H16 γ-terpinene | 0.0013 ± 0.0002 | * 0.0048 ± 0.0009 | C15H24 caryophyllene | 0.023 ± 0.00001 | |||||||
| C10H16 α-terpinene | 0.00093 ± 0.00006 | * 0.0032 ± 0.0005 | C15H24 bicyclogermacrene | * 0.015 ± 0.0041 | 0.0014 ± 0.0005 | ||||||
| C10H14 p-cymene | 0.0021 ± 0.0005 | 0.0031 ± 0.0012 | C15H24 γ-gurjunene | 0.015 ± 0.0062 | 0.0087 ± 0.0028 | ||||||
| C10H16 α-fenchene | 0.0014 ± 0.0002 | ||||||||||
| C10H16O Camphor | 0.058 ± 0.0311 | ||||||||||
| C10H16 α-pinene | 0.042 ± 0.0069 | 0.040 ± 0.016 | |||||||||
| C10H16 α-thujene | 0.0025 ± 0.0002 | ||||||||||
| Magnolia liliflora | C10H16 α-pinene | 0.076 ± 0.032 | 0.13 ± 0.018 | C15H24 γ-gurjunene | 0.14 ± 0.038 | C14H18O2 2-phenylethyl benzoate | 0.013 ± 0.0012 | 0.014 ± 0.0031 | |||
| C10H16 Pseudolimonene | 0.063 ± 0.035 | * 0.18 ± 0.019 | C15H24 γ-humulene | 0.040 ± 0.0075 | C10H12 3,4-dimethylstyrene | 0.0036 ± 0.0014 | 0.0037 ± 0.0006 | ||||
| C10H16 γ-terpinene | 0.035 ± 0.016 | * 0.14 ± 0.031 | C15H24 β-longipinene | 0.11 ± 0.0002 | C9H12O 3,4-dimethylbenzyl alcohol | 0.011 ± 0.0033 | |||||
| C10H16 Limonene | 0.027 ± 0.011 | * 0.068 ± 0.0098 | C15H24 δ-guaiene | 0.016 ± 0.0037 | |||||||
| C10H16 camphene | 0.025 ± 0.011 | 0.040 ± 0.0061 | C15H24 germacrene D | 0.012 ± 0.0031 | |||||||
| C10H16 (E)-β-ocimene | 0.0035 ± 0.0004 | * 0.011 ± 0.0034 | C15H24 β-bourbonene | 0.0032 ± 0.0004 | |||||||
| C10H16 α-terpinolene | 0.0029 ± 0.0003 | 0.0044 ± 0.0008 | |||||||||
| C10H16 α-thujene | 0.0013 ± 0.00006 | 0.0025 ± 0.0004 | |||||||||
| C10H16 β-phellandrene | 0.00063 ± 0.0001 | 0.0014 ± 0.0003 | |||||||||
| C10H18O eucalyptol | 0.012 ± 0.0019 | 0.020 ± 0.0019 | |||||||||
| C10H14 p-cymene | 0.047 ± 0.022 | * 0.13 ± 0.017 | |||||||||
| C10H16 α-terpinene | 0.022 ± 0.0052 | ||||||||||
| C10H16O Camphor | 0.13 ± 0.042 | ||||||||||
| C10H18O α-terpineol | 0.0090 ± 0.0003 | ||||||||||
| Melia azedarach | C10H16 α-pinene | 0.00052 ± 0.00009 | 0.00094 ± 0.0002 | C8H10 p-xylene | 0.0032 ± 0.0002 | ||||||
| C10H16O camphor | 0.00062 ± 0.00001 | C10H18O2 cis-3-Hexenyl iso-butyrate | 0.00093 ± 0.00005 | ||||||||
| C15H14O2 2-phenylethyl benzoate | 0.0014 ± 0.0030 | ||||||||||
| Michelia chapensis | C10H16 α-pinene | 0.090 ± 0.031 | 0.11 ± 0.027 | C10H12 3,4-dimethylstyrene | 0.013 ± 0.0050 | * 0.049 ± 0.021 | |||||
| C10H16 α-terpinene | 0.061 ± 0.029 | 0.11 ± 0.042 | C9H12O 3,4-dimethylbenzyl alcohol | 0.082 ± 0.032 | |||||||
| C10H16 camphene | 0.054 ± 0.017 | 0.055 ± 0.023 | C9H10O2 (2-methylphenyl)methyl formate | 0.0031 ± 0.0003 | |||||||
| C10H16 Limonene | 0.041 ± 0.019 | 0.061 ± 0.027 | |||||||||
| C10H16 α-thujene | 0.029 ± 0.018 | * 0.11 ± 0.031 | |||||||||
| C10H16 Pseudolimonene | 0.027 ± 0.013 | 0.052 ± 0.025 | |||||||||
| C10H16 γ-terpinene | 0.018 ± 0.0068 | * 0.13 ± 0.048 | |||||||||
| C10H16 β-phellandrene | 0.013 ± 0.0045 | * 0.038 ± 0.016 | |||||||||
| C10H16 α-phellandrene | 0.0087 ± 0.0043 | 0.015 ± 0.0093 | |||||||||
| C10H16 α-fenchene | 0.0066 ± 0.0021 | 0.0087 ± 0.0036 | |||||||||
| C10H16 α-terpinolene | 0.0036 ± 0.0013 | * 0.040 ± 0.015 | |||||||||
| C10H18O eucalyptol | 0.031 ± 0.0096 | 0.037 ± 0.014 | |||||||||
| C10H14 p-cymene | 0.10 ± 0.028 | * 0.20 ± 0.033 | |||||||||
| C10H16 3-carene | 0.015 ± 0.0026 | ||||||||||
| C10H18O trans-4-thujanol | 0.010 ± 0.0011 | ||||||||||
| Michelia figo | C10H16 α-pinene | 0.00043 ± 0.00008 | * 0.0039 ± 0.0003 | C15H24 germacrene D | 0.0052 ± 0.00001 | C11H14O2 2-phenylethyl benzoate | 0.0011 ± 0.00041 | ||||
| C10H16 camphene | 0.013 ± 0.0014 | C15H24 cis-muurola-4(14),5-diene | 0.0023 ± 0.0002 | ||||||||
| C10H16 Limonene | 0.00076 ± 0.0003 | ||||||||||
| Michelia foveolata | C10H16 α-thujene | 0.039 ± 0.000022 | 0.068 ± 0.012 | C15H24 β-copaene | 0.036 ± 0.000011 | C14H18O2 4-cyclopentyl ethylbenzoate | * 0.14 ± 0.0087 | 0.064 ± 0.023 | |||
| C10H16 γ-terpinene | 0.068 ± 0.0052 | 0.11 ± 0.024 | C15H24 γ-elemene | 0.034 ± 0.0056 | C10H12 2,5-dimethylstyrene | 0.010 ± 0.0015 | 0.0096 ± 0.0054 | ||||
| C10H16 α-terpinene | 0.028 ± 0.0049 | 0.046 ± 0.0190 | C15H24 bicyclogermacrene | 0.026 ± 0.0010 | C9H12O 3,4-dimethylbenzyl alcohol | 0.027 ± 0.0028 | |||||
| C10H16 Limonene | 0.025 ± 0.0028 | 0.029 ± 0.017 | C15H24 α-muurolene | 0.021 ± 0.0060 | C9H10O2 2-phenylethyl benzoate | 0.0021 ± 0.00022 | |||||
| C10H16 camphene | 0.020 ± 0.0002 | * 0.055 ± 0.014 | C15H24 α-copaene | 0.017 ± 0.0033 | |||||||
| C10H16 α-terpinolene | 0.0073 ± 0.0012 | 0.016 ± 0.0078 | C15H24 -guaiene | 0.014 ± 0.00041 | |||||||
| C10H16 α-phellandrene | 0.0062 ± 0.0013 | 0.019 ± 0.0035 | C15H24 (-)-aristolene | 0.0097 ± 0.0022 | |||||||
