Salinity as an Inducer of Antioxidant Activity Exerted by Mangrove Species from Campeche, Mexico
Abstract
1. Introduction
2. Materials and Methods
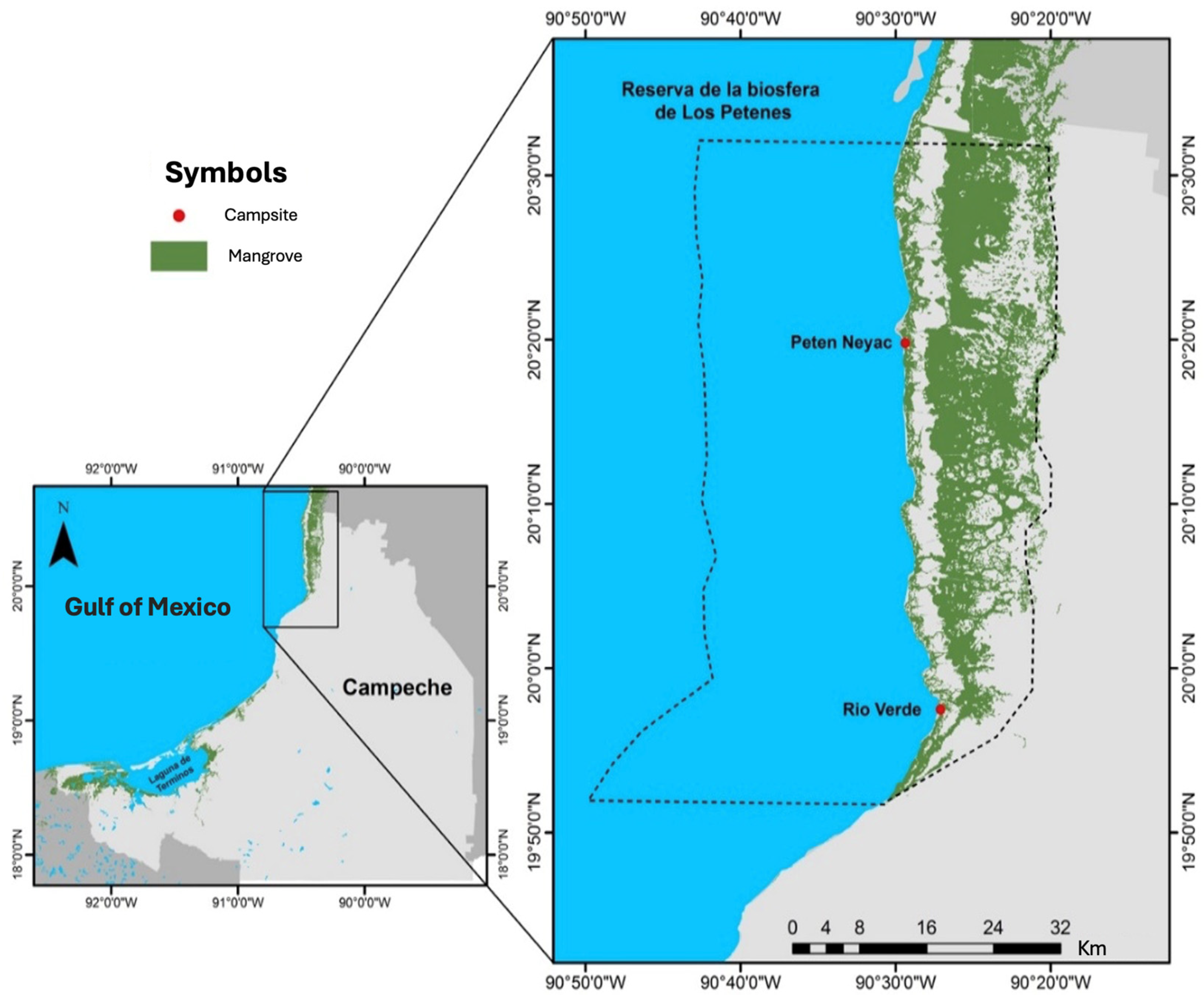
2.1. Soil Interstitial Water Salinity
2.2. Collection of Plant Material
2.3. Extraction
2.4. Qualitative Phytochemistry
2.5. Spectroscopic Analysis
2.6. Chromatographic Profile
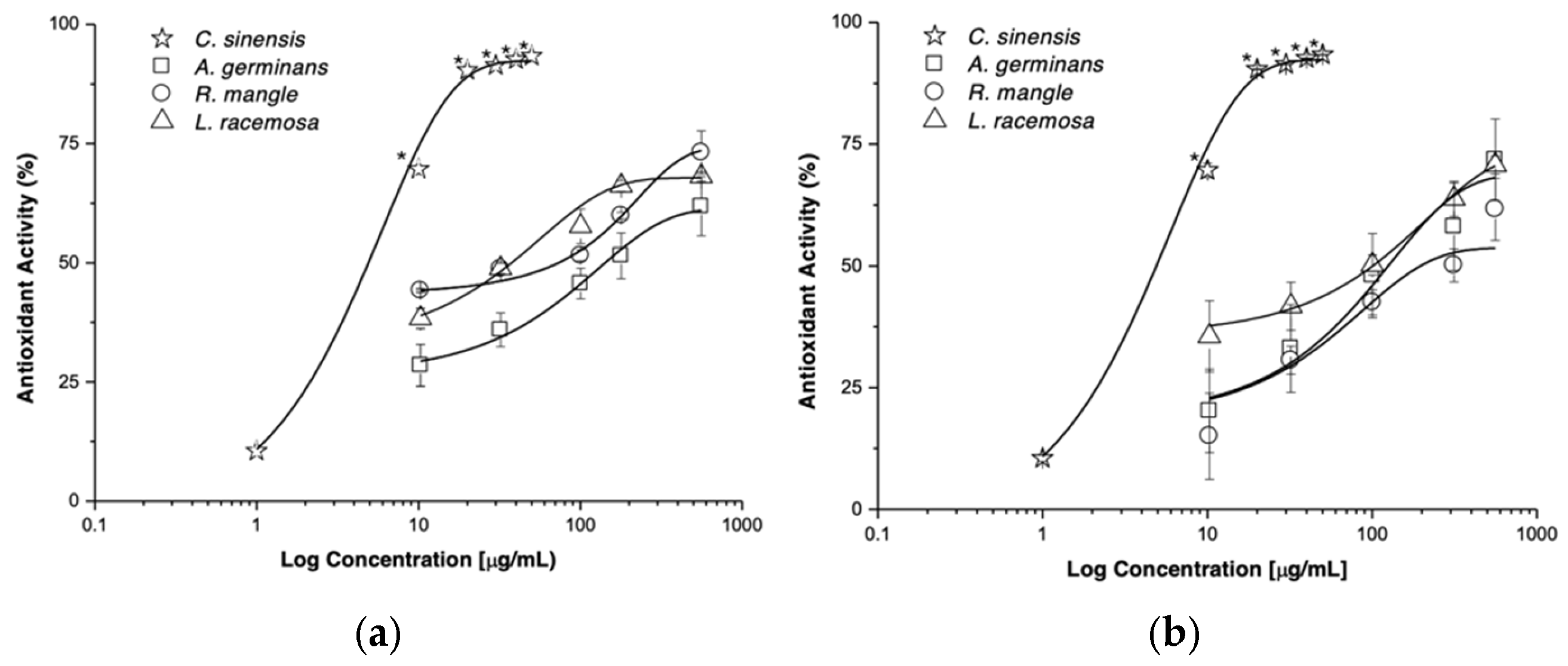
2.7. In Vitro Test
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kathiresan, K.; Bingham, B.L. Biology of Mangroves and Mangrove Ecosystems. Adv. Mar. Bio. 2001, 40, 81–251. [Google Scholar] [CrossRef]
- Chan-Keb, C.A.; Agraz-Hernández, C.M.; Muñiz-Salazar, R.; Posada-Vanegas, G.; Osti-Sáenz, J.; Reyes Castellano, J.E.; Conde-Molina, K.P.; Vega-Serratos, B.E. Ecophysiological response of Rhizophora mangle to the variation in hydrochemistry during five years along the coast of Campeche, México. Diversity 2018, 10, 9. [Google Scholar] [CrossRef]
- Mitra, A.; Chowdhury, R.; Sengupta, K.; Banerjee, K. Impact of salinity on mangroves of Indian Sundarbans. J. Coast. Environ. 2010, 1, 71–82. [Google Scholar]
- Mariam, H.; Alamgir, A.N.M. Flowering and Fruiting Behavior, Seed Germination, and Survival Status of Heritiera fomes Buch-Ham and Excoecaria agallocha L. Species in the Sundarban Mangrove Forest of Bangladesh. Open Access Libr. J. 2022, 9, 1–11. [Google Scholar] [CrossRef]
