Effects of Microalgae as Biostimulants on Plant Growth, Content of Antioxidant Molecules and Total Antioxidant Capacity in Chenopodium quinoa Exposed to Salt Stress
Abstract
1. Introduction
2. Results
2.1. Effects of Microalgae on Germination and Seedlings Length in Quinoa Exposed to Salt Stress
2.2. Effects of Microalgae on Total Phenolics and Total Flavonoids Content in Quinoa Sprouts Exposed to Salt Stress
2.3. Effects of Microalgae on Antioxidant Activity and ROS Content in Quinoa Sprouts Exposed to Salt Stress
2.4. Effects of Microalgae on Pigments Content in Quinoa Sprouts Exposed to Salt Stress
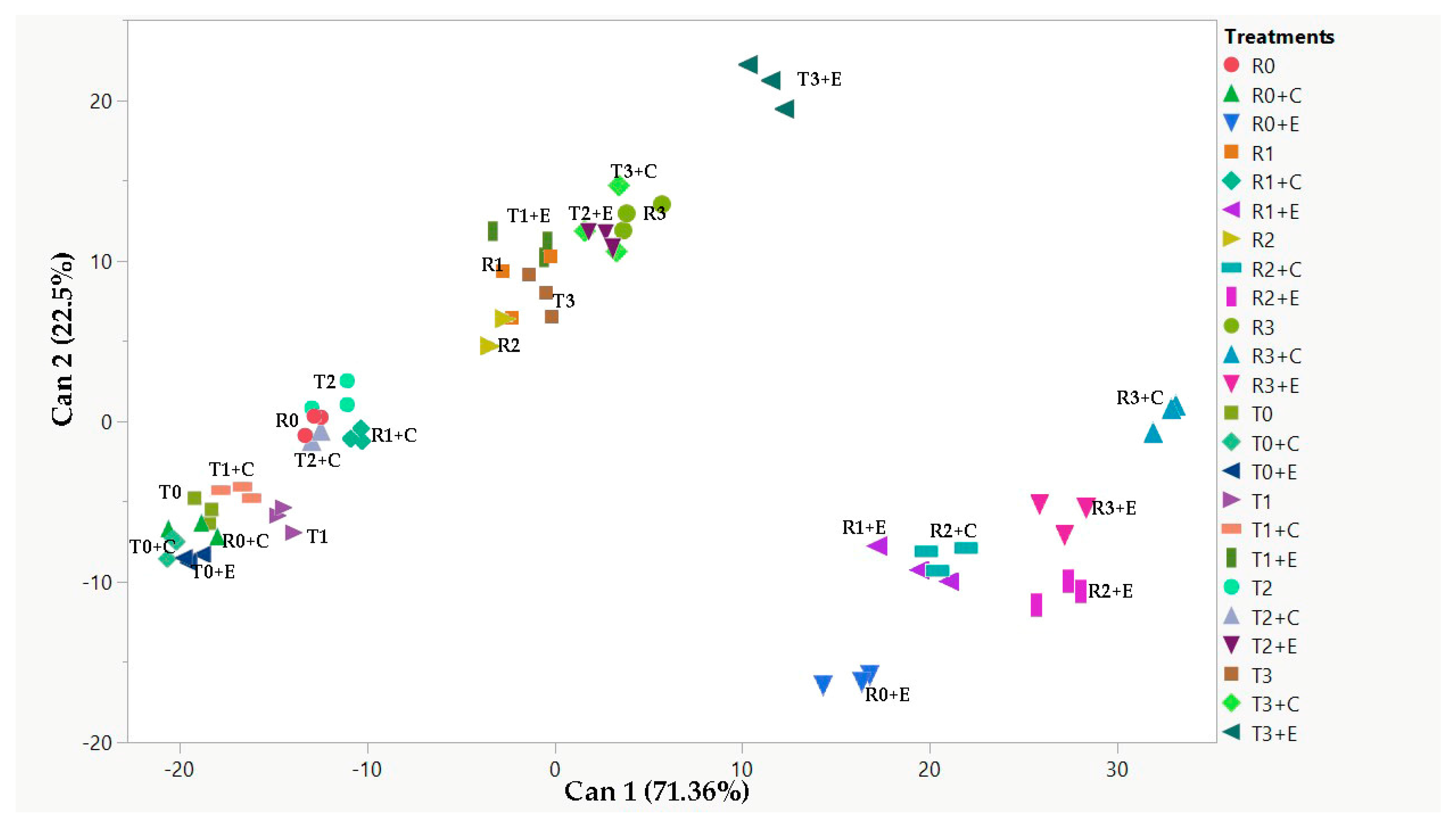
2.5. Canonical Discriminant Analysis (CDA) and Pearson’s Correlation
3. Discussion
4. Materials and Methods
4.1. Plant Material and Treatments
4.2. Preparation of Quinoa Extracts
4.3. Determination of Total Phenolics Content
4.4. Determination of Total Flavonoid Content
4.5. DPPH Assay
4.6. FRAP Assay
4.7. Quantification of ROS (Reactive Oxygen Species)
4.8. HPLC (High Performance Liquid Chromatography)
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Souid, A.; Giorgetti, L.; Smaoui, A.; Abdelly, C.; Magné, C.; Ben Hamed, K.; Longo, V.; Bellani, L. Germination and antioxidant responses to salt stress of Tunisian. Acta Physiol. Plant. 2024, 46, 7. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of Plant Responses to Salt Stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef]
- Zhou, H.; Shi, H.; Yang, Y.; Feng, X.; Chen, X.; Xiao, F.; Lin, H.; Guo, Y. Insights into plant salt stress signaling and tolerance. J. Genet. Genom. 2024, 51, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Prisa, D.; Spagnuolo, D. Plant Production with Microalgal Biostimulants. Horticulturae 2023, 9, 829. [Google Scholar] [CrossRef]
- Bhupenchandra, I.; Chongtham, S.; Devi, E.; Ramesh, R.; Choudhary, A.; Salam, M.; Sahoo, M.; Bhutia, T.; Devi, S.; Thounaojam, A.; et al. Role of biostimulants in mitigating the effects of climate change on crop performance. Front. Plant Sci. 2022, 13, 967665. [Google Scholar] [CrossRef] [PubMed]
- El-Baky, H.H.A.; El Baz, F.K.; El-Baroty, G.S. Production of phenolic compounds from Spirulina maxima microalgae and its protective effects. Afr. J. Biotechnol. 2009, 8, 7059–7067. [Google Scholar]
- Chabili, A.; Minaoui, F.; Hakkoum, Z.; Douma, M.; Meddich, A.; Loudiki, M.A. Comprehensive Review of Microalgae and Cyanobacteria-Based Biostimulants for Agriculture Use. Plants 2024, 13, 159. [Google Scholar] [CrossRef] [PubMed]
- Nezamdoost, D.; Chahremani, Z.; Ranjbar, M.E. Seed priming with Chlorella vulgaris extract increases salt tolerance threshold of leafy lettuce more than two times based on germination indices (a modeling approach). J. Appl. Phycol. 2024, 36, 1247–1258. [Google Scholar] [CrossRef]