| C10H16 β-phellandrene | 0.0060 ± 0.0008 | * 0.020 ± 0.0043 | C15H24 β-longipinene | 0.0084 ± 0.00008 | |||||||
| C10H16 α-fenchene | 0.0013 ± 0.00002 | * 0.0080 ± 0.0019 | C15H24 γ-humulene | 0.0065 ± 0.00004 | |||||||
| C10H18O eucalyptol | 0.0070 ± 0.0005 | 0.020 ± 0.0030 | C15H24 β-maaliene | 0.0045 ± 0.00005 | |||||||
| C10H16O Camphor | 0.012 ± 0.0020 | C15H24 isoledene | 0.0029 ± 0.00052 | ||||||||
| C10H14 p-cymene | 0.055 ± 0.0097 | 0.040 ± 0.020 | |||||||||
| C10H16 α-pinene | 0.14 ± 0.019 | 0.082 ± 0.029 | |||||||||
| C10H16 (E)-β-ocimene | 0.084 ± 0.0052 | 0.049 ± 0.023 | |||||||||
| Michelia maudiae | C10H16 camphene | 0.18 ± 0.041 | * 1.0 ± 0.19 | C10H12 2,5-dimethylstyrene | 0.018 ± 0.054 | * 0.15 ± 0.027 | |||||
| C10H16 α-pinene | 0.12 ± 0.031 | * 0.45 ± 0.079 | C13H18O2 2,4-butylethylbenzoate | 0.031 ± 0.0042 | |||||||
| C10H16 (E)-β-ocimene | 0.11 ± 0.037 | 0.12 ± 0.027 | C9H12O 3,4-dimethylbenzyl alcohol | 0.077 ± 0.029 | |||||||
| C10H16 Cyclofeuchene | 0.10 ± 0.023 | * 0.32 ± 0.021 | |||||||||
| C10H16 Limonene | 0.086 ± 0.032 | * 0.43 ± 0.090 | |||||||||
| C10H16 Pseudolimonene | 0.082 ± 0.028 | * 0.44 ± 0.052 | |||||||||
| C10H16 γ-terpinene | 0.036 ± 0.015 | * 0.17 ± 0.045 | |||||||||
| C10H16 α-terpinene | 0.028 ± 0.0095 | * 0.15 ± 0.051 | |||||||||
| C10H16 α-thujene | 0.019 ± 0.0074 | * 0.10 ± 0.037 | |||||||||
| C10H16 α-terpinolene | 0.013 ± 0.0039 | * 0.16 ± 0.054 | |||||||||
| C10H16 α-phellandrene | 0.0051 ± 0.00023 | * 0.11 ± 0.0092 | |||||||||
| C10H16 β-phellandrene | 0.0043 ± 0.0013 | * 0.076 ± 0.020 | |||||||||
| C10H18O eucalyptol | 0.023 ± 0.0093 | * 0.23 ± 0.068 | |||||||||
| C10H16O Camphor | 0.0017 ± 0.00072 | * 0.21 ± 0.030 | |||||||||
| C10H14 p-cymene | 0.083 ± 0.031 | * 0.23 ± 0.057 | |||||||||
| C10H16 β-fenchene | 0.17 ± 0.11 | ||||||||||
| C10H16 3-carene | 0.12 ± 0.0048 | ||||||||||
| C10H18O Fenchol | 0.053 ± 0.0017 | ||||||||||
| C10H18O endo-borneol | 0.050 ± 0.0017 | ||||||||||
| C10H18O trans-4-thujanol | 0.024 ± 0.0.0041 | ||||||||||
| C10H18O α-terpineol | 0.054 ± 0.016 | ||||||||||
| Osmanthus fragrans | C10H16O Camphor | 0.024 ± 0.000090 | C15H24 β-longipinene | 0.13 ± 0.021 | C14H18O2 4-cyclopentyl ethylbenzoate | * 0.017 ± 0.0038 | 0.0064 ± 0.00054 | ||||
| C10H16 α-pinene | 0.0049 ± 0.00051 | C10H12 3,4-dimethylstyrene | * 0.14 ± 0.039 | 0.029 ± 0.0069 | |||||||
| C10H16 Pseudolimonene | 0.0099 ± 0.0014 | ||||||||||
| C10H16 camphene | * 0.55 ± 0.047 | 0.20 ± 0.059 | |||||||||
| C10H16 Cyclofeuchene | * 0.32 ± 0.051 | 0.13 ± 0.00001 | |||||||||
| C10H16 Limonene | * 0.28 ± 0.00013 | 0.044 ± 0.0077 | |||||||||
| C10H16 α-terpinolene | * 0.020 ± 0.00014 | 0.0041 ± 0.0010 | |||||||||
| C10H14 p-cymene | * 0.36 ± 0.11 | 0.14 ± 0.058 | |||||||||
| C10H16 bornylene | 0.031 ± 0.0090 | ||||||||||
| C10H16 α-fenchene | 0.020 ± 0.000013 | ||||||||||
| Parakmeria lotungensis | C10H16 α-pinene | 0.028 ± 0.012 | * 0.45 ± 0.16 | C15H24 β-longipinene | 0.17 ± 0.036 | C10H12 3,4-dimethylstyrene | 0.012 ± 0.000014 | * 0.14 ± 0.037 | |||
| C10H16 Limonene | 0.021 ± 0.0077 | * 0.47 ± 0.21 | C15H24 α-copaene | 0.0040 ± 0.00011 | C14H18O2 4-cyclopentyl ethylbenzoate | 0.0029 ± 0.00020 | |||||
| C10H16 Pseudolimonene | 0.019 ± 0.0099 | * 0.58 ± 0.19 | C9H12O p-cumenol | 0.022 ± 0.0048 | |||||||
| C10H16 β-phellandrene | 0.0020 ± 0.00051 | * 0.14 ± 0.052 | |||||||||
| C10H18O eucalyptol | 0.0057 ± 0.00062 | * 0.28 ± 0.051 | |||||||||
| C10H16 camphene | 0.46 ± 0.11 | ||||||||||
| C10H16 α-terpinene | 0.54 ± 0.19 | ||||||||||
| C10H16 γ-terpinene | 0.32 ± 0.094 | ||||||||||
| C10H16 α-fenchene | 0.17 ± 0.047 | ||||||||||
| C10H16 (E)-β-ocimene | 0.10 ± 0.041 | ||||||||||
| C10H16 3-carene | 0.18 ± 0.059 | ||||||||||
| C10H16 Cyclofeuchene | 0.18 ± 0.061 | ||||||||||
| C10H16 α-phellandrene | 0.15 ± 0.059 | ||||||||||
| C10H16 β-fenchene | 0.068 ± 0.024 | ||||||||||
| C10H14 p-cymene | 0.41 ± 0.084 | ||||||||||
| C10H16O Camphor | 0.12 ± 0.047 | ||||||||||
| C10H18O endo-borneol | 0.10 ± 0.022 | ||||||||||
| C10H18O trans-4-thujanol | 0.082 ± 0.017 | ||||||||||
| C10H18O Fenchol | 0.064 ± 0.021 | ||||||||||
| C10H18O α-terpineol | 0.034 ± 0.0082 | ||||||||||
| C10H18O Linalool | 0.29 ± 0.16 | ||||||||||
| Photinia × fraseri | C10H16 α-pinene | 0.0056 ± 0.00080 | 0.0068 ± 0.0010 | C7H6O benzaldehyde | 0.0018 ± 0.00042 | * 0.016 ± 0.0047 | |||||
| C10H16O Camphor | 0.019 ± 0.0023 | 0.021 ± 0.0016 | C11H14O2 2-phenylethyl benzoate | 0.0042 ± 0.00071 | 0.0038 ± 0.00070 | ||||||
| C10H16 (E)-β-ocimene | 0.0068 ± 0.0010 | C8H10 p-xylene | 0.013 ± 0.0034 | ||||||||
| Photinia serrulata | C10H16 Limonene | 0.00073 ± 0.00005 | 0.00091 ± 0.0002 | C14H18O2 4-cyclopentyl ethylbenzoate | 0.015 ± 0.0054 | 0.016 ± 0.0020 | |||||