- Nabeelah Bibi, S.; Fawzi, M.M.; Gokhan, Z.; Rajesh, J.; Nadeem, N.; Kannan, R.R.R.; Albuquerque, R.D.D.G.; Pandian, S.K. Ethnopharmacology, Phytochemistry, and Global Distribution of Mangroves―A Comprehensive Review. Mar. Drugs 2019, 17, 231. [Google Scholar] [CrossRef] [PubMed]
- Pastene, E.R. Current state of the search for plants with antioxidant activity. Bol. Latinoam. Caribe Plant. Med. Aromat. 2009, 8, 449–455. [Google Scholar]
- Flores-Verdugo, F.; González-Farías, F.; Ramírez-Flores, O.; Amezcua-Linares, F.; Yáñez-Arancibia, A.; Day, J.W. Mangrove ecology, aquatic primary productivity, and fish community dynamics in the Teacapán-Agua Brava lagoon-estuarine system (Mexican Pacific). Estuaries 1997, 20, 276–290. [Google Scholar] [CrossRef]
- Acosta-Lugo, E.; Alonso-Parra, D.; Andrade-Hernández, M.; Castillo-Tzab, D.; Chablé-Santos, J.; Durán-García, R.; Espadas-Manrique, C.; Fernández-Stohanzlova, I.; Fraga-Berdugo, J.; Galicia, E.; et al. Plan de Conservación de la eco-región Petenes-Celestún-Palmar. Universidad Autónoma de Campeche, Pronatura Peninsula de Yucatan AC: Campeche, México, 2010. Available online: http://etzna.uacam.mx/epomex/pdf/Eco-region.pdf#page=9 (accessed on 19 February 2025).
- Zamora-Crescencio, P.; Mas, J.F.; Rico-Gray, V.; del Rosario Domínguez-Carrasco, M.; Villegas, P.; Gutiérrez-Báez, C.; Barrientos-Medina, R.C. Arboreal composition and structure of the petenes of the Petenes Biosphere Reserve, Campeche, Mexico. Polibotánica 2015, 39, 1–19. Available online: http://www.polibotanica.mx/pdf/pb39/compo.pdf (accessed on 19 February 2025).
