Elemental Screening and Nutritional Strategies of Gypsophile Flora in Sicily
Abstract
1. Introduction
2. Results and Discussion
2.1. Soil Analyses
2.2. Foliar Analyses
2.2.1. C Contents
2.2.2. N Contents
2.2.3. N:P Ratio
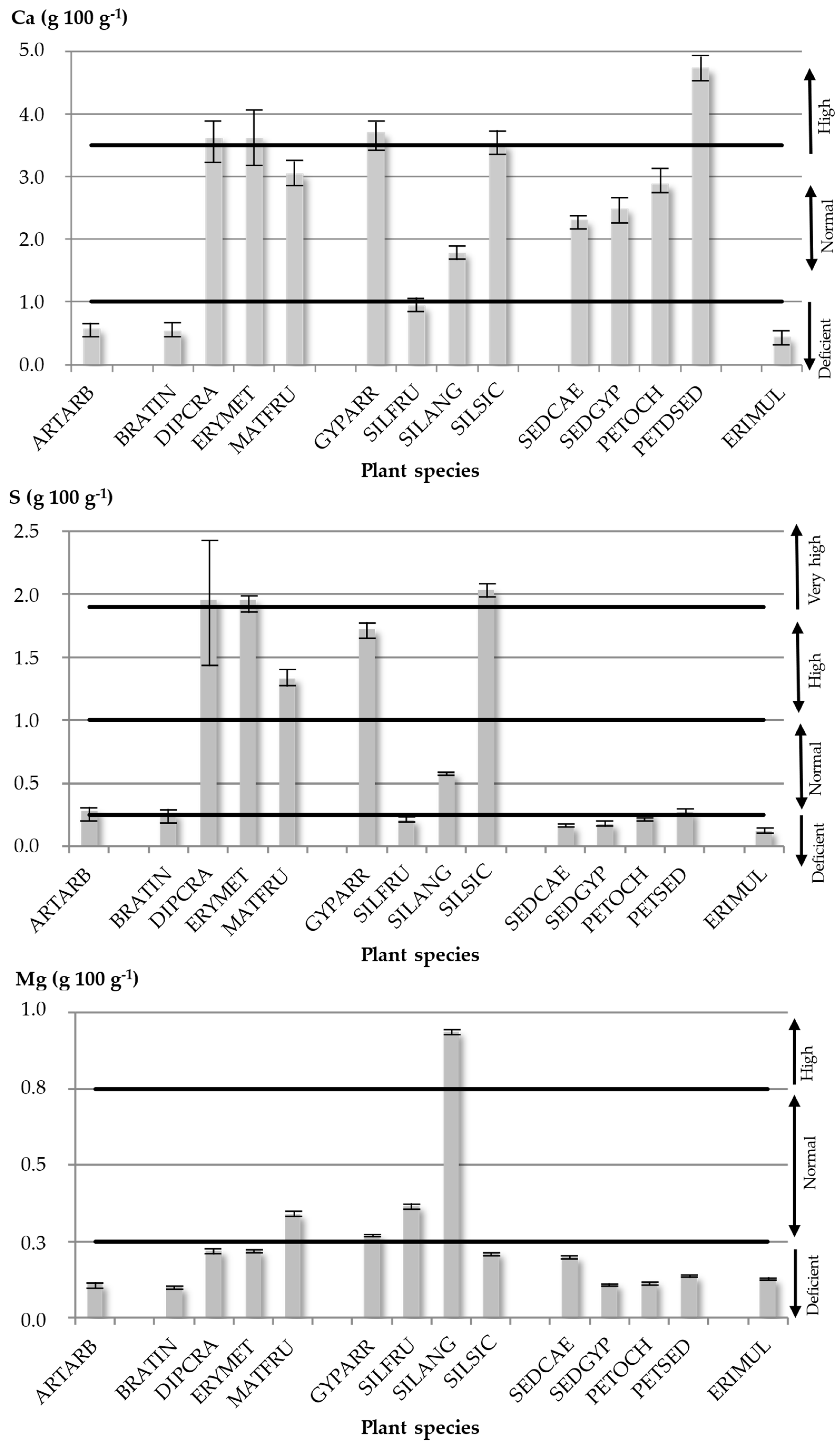
2.2.4. Ca Contents
2.2.5. S Contents
2.2.6. Mg Contents
2.2.7. Other Elements: Al, Fe and Sr
2.3. Bioconcentration Factors (BCFs)
2.4. Statistical Tests
2.4.1. ANOVA
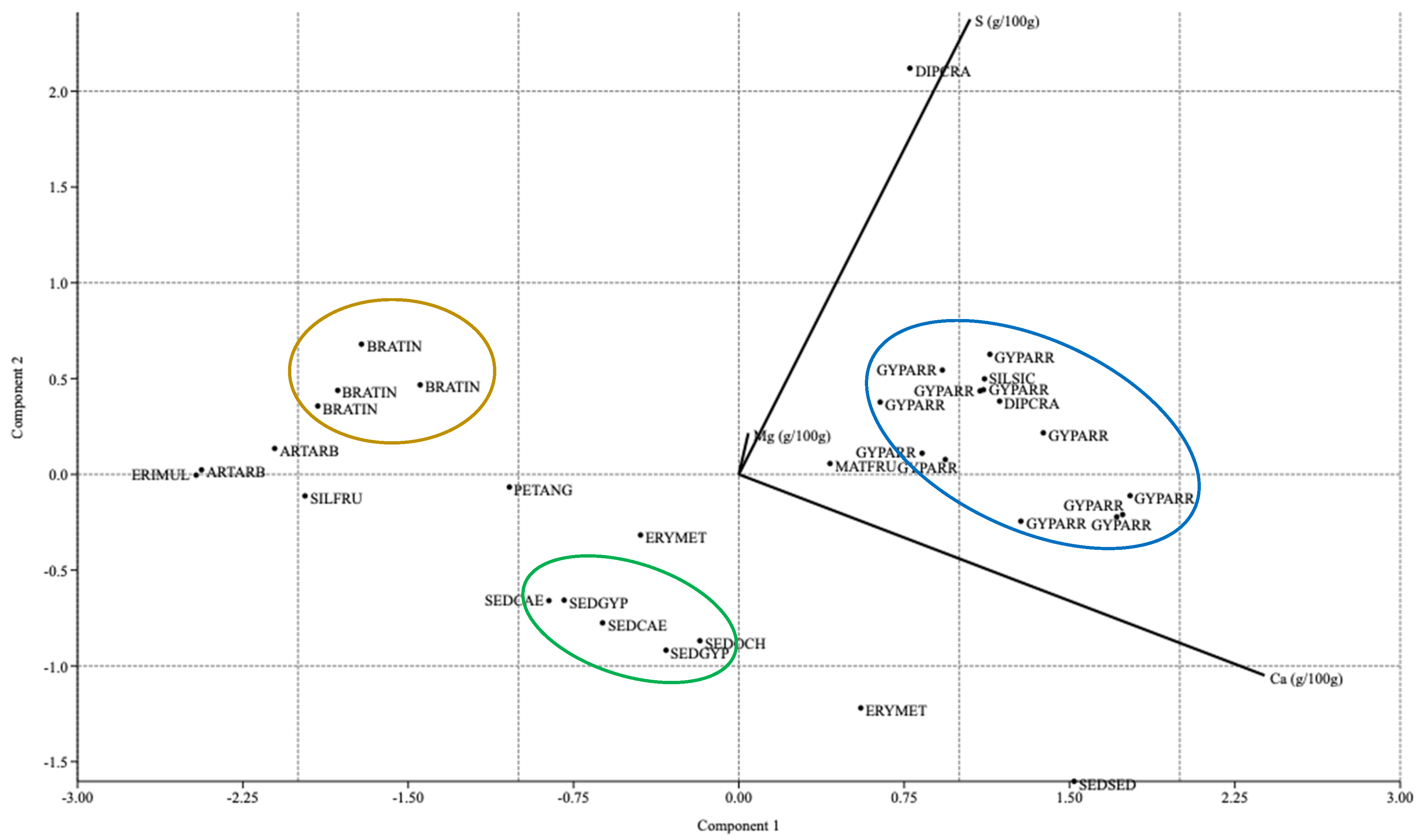
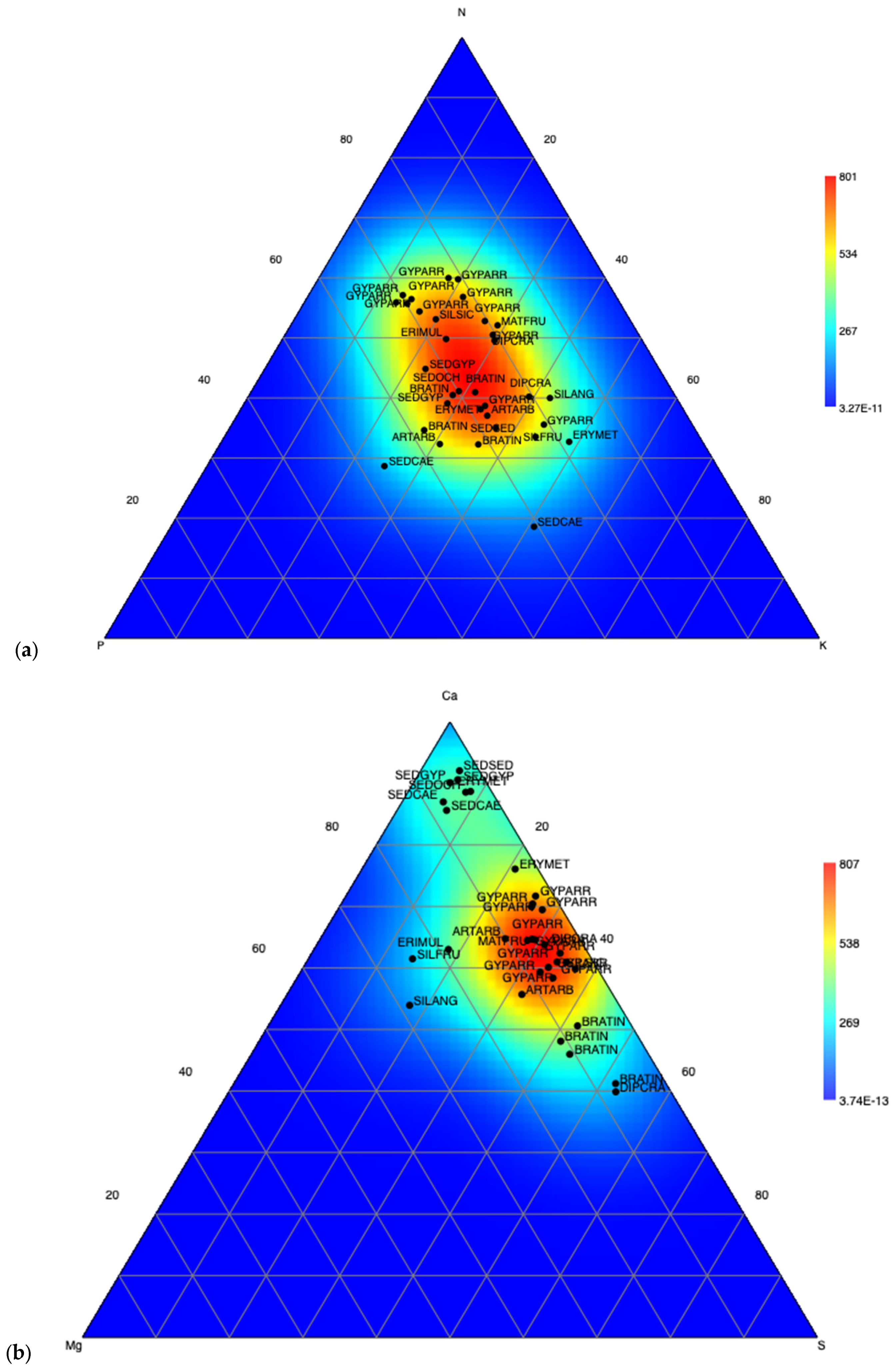
2.4.2. PCA and Terniary Plots
3. Materials and Methods
3.1. Study Area
3.2. Fieldwork
3.3. Selected Plants
3.4. Soil Samples
3.5. Elemental Analysis
3.6. Bioconcentration Factor
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Font Quer, P. Diccionario de Botánica, 6th ed.; Labor: Barcelona, Spain, 1977. [Google Scholar]
- Sarmiento, F.O. Diccionario de Ecología: Paisajes, Conservación y Desarrollo Sustentable Para Latinoamérica; Ediciones Abya–Yala: Quito, Ecuador, 2001; pp. 1–226. [Google Scholar]
- Géze, J.B. Notes d’édaphisme chimique-distribution de l’anjonc Ulex europaeus aux environs de Villefranche de Roverque. Bull. Soc. Bot. Fr. 1908, 55, 462–466. [Google Scholar] [CrossRef]
- Gola, G. Saggio di una teoria osmotica dell’edafismo. Ann. Bot. 1910, 3, 455–512. [Google Scholar]
- Cavers, F. Gola’s osmotic theory of edaphsim. J. Ecol. 1914, 2, 209–231. [Google Scholar] [CrossRef]
- Rivas Goday, S. Flora serpentinícola española. Nota primera. Edafismos endémicos del Reino de Granada. An. La Real Acad. Farm. 1969, 353, 297–304. [Google Scholar]
- Kruckeberg, A.R. Geology and Plant Life: The Effects of Landforms and Rock Types on Plants; University of Washington Press: Washington, DC, USA, 2002. [Google Scholar]
- Cesalpino, A. De Plantis Libri XVI; Apud Georgium Marescottum: Florentiae, Italy, 1583. [Google Scholar]
- Parsons, R.F. Gypsophily in plants–a review. Am. Midl. Nat. 1976, 96, 1–20. [Google Scholar] [CrossRef]
- Mota, J.F.; Sánchez–Gómez, P.; Guirado Romero, J.S. Diversidad Vegetal de las Yeseras Ibéricas: El Reto de los Archipiélagos Edáficos Para la Biología de la Conservación; ADIF–Mediterráneo Asesores Consultores: Almería, Spain, 2011. [Google Scholar]
- Pérez-García, F.J.; Martínez-Hernández, F.; Mendoza-Fernández, A.J.; Merlo, M.E.; Sola, F.; Salmerón-Sánchez, E.; Garrido-Becerra, J.A.; Mota, J.F. Towards a global checklist of the of world gypsophytes: A qualitative approach. Plant Sociol. 2017, 54, 61–76. [Google Scholar] [CrossRef]
- Pérez-García, F.J.; Akhani, H.; Parsons, R.F.; Silcock, J.L.; Kurt, L.; Özdeniz, E.; Spampinato, G.; Musarella, C.; Salmerón-Sánchez, E.; Sola, F.; et al. A first inventory of gypsum flora in the Palearctic and Australia. Mediterr. Bot. 2018, 39, 35–49. [Google Scholar] [CrossRef]
- Macchiati, L. Contribuzione alla flora del gesso. Nuevo Giorn. Botan. Ital. 1888, 20, 418–422. [Google Scholar]
- Macchiati, L. Seconda contribuzione alla flora del gesso. Nuovo Giorn. Botan. Ital. 1891, 23, 171–175. [Google Scholar]
- Macchiati, L. Terza contribuzione alla flora del gesso. Bull. Soc. Bot. Ital. 1892, 120–122. [Google Scholar]
- Braun-Blanquet, J. Plant Sociology: The Study of Plant Communities; McGraw Hill: London, UK, 1932. [Google Scholar]
- Ferro, G.; Coniglione, P.; Oliveri, S. I praticelli effimeri su gesso nel territorio di Caltanissetta, Sicilia. Atti Accad. Gioenia Sci. Nat. Catania 1979, 64, 137–141. [Google Scholar]