| C10H16O Camphor | 0.0032 ± 0.0010 | 0.0042 ± 0.00065 | C15H14O2 2-phenylethyl benzoate | 0.00033 ± 0.000022 | * 0.00081 ± 0.000041 | ||||||
| C10H16 α-pinene | 0.0044 ± 0.0021 | 0.0032 ± 0.00071 | C7H6O benzaldehyde | 0.21 ± 0.042 | |||||||
| C7H8O benzyl alcohol | 0.050 ± 0.0081 | ||||||||||
| Pittosporum tobira | C10H16 camphene | 0.079 ± 0.012 | * 0.32 ± 0.082 | C15H24 thujopsene | 0.044 ± 0.0026 | C10H12 2,5-dimethylstyrene | 0.065 ± 0.0097 | * 0.17 ± 0.043 | |||
| C10H16 Cyclofeuchene | 0.025 ± 0.0040 | * 0.22 ± 0.012 | C15H24 β-longipinene | 0.14 ± 0.0020 | C14H18O2 4-cyclopentyl ethylbenzoate | 0.018 ± 0.0023 | |||||
| C10H16 α-pinene | 0.039 ± 0.022 | * 0.93 ± 0.25 | C15H24 cis-thujopsene | 0.049 ± 0.0036 | C9H12O 3,4-dimethylbenzyl alcohol | 0.090 ± 0.0016 | |||||
| C10H16 bornylene | 0.0053 ± 0.00061 | * 0.10 ± 0.022 | C15H24 bicyclogermacrene | 0.0043 ± 0.0001 | |||||||
| C10H16 β-fenchene | 0.0038 ± 0.00064 | * 0.47 ± 0.19 | C15H24 γ-amorphene | 0.051 ± 0.0125 | |||||||
| C10H16 Limonene | 0.021 ± 0.0047 | * 0.18 ± 0.048 | C15H24 γ-cadinene | 0.036 ± 0.00030 | |||||||
| C10H16 α-fenchene | 0.12 ± 0.034 | C15H24 cis-muurola-4(14),5-diene | 0.012 ± 0.0028 | ||||||||
| C10H16 α-terpinene | 0.12 ± 0.041 | C15H24 γ-elemene | 0.013 ± 0.0028 | ||||||||
| C10H16 Pseudolimonene | 0.13 ± 0.029 | C15H24 β-copaene | 0.17 ± 0.016 | ||||||||
| C10H16 α-terpinolene | 0.12 ± 0.033 | C15H24 cis-muurola-3,5-diene | * 0.057 ± 0.0074 | 0.017 ± 0.0070 | |||||||
| C10H16 γ-terpinene | 0.075 ± 0.030 | C15H24 isoledene | 0.035 ± 0.016 | 0.028 ± 0.018 | |||||||
| C10H16 α-phellandrene | 0.050 ± 0.013 | ||||||||||
| C10H16 β-phellandrene | 0.030 ± 0.0058 | ||||||||||
| C10H16 3-carene | 0.12 ± 0.00050 | ||||||||||
| C10H16 (E)-β-ocimene | 0.0044 ± 0.0017 | ||||||||||
| C10H18O Fenchol | 0.049 ± 0.016 | ||||||||||
| C10H18O endo-borneol | 0.049 ± 0.0091 | ||||||||||
| C10H16O Camphor | 0.025 ± 0.00051 | ||||||||||
| C10H18O fenchone | 0.015 ± 0.0015 | ||||||||||
| C10H14 p-cymene | 0.21 ± 0.012 | ||||||||||
| Platanus orientalis | 0.13 ± 0.044 | * 0.37 ± 0.090 | C10H16 α-pinene | 0.0018 ± 0.00001 | C13H18O2 2,4-butylethylbenzoate | 0.064 ± 0.030 | 0.13 ± 0.057 | ||||
| C10H16O Camphor | 0.0085 ± 0.00062 | ||||||||||
| Prunus mume | C10H16 α-pinene | 0.0032 ± 0.00020 | 0.0048 ± 0.00063 | C15H14O2 2-phenylethyl benzoate | 0.0021 ± 0.000024 | ||||||
| C10H16O Camphor | 0.0086 ± 0.0010 | 0.011 ± 0.0018 | |||||||||
| Pyracantha fortuneana | C10H16 camphene | 0.011 ± 0.0044 | 0.012 ± 0.0018 | C13H18O2 2,4-butylethylbenzoate | 0.072 ± 0.015 | 0.064 ± 0.014 | |||||
| C10H16 (E)-β-ocimene | 0.13 ± 0.00033 | C16H16O2 2-phenylethyl benzoate | 0.0034 ± 0.0013 | ||||||||
| C10H16 α-pinene | 0.017 ± 0.0061 | 0.015 ± 0.0015 | |||||||||
| C10H16O Camphor | 0.012 ± 0.0050 | 0.010 ± 0.0013 | |||||||||
| Quercus serrata var. brevipetiolata | 1.4 ± 0.3024 | 1.3 ± 0.5391 | C10H16 α-pinene | 0.0074 ± 0.00060 | * 0.023 ± 0.0010 | C14H18O2 4-cyclopentyl ethylbenzoate | 0.019 ± 0.0064 | 0.023 ± 0.0026 | |||
| C10H16 Limonene | 0.0019 ± 0.00027 | * 0.0050 ± 0.0013 | |||||||||
| C10H16O Camphor | 0.013 ± 0.00073 | 0.023 ± 0.00066 | |||||||||
| C10H16 Sabinene | 0.0020 ± 0.00090 | ||||||||||
| C10H16 Pseudolimonene | 0.0018 ± 0.00006 | ||||||||||
| Sapindus mukorossi | C10H16 α-pinene | 0.0020 ± 0.00011 | 0.0034 ± 0.00070 | C13H18O2 2,4-butylethylbenzoate | 0.012 ± 0.0031 | 0.0057 ± 0.0015 | |||||
| C10H16 Limonene | 0.00053 ± 0.0001 | C10H18O2 4-hexen-1 butylethylbenzoate | 0.00063 ± 0.00013 | ||||||||
| C10H16O Camphor | 0.023 ± 0.0024 | 0.021 ± 0.0061 | |||||||||
| Sophora japonica | 1.7 ± 0.29 | 0.76 ± 0.29 | C10H14 p-cymene | 0.00084 ± 0.0002 | C15H24 cis-muurola-4(14),5-diene | 0.00063 ± 0.000023 | |||||
| Sophora japonica var. japonica f. pendula | 0.87 ± 0.22 | 0.49 ± 0.21 | C10H16 Cyclofeuchene | * 0.049 ± 0.00013 | 0.012 ± 0.0020 | C15H24 α-muurolene | 0.1498 ± 0.0024 | ||||
| C10H16 α-pinene | 0.0049 ± 0.0026 | 0.0054 ± 0.00011 | C15H24 cis-muurola-4(14),5-diene | 0.089 ± 0.040 | |||||||
| C10H16O Camphor | 0.016 ± 0.0056 | 0.028 ± 0.00053 | C15H24 β-copaene | 0.060 ± 0.000021 | |||||||
| C15H24 σ-cadinene | 0.0356 ± 0.0080 | ||||||||||
* indicates significant differences between intact and injured leaf blades as assessed by paired-samples t-tests (p < 0.05). All reported values are the averages of three independent replicates per species. The emission rates were averaged for each plant individual (two replicate samples taken from each species), and then the species average (three replicate specimens) was calculated.