- Oliva, M.; Montiel, S. Stakeholder linkage in conservation strategies: A qualitative tool for improving the management of a biosphere reserve in the Yucatan Peninsula, Mexico. Trop. Conserv. Sci. 2016, 9, 423–438. [Google Scholar] [CrossRef]
- Méndez-Cabrera, F.; Montiel, S. Preliminary assessment of the wild fauna and flora used by the Mayan populations of two coastal communities in Campeche, Mexico. Univ. Cienc. 2007, 23, 127–139. [Google Scholar]
- Agraz-Hernández, C.M. Estudio de caso: Usos y beneficios ecológicos, económicos y sociales que proporcionan los ecosistemas de manglar en el estado de Campeche. In La Biodiversidad en Campeche: Estudio de Estado; Villalobos-Zapata, G.J., Mendoza-Vega, J., Eds.; Comisión Nacional Para el Conocimiento y Uso de la Biodiversidad, Gobierno del Estado de Campeche, Universidad Autónoma de Campeche, El Colegio de la Frontera Sur: Campeche, México, 2010; pp. 470–475. [Google Scholar]
- Agraz-Hernández, C.M.; García-Zaragoza, C.; Iriarte-Vivar, S.; Flores-Verdugo, F.J.; Moreno-Casasola, P. Forest structure, productivity and species phenology of mangroves in the La Mancha lagoon in the Atlantic coast of Mexico. Wetl. Ecol. Manag. 2011, 19, 273–293. [Google Scholar] [CrossRef]
- Bandaranayake, W.M. Bioactivities, bioactive compounds, and chemical constituents of mangrove plants. Wetl. Ecol. Manag. 2002, 10, 421–452. [Google Scholar] [CrossRef]
- Rafii, Z.A.; Dodd, R.S.; Fromard, F. Biogeographic variation in foliar waxes of mangrove species. Biochem. Syst. Ecol. 1996, 24, 341–345. [Google Scholar] [CrossRef]
- Neves-Costa, F.; Jerz, G.; Hewitson, P.; de Souza-Figueiredo, F.; Ignatova, S. Laguncularia racemosa phenolics profiling by three-phase solvent system step-gradient using high-performance countercurrent chromatography with off-line electrospray mass-spectrometry detection. Molecules 2021, 26, 2284. [Google Scholar] [CrossRef]
- Glasenapp, Y.; Korth, I.; Nguyen, X.V.; Papenbrock, J. Sustainable use of mangroves as sources of valuable medicinal compounds: Species identification, propagation and secondary metabolite composition. S. Afr. J. Bot. 2019, 121, 317–328. [Google Scholar] [CrossRef]
- Koch, B.P.; Rullkötter, J.; Lara, R.J. Evaluation of triterpenols and sterols as organic matter biomarkers in a mangrove ecosystem in northern Brazil. Wetl. Ecol. Manag. 2003, 11, 257–263. [Google Scholar] [CrossRef]
- Koch, B.P.; Harder, J.; Lara, R.J.; Kattner, G. The effect of selective microbial degradation on the composition of mangrove derived pentacyclic triterpenols in surface sediments. Org. Geochem. 2005, 36, 273–285. [Google Scholar] [CrossRef]
- He, D.; Ladd, S.N.; Sachs, J.P.; Jaffé, R. Inverse relationship between salinity and 2H/1H fractionation in leaf wax n-alkanes from Florida mangroves. Org. Geochem. 2017, 110, 1–12. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, Q.; Xu, J.; Li, M.Y.; Pan, J.Y.; Yang, M.H. Natural products from true mangrove flora: Source, chemistry and bioactivities. Nat. Prod. Rep. 2008, 25, 955–981. [Google Scholar] [CrossRef]
- Rodrigues, C.F.B.; Gaeta, H.H.; Belchor, M.N.; Ferreira, M.J.P.; Pinho, M.V.T.; de Oliveira Toyama, D.; Toyama, M.H. Evaluation of potential thrombin inhibitors from the white mangrove (Laguncularia racemosa (L.) CF Gaertn.). Mar. Drugs 2015, 13, 4505–4519. [Google Scholar] [CrossRef]
- Agraz-Hernández, C.; Noriega-Trejo, R.; López-Portillo, J.; Flores-Verdugo, F.; Jiménez-Zacarías, J. Guía de Campo. Identificación de los Manglares en México; Centro EPOMEX-UAC: Campeche, México, 2006. [Google Scholar]
- Comisión Permanente de la Farmacopea de los Estados Unidos Mexicanos. Farmacopea Herbolaria de los Estados Unidos Mexicanos. 3.0; Secretaría de Salud CDMX México: Ciudad de México, México, 2021. [Google Scholar]
- Robles-García, M.A.; Aguilar-Antonio, J.; Gutiérrez-Lomelí, M.; Rodríguez-Félix, F.; Morales-Del-Río, J.A.; Guerrero-Medina, P.J.; Madrigal-Pulido, J.A.; Del-Toro-Sánchez, C.L. Qualitative identification of secondary metabolites and cytotoxicity determination of tempisque extracts (Sideroxylum capiri pittier). Biotecnia 2016, 18, 3–8. [Google Scholar] [CrossRef]
- García-Granados, R.U.; Cruz-Sosa, F.; Alarcón-Aguilar, F.J.; Nieto-Trujillo, A.; Gallegos-Martínez, M.E. Qualitative phytochemical analysis of the aqueous extracts of Thalassia testudinum Banks ex Köning et Sims from the area of Champoton, Campeche, Mexico, during the annual cycle 2016–2017. Polibotánica 2019, 48, 151–168. [Google Scholar] [CrossRef]
- Mohy El-Din, S.M.; Mohyeldin, M.M. Component analysis and antifungal activity of the compounds extracted from four brown seaweeds with different solvents at different seasons. J. Ocean Univ. China 2018, 17, 1178–1188. [Google Scholar] [CrossRef]
- Aguirre-Crespo, F.J.; Cerino-Pérez, E.; Valdovinos-Estrella, J.D.C.; Maldonado-Velázquez, M.G.; Ortega-Morales, B.O.; Zamora-Crescencio, P.; Hernandez-Nuñez, E.; Estrada-Soto, S.E. Vasorelaxant and Antioxidant Activity of Some Medicinal Plants from Campeche, Mexico. Pharmacogn. Mag. 2021, 17, 23–30. [Google Scholar] [CrossRef]
- Cu-Quiñones, L.D. Desarrollo de un Prototipo de Forma Farmacéutica a Partir de Las Hojas de Jatropha gaumeri Greenm (pomol che). Master’s Thesis, Universidad Autónoma de Campeche, Campeche, México, 2021. [Google Scholar]
- Seal, T. Quantitative HPLC analysis of phenolic acids, flavonoids and ascorbic acid in four different solvent extracts of two wild edible leaves, Sonchus arvensis and Oenanthe linearis of North-Eastern region in India. J. Appl. Pharm. Sci. 2016, 6, 157–166. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4-3. [Google Scholar] [CrossRef]