- Biondi, E.; Blasi, C.; Allegrezza, M.; Anzellotti, I.; Azzella, M.M.; Carli, E.; Casavecchia, S.; Copiz, R.; Del Vico, E.; Facioni, L.; et al. Plant communities of Italy: The Vegetation Prodrome. Plant Biosyst. 2014, 1484, 728–814. [Google Scholar] [CrossRef]
- Cobau, R. Su la flora dei “gessi” bolognesi. Nuevo Giorn. Botan. Ital. 1932, 392, 313–345. [Google Scholar] [CrossRef]
- Pasquini, D. La vegetazione dei gessi reggiani. Atti Soc. Naturalisti Mat. Modena 1944, 75, 264–282. [Google Scholar]
- Corbetta, F. Alcuni aspetti della vegetazione dei gessi Bolognesi. Nat. E Mont. 1964, 24, 30–37. [Google Scholar]
- Ferrari, C. La vegetazione delle rupi gessose di Miserazzano e della Croara, Bologna. Notes Fitosociol. 1974, 8, 65–74. [Google Scholar]
- Aleffi, M.; Pellis, G.; Puglisi, M. The bryophyte flora of six gypsum outcrops in the Northern Apennines Nature 2000 Network, Emilia Romagna Region, Italy. Plant Biosyst. 2014, 1484, 825–836. [Google Scholar] [CrossRef]
- Musarella, C.M.; Mendoza-Fernández, A.J.; Mota, J.F.; Alessandrini, A.; Bacchetta, G.; Brullo, S.; Caldarella, O.; Ciaschetti, G.; Conti, F.; Di Martino, L.; et al. Checklist of gypsophilous vascular flora in Italy. PhytoKeys 2018, 103, 61–82. [Google Scholar] [CrossRef]
- Di Martino, A.; Marcenò, C.; Raimondo, F.M. Nota preliminare sulla vegetazione gipsofila della Sicilia centro–meridionale. Giorn. Botan. Ital. 1976, 111, 369–370. [Google Scholar]
- Brullo, S.; Marcenò, C.; Minissale, P.; Spampinato, G. Su una nuova associazione del Sedo–Ctenopsion gypsophilae rinvenuta in Sicilia. Arch. Bot. Biogeogr. Ital. 1989, 651, 100–108. [Google Scholar]
- Privitera, M. La vegetazione muscinale dei gessi dell’Agrigentino, Sicilia occidentale. Boll. Della Accad. Gioenia Sci. Nat. 1989, 22, 105–113. [Google Scholar]
- Bazan, G.; Ilardi, V.; Minissale, P.; Sciandrello, S. La biodiversità vegetale di Monte Gibliscemi Mazzarino Sicilia. Quad. Bot. Ambient. E Appl. 2006, 172, 121–140. [Google Scholar]
- Giusso del Galdo, G.; Marcenò, C.; Musarella, C.M.; Sciandrello, S. La vegetazione costiera R.N.O. ‘Torre Salsa’ Siculiana-AG. Inform. Bot. Ital. 2008, 401, 73–89. [Google Scholar]
- Gianguzzi, L.; D’Amico, A.; Caldarella, O.; Romano, S. Note distributive ed ecologiche su alcune rare entità della flora vascolare siciliana. Nat. Sicil. 2010, 342, 227–244. [Google Scholar]
- Gianguzzi, L.; D’Amico, A.; Caldarella, O.; Romano, S. La flora vascolare delle Rocche di Entella entroterra della Sicilia occidentale. Nat. Sicil. 2011, 353, 363–405. [Google Scholar]
- Marcenò, C.; Falci, A.; Pasta, S. Su alcuni lembi di vegetazione pre–forestale e forestale della provincia di Enna Sicilia centrale. Nat. Sicil. 2011, 352, 295–312. [Google Scholar]
- Loidi, J. F6.7 Mediterranean gypsum scrub. In European Red List of Habitats. Part 2. Terrestrial and Freshwater Habitats; Janssen, J.A.M., Rodwell, J.S., García Criado, M., Gubbay, S., Haynes, T., Nieto, A., Eds.; Directorate-General for Environment (European Commission), Publications Office: Luxembourg, 2016; Available online: https://data.europa.eu/doi/10.2779/091372 (accessed on 10 December 2024).
- Habitats Directive. Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. DOCE 1992, 206, 7–50. [Google Scholar]
- Mota, J.F.; Sánchez-Gómez, P.; Merlo, M.E.; Catalán Rodríguez, P.; Laguna Lumbreras, E.; De la Cruz Rot, M.; Reyes, F.B.N.; Gallardo, F.M.; Estaban, C.B.; Labarga, J.M.M. Aproximación a la checklist de los gipsófitos ibéricos. An. Biol. 2009, 31, 71–80. [Google Scholar]