Appendix B

Table A2.
Detailed list of calculated retention indices of target compounds detected in the analysis. The tRx, tRn, and tRn+1 in the table are the retention times of the measured compounds and the peaks of normal alkanes with carbon atoms between n and n + 1 (tRn < tRx < tRn+1).
Table A2.
Detailed list of calculated retention indices of target compounds detected in the analysis. The tRx, tRn, and tRn+1 in the table are the retention times of the measured compounds and the peaks of normal alkanes with carbon atoms between n and n + 1 (tRn < tRx < tRn+1).
| Compounds | Molecular Formula | tx/min | Carbon Number (n) | tn/min | tn+1/min | Retention Index (RI) |
|---|---|---|---|---|---|---|
| benzyl alcohol | C7H8O | 20.872 | C8 | 25.521 | 29.855 | 692.7319 |
| p-xylene | C8H10 | 29.507 | C8 | 25.521 | 29.855 | 891.9705 |
| 2-phenylethyl benzoate | C11H14O2 | 30.714 | C9 | 29.855 | 34.8 | 917.3711 |
| 2-phenylethyl benzoate | C15H14O2 | 30.726 | C9 | 29.855 | 34.8 | 917.6138 |
| 2-phenylethyl benzoate | C9H10O2 | 30.735 | C9 | 29.855 | 34.8 | 917.7958 |
| cyclofeuchene | C10H16 | 31.294 | C9 | 29.855 | 34.8 | 929.1001 |
| α-pinene | C10H16 | 31.695 | C9 | 29.855 | 34.8 | 937.2093 |
| α-bulnesene | C15H24 | 32.283 | C9 | 29.855 | 34.8 | 949.1001 |
| α-fenchene | C10H16 | 32.425 | C9 | 29.855 | 34.8 | 951.9717 |
| camphene | C10H16 | 32.538 | C9 | 29.855 | 34.8 | 954.2568 |
| thujopsene | C15H24 | 33.098 | C9 | 29.855 | 34.8 | 965.5814 |
| 4-cyclopentyl ethylbenzoate | C14H18O2 | 33.532 | C9 | 29.855 | 34.8 | 974.3579 |
| 2,4-butylethylbenzoate | C13H18O2 | 33.561 | C9 | 29.855 | 34.8 | 974.9444 |
| pseudolimonene | C10H16 | 33.804 | C9 | 29.855 | 34.8 | 979.8584 |
| β-longipinene | C15H24 | 34.35 | C9 | 29.855 | 34.8 | 990.8999 |
| aciphyllene | C15H24 | 34.722 | C9 | 29.855 | 34.8 | 998.4226 |
| α-phellandrene | C10H16 | 34.809 | C10 | 38.719 | 34.8 | 1000.2297 |
| benzaldehyde | C7H6O | 35.052 | C10 | 38.719 | 34.8 | 1006.4302 |
| 3,4-dimethylbenzyl alcohol | C9H12O | 35.399 | C10 | 38.719 | 34.8 | 1015.2845 |
| limonene | C10H16 | 35.912 | C10 | 38.719 | 34.8 | 1028.3746 |
| p-cymene | C10H14 | 36.137 | C10 | 38.719 | 34.8 | 1034.1158 |
| β-phellandrene | C10H16 | 36.309 | C10 | 38.719 | 34.8 | 1038.5047 |
| (E)-β-ocimene | C10H16 | 36.451 | C10 | 38.719 | 34.8 | 1042.1281 |
| eucalyptol | C10H18O | 36.68 | C10 | 38.719 | 34.8 | 1047.9714 |
| 3-carene | C10H16 | 37.089 | C10 | 38.719 | 34.8 | 1058.4078 |
| γ-terpinene | C10H16 | 37.319 | C10 | 38.719 | 34.8 | 1064.2766 |
| aromadendrene | C15H24 | 37.745 | C11 | 42.954 | 38.719 | 1077.0012 |
| α-terpinolene | C10H16 | 38.885 | C11 | 42.954 | 38.719 | 1103.9197 |
| p-cumenol | C9H12O | 38.885 | C11 | 42.954 | 38.719 | 1103.9197 |
| γ-humulene | C15H24 | 39.052 | C11 | 42.954 | 38.719 | 1107.8630 |
| γ-himachalene | C15H24 | 39.086 | C11 | 42.954 | 38.719 | 1108.6659 |
| (-)-aristolene | C15H24 | 39.327 | C11 | 42.954 | 38.719 | 1114.3566 |
| 2,5-dimethylstyrene | C10H12 | 39.433 | C11 | 42.954 | 38.719 | 1116.8595 |
| β-maaliene | C15H24 | 40.158 | C11 | 42.954 | 38.719 | 1133.9787 |
| α-ylangene | C15H24 | 41.436 | C11 | 42.954 | 38.719 | 1164.1558 |
| β-copaene | C15H24 | 41.736 | C11 | 42.954 | 38.719 | 1171.2397 |
| δ-selinene | C15H24 | 41.828 | C11 | 42.954 | 38.719 | 1173.4120 |
| cis-muurola-3,5-diene | C15H24 | 41.837 | C11 | 42.954 | 38.719 | 1173.6246 |
| germacrene D | C15H24 | 42.338 | C11 | 42.954 | 38.719 | 1185.4545 |
| cis-muurola-4(14),5-diene | C15H24 | 42.392 | C11 | 42.954 | 38.719 | 1186.7296 |
| isoledene | C15H24 | 42.66 | C11 | 42.954 | 38.719 | 1193.0579 |
| valerena-4,7(11)-diene | C15H24 | 42.78 | C11 | 42.954 | 38.719 | 1195.8914 |
| 4-hexen-1-butylethylbenzoate | C10H18O2 | 42.868 | C11 | 42.954 | 38.719 | 1197.9693 |
| β-bourbonene | C15H24 | 43.123 | C12 | 44.8 | 42.954 | 1209.1549 |
| β-elemene | C15H24 | 43.511 | C12 | 44.8 | 42.954 | 1230.1733 |
| camphor | C10H16O | 43.515 | C12 | 44.8 | 42.954 | 1230.3900 |
| bicyclogermacrene | C15H24 | 44.246 | C12 | 44.8 | 42.954 | 1269.9892 |
| α-muurolene | C15H24 | 44.262 | C12 | 44.8 | 42.954 | 1270.8559 |
| endo-borneol | C10H18O | 44.308 | C12 | 44.8 | 42.954 | 1273.3478 |
| caryophyllene | C15H24 | 44.492 | C12 | 44.8 | 42.954 | 1283.3153 |
| α-terpineol | C10H18O | 44.731 | C12 | 44.8 | 42.954 | 1296.2622 |
References
- Loreto, F.; Schnitzler, J.P. Abiotic stresses and induced BVOCs. Trends Plant Sci. 2010, 15, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Fineschi, S.; Loreto, F.; Staudt, M.; Peñuelas, J. Diversification of volatile isoprenoid emissions from trees: Evolutionary and ecological perspectives. In Biology, Controls and Models of Tree Volatile Organic Compound Emissions; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–20. [Google Scholar]
- Peñuelas, J.; Llusià, J. BVOCs: Plant defense against climate warming? Trends Plant Sci. 2003, 8, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Fehsenfeld, F.; Calvert, J.; Fall, R.; Goldan, P.; Guenther, A.B.; Hewitt, C.N.; Lamb, B.; Liu, S.; Trainer, M.; Westberg, H. Emissions of volatile organic compounds from vegetation and the implications for atmospheric chemistry. Glob. Biogeochem. Cycles 1992, 6, 389–430. [Google Scholar] [CrossRef]
- Calfapietra, C.; Fares, S.; Manes, F.; Morani, A.; Sgrigna, G.; Loreto, F. Role of Biogenic Volatile Organic Compounds (BVOC) emitted by urban trees on ozone concentration in cities: A review. Environ. Pollut. 2013, 183, 71–80. [Google Scholar] [CrossRef]
- Kulmala, M.; Nieminen, T.; Chellapermal, R.; Makkonen, R.; Bäck, J.; Kerminen, V.-M. Climate feedbacks linking the increasing atmospheric CO2 concentration, BVOC emissions, aerosols and clouds in forest ecosystems. In Biology, Controls and Models of Tree Volatile Organic Compound Emissions; Springer: Berlin/Heidelberg, Germany, 2013; pp. 489–508. [Google Scholar]