- Mediani, A.; Abas, F.; Tan, C.P.; Khatib, A. Effects of different drying methods and storage time on free radical scavenging activity and total phenolic content of Cosmos caudatus. Antioxidants 2014, 3, 358–370. [Google Scholar] [CrossRef]
- Aguirre-Crespo, F.J.; Aragón-Gastélum, J.L.; Gutiérrez-Alcántara, E.J.; Zamora-Crescencio, P.; Gómez-Galicia, D.L.; Alatriste-Kurzel, D.R.; Alvarez, G.; Hernández-Núñez, E. β-Sitosterol Mediates Gastrointestinal Smooth Muscle Relaxation Induced by Coccoloba uvifera via Muscarinic Acetylcholine Receptor Subtype 3. Sci. Pharm. 2024, 92, 19. [Google Scholar] [CrossRef]
- Stratil, P.; Klejdus, B.; Kubáň, V. Determination of total content of phenolic compounds and their antioxidant activity in vegetables evaluation of spectrophotometric methods. J. Agric. Food Chem. 2006, 54, 607–616. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 3rd ed.; Prentice Hall: Upper Anhe, NJ, USA, 1996; p. 662. [Google Scholar]
- Castorena-García, J.H.; Rojas-López, M.; Delgado-Macuil, R.; Robles de la Torre, R.R. Análisis de pulpa y aceite de aguacate con espectroscopia infrarroja. Concienc. Tecnológica 2011, 42, 5–10. [Google Scholar]
- Zhang, J.; Kamdem, D. FTIR Characterization of Copper Ethanolamine—Wood Interaction for Wood Preservation. Holzforschung 2000, 54, 119–122. [Google Scholar] [CrossRef]
- Hu, Y.; Pan, Z.J.; Liao, W.; Li, J.; Gruget, P.; Kitts, D.D.; Lu, X. Determination of antioxidant capacity and phenolic content of chocolate by attenuated total reflectance-Fourier transformed-infrared spectroscopy. Food Chem. 2016, 202, 254–261. [Google Scholar] [CrossRef]
- Ng, S.; Lasekan, O.; Muhammad, K.; Sulaiman, R.; Hussain, N. Effect of roasting conditions on color development and Fourier transform infrared spectroscopy (FTIR-ATR) analysis of Malaysian-grown tropical almond nuts (Terminalia catappa L.). Chem. Cent. J. 2014, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Rivera, W.; Velasco, X.; Rincón, C.A. TGA and FTIR Evaluation of Composition Changes Produced by Roasting of Coffee Beans. Rev. Colomb. Fís. 2013, 45, 205–208. [Google Scholar]
- Movasaghi, Z.; Rehman, S.; ur Rehman, I. Fourier Transform Infrared (FTIR) Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to read and interpret FTIR spectroscope of organic material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Mondragon-Cortez, P. Espectroscopía de Infrarrojo Para Todos…y 51 Espectros de Alimentos Consumidos en México; Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco, AC.: Jalisco, México, 2017; pp. 115–162. Available online: https://ciatej.mx/files/el_ciatej/comunicacion/divulgacion/espectroscopia.pdf (accessed on 19 February 2025).
- Lewis, N.H.; Fleming, G.R. Two-dimensional electronic-vibrational spectroscopy of chlorophyll a, and b. J. Phys. Chem. Lett. 2016, 7, 831–837. [Google Scholar] [CrossRef]
- Singh, A.; Ahmad, S.; Ahmad, A. Green extraction methods and environmental applications of carotenoids-a review. RSC Adv. 2015, 5, 62358–62393. [Google Scholar] [CrossRef]
- Guzansky-Milanezi, F.; Martins-Meireles, L.; Malavazi de Christo-Scherer, M.; de Oliveira, J.P.; Romero da Silva, A.; Lamas de Araujo, M.; Coutinho-Endringer, D.; Fronza, M.; Cunegundes-Guimarães, M.C.; Scherer, R. Antioxidant, antimicrobial and cytotoxic activities of gold nanoparticles capped with quercetin. Saudi Pharm. J. 2019, 27, 968–974. [Google Scholar] [CrossRef]
- Vijayalakshmi, R.; Ravindhran, R. Comparative fingerprint and extraction yield of Diospyrus ferrea (willd.) Bakh. root with phenol compounds (gallic acid), as determined by uv–vis and ft–ir spectroscopy. Asian Pac. J. Trop. Biomed. 2012, 2, S1367–S1371. [Google Scholar] [CrossRef]
- Catauro, M.; Barrino, F.; Dal Poggetto, G.; Crescente, G.; Piccolella, S.; Pacifico, S. New SiO2/caffeic acid hybrid materials: Synthesis, spectroscopic characterization, and bioactivity. Materials 2020, 13, 394. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Crespo, F.J.; Cu-Quiñones, L.D.; Popoca-Cuaya, M.A.; Maldonado-Velázquez, M.G.; Chan-Keb, C.; Graz-Hernández, C.M.; Hernández-Núñez, E. Análisis fitoquímico preliminar y valoración de la actividad antioxidante de tres especies de Mangle de Campeche, México. Quim. Hoy 2022, 10, 1–5. [Google Scholar] [CrossRef]
- Lopes, D.M.S.; Tognella, M.M.P.; Falqueto, A.R.; Soares, M.L.G. Salinity variation effects on photosynthetic responses of the mangrove species Rhizophora mangle L. growing in natural habitats. Photosynthetica 2019, 57, 1142–1155. [Google Scholar] [CrossRef]
- Agraz Hernández, C.M.; Chan Keb, C.A.; Iriarte-Vivar, S.; Posada Venegas, G.; Vega Serratos, B.; Osti Sáenz, J. Phenological variation of Rhizophora mangle and ground water chemistry associated to changes of the precipitation. Hidrobiológica 2015, 25, 49–61. [Google Scholar]
- Agraz-Hernández, C.M.; Chan-Keb, C.A.; Muñiz-Salazar, R.; Pérez-Balan, R.A.; Vanegas, G.P.; Manzanilla, H.G.; Osti-Sáenz, J.; del Río Rodríguez, R. Pore water chemical variability and its effect on phenological production in three mangrove species under drought conditions in southeastern Mexico. Diversity 2022, 14, 668. [Google Scholar] [CrossRef]
- Diaz-Villagómez, D.G.; Hernández-Ramírez, R.E.; Cruz-Cruz, D.; Villegas-Gómez, C. Análisis fitoquímico: Una visión integral de los métodos de extracción de productos naturales. Nat. Tecnol. 2023, 10, 22–35. Available online: http://repositorio.ugto.mx/bitstream/20.500.12059/10156/1/473-1463-1-PB.pdf (accessed on 19 February 2025).