- Mota, J.; Garrido-Becerra, J.; Merlo, M.; Medina-Cazorla, J.; Sánchez-Gómez, P. The edaphism: Gypsum, dolomite and serpentine flora and vegetation. In The Vegetation of the Iberian Peninsula; Loidi, J., Ed.; Springer Nature: Basel, Switzerland, 2017. [Google Scholar] [CrossRef]
- Merlo, M.E.; Rodríguez-Tamayo, M.L.; Jiménez, M.L.; Mota, J.F. Recapitulación sobre el comportamiento biogeoquímico de algunos gipsófitos y halófitos ibéricos. Monogr. Flora Y Veg. Béticas 2001, 12, 77–95. [Google Scholar]
- Merlo, M.E.; Garrido-Becerra, J.A.; Mota, J.F.; Salmerón-Sánchez, E.; Martínez-Hernández, F.; Mendoza-Fernández, A.; Pérez-García, F.J. Threshold ionic contents for defining the nutritional strategies of gypsophile flora. Ecol. Indic. 2019, 97, 247–259. [Google Scholar] [CrossRef]
- Bolukbasi, A.; Kurt, L.; Palacio, S. Unravelling the mechanisms for plant survival on gypsum soils: An analysis of the chemical composition of gypsum plants from Turkey. Plant Biol. 2016, 18, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Palacio, S.; Cera, A.; Escudero, A.; Luzuriaga, A.L.; Sánchez, A.M.; Mota, J.F.; Pérez-Serrano Serrano, M.; Merlo, M.E.; Martínez-Hernández, F.; Salmerón-Sánchez, E.; et al. Recent and ancient evolutionary events shaped plant elemental composition of edaphic endemics: A phylogeny-wide analysis of Iberian gypsum plants. New Phytol. 2022, 235, 2406–2423. [Google Scholar] [CrossRef] [PubMed]
- Mota, J.F.; Garrido-Becerra, J.A.; Pérez-García, F.J.; Salmerón-Sánchez, E.; Sánchez-Gómez, P.; Merlo, E. Conceptual baseline for a global checklist of gypsophytes. Lazaroa 2016, 37, 7–30. [Google Scholar]
- Merlo, M.; Mota, J.; Cabello, J.; Alemán, M. La gipsofilia en plantas: Un apasionante edafismo. Investig. Gestión 1998, 3, 103–112. [Google Scholar]
- Boukhris, M.; Lossaint, P. Sur la teneur en soufre de quelques plantes gypsophiles de Tunisie. Oecol. Plant. 1970, 5, 345–354. [Google Scholar]
- Boukhris, M.; Lossaint, P. Aspects écologiques de la nutrition minérale des plantes gypsicoles de Tunisie. Rev. Ecol. Biol. Sol. 1975, 12, 329–348. [Google Scholar]
- Alvarado, J.J.; Ruiz, J.J.; López-Cantarero, I.; Molero, J.; Romero, L. Nitrogen metabolism in five plant species characteristic of gypsiferous soils. J. Plant Physiol. 2000, 156, 612–616. [Google Scholar] [CrossRef]
- Duvigneaud, P.; Denaeyer-De Smet, S. Accumulation du soufre dans quelques espèces gypsophiles d’Espagne. Bull. Soc. Roc. Bot. Belg. 1966, 99, 263–269. [Google Scholar]
- Duvigneaud, P.; Denaeyer-De Smet, S. Essai de classification chimique (elements mineraux) des plantes gypsicoles. Bull. Soc. Roy. Bot. Belg. 1968, 101, 279–291. [Google Scholar]
- Denayer-De Smet, S. Note sur la composition chimique des sels recrétés par diverses especes gypsohalophytes d’Espagne. Bull. Soc. Roy. Bot. Belg. 1970, 103, 273–278. [Google Scholar]
- Merlo, M.E.; Cabello, J.; Márquez, M.M.; Alemán, M.M. On the germination of plants of gypseous soils in relation to the medium calcium content. In Proceedings of the 36th IAVS Symposium. Island and High Mountain Vegetation: Biodiversity, Bioclimate and Conservation, Serie Informes, Canary Islands, Spain, June 1993; Volume 40, pp. 197–206. [Google Scholar]
- Palacio, S.; Escudero, A.; Montserrat-Martí, G.; Maestro-Martínez, M.; Milla, R.; Albert, M.J. Plants living on gypsum: Beyond the specialist model. Ann. Bot. 2007, 99, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Cera, A.; Montserrat-Martí, G.; Ferrio, J.P.; Drenovsky, R.E.; Palacio, S. Gypsum-exclusive plants accumulate more leaf S than non-exclusive species both in and off gypsum. Environ. Exp. Bot. 2021, 182, 104294. [Google Scholar] [CrossRef]