- Vornoli, A.; Grande, T.; Lubrano, V.; Vizzarri, F.; Gorelli, C.; Raffaelli, A.; Della Croce, M.C.; Zarate Baca, S.; Sandoval, C.; Longo, V.; et al. In Vitro Characterization of Antioxidant, Antibacterial and Antimutagenic Activities of the Green Microalga Ettlia pseudoalveolaris. Antioxidants 2023, 12, 1308. [Google Scholar] [CrossRef]
- Jan, N.; Hussain, S.Z.; Naseer, B.; Bhat, T.A. Amaranth and quinoa as potential nutraceuticals: A review of anti-nutritional factors, health benefits and their applications in food, medicinal and cosmetic sectors. Food Chem. 2023, 18, 100687. [Google Scholar] [CrossRef]
- Ranjan, S.; Sow, S.; Ghosh, M.; Padhan, S.R.; Kumar, S.; Gitari, H.; Mirriam, A.; Nath, D. Nutraceutical properties and secondary metabolites of quinoa (Chenopodium quinoa Willd.): A review. Int. J. Food Prop. 2023, 26, 3477–3491. [Google Scholar] [CrossRef]
- Pathan, S.; Siddiqui, R.A. Nutritional Composition and Bioactive Components in Quinoa (Chenopodium quinoa Willd.) Greens: A Review. Nutrients 2022, 14, 558. [Google Scholar] [CrossRef]
- Enciso-Roca, E.C.; Aguilar-Felices, E.J.; Tinco-Jayo, J.A.; Arroyo-Acevedo, J.L.; Herrera-Calderon, O. Biomolecules with Antioxidant Capacity from the Seeds and Sprouts of 20 Varieties of Chenopodium quinoa Willd. (Quinoa). Plants 2021, 10, 2417. [Google Scholar] [CrossRef] [PubMed]
- Siemińska-Kuczer, A.; Szymańska-Chargot, M.; Zdunek, A. Recent advances in interactions between polyphenols and plant cell wall polysaccharides as studied using an adsorption technique. Food Chem. 2022, 373, 131487. [Google Scholar] [CrossRef] [PubMed]
- Melini, V.; Melini, F. Functional Components and Anti-Nutritional Factors in Gluten-Free Grains: A Focus on Quinoa Seeds. Foods 2020, 10, 351. [Google Scholar] [CrossRef]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef]
- Angeli, V.; Miguel Silva, P.; Crispim Massuela, D.; Khan, M.W.; Hamar, A.; Khajehei, F.; Graeff-Hönninger, S.; Piatti, C. Quinoa (Chenopodium quinoa Willd.): An Overview of the Potentials of the “Golden Grain” and Socio-Economic and Environmental Aspects of Its Cultivation and Marketization. Foods 2020, 9, 216. [Google Scholar] [CrossRef]
- Noulas, C.; Vlachostergios, D.N.; Tziouvalekas, M.; Baxevanos, D. Adaptation, Agronomic Potential, and Current Perspectives of Quinoa Under Mediterranean Conditions: Case Studies from the Lowlands of Central Greece. Commun. Soil Sci. Plant Anal. 2017, 48, 2612–2629. [Google Scholar]
- Hirich, A.; Choukr-Allah, R.; Jacobsen, S.E.; Benlhabib, O. Could Quinoa be an alternative crop of wheat in the Mediterranean region: Case of Morocco? Les Notes D’alerte CIHEAM 2012, 86, 1–8. [Google Scholar]
- Souid, A.; Bellani, L.; Lanfranca Tassi, E.; Hamed, K.B.; Longo, V.; Giorgetti, L. Early Physiological, Cytological and Antioxidative Responses of the Edible Halophyte Chenopodium quinoa exposed to Salt Stress. Antioxidants 2023, 12, 1060. [Google Scholar] [CrossRef]
- Hinojosa, L.; Luguizamo, A.; Carpio, C.; Muñoz, D.; Mestanza, C.; Ochoa, J.; Castillo, C.; Murillo, A.; Villacréz, E.; Monar, C.; et al. Quinoa in Ecuador: Recent Advances under Global Expansion. Plants 2021, 10, 298. [Google Scholar] [CrossRef] [PubMed]