- Guenther, A.B.; Jiang, X.; Heald, C.L.; Sakulyanontvittaya, T.; Duhl, T.; Emmons, L.K.; Wang, X. The Model of Emissions of Gases and Aerosols from Nature version 2.1 (MEGAN2.1): An extended and updated framework for modeling biogenic emissions. Geosci. Model Dev. 2012, 5, 1471–1492. [Google Scholar] [CrossRef]
- Calfapietra, C.; Pallozzi, E.; Lusini, I.; Velikova, V. Modification of BVOC emissions by changes in atmospheric [CO2] and air pollution. In Biology, Controls and Models of Tree Volatile Organic Compound Emissions; Springer: Berlin/Heidelberg, Germany, 2013; pp. 253–284. [Google Scholar]
- Tani, A.; Masui, N.; Chang, T.; Okumura, M.; Kokubu, Y. Basal emission rates of isoprene and monoterpenes from major tree species in Japan: Interspecies and intraspecies variabilities. Prog. Earth Planet. Sci. 2024, 11, 42. [Google Scholar] [CrossRef]
- Yuan, Y.; Sun, Z.; Kännaste, A.; Guo, M.; Zhou, G.; Niinemets, Ü. Isoprenoid and aromatic compound emissions in relation to leaf structure, plant growth form and species ecology in 45 East-Asian urban subtropical woody species. Urban For. Urban Green. 2020, 53, 126705. [Google Scholar] [CrossRef]
- Copolovici, L.; Niinemets, Ü. Environmental impacts on plant volatile emission. In Deciphering Chemical Language of Plant Communication; Springer: Berlin/Heidelberg, Germany, 2016; pp. 35–59. [Google Scholar]
- Brilli, F.; Ciccioli, P.; Frattoni, M.; Prestininzi, M.; Spanedda, A.F.; Loreto, F. Constitutive and herbivore-induced monoterpenes emitted by Populus × euroamericana leaves are key volatiles that orient Chrysomela populi beetles. Plant Cell Environ. 2009, 32, 542–552. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Arneth, A.; Kuhn, U.; Monson, R.; Peñuelas, J.; Staudt, M. The emission factor of volatile isoprenoids: Stress, acclimation, and developmental responses. Biogeosciences 2010, 7, 2203–2223. [Google Scholar] [CrossRef]
- Guenther, A.B. Biological and chemical diversity of biogenic volatile organic emissions into the atmosphere. Int. Sch. Res. Not. 2013, 2013, 786290. [Google Scholar] [CrossRef]
- Fernández-Martínez, M.; Llusià, J.; Filella, I.; Niinemets, Ü.; Arneth, A.; Wright, I.J.; Loreto, F.; Peñuelas, J. Nutrient-rich plants emit a less intense blend of volatile isoprenoids. New Phytol. 2018, 220, 773–784. [Google Scholar] [CrossRef]
- Ciccioli, P.; Centritto, M.; Loreto, F. Biogenic volatile organic compound emissions from vegetation fires. Plant Cell Environ. 2014, 37, 1810–1825. [Google Scholar] [CrossRef] [PubMed]
- Hanson, D.T.; Swanson, S.; Graham, L.E.; Sharkey, T.D. Evolutionary significance of isopreneemission from mosses. Am. J. Bot. 1999, 86, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, T.D.; Chen, X.; Yeh, S. Isoprene increases thermotolerance of fosmidomycin-fed leaves. Plant Physiol. 2001, 125, 2001–2006. [Google Scholar] [CrossRef]
- Salomón, R.L.; Rodríguez-Calcerrada, J.; Staudt, M. Carbon losses from respiration and emission of volatile organic compounds—The overlooked side of tree carbon budgets. In Oaks Physiological Ecology. Exploring the Functional Diversity of Genus Quercus L.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 327–359. [Google Scholar]
- Niinemets, Ü.; Rasulov, B.; Talts, E. CO2-responsiveness of leaf isoprene emission: Why do species differ? Plant Cell Environ. 2021, 44, 3049–3063. [Google Scholar] [CrossRef]
- Fischbach, R.J.; Staudt, M.; Zimmer, I.; Rambal, S.; Schnitzler, J.P. Seasonal pattern of monoterpene synthase activities in leaves of the evergreen tree Quercus ilex. Physiol. Plant. 2002, 114, 354–360. [Google Scholar] [CrossRef]
- Niinemets, Ü. What are plant-released biogenic volatiles and how they participate in landscape-to global-level processes? In Ecosystem Services from Forest Landscapes: Broadscale Considerations; Springer: Berlin/Heidelberg, Germany, 2018; pp. 29–56. [Google Scholar]
- Loreto, F.; Förster, A.; Dürr, M.; Csiky, O.; Seufert, G. On the monoterpene emission under heat stress and on the increased thermotolerance of leaves of Quercus ilex L. fumigated with selected monoterpenes. Plant Cell Environ. 1998, 21, 101–107. [Google Scholar] [CrossRef]
- Misztal, P.K.; Hewitt, C.N.; Wildt, J.; Blande, J.D.; Eller, A.S.D.; Fares, S.; Gentner, D.R.; Gilman, J.B.; Graus, M.; Greenberg, J. Atmospheric benzenoid emissions from plants rival those from fossil fuels. Sci. Rep. 2015, 5, 12064. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, Ü. Storage of defense metabolites in the leaves of Myrtaceae: News of the eggs in different baskets. Tree Physiol. 2018, 38, 1445–1450. [Google Scholar] [CrossRef]
- Grote, R.; Sharma, M.; Ghirardo, A.; Schnitzler, J.P. A new modeling approach for estimating abiotic and biotic stress-induced de novo emissions of biogenic volatile organic compounds from plants. Front. For. Glob. Chang. 2019, 2, 26. [Google Scholar] [CrossRef]
- Pazouki, L.; Kanagendran, A.; Li, S.; Kännaste, A.; Memari, H.R.; Bichele, R.; Niinemets, Ü. Mono-and sesquiterpene release from tomato (Solanum lycopersicum) leaves upon mild and severe heat stress and through recovery: From gene expression to emission responses. Environ. Exp. Bot. 2016, 132, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kanagendran, A.; Pazouki, L.; Li, S.; Liu, B.; Kännaste, A.; Niinemets, Ü. Ozone-triggered surface uptake and stress volatile emissions in Nicotiana tabacum ‘Wisconsin’. J. Exp. Bot. 2018, 69, 681–697. [Google Scholar] [CrossRef] [PubMed]
- Vickers, C.E.; Gershenzon, J.; Lerdau, M.T.; Loreto, F. A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat. Chem. Biol. 2009, 5, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Holopainen, J.K.; Gershenzon, J. Multiple stress factors and the emission of plant VOCs. Trends Plant Sci. 2010, 15, 176–184. [Google Scholar] [CrossRef]
- Rasulov, B.; Talts, E.; Niinemets, Ü. A novel approach for real-time monitoring of leaf wounding responses demonstrates unprecedently fast and high emissions of volatiles from cut leaves. Plant Sci. 2019, 283, 256–265. [Google Scholar] [CrossRef]