- Cera, A.; Montserrat-Martí, G.; Drenovsky, R.E.; Ourry, A.; Brunel-Muguet, S.; Palacio, S. Gypsum endemics accumulate excess nutrients in leaves as a potential constitutive strategy to grow in grazed extreme soils. Physiol. Plant. 2022, 174, e13738. [Google Scholar] [CrossRef] [PubMed]
- Cera, A.; Montserrat-Martí, G.; Palacio, S. Nutritional strategy underlying plant specialization to gypsum soils. AoB Plants 2023, 15, plad041. [Google Scholar] [CrossRef]
- Mota, J.; Merlo, E.; Martínez-Hernández, F.; Mendoza-Fernández, A.; Pérez-García, F.; Salmerón-Sánchez, E. Plants on Rich-Magnesium Dolomite Barrens: A Global Phenomenon. Biology 2021, 10, 38. [Google Scholar] [CrossRef]
- Van Alphen, J.G.; de los Rios Romero, F. Gypsiferous soils, notes on their characteristics and management. Bull./Int. Inst. Land Reclam. Improv. 1971, 12, 1–44. [Google Scholar]
- Bridges, E.M.; Burnham, C.P. Soils of the state of Bahrain. J. Soil Sci. 1980, 31, 689–707. [Google Scholar] [CrossRef]
- Verheye, W.H.; Boyadgiev, T.G. Evaluating the land use potential of gypsiferous soils from field pedogenic characteristics. Soil Use Manag. 1997, 13, 97–103. [Google Scholar] [CrossRef]
- Escudero, A.; Somolinos, R.C.; Olano, J.; Rubio, A. Factors controlling the establishment of Helianthemum squamatum, an endemic gypsophile of semi-arid Spain. J. Ecol. 1999, 87, 290–302. [Google Scholar] [CrossRef]
- Mardoud, T. Gypsiferous soils in the Balikh basin: Characteristics and productivity. In Proceedings of the Soil Taxonomy Workshop, ACSAD, Damascus, Syria, 1980; pp. 308–320. [Google Scholar]
- Herrero, J.; Artieda, O.; Hudnall, W.H. Gypsum, a tricky material. Soil Sci. Soc. Am. J. 2009, 73, 1757–1763. [Google Scholar] [CrossRef]
- Merlo, M.E.; Mota, J.F.; Sánchez Gómez, P. Ecofisiología y adaptaciones de las plantas vasculares a las características físicas y químicas de sustratos especiales. In Diversidad Vegetal de las Yeseras Ibéricas; Mota, J.F., Sánchez-Gómez, P., Guirado, J.S., Eds.; ADIF-Mediterráneo Asesores Consultores: Almería, Spain, 2011; pp. 51–74. [Google Scholar]
- Salmerón-Sánchez, E.; Martínez-Nieto, M.I.; Martínez-Hernández, F.; Garrido-Becerra, J.A.; Mendoza-Fernández, A.J.; Gil de Carrasco, C.; Ramos-Miras, J.J.; Lozano, R.; Merlo, M.E.; Mota, J.F. Ecology, genetic diversity and phylogeography of the Iberian endemic plant Jurinea pinnata (Lag.) DC. (Compositae) on two special edaphic substrates: Dolomite and gypsum. Plant Soil 2014, 374, 233–250. [Google Scholar] [CrossRef]
- USDA. National Soil Survey Handbook, Title 430-VI; Department of Agriculture, Natural Resources Conservation Service: Washington, DC, USA, 2018. [Google Scholar]
- Boyadgiev, T.G.; Verheye, W.H. Contribution to an utilitarian classification of gypsiferous soil. Geoderma 1996, 74, 321–338. [Google Scholar] [CrossRef]
- Herrero, J.; Porta, J. The terminology and the concepts of gypsum-rich soils. Geoderma 2000, 96, 47–61. [Google Scholar] [CrossRef]
- Escudero, A.; Palacio, S.; Maestre, F.T.; Luzuriaga, A.L. Plant life on gypsum: A review of its multiple facets. Biol. Rev. Camb. Philos. Soc. 2015, 90, 1–18. [Google Scholar] [CrossRef]
- Muller, C. Plant-soil relations on gypsum and non-gypsum soils of the Chihuahuan Desert. Sr. Honor. Proj. 2015, 75. Available online: https://collected.jcu.edu/honorspapers/75 (accessed on 21 November 2024).