- Chiaiese, P.; Corrado, G.; Colla, G.; Kyriacou, M.C.; Rouphael, Y. Renewable Sources of Plant Biostimulation: Microalgae as a Sustainable Means to Improve Crop Performance. Front. Plant Sci. 2018, 9, 1782. [Google Scholar] [CrossRef] [PubMed]
- Lavini, A.; Pulvento, C.; D’Andria, R.; Riccardi, M.; Choukr-Allah, R.; Belhabib, O.; Yazar, A.; İncekaya, Ç.; Metin Sezen, S.; Qadir, M.; et al. Quinoa’s Potential in the Mediterranean Region. J. Agron. Crop Sci. 2014, 200, 344–360. [Google Scholar] [CrossRef]
- Parmar, P.; Kumar, R.; Neha, Y.; Srivatsan, V. Microalgae as next generation plant growth additives: Functions, applications, challenges and circular bioeconomy-based solutions. Front. Plant Sci. 2023, 14, 1073546. [Google Scholar] [CrossRef]
- Ma, C.; Cui, H.; Ren, C.; Yang, J.; Liu, Z.; Tang, T.; Ji, C.; Zhang, C.; Xue, J.; Li, R. The seed primer and biofertilizer performances of living Chlorella pyrenoidosa on Chenopodium quinoa under saline-alkali condition. J. Appl. Phycol. 2022, 34, 1621–1634. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- D’Amato, R.; Del Buono, D. Use of a Biostimulant to Mitigate Salt Stress in Maize Plants. Agronomy 2021, 11, 1755. [Google Scholar] [CrossRef]
- Minaoui, F.; Hakkoum, Z.; Chabili, A.; Douma, M.; Mouhri, K.; Loudiki, M. Biostimulant effect of green soil microalgae Chlorella vulgaris suspensions on germination and growth of wheat (Triticum aestivum var. Achtar) and soil fertility. Algal Res. 2024, 82, 103655. [Google Scholar] [CrossRef]
- Mendes, A.R.; Spínola, M.P.; Lordelo, M.; Prates, J.A.M. Chemical Compounds, Bioactivities, and Applications of Chlorella vulgaris in Food, Feed and Medicine. Appl. Sci. 2024, 14, 10810. [Google Scholar] [CrossRef]
- Gitau, M.M.; Farkas, A.; Balla, B.; Ördög, V.; Futó, Z.; Maróti, G. Strain-Specific Biostimulant Effects of Chlorella and Chlamydomonas Green Microalgae on Medicago truncatula. Plants 2021, 10, 1060. [Google Scholar] [CrossRef]
- Gitau, M.M.; Farkas, A.; Ördög, V.; Maróti, G. Evaluation of the biostimulant effects of two Chlorophyta microalgae on tomato (Solanum lycopersicum). J. Clean. Prod. 2022, 364, 132689. [Google Scholar] [CrossRef]
- Dehghanian, Z.; Habibi, K.; Dehghanian, M.; Aliyar, S.; Lajayer, B.A.; Astatkie, T.; Minkina, T.; Keswani, C. Reinforcing the bulwark: Unravelling the efficient applications of plant phenolics and tannins against environmental stresses. Heliyon 2022, 8, e09094. [Google Scholar] [CrossRef]
- Zeeshan, M.; Lu, M.; Sehar, S.; Holford, P.; Wu, F. Comparison of biochemical, anatomical, morphological, and physiological responses to salinity stress in wheat and barley genotypes deferring in salinity tolerance. Agronomy 2020, 10, 127. [Google Scholar] [CrossRef]