- Portillo-Estrada, M.; Niinemets, Ü. Massive release of volatile organic compounds due to leaf midrib wounding in Populus tremula. Plant Ecol. 2018, 219, 1021–1028. [Google Scholar] [CrossRef]
- Portillo-Estrada, M.; Okereke, C.N.; Jiang, Y.; Talts, E.; Kaurilind, E.; Niinemets, Ü. Wounding-induced VOC emissions in five tropical agricultural species. Molecules 2021, 26, 2602. [Google Scholar] [CrossRef]
- Kishimoto, K.; Matsui, K.; Ozawa, R.; Takabayashi, J. Direct fungicidal activities of C6-aldehydes are important constituents for defense responses in Arabidopsis against Botrytis cinerea. Phytochemistry 2008, 69, 2127–2132. [Google Scholar] [CrossRef]
- Kanagendran, A.; Pazouki, L.; Bichele, R.; Külheim, C.; Niinemets, Ü. Temporal regulation of terpene synthase gene expression in Eucalyptus globulus leaves upon ozone and wounding stresses: Relationships with stomatal ozone uptake and emission responses. Environ. Exp. Bot. 2018, 155, 552–565. [Google Scholar] [CrossRef]
- Kanagendran, A.; Pazouki, L.; Niinemets, Ü. Differential regulation of volatile emission from Eucalyptus globulus leaves upon single and combined ozone and wounding treatments through recovery and relationships with ozone uptake. Environ. Exp. Bot. 2018, 145, 21–38. [Google Scholar] [CrossRef]
- Arimura, G.i.; Ozawa, R.; Kugimiya, S.; Takabayashi, J.; Bohlmann, J.r. Herbivore-induced defense response in a model legume. Two-spotted spider mites induce emission of (E)-β-ocimene and transcript accumulation of (E)-β-ocimene synthase in Lotus japonicus. Plant Physiol. 2004, 135, 1976–1983. [Google Scholar] [CrossRef] [PubMed]
- Brilli, F.; Ruuskanen, T.M.; Schnitzhofer, R.; Müller, M.; Breitenlechner, M.; Bittner, V.; Wohlfahrt, G.; Loreto, F.; Hansel, A. Detection of plant volatiles after leaf wounding and darkening by proton transfer reaction “time-of-flight” mass spectrometry (PTR-TOF). PLoS ONE 2011, 6, e20419. [Google Scholar] [CrossRef] [PubMed]
- Peñuelas, J.; Staudt, M. BVOCs and global change. Trends Plant Sci. 2010, 15, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Männistö, E.; Ylänne, H.; Kokkonen, N.; Korrensalo, A.; Laine, A.M.; Yli-Pirilä, P.; Keinänen, M.; Tuittila, E.S. Impact of severe drought on biogenic volatile organic compounds emissions from Sphagnum mosses in boreal peatlands. Sci. Total Environ. 2024, 951, 175738. [Google Scholar] [CrossRef]
- Alamgir, A. Pharmacognostical Botany: Classification of medicinal and aromatic plants (MAPs), botanical taxonomy, morphology, and anatomy of drug plants. In Therapeutic Use of Medicinal Plants and Their Extracts: Volume 1: Pharmacognosy; Springer: Cham, Switzerland, 2017; pp. 177–293. [Google Scholar]
- Huang, X.; Jiang, H.; Hao, G. Direct HPLC detection of benzodilactones and quinones in glands of Lysimachia fordiana. Fitoterapia 2009, 80, 173–176. [Google Scholar] [CrossRef]
- Lersten, N.R.; Czlapinski, A.R.; Curtis, J.D.; Freckmann, R.; Horner, H.T. Oil bodies in leaf mesophyll cells of angiosperms: Overview and a selected survey. Am. J. Bot. 2006, 93, 1731–1739. [Google Scholar] [CrossRef]
- Tang, B.; Han, M.; Xu, Q.; Jin, J. Leaf cuticle microstructure of Machilus maomingensis sp. nov. (Lauraceae) from the Eocene of the Maoming Basin, South China. Acta Geol. Sin.-Engl. Ed. 2016, 90, 1561–1571. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, X.; Lai, Y.; Li, X.; Zhang, D.; Chen, Y. Efficient extraction of bioenergy from Cinnamomum camphora leaves. Front. Energy Res. 2020, 8, 90. [Google Scholar] [CrossRef]
- Shang, A.; Gan, R.; Zhang, J.; Xu, X.; Luo, M.; Liu, H.; Li, H. Optimization and characterization of microwave-assisted hydro-distillation extraction of essential oils from Cinnamomum camphora leaf and recovery of polyphenols from extract fluid. Molecules 2020, 25, 3213. [Google Scholar] [CrossRef]
- Peng, F.; Guo, H.; Hao, M.; Guo, J.; Yang, Y.; Tan, P. The ultrastructure characteristics of secretory cavities associated with the secretory products of Ginkgo biloba. Am. J. Plant Sci. 2011, 3, 102–109. [Google Scholar] [CrossRef]
- Ali, J.K.; Sosa, A.A. Anatomical study of some characters in certain species of genus Jasminum L. growing in Iraq. Int. J. Sci. Res. 2015, 5, 1137–1140. [Google Scholar]
- Kröber, W.; Heklau, H.; Bruelheide, H. Leaf morphology of 40 evergreen and deciduous broadleaved subtropical tree species and relationships to functional ecophysiological traits. Plant Biol. 2015, 17, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Xia, N. Leaf epidermal features of Lithocarpus (Fagaceae) from China and their systematic significance. Bot. J. Linn. Soc. 2012, 168, 216–228. [Google Scholar] [CrossRef]
- Marinho, C.R.; Zacaro, A.A.; Ventrella, M.C. Secretory cells in Piper umbellatum (Piperaceae) leaves: A new example for the development of idioblasts. Flora 2011, 206, 1052–1062. [Google Scholar] [CrossRef]
- Turner, G.W. A brief history of the lysigenous gland hypothesis. Bot. Rev. 1999, 65, 76–88. [Google Scholar] [CrossRef]
- Deng, M.; Hipp, A.; Song, Y.; Li, Q.; Coombes, A.; Cotton, A. Leaf epidermal features of Quercus subgenus Cyclobalanopsis (Fagaceae) and their systematic significance. Bot. J. Linn. Soc. 2014, 176, 224–259. [Google Scholar] [CrossRef]
- Sun, Z.; Niinemets, Ü.; Hüve, K.; Rasulov, B.; Noe, S.M. Elevated atmospheric CO2 concentration leads to increased whole-plant isoprene emission in hybrid aspen (Populus tremula × Populus tremuloides). New Phytol. 2013, 198, 788–800. [Google Scholar] [CrossRef]
- Velikova, V.; Loreto, F.; Tsonev, T.; Brilli, F.; Edreva, A. Isoprene prevents the negative consequences of high temperature stress in Platanus orientalis leaves. Funct. Plant Biol. 2006, 33, 931–940. [Google Scholar] [CrossRef]
- Velikova, V.; Sharkey, T.D.; Loreto, F. Increased Thermostability of Thylakoid Membranes in Isoprene-Emitting Leaves Probed with Three Biophysical Techniques. Plant Physiol. 2012, 157, 905–916. [Google Scholar] [CrossRef]