- Rabizadeh, F.; Zare-Maivan, H.; Kazempour, S. Endemic Gypsophytes Composition Delimitated by Soil Properties and Altitude from Calciphytes and Halophytes in the South-Central Alborz Ranges. Nord. J. Bot. 2017, 36, e01568. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen-phosphorus interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef]
- Radabaugh, K.R.; Powell, C.E.; Bociu, I.; Clark, B.C.; Moyer, R.P. Plant size metrics and organic carbon content of Florida salt marsh vegetation. Wetl. Ecol. Manag. 2017, 25, 443–455. [Google Scholar] [CrossRef]
- Starry, O.; Lea-Cox, J.D.; Kim, J.; van Iersel, M.W. Photosynthesis and water use by two Sedum species in green roof substrate. Environ. Exp. Bot. 2014, 107, 105–112. [Google Scholar] [CrossRef]
- Merlo, E.; Mendoza-Fernández, A.J.; Salmerón-Sánchez, E.; Martínez-Hernández, F.; Ortiz-Úbeda, A.; Mota, J. Elementome of Endemic Dolomitic Flora: Pterocephalus spathulatus (Lag.) Coult. Land 2021, 10, 1253. [Google Scholar] [CrossRef]
- Güsewell, S.; Koerselman, W. Variation in nitrogen and phosphorus concentrations of wetland plants. Perspec. Plant Ecol. 2002, 5, 37–61. [Google Scholar]
- Olde Venterink, H.; Wassen, M.J.; Verkroost, A.W.M.; de Ruiter, P.C. Species richness-productivity patterns differ between N-, P-, and K-limited wetlands. Ecology 2003, 84, 2191–2199. [Google Scholar] [CrossRef]
- Kalra, Y. Handbook of Reference Methods for Plant Analysis; CRC Press, Taylor and Francis Group: London, UK, 1998. [Google Scholar]
- Watanabe, T.; Maejima, E.; Yoshimura, T.; Urayama, M.; Yamauchi, A.; Owadano, M.; Shinano, T. The ionomic study of vegetable crops. PLoS ONE 2016, 11, e0160273. [Google Scholar] [CrossRef] [PubMed]
- Elser, J.J.; Fagan, W.F.; Kerkhoff, A.J.; Swenson, N.G.; Enquist, B.J. Biological stoichiometry of plant production: Metabolism, scaling and ecological response to global change. New Phytol. 2010, 186, 593–608. [Google Scholar] [CrossRef]
- Sardans, J.; Rivas-Ubach, A.; Penuelas, J. The elemental stoichiometry of aquatic and terrestrial ecosystems and its relationships with organismic lifestyle and ecosystem structure and function: A review and perspectives. Biogeochemistry 2012, 111, 1–39. [Google Scholar] [CrossRef]
- Güsewell, S. N:P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef]
- Drohan, P.J.; Merkler, D.J. How do we find a true gypsophile? Geoderma 2009, 150, 96–105. [Google Scholar] [CrossRef]
- Ramírez, I.; Vicente, M.; García, J.; Vaquero, A. Mapa Digital de Suelos de la Región de Murcia; Consejería de Agricultura, Agua y Medio Ambiente: Murcia, Spain, 1999. [Google Scholar]
- Emerson, F.W. An ecological reconoissance in the White Sands, New Mexico. Ecology 1935, 16, 226–233. [Google Scholar] [CrossRef]
- Campbell, R.S.; Campbell, I.F. Vegetation on gypsum soil of the Jornada Plain, New Mexico. Ecology 1938, 19, 572–577. [Google Scholar] [CrossRef]
- Boukhris, M.; Lossaint, P. Spécificité biogeochimique des plantes gypsophiles de Tunisie. Oecol. Plant. 1972, 7, 45–68. [Google Scholar]
- Franceschi, V.R.; Nakata, P.A. Calcium oxalate in plants: Formation and function. Annu. Rev. Plant Biol. 2005, 56, 41–71. [Google Scholar] [CrossRef] [PubMed]
- Doege, S.J. The role of natural calcium oxalate crystals in plant defense against chewing insects. Inquiry 2003, 4, 15. [Google Scholar]
- Palacio, S.; Aitkenhead, M.; Escudero, A.; Montserrat–Martí, G.; Maestro, M.; Robertson, A.J. Gypsophile chemistry unveiled: Fourier transform infrared (FTIR) spectroscopy provides new insight into plant adaptations to gypsum soils. PLoS ONE 2014, 9, e107285. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.A.; Gioia, F.D.; Ntatsi, G. Vegetable Organosulfur Compounds and their Health Promoting Effects. Curr. Pharm. Des. 2017, 23, 2850–2875. [Google Scholar] [CrossRef]
- Casby-Horton, S.; Herrero, J.; Rolong, N.A. Chapter four: Gypsum soils, their morphology, classification, function, and landscapes. Adv. Agron. 2015, 130, 231–290. [Google Scholar] [CrossRef]
- Moore, M.J.; Mota, J.F.; Douglas, N.A.; Olvera, H.F.; Ochoterena, H. The ecology, assembly, and evolution of gypsophile floras. In Plant Ecology and Evolution in Harsh Environments; Rajakaruna, N., Boyd, R.S., Harris, T.B., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2014; pp. 97–128. [Google Scholar]
- White, P.J.; Broadley, M.R.; El-Serehy, H.A.; George, T.S.; Neugebauer, K. Linear relationships between shoot magnesium and calcium concentrations among angiosperm species are associated with cell wall chemistry. Ann. Bot. 2018, 122, 221–226. [Google Scholar] [CrossRef]
- Chen, Z.C.; Liao, H. Organic acid anions: An effective defensive weapon for plants against aluminium toxicity and phosphorus deficiency in acidic soils. J. Genet. Genomics 2016, 43, 631–638. [Google Scholar] [CrossRef]
- Heuer, S.; Gaxiola, R.; Schilling, R.; Herrera-Estrella, L.; López-Arredondo, D.; Wissuwa, M.; Rouached, H. Improving phosphorus use efficiency: A complex trait with emerging opportunities. Plant J. 2017, 90, 868–885. [Google Scholar] [CrossRef]
- Bartolucci, F.; Peruzzi, L.; Galasso, G.; Albano, A.; Alessandrini, A.; Ardenghi, N.M.G.; Astuti, G.; Bacchetta, G.; Conti, F. An updated checklist of the vascular flora native to Italy. Plant Biosyst. 2018, 152, 179–303. [Google Scholar] [CrossRef]
- Babaoğlu, M.; Gezgin, S.; Topal, A.; Sade, B.; Dural, H. Gypsophila sphaerocephala Fenzl ex Tchihat: A boron hyperaccumulator plant species that may phytoremediate soils with toxic B levels. Turk J. Agric For. 2004, 28, 273–278. [Google Scholar]