- Grassino, M.; Batstone, D.J.; Yong, K.W.; Capson-Tojo, G.; Hülsen, T. Method development for PPB culture screening, pigment analysis with UPLC-UV-HRMS vs. spectrophotometric methods, and spectral decomposition-based analysis. Talanta 2022, 246, 123490. [Google Scholar] [CrossRef]
- Milenković, S.M.; Zvezdanović, J.B.; Anđelković, T.D.; Marković, D.Z. The identification of chlorophyll and its derivatives in the pigment mixtures: HPLC-chromatography, visible and mass spectroscopy studies. Adv. Technol. 2012, 1, 16–24. [Google Scholar]
- Eckardt, N.A.A. A new chlorophyll degradation pathway. Plant Cell 2009, 21, 700. [Google Scholar] [CrossRef]
- Pelozo, A.; T-Boeger, M.R.; Sereneski-de-Lima, C.; Soffiatti, P. Leaf morphological strategies of seedlings and saplings of Rhizophora mangle (Rhizophoraceae), Laguncularia racemosa (Combretaceae) and Avicennia schaueriana (Acanthaceae) from Southern Brazil. Rev. Biol. Trop. 2016, 64, 305–317. [Google Scholar] [CrossRef][Green Version]
- Erickson, A.A.; Saltis, M.; Bell, S.S.; Dawes, C.J. Herbivore feeding preferences as measured by leaf damage and stomatal ingestion: A mangrove crab example. J. Exp. Mar. Biol. Ecol. 2003, 289, 123–138. [Google Scholar] [CrossRef]
- Sobrado, M. Leaf characteristics and gas exchange of the mangrove Laguncularia racemosa as affected by salinity. Photosynthetica 2005, 43, 217–221. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology; Sinauer Associates: Sindiran, MA, USA, 2010. [Google Scholar]