- Singsaas, E.L.; Lerdau, M.; Winter, K.; Sharkey, T.D. Isoprene increases thermotolerance of isoprene-emitting species. Plant Physiol. 1997, 115, 1413–1420. [Google Scholar] [CrossRef]
- Siwko, M.E.; Marrink, S.J.; de Vries, A.H.; Kozubek, A.; Uiterkamp, A.J.S.; Mark, A.E. Does isoprene protect plant membranes from thermal shock? A molecular dynamics study. Biochim. Biophys. Acta (BBA)-Biomembr. 2007, 1768, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Loreto, F.; Mannozzi, M.; Maris, C.; Nascetti, P.; Ferranti, F.; Pasqualini, S. Ozone quenching properties of isoprene and its antioxidant role in leaves. Plant Physiol. 2001, 126, 993–1000. [Google Scholar] [CrossRef]
- Affek, H.P.; Yakir, D. Protection by isoprene against singlet oxygen in leaves. Plant Physiol. 2002, 129, 269–277. [Google Scholar] [CrossRef]
- Possell, M.; Loreto, F. The role of volatile organic compounds in plant resistance to abiotic stresses: Responses and mechanisms. In Biology, Controls and Models of Tree Volatile Organic Compound Emissions; Springer: Berlin/Heidelberg, Germany, 2013; pp. 209–235. [Google Scholar]
- Sun, Z.; Copolovici, L.; Niinemets, Ü. Can the capacity for isoprene emission acclimate to environmental modifications during autumn senescence in temperate deciduous tree species Populus tremula? J. Plant Res. 2012, 125, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, T.D.; Loreto, F. Water stress, temperature, and light effects on isoprene emission and photosynthesis of Kudzu leaves. Oecologia 1993, 95, 328–333. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Sun, Z.; Talts, E. Controls of the quantum yield and saturation light of isoprene emission in different-aged aspen leaves. Plant Cell Environ. 2015, 38, 2707–2720. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H. Non-mevalonate isoprenoid biosynthesis: Enzymes, genes and inhibitors. Biochem. Soc. Trans. 2000, 28, 785–789. [Google Scholar] [CrossRef]
- Chen, F.; Tholl, D.; Bohlmann, J.; Pichersky, E. The family of terpene synthases in plants: A mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J. 2011, 66, 212–229. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Yeh, S. Isoprene emission from plants. Annu. Rev. Plant Biol. 2001, 52, 407–436. [Google Scholar] [CrossRef]
- Velikova, V.; Pinelli, P.; Pasqualini, S.; Reale, L.; Ferranti, F.; Loreto, F. Isoprene decreases the concentration of nitric oxide in leaves exposed to elevated ozone. New Phytol. 2005, 166, 419–426. [Google Scholar] [CrossRef]
- Niinemets, Ü. Mild versus severe stress and BVOCs: Thresholds, priming and consequences. Trends Plant Sci. 2010, 15, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Shen, Y.; Niinemets, Ü. Responses of isoprene emission and photochemical efficiency to severe drought combined with prolonged hot weather in hybrid Populus. J. Exp. Bot. 2020, 71, 7364–7381. [Google Scholar] [CrossRef] [PubMed]
- Oku, H.; Mutanda, I.; Inafuku, M. Molecular characteristics of isoprene synthase and its control effects on isoprene emissions from tropical trees. J. Plant Res. 2023, 136, 63–82. [Google Scholar] [CrossRef] [PubMed]
- Aldea, M.; Frank, T.D.; DeLucia, E.H. A method for quantitative analysis of spatially variable physiological processes across leaf surfaces. Photosynth. Res. 2006, 90, 161–172. [Google Scholar] [CrossRef]
- Tritsch, D.; Hemmerlin, A.; Bach, T.J.; Rohmer, M. Plant isoprenoid biosynthesis via the MEP pathway: In vivo IPP/DMAPP ratio produced by (E)-4-hydroxy-3-methylbut-2-enyl diphosphate reductase in tobacco BY-2 cell cultures. FEBS Lett. 2010, 584, 129–134. [Google Scholar] [CrossRef]
- Vranová, E.; Coman, D.; Gruissem, W. Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Annu. Rev. Plant Biol. 2013, 64, 665–700. [Google Scholar] [CrossRef]
- Harrison, S.P.; Morfopoulos, C.; Dani, K.S.; Prentice, I.C.; Arneth, A.; Atwell, B.J.; Barkley, M.P.; Leishman, M.R.; Loreto, F.; Medlyn, B.E. Volatile isoprenoid emissions from plastid to planet. New Phytol. 2013, 197, 49–57. [Google Scholar] [CrossRef]
- Joó, É.; Dewulf, J.; Amelynck, C.; Schoon, N.; Pokorska, O.; Šimpraga, M.; Steppe, K.; Aubinet, M.; Van Langenhove, H. Constitutive versus heat and biotic stress induced BVOC emissions in Pseudotsuga menziesii. Atmos. Environ. 2011, 45, 3655–3662. [Google Scholar] [CrossRef]
- Peer, W.A.; Langenheim, J.H. Influence of phytochrome on leaf monoterpene variation in Satureja douglasii. Biochem. Syst. Ecol. 1998, 26, 25–34. [Google Scholar] [CrossRef]
- Loreto, F.; Nascetti, P.; Graverini, A.; Mannozzi, M. Emission and content of monoterpenes in intact and wounded needles of the Mediterranean pine, Pinus pinea. Funct. Ecol. 2000, 14, 589–595. [Google Scholar] [CrossRef]
- Zabaras, D.; Spooner-Hart, R.; Wyllie, S.G. Effects of mechanical wounding on concentration and composition of essential oil from Melaleuca alternifolia leaves. Biochem. Syst. Ecol. 2002, 30, 399–412. [Google Scholar] [CrossRef]
- Kask, K.; Kaurilind, E.; Talts, E.; Kännaste, A.; Niinemets, Ü. Combined acute ozone and water stress alters the quantitative relationships between O3 uptake, photosynthetic characteristics and volatile emissions in Brassica nigra. Molecules 2021, 26, 3114. [Google Scholar] [CrossRef]
- Goodger, J.Q.; Senaratne, S.L.; Nicolle, D.; Woodrow, I.E. Differential metabolic specialization of foliar oil glands in Eucalyptus brevistylis Brooker (Myrtaceae). Tree Physiol. 2018, 38, 1451–1460. [Google Scholar] [CrossRef] [PubMed]
- Goodger, J.Q.; Seneratne, S.L.; Nicolle, D.; Woodrow, I.E. Foliar essential oil glands of Eucalyptus subgenus Eucalyptus (Myrtaceae) are a rich source of flavonoids and related non-volatile constituents. PLoS ONE 2016, 11, e0151432. [Google Scholar]
- Ekanayaka, E.P.; Li, C.; Jones, A.D. Sesquiterpenoid glycosides from glandular trichomes of the wild tomato relative Solanum habrochaites. Phytochemistry 2014, 98, 223–231. [Google Scholar] [CrossRef]