- Mota, J.F.; Martínez-Hernández, F.; Salmerón-Sánchez, E.; Mendoza-Fernández, A.J.; Pérez-García, F.J.; Merlo, M.E. Spontaneous Primary Succession and Vascular Plant Recovery in the Iberian Gypsum Quarries: Insights for Ecological Restoration in an EU Priority Habitat. Plants 2023, 12, 1162. [Google Scholar] [CrossRef]
- Mota, J.F.; Martínez-Hernández, F.; Pérez-García, F.J.; Mendoza-Fernández, A.J.; Salmerón-Sánchez, E.; Merlo, M.E. Shipwrecked on the Rock, or Not Quite: Gypsophytes and Edaphic Islands. Plants 2024, 13, 970. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.T.; Moore, M.J.; Feder, Z.; Tiley, H.; Drenovsky, R.E. Phylogenetic patterns of foliar mineral nutrient accumulation among gypsophiles and their relatives in the Chihuahuan Desert. Am. J. Bot. 2017, 104, 1442–1450. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Kirilak, Y.; Kuo, J.; Lambers, H. Accumulation and precipitation of magnesium, calcium, and sulfur in two Acacia (Leguminosae; Mimosoideae) species grown in different substrates proposed for mine-site rehabilitation. Am. J. Bot. 2015, 102, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Allahham, A.; Kanno, S.; Zhang, L.; Maruyama-Nakashita, A. Sulfur deficiency increases phosphate accumulation, uptake, and transport in Arabidopsis thaliana. Int. J. Mol. Sci. 2020, 21, 2971. [Google Scholar] [CrossRef]
- Shaul, O. Magnesium transport and function in plants: The tip of the iceberg. Biometals 2002, 15, 307–321. [Google Scholar] [CrossRef]
- Tyndall, R.W.; Hull, J.C. Vegetation, flora, and plant physiological ecology of serpentine barrens of eastern North America. In Savannas, Barrens, and Rock Outcrop Plant Communities of North America; Anderson, R.C., Fralish, J.S., Baskin, J.M., Eds.; Cambridge University Press: Cambridge, UK, 1999; pp. 67–82. [Google Scholar]
- Brady, K.U.; Kruckeberg, A.R.; Bradshaw, H.D., Jr. Evolutionary ecology of plant adaptation to serpentine soils. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 243–266. [Google Scholar] [CrossRef]
- Oze, C.; Skinner, C.; Schroth, A.W.; Coleman, R.G. Growing up green on serpentine soils: Biogeochemistry of serpentine vegetation in the Central Coast Range of California. Appl. Geochem. 2008, 23, 3391–3403. [Google Scholar] [CrossRef]
- Visscher, A.M.; Paul, A.L.; Kirst, M.; Guy, C.L.; Schuerger, A.C.; Ferl, R.J. Growth performance and root transcriptome remodeling of Arabidopsis in response to Mars–like levels of magnesium sulfate. PLoS ONE 2010, 5, e12348. [Google Scholar] [CrossRef]
- Muller, C.T.; Cera, A.; Palacio, S.; Moore, M.J.; Tejero, P.; Mota, J.F.; Drenovsky, R.E. Nutritional convergence in plants growing on gypsum soils in two distinct climatic regions. Ann. Bot. 2024, 134, mcae127. [Google Scholar] [CrossRef]
- Médail, F.; Quézel, P. Biodiversity hotspots in the Mediterranean basin: Setting global conservation priorities. Conserv. Biol. 1999, 13, 1510–1513. [Google Scholar] [CrossRef]
- Cañadas, E.M.; Giuseppe, F.; Peñas, J.; Lorite, J.; Mattana, E.; Bacchetta, G. Hotspots within hotspots: Endemic plant richness, environmental drivers, and implications for conservation. Biol. Conserv. 2014, 170, 282–291. [Google Scholar] [CrossRef]
- Antolini, P. Rassegna dei principali affioramenti di gesso in Italia. Atti Della Accad. Roveretana Degli Agiati 1984, 24, 83–117. [Google Scholar]
- Cucchi, F.; Forti, P.; Finocchiaro, F. Gypsum degradation in Italy with respect to climatic, textural and erosional conditions. Suppl. Geogr. Fis. Dinam. Quat. 1998, 3, 41–49. [Google Scholar]
- Cita, M.B.; Abbate, E.; Balini, M.; Conti, M.A.; Falorni, P.; Germani, D.; Groppelli, G.; Manetti, P.; Petti, F.M. Carta Geologica d’Italia 1:50.000. Catalogo delle Formazioni-Unità Tradizionali 2; Quaderni del servizio geologico d’Italia, serie III, VII; ISPRA: Roma, Italy, 2007. [Google Scholar]
- Butler, R.W.H.; Maniscalco, R.; Sturiale, G.; Grasso, M. Stratigraphic variations control deformation patterns in evaporite basins: Messinian examples, onshore and offshore Sicily Italy. J. Geol. Soc. London 2014, 172, 113–124. [Google Scholar] [CrossRef]
- Madonia, G.; Panzica La Manna, M.; Vattano, M. Trent’anni di ricerche carsologiche nelle evaporiti della Sicilia. In Proceedings of the Atti del Convegno Nazionale “La ricerca carsologica in Italia”, Frabosa Soprana, Italy, 22–23 June 2013; Laboratorio carsologico sotterraneo di Bossea: Frabosa Soprana, Italy, 2016; pp. 37–48. [Google Scholar]
- Selli, R. Il Messiniano Mayer-Eymar 1867. Proposta di un neostratotipo. G. Geol. G. 1960, 28. [Google Scholar]
- Cucchi, F.; Piano, C. Inquadramento geografico e geologico dei gessi in Italia. In Le Aree Carsiche Gessose d’Italia; Madonia, G., Forti, P., Eds.; Memorie dell’Istituto Italiano di Speleologia: Bologna, Italy, 2003; pp. 2–14, 17–26. [Google Scholar]
- Pesaresi, S.; Galdenzi, D.; Biondi, E.; Casavecchia, S. Bioclimate of Italy: Application of the worldwide bioclimatic classification system. J. Maps 2014, 104, 538–553. [Google Scholar] [CrossRef]
- Martínez-Hernández, F.; Pérez-García, F.J.; Garrido-Becerra, J.A.; Mendoza-Fernández, A.J.; Medina-Cazorla, J.M.; Martínez-Nieto, M.I.; Merlo Calvente, M.E.; Mota Poveda, J.F. The distribution of Iberian gypsophilous flora as a criterion for conservation policy. Biodiver. Conserv. 2011, 20, 1353–1364. [Google Scholar] [CrossRef]
- USGS. 2013 Mineral Year Book. Available online: https://www.usgs.gov/centers/national-minerals-information-center/publications (accessed on 30 September 2016).
- QGIS.org. QGIS Geographic Information System. QGIS Association, 2024. Available online: http://www.qgis.org (accessed on 24 June 2024).