- Lee, S.J.; Jeong, E.M.; Ki, A.Y.; Oh, K.S.; Kwon, J.; Jeong, J.H.; Chung, N.J. Oxidative defense metabolites induced by salinity stress in roots of Salicornia herbacea. J. Plant Physiol. 2016, 206, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, M.; Miguel, M.; Aleixandre, A. Los polifenoles, compuestos de origen natural con efectos saludables sobre el sistema cardiovascular. Nutr. Hosp. 2012, 27, 76–89. [Google Scholar] [CrossRef]
- Aarland, R.; Castellanos-Hernandez, O.; Rodriguez-Sahagun, A.; Acevedo-Hernandez, G. Efecto del estrés salino sobre la morfología y fitoquímica de orégano mexicano (Lippia graveolens Kunth) cultivado in vitro. Biotecnia 2020, 22, 131–137. [Google Scholar] [CrossRef]
- Mzabri, I.; Legsayer, M.; Kouddane, N.; Boukroute, A.; Berrichi, A. Salt Stress Effects on Some Morphological, Physiological and Biochemical Parameters of Saffron Plant (Crocus sativus L.) in Eastern Morocco. J. Mater. 2017, 8, 4894–4901. Available online: http://www.jmaterenvironsci.com/Document/vol8/vol8_NS/519-JMES-3824-Mzabri.pdf (accessed on 21 June 2024).
- Zhou, Y.; Tang, N.; Huang, L.; Zhao, Y.; Tang, X.; Wang, K. Effects of Salt Stress on Plant Growth, Antioxidant Capacity, Glandular Trichome Density, and Volatile Exudates of Schizonepeta tenuifolia Briq. Int. J. Mol. Sci. 2018, 19, 252. [Google Scholar] [CrossRef]
- Escribano-Bailón, M.T.; Rivas-Gonzalo, J.C.; García-Estévez, I. Wine color evolution and stability. In Red Wine Technology; Academic Press: Cambridge, MA, USA, 2019; pp. 195–205. [Google Scholar] [CrossRef]
- Foti, M.C.; Daquino, C.; Geraci, C. Electron-transfer reaction of cinnamic acids and their methyl esters with the DPPH· radical in alcoholic solutions. J. Org. Chem. 2004, 69, 2309–2314. [Google Scholar] [CrossRef]
- Gülçin, İ. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef]
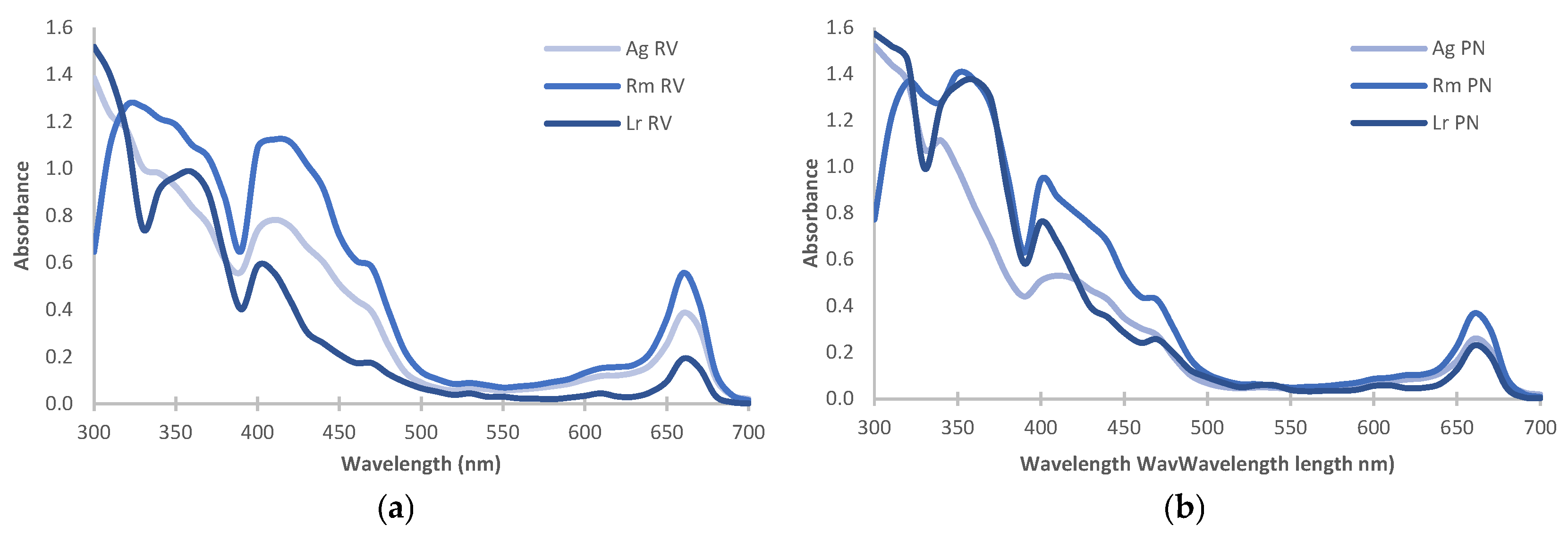