- Paré, P.W.; Tumlinson, J.H. Plant volatiles as a defense against insect herbivores. Plant Physiol. 1999, 121, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Piesik, D.; Łyszczarz, A.; Tabaka, P.; Lamparski, R.; Bocianowski, J.; Delaney, K. Volatile induction of three cereals: Influence of mechanical injury and insect herbivory on injured plants and neighbouring uninjured plants. Ann. Appl. Biol. 2010, 157, 425–434. [Google Scholar] [CrossRef]
- Kopaczyk, J.M.; Warguła, J.; Jelonek, T. The variability of terpenes in conifers under developmental and environmental stimuli. Environ. Exp. Bot. 2020, 180, 104197. [Google Scholar] [CrossRef]
- Grote, R.; Monson, R.K.; Niinemets, Ü. Leaf-level models of constitutive and stress-driven volatile organic compound emissions. In Biology, Controls and Models of Tree Volatile Organic Compound Emissions; Springer: Berlin/Heidelberg, Germany, 2013; pp. 315–355. [Google Scholar]
- Monson, R.K. Metabolic and gene expression controls on the production of biogenic volatile organic compounds. In Biology, Controls and Models of Tree Volatile Organic Compound Emissions; Springer: Berlin/Heidelberg, Germany, 2013; pp. 153–179. [Google Scholar]
- Farré-Armengol, G.; Filella, I.; Llusià, J.; Peñuelas, J. β-Ocimene, a key floral and foliar volatile involved in multiple interactions between plants and other organisms. Molecules 2017, 22, 1148. [Google Scholar] [CrossRef]
- Smith, W.; Shivaji, R.; Williams, W.; Luthe, D.; Sandoya, G.; Smith, C.; Sparks, D.; Brown, A. A maize line resistant to herbivory constitutively releases (E)-β-caryophyllene. J. Econ. Entomol. 2012, 105, 120–128. [Google Scholar] [CrossRef]
- Holopainen, J.K.; Nerg, A.M.; Blande, J.D. Multitrophic signalling in polluted atmospheres. In Biology, Controls and Models of Tree Volatile Organic Compound Emissions; Springer: Berlin/Heidelberg, Germany, 2013; pp. 285–314. [Google Scholar]
- Blande, J.D.; Holopainen, J.K.; Niinemets, Ü. Plant volatiles in polluted atmospheres: Stress responses and signal degradation. Plant Cell Environ. 2014, 37, 1892–1904. [Google Scholar] [CrossRef]
- Wang, F.; Park, Y.L.; Gutensohn, M. Glandular trichome-derived mono-and sesquiterpenes of tomato have contrasting roles in the interaction with the potato aphid Macrosiphum euphorbiae. J. Chem. Ecol. 2021, 47, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Cui, Z.; Shu, B.; Zhao, S.; Yu, X. Histochemistry and cell wall specialization of oil cells related to the essential oil accumulation in the bark of Cinnamomum cassia Presl. (Lauraceae). Plant Prod. Sci. 2012, 15, 1–9. [Google Scholar] [CrossRef]
- Barbosa, L.C.A.; Filomeno, C.A.; Teixeira, R.R. Chemical variability and biological activities of Eucalyptus spp. essential oils. Molecules 2016, 21, 1671. [Google Scholar] [CrossRef]
- Serrato-Valenti, G.; Bisio, A.; Cornara, L.; Ciarallo, G. Structural and histochemical investigation of the glandular trichomes of Salvia aurea L. leaves, and chemical analysis of the essential oil. Ann. Bot. 1997, 79, 329–336. [Google Scholar] [CrossRef]
- Stefanello, M.É.A.; Pascoal, A.C.; Salvador, M.J. Essential oils from neotropical Myrtaceae: Chemical diversity and biological properties. Chem. Biodivers. 2011, 8, 73–94. [Google Scholar] [CrossRef] [PubMed]
- Kännaste, A.; Copolovici, L.; Niinemets, Ü. Gas chromatography–mass spectrometry method for determination of biogenic volatile organic compounds emitted by plants. In Plant Isoprenoids: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2014; pp. 161–169. [Google Scholar]
- Tzin, V.; Galili, G. New insights into the shikimate and aromatic amino acids biosynthesis pathways in plants. Mol. Plant 2010, 3, 956–972. [Google Scholar] [CrossRef]
- Nagesh, K.M.; Piovene, C. Emission and function of volatile organic compounds in response to abiotic stress. In Abiotic Stress in Plants: Mechanisms and Adaptations; IntechOpen: London, UK, 2011; 367p. [Google Scholar]
- Liu, B.; Kaurilind, E.; Jiang, Y.; Niinemets, Ü. Methyl salicylate differently affects benzenoid and terpenoid volatile emissions in Betula pendula. Tree Physiol. 2018, 38, 1513–1525. [Google Scholar] [CrossRef]
- Nagel, J.; Culley, L.K.; Lu, Y.; Liu, E.; Matthews, P.D.; Stevens, J.F.; Page, J.E. EST analysis of hop glandular trichomes identifies an O-methyltransferase that catalyzes the biosynthesis of xanthohumol. Plant Cell 2008, 20, 186–200. [Google Scholar] [CrossRef]
- Zager, J.J.; Lange, B.M. Assessing flux distribution associated with metabolic specialization of glandular trichomes. Trends Plant Sci. 2018, 23, 638–647. [Google Scholar] [CrossRef]
- Bartwal, A.; Mall, R.; Lohani, P.; Guru, S.; Arora, S. Role of secondary metabolites and brassinosteroids in plant defense against environmental stresses. J. Plant Growth Regul. 2013, 32, 216–232. [Google Scholar] [CrossRef]
- Okereke, C.N.; Liu, B.; Kaurilind, E.; Niinemets, Ü. Heat stress resistance drives coordination of emissions of suites of volatiles after severe heat stress and during recovery in five tropical crops. Environ. Exp. Bot. 2021, 184, 104375. [Google Scholar] [CrossRef]
- Nagalingam, S.; Seco, R.; Kim, S.; Guenther, A. Heat stress strongly induces monoterpene emissions in some plants with specialized terpenoid storage structures. Agric. For. Meteorol. 2023, 333, 109400. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.; Diemer, M. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Onoda, Y.; Wright, I.J.; Evans, J.R.; Hikosaka, K.; Kitajima, K.; Niinemets, Ü.; Poorter, H.; Tosens, T.; Westoby, M. Physiological and structural tradeoffs underlying the leaf economics spectrum. New Phytol. 2017, 214, 1447–1463. [Google Scholar] [CrossRef]
- Tilney, P.M.; Nel, M.; van Wyk, A.E. Foliar secretory structures in Melia azedarach (Meliaceae), a widely cultivated and often invasive tree. N. Z. J. Bot. 2018, 56, 198–215. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).