- Artieda, O.; Herrero, J.; Drohan, P.J. Refinement of the differential water loss method for gypsum determination in soils. Soil Sci. Soc. Am. J. 2006, 70, 1932–1935. [Google Scholar] [CrossRef]
- MAPA. Métodos Oficiales de Análisis de Suelos y Aguas; Ministerio de Agricultura, Pesca y Alimentación: Madrid, Spain, 1994. [Google Scholar]
- Ali, A.; Said, K.; Messaoudi, I. Cadmium: Bioaccumulation, histopathology and detoxifying mechanisms in fish. Am. J. Res. Commun. 2013, 1, 60–79. [Google Scholar]
- Brooks, R. Plants that Hyperaccumulate Heavy Metals, Their Role in Phytoremediation, Microbiology, Archaeology, Mineral Exploration and Phytomining; Cab International: New York, NY, USA, 1998. [Google Scholar]
- Zhuang, P.; Yang, Q.W.; Wang, H.B.; Shu, W.S. Phytoextraction of heavy metals by eight plant species in the field. Water Air Soil Pollut. 2007, 184, 235–242. [Google Scholar] [CrossRef]
- Buscaroli, A. An overview of indexes to evaluate terrestrial plants for phytoremediation purposes. Ecol. Indic. 2017, 82, 367–380. [Google Scholar] [CrossRef]
- Baker, A. Accumulators and excluders-strategies in the response of plants to heavy metals. J. Plant Nutr. 1981, 3, 643–654. [Google Scholar] [CrossRef]
- IBM Corporation. Released. SPSS Software Version 26.0; IBM: Armonk, NY, USA, 2019. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Mota, J.F. Vegetación de escarpes, gleras y rocas. In Proyecto Andalucía Botánica V; Publicaciones Comunitarias: Sevilla, Spain, 2007; Volume 24, pp. 139–162. [Google Scholar]
| Site | Dominant Plants | MGRS (ZONE 33S) | H (m) | % Gypsum | EC dS m−1 | pHW | |
|---|---|---|---|---|---|---|---|
| X | Y | ||||||
| Glibiscemi | G. arrostii subsp. arrostii | 435419 | 4118582 | 440 | 78.33 | 2.78 | 7.84 |
| Glibiscemi | G. arrostii subsp. arrostii | 435511 | 4118308 | 519 | 44.52 | 2.82 | 7.90 |
| Glibiscemi | Sedum gypsicola subsp. trinacriae | 435509 | 4118310 | 517 | 55.91 | 2.94 | 8.00 |
| Glibiscemi | Sedum gypsicola subsp. trinacriae | 435419 | 4118582 | 438 | 72.46 | 2.97 | 7.91 |
| Glibiscemi | Quercus ilex | 435419 | 4118582 | 435 | 61.18 | 2.90 | 7.76 |
| Realmonte | Ampelodesmos mauritanica | 364177 | 4132388 | 220 | 44.16 | 2.03 | 8.10 |
| Realmonte | Erica multiflora subsp. multiflora | 364165 | 4132394 | 209 | 36.75 | 1.69 | 8.59 |
| Realmonte | G. arrostii subsp. arrostii | 364269 | 4132213 | 279 | 50.77 | 3.03 | 7.51 |
| Castelmola | G. arrostii subsp. arrostii | 523959 | 4191241 | 639 | 2.96 | 1.25 | 7.36 |
| R. Entella | G. arrostii subsp. arrostii | 333979 | 4182564 | 424 | 95.64 | 3.17 | 7.70 |
| Site | Macronutrients (g 100 g−1) | Micronutrients (mg kg−1) | Other Elements (mg kg−1) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | N | Ca | Mg | K | P | S | Na | B | Mn | Ni | Zn | Cr | Co | Cu | Pb | Sr | Ti | Tl | V | |
| Realmonte | 9.80 | 0.40 | 12.87 | 0.52 | 0.29 | 0.05 | 1.50 | 0.03 | 17.52 | 281.24 | 11.36 | 28.02 | 18.15 | 3.25 | 9.24 | 16.33 | 526.85 | 136.97 | 10.02 | 31.33 |
| R. Entella | 12.09 | 0.15 | 4.58 | 0.32 | 0.16 | 0.01 | 3.22 | 0.04 | 9.34 | 105.56 | 9.25 | 24.39 | 6.51 | 3.41 | 4.95 | 18.19 | 341.61 | 137.17 | 1.36 | 9.05 |
| Glibiscemi | 3.29 | 0.13 | 8.80 | 0.18 | 0.24 | 0.02 | 3.92 | 0.01 | 12.73 | 381.96 | 14.92 | 29.34 | 13.37 | 3.01 | 10.62 | 9.48 | 208.17 | 102.27 | 7.33 | 21.30 |
| Castelmola | 5.36 | 0.09 | 9.47 | 0.36 | 0.65 | 0.04 | 0.13 | 0.04 | 24.58 | 163.52 | 20.84 | 47.79 | 26.68 | 2.63 | 20.91 | 18.81 | 237.06 | 64.98 | 13.34 | 39.47 |
| Species | Functional Type | C | N | P | N:P | K | Ca | S | Mg |
|---|---|---|---|---|---|---|---|---|---|
| Artarb | Gypsovag | 44.94 | 2.29 | 0.21 | 10.72 | 2.17 | 0.57 | 0.28 | 0.11 |
| Erimul | Gypsovag | 55.91 | 0.72 | 0.04 | 18.23 | 0.33 | 0.43 | 0.13 | 0.13 |
| Petang | Gypsovag | 38.19 | 2.50 | 0.11 | 22.60 | 2.65 | 1.77 | 0.58 | 0.94 |
| Petsed | Gypsovag | 37.62 | 0.76 | 0.06 | 12.62 | 0.80 | 4.72 | 0.27 | 0.14 |
| Sedcae | Gypsovag | 40.33 | 1.15 | 0.19 | 6.13 | 1.61 | 2.30 | 0.16 | 0.20 |
| Silfru | Gypsovag | 42.60 | 2.01 | 0.14 | 14.63 | 2.61 | 0.93 | 0.21 | 0.37 |
| Silsic | Gypsovag | 39.38 | 3.35 | 0.17 | 19.59 | 1.25 | 3.50 | 2.03 | 0.21 |
| Bratin | Narrow gypsophile | 40.99 | 1.92 | 0.16 | 11.76 | 1.55 | 0.91 | 0.87 | 0.17 |
| Erymet | Narrow gypsophile | 37.41 | 1.45 | 0.10 | 14.45 | 1.53 | 3.04 | 0.45 | 0.12 |
| Gyparr | Narrow gypsophile | 38.25 | 3.13 | 0.15 | 20.51 | 1.35 | 3.73 | 1.76 | 0.28 |
| Dipcra | Wide gypsophile | 34.19 | 2.56 | 0.12 | 22.19 | 1.88 | 3.07 | 2.65 | 0.35 |
| Matfru | Wide gypsophile | 37.77 | 2.24 | 0.08 | 27.46 | 1.25 | 3.04 | 1.33 | 0.34 |
| Petoch | Wide gypsophile | 41.20 | 0.67 | 0.05 | 13.77 | 0.47 | 2.87 | 0.26 | 0.12 |
| Sedgyp | Wide gypsophile | 42.27 | 0.80 | 0.06 | 12.72 | 0.50 | 2.48 | 0.18 | 0.11 |
| Macronutrients | Micronutrients | Another | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Functional Type | Site | C | N | Ca | K | Mg | P | S | B | Cu | Fe | Na | Al | Cr | Sr |
| Bratin | Narrow gyps. | R. Entella | 3.39 | 12.20 | 0.20 | 9.80 | 0.54 | 13.46 | 0.27 | 2.42 | 0.38 | 0.02 | 1.05 | 0.02 | 0.22 | 0.70 |
| Dipcra | Wide gyps. | 2.81 | 11.65 | 0.55 | 11.32 | 1.50 | 7.72 | 1.04 | 2.59 | 0.33 | 0.03 | 19.40 | 0.04 | 0.04 | 3.46 | |
| Erymet | Narrow gyps. | 3.09 | 9.19 | 0.66 | 9.68 | 0.38 | 8.25 | 0.14 | 2.91 | 0.31 | 0.06 | 2.98 | 0.08 | 0.07 | 3.77 | |