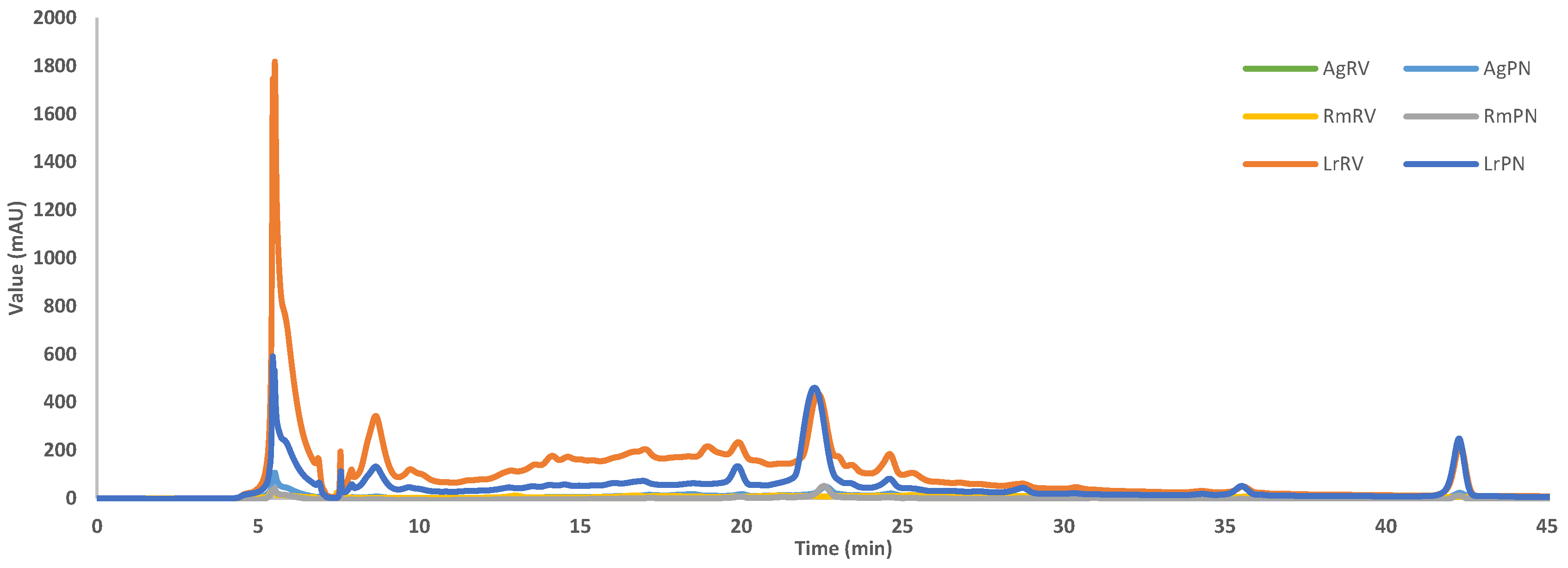
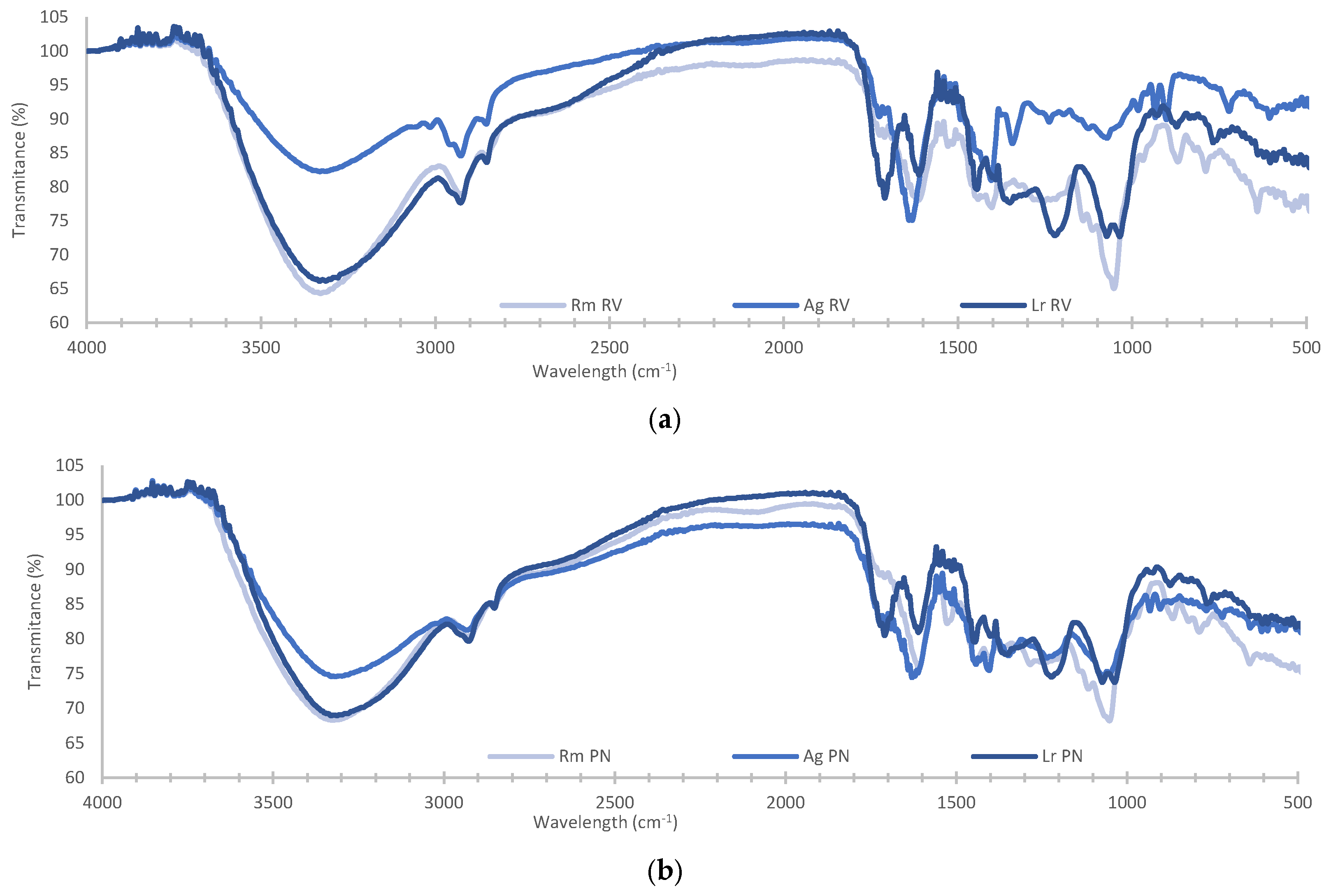
| Species | Site | Salinity (PSU) | Yield (%) | Chlorophyll | Carotenoids (mg/g) | |||
|---|---|---|---|---|---|---|---|---|
| A (mg/g) | B (mg/g) | Ratio a/b | Total (mg/g) | |||||
| Ag | RV | 74.87 ± 5 | 21.98 ± 0.94 | 0.16 ± 0.06 | 0.07 ± 0.03 | 2.3 | 0.23 ± 0.05 | 0.01 ± 0.0030 |
| PN | 58.93 ± 3 | 18.04 ± 2.07 | 0.12 ± 0.03 | 0.05 ± 0.01 | 2.4 | 0.17 ± 0.04 | 0.008 ± 0.0010 | |
| Rm | RV | 42.12 ± 4 | 17.46 ± 0.46 | 0.26 ± 0.02 | 0.10 ± 0.01 | 2.6 | 0.35 ± 0.02 | 0.018 ± 0.0004 |
| PN | 42.25 ± 2 | 21.52 ± 2.22 | 0.17 ± 0.02 | 0.06 ± 0.01 | 2.8 | 0.23 ± 0.03 | 0.013 ± 0.0010 | |
| Lr | RV | 43.25 ± 4 | 35.21 ± 0.76 | 0.09 ± 0.03 | 0.01 ± 0.01 | 9 | 0.10 ± 0.04 | 0.005 ± 0.0040 |
| PN | 40.59 ± 2 | 35.38 ± 0.14 | 0.11 ± 0.04 | 0.02 ± 0.01 | 5.5 | 0.13 ± 0.05 | 0.009 ± 0.0020 | |
| Species | Site | Phenols (mg/g) | Flavonoids (mg/g) | Potency EC50 (μg/mL) | Efficacy Emax (%) |
|---|---|---|---|---|---|
| Ag | RV | 0.42 ± 0.2 | 0.26 ± 0.08 | 157.83 ± 5.44 | 61.44 ± 3.45 |
| PN | 0.63 ± 0.3 | 0.20 ± 0.08 | 194.76 ± 16.98 | 68.75 ± 12.5 | |
| Rm | RV | 0.64 ± 0.2 | 0.14 ± 0.04 | 59.81 ± 5.35 | 74.42 ± 8.95 |
| PN | 0.86 ± 0.3 | 0.23 ± 0.07 | 294.47 ± 14.19 | 53.69 ± 9.17 | |
| Lr | RV | 0.54 ± 0.3 | 0.15 ± 0.02 | 39.74 ± 0.91 | 67.82 ± 1.00 |
| PN | 0.75 ± 0.2 | 0.16 ± 0.13 | 123.47 ± 4.87 | 73.27 ± 4.56 | |
| C. sinensis | - | - | - | 8.69 ± 0.34 * | 92.36 ± 0.87 * |
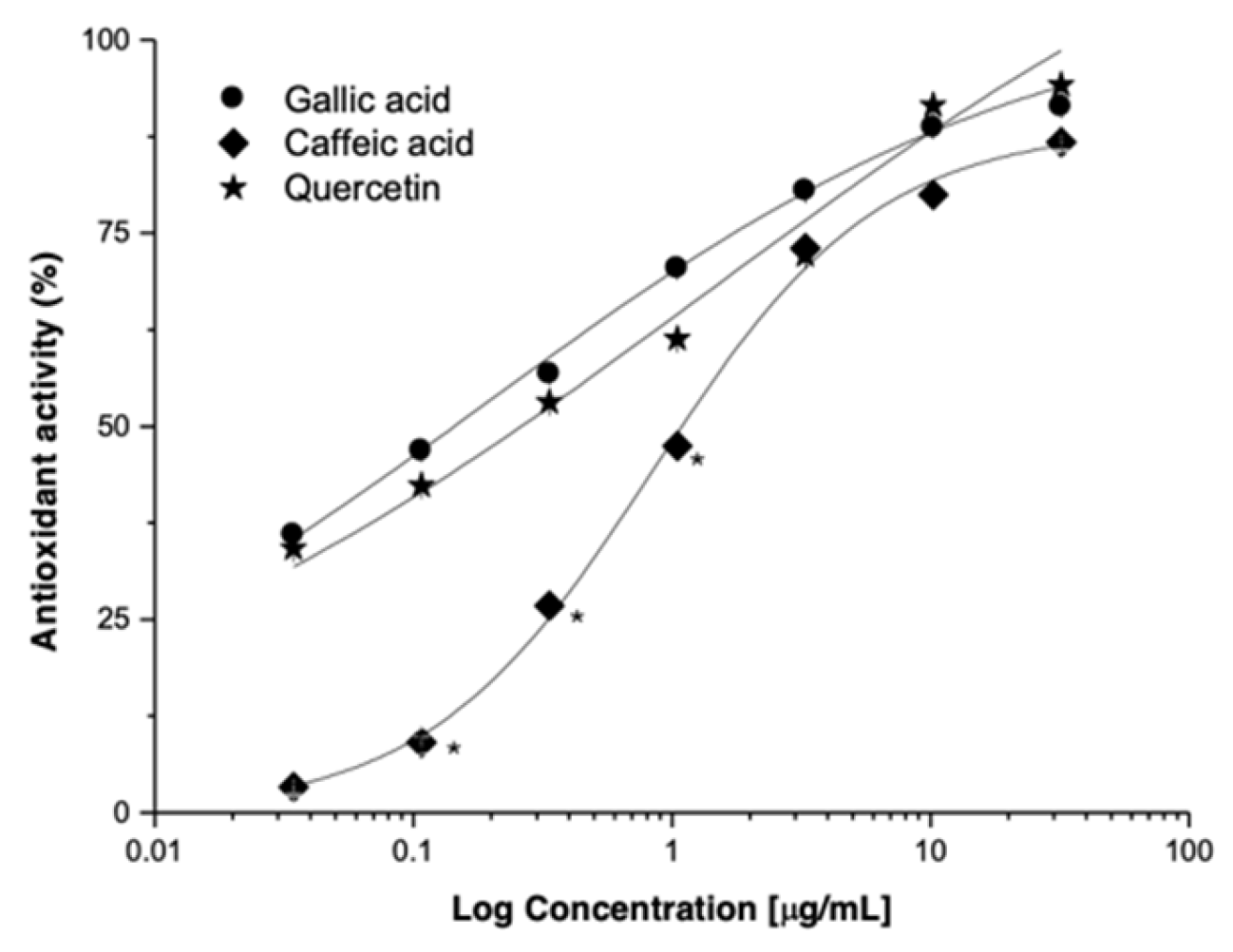
| Activity | Caffeic Acid | Gallic Acid | Quercetin | L. racemosa-RV |
|---|---|---|---|---|
| EC50 (μg/mL) | 1.09 ± 0.06 | 0.22 ± 0.01 | 0.12 ± 0.01 * | 39.74 ± 0.91 |
| Emax (%) | 86.75 ± 0.20 | 91.35 ± 0.14 | 94.15 ± 0.11 * | 67.82 ± 1.00 |
| Relative Potency | −9.08 | −1.83 | 1 | −331.2 |
| Relative Efficacy | 91.1 | 97.0 | 100 | 72.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan-Keb, C.A.; Aragón-Gastélum, J.L.; Agraz-Hernández, C.M.; Pérez-Balan, R.A.; Gutiérrez Alcántara, E.J.; Popoca-Cuaya, M.A.; Guillen-Poot, M.A.; Hernández-Núñez, E.; Aguirre-Crespo, F.J. Salinity as an Inducer of Antioxidant Activity Exerted by Mangrove Species from Campeche, Mexico. Plants 2025, 14, 800. https://doi.org/10.3390/plants14050800
Chan-Keb CA, Aragón-Gastélum JL, Agraz-Hernández CM, Pérez-Balan RA, Gutiérrez Alcántara EJ, Popoca-Cuaya MA, Guillen-Poot MA, Hernández-Núñez E, Aguirre-Crespo FJ. Salinity as an Inducer of Antioxidant Activity Exerted by Mangrove Species from Campeche, Mexico. Plants. 2025; 14(5):800. https://doi.org/10.3390/plants14050800
Chicago/Turabian StyleChan-Keb, Carlos A., José L. Aragón-Gastélum, Claudia M. Agraz-Hernández, Román A. Pérez-Balan, Eduardo J. Gutiérrez Alcántara, Marco A. Popoca-Cuaya, Mónica A. Guillen-Poot, Emanuel Hernández-Núñez, and Francisco J. Aguirre-Crespo. 2025. "Salinity as an Inducer of Antioxidant Activity Exerted by Mangrove Species from Campeche, Mexico" Plants 14, no. 5: 800. https://doi.org/10.3390/plants14050800
APA StyleChan-Keb, C. A., Aragón-Gastélum, J. L., Agraz-Hernández, C. M., Pérez-Balan, R. A., Gutiérrez Alcántara, E. J., Popoca-Cuaya, M. A., Guillen-Poot, M. A., Hernández-Núñez, E., & Aguirre-Crespo, F. J. (2025). Salinity as an Inducer of Antioxidant Activity Exerted by Mangrove Species from Campeche, Mexico. Plants, 14(5), 800. https://doi.org/10.3390/plants14050800