| Gyparr | Narrow gyps. | 3.12 | 16.73 | 0.70 | 17.23 | 1.27 | 14.15 | 0.57 | 3.00 | 0.61 | 0.02 | 2.15 | 0.01 | 0.04 | 0.87 | |
| Matfru | Wide gyps. | 3.12 | 14.22 | 0.66 | 7.86 | 1.06 | 6.72 | 0.41 | 6.20 | 0.65 | 0.11 | 5.60 | 0.16 | 0.14 | 3.59 | |
| Sedcae | Gypsovag | 3.34 | 7.33 | 0.50 | 10.14 | 0.63 | 15.49 | 0.05 | 2.13 | 0.84 | 0.12 | 2.11 | 0.16 | 0.17 | 0.97 | |
| Sedgyp | Wide gyps. | 3.50 | 5.66 | 0.60 | 4.09 | 0.47 | 6.11 | 0.05 | 1.63 | 0.67 | 0.03 | 0.50 | 0.04 | 0.06 | 1.18 | |
| Petoch | Wide gyps. | 3.41 | 4.25 | 0.63 | 2.98 | 0.36 | 4.00 | 0.08 | 1.63 | 0.46 | 0.02 | 0.35 | 0.03 | 0.03 | 1.90 | |
| Petsed | Gypsovag | 3.11 | 4.81 | 1.03 | 5.08 | 0.43 | 4.94 | 0.08 | 2.34 | 0.71 | 0.02 | 0.78 | 0.04 | 0.14 | 3.36 | |
| Dipcra | Wide gyps. | Glibiscemi | 10.43 | 25.51 | 0.41 | 8.04 | 1.23 | 6.09 | 0.50 | 1.24 | 0.25 | 0.01 | 28.70 | 0.01 | 0.04 | 2.25 |
| Gyparr | Narrow gyps. | 12.10 | 24.99 | 0.45 | 3.96 | 0.77 | 5.22 | 0.36 | 2.09 | 0.40 | 0.01 | 1.65 | 0.01 | 0.02 | 0.66 | |
| Sedgyp | Wide gyps. | 12.82 | 5.51 | 0.25 | 1.45 | 0.40 | 2.30 | 0.05 | 0.90 | 0.24 | 0.01 | 2.09 | 0.02 | 0.02 | 0.38 | |
| Gyparr | Narrow gyps. | Realmonte | 4.57 | 5.65 | 0.36 | 4.74 | 0.50 | 1.90 | 0.48 | 1.89 | 0.31 | 0.01 | 1.21 | 0.00 | 0.03 | 0.26 |
| Gyparr | Narrow gyps. | Castelmola | 7.13 | 36.79 | 0.40 | 1.67 | 0.75 | 3.67 | 14.06 | 1.54 | 0.21 | 0.00 | 0.50 | 0.00 | 0.03 | 0.36 |
| Petang | Gypsovag | 7.13 | 28.49 | 0.19 | 4.09 | 2.58 | 2.67 | 4.55 | 0.71 | 0.25 | 0.01 | 22.08 | 0.01 | 0.03 | 0.20 | |
| Silsic | Gypsovag | 7.35 | 38.23 | 0.37 | 1.93 | 0.58 | 4.13 | 16.02 | 1.25 | 0.23 | 0.01 | 0.54 | 0.01 | 0.09 | 0.14 | |
| Site | Substrate | N | K | Na | S |
|---|---|---|---|---|---|
| Castelmola | Limestone | 47.02 | 1.66 | 0.58 | 15.55 |
| 40.79 | 1.71 | 0.43 | 12.34 | ||
| 36.75 | 1.14 | 0.13 | 12.85 | ||
| 39.93 | 1.41 | 0.31 | 12.20 | ||
| 37.03 | 1.16 | 0.28 | 12.68 | ||
| Glibiscemi | Gypsum | 24.99 | 3.96 | 1.65 | 0.36 |
| R. Entella | 18.27 | 15.91 | 1.61 | 0.53 | |
| R. Entella | 15.19 | 18.55 | 2.69 | 0.61 | |
| Realmonte | 6.42 | 5.12 | 1.04 | 0.49 | |
| Realmonte | 4.89 | 4.36 | 1.37 | 0.48 | |
| p-value | 0.00 | 0.01 | 0.00 | 0.00 |
| Plant Species | Abrev. | Family | Site | Succulent | Functional Type |
|---|---|---|---|---|---|
| Artemisia arborescens | Artarb | Asteraceae | R. Entella | No | Gypsovag |
| Artemisia arborescens | Artarb | Asteraceae | Glibiscemi | No | Gypsovag |
| Brassica villosa subsp. tineoi | Bratin | Brassicaceae | R. Entella | Yes | Narrow gyps. |
| Diplotaxis harra subsp. crassifolia | Dipcra | Brassicaceae | R. Entella | Yes | Wide gyps. |
| Diplotaxis harra subsp. crassifolia | Dipcra | Brassicaceae | Glibiscemi | Yes | Wide gyps. |
| Erysimum metlesicsii | Erymet | Brassicaceae | R. Entella | No | Narrow gyps. |
| Matthiola fruticulosa subsp. fruticulosa | Matfru | Brassicaceae | R. Entella | No | Wide gyps. |
| Gypsophila arrostii subsp. arrostii | Gyparr | Cariophyllaceae | R. Entella | Yes | Narrow gyps. |
| Gypsophila arrostii subsp. arrostii | Gyparr | Cariophyllaceae | Monte Conca | Yes | Narrow gyps. |
| Gypsophila arrostii subsp. arrostii | Gyparr | Cariophyllaceae | Castelmola | Yes | Narrow gyps. |
| Gypsophila arrostii subsp. arrostii | Gyparr | Cariophyllaceae | Glibiscemi | Yes | Narrow gyps. |
| Petrorhagia illyrica subsp. angustifolia | Petang | Cariophyllaceae | Castelmola | No | Gipsovag |
| Silene fruticosa | Silfru | Cariophyllaceae | R. Entella | No | Gypsovag |
| Silene italica subsp. sicula | Silsic | Cariophyllaceae | Castelmola | No | Gipsovag |
| Petrosedum ochroleucum subsp. mediterraneum | Petoch | Crassulaceae | R. Entella | Yes | Wide gyps. |
| Petrosedum sediforme | Petsed | Crassulaceae | R. Entella | Yes | Gypsovag |
| Sedum caeruleum | Sedcae | Crassulaceae | R. Entella | Yes | Gypsovag |
| Sedum gypsicola subsp. trinacriae | Sedgyp | Crassulaceae | R. Entella | Yes | Wide gyps. |
| Sedum gypsicola subsp. trinacriae | Sedgyp | Crassulaceae | Glibiscemi | Yes | Wide gyps. |
| Erica multiflora subsp. multiflora | Erimul | Ericaceae | Glibiscemi | No | Gypsovag |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendoza-Fernández, A.J.; Merlo, E.; Musarella, C.M.; Salmerón-Sánchez, E.; Martínez-Hernández, F.; Pérez-García, F.J.; Spampinato, G.; Mota, J. Elemental Screening and Nutritional Strategies of Gypsophile Flora in Sicily. Plants 2025, 14, 804. https://doi.org/10.3390/plants14050804
Mendoza-Fernández AJ, Merlo E, Musarella CM, Salmerón-Sánchez E, Martínez-Hernández F, Pérez-García FJ, Spampinato G, Mota J. Elemental Screening and Nutritional Strategies of Gypsophile Flora in Sicily. Plants. 2025; 14(5):804. https://doi.org/10.3390/plants14050804
Chicago/Turabian StyleMendoza-Fernández, Antonio J., Encarna Merlo, Carmelo M. Musarella, Esteban Salmerón-Sánchez, Fabián Martínez-Hernández, Francisco J. Pérez-García, Giovanni Spampinato, and Juan Mota. 2025. "Elemental Screening and Nutritional Strategies of Gypsophile Flora in Sicily" Plants 14, no. 5: 804. https://doi.org/10.3390/plants14050804
APA StyleMendoza-Fernández, A. J., Merlo, E., Musarella, C. M., Salmerón-Sánchez, E., Martínez-Hernández, F., Pérez-García, F. J., Spampinato, G., & Mota, J. (2025). Elemental Screening and Nutritional Strategies of Gypsophile Flora in Sicily. Plants, 14(5), 804. https://doi.org/10.3390/plants14050804