- Naz, T.; Alihtar, J.; Iqbal, M.M.; Anwar-ul-Haq, M.; Murtaza, G.; Niazi, N.K.; Farooq, O.; Ali, M.; Dell, B. Assessment of gas exchange attributes, chlorophyll contents, ionic composition and antioxidant enzymes of bread wheat genotypes in boron toxic, saline and boron toxic-saline soils. J. Appl. Phycol. 2019, 21, 1271–1281. [Google Scholar]
- Hosseini, S.J.; Tahmasebi-Sarvestani, Z.; Pirdashti, H.; Modarres-Sanavy, S.A.M.; Mokhtassi-Bidgoli, A.; Hazrati, S.; Nicola, S. Investigation of yield, phytochemical composition, and photosynthetic pigments in different mint ecotypes under salinity stress. Food Sci. Nutr. 2021, 9, 2620–2643. [Google Scholar] [CrossRef]
- Toscano, S.; Romano, D.; Patanè, C. Effect of application of biostimulants on the biomass, nitrate, pigments and antioxidant content in radish and turnip microgreens. Agronomy 2023, 13, 145. [Google Scholar] [CrossRef]
- Kusvuran, S. Microalgae (Chlorella vulgaris Beijerinck) alleviates drought stress of broccoli plants by improving nutrient uptake, secondary metabolites, and antioxidative defense system. Hortic. Plant J. 2021, 7, 221–231. [Google Scholar] [CrossRef]
- Moon, J.; Park, Y.J.; Choi, Y.B.; Truong, T.Q.; Huynh, P.K.; Kim, Y.B.; Kim, S.M. Physiological Effects and Mechanisms of Chlorella vulgaris as a Biostimulant on the Growth and Drought Tolerance of Arabidopsis thaliana. Plants 2024, 13, 3012. [Google Scholar] [CrossRef]
- Dehghanian, Z.; Ahmadabadi, M.; Asgari Lajayer, B.; Gougerdchi, V.; Hamedpour-Darabi, M.; Bagheri, N.; Sharma, R.; Vetukuri, R.R.; Astatkie, T.; Dell, B. Quinoa: A Promising Crop for Resolving the Bottleneck of Cultivation in Soils Affected by Multiple Environmental Abiotic Stresses. Plants 2024, 13, 2117. [Google Scholar] [CrossRef]
- Mareri, L.; Parrotta, L.; Cai, G. Environmental Stress and Plants. Int. J. Mol. Sci. 2022, 23, 5416. [Google Scholar] [CrossRef]
- Lahlali, R.; Taoussi, M.; Laasli, S.-E.; Gachara, G.; Ezzouggari, R.; Belabess, Z.; Aberkani, K.; Assouguem, A.; Meddich, A.; El Jarroudi, M.; et al. Effects of climate change on plant pathogens and host-pathogen interactions. Crop Environ. 2024, 3, 159–170. [Google Scholar] [CrossRef]
- Sosa-Zuniga, V.; Brito, V.; Fuentes, F.; Steinfort, U. Phenological growth stages of quinoa (Chenopodium quinoa) based on the BBCH scale. Ann. Appl. Biol. 2017, 171, 117–124. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Heimler, D.; Vignolini, P.; Dini, M.G.; Romani, A. Rapid tests to assess the antioxidant activity of Phaseolus vulgaris L. dry beans. J. Agric. Food Chem. 2005, 53, 3053–3056. [Google Scholar] [CrossRef]
- Boudjou, S.; Oomah, B.D.; Zaidi, F.; Hosseinian, F. Phenolics content and antioxidant and anti-inflammatory activities of legume fractions. Food Chem. 2013, 138, 1543–1550. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar]
- Bellani, L.M.; Salvini, L.; Dell’Aquila, A.; Scialabba, A. Reactive oxygen species release, vitamin E, Fatty acid and phytosterol contents of artificially aged radish (Raphanus sativus L.) seeds during germination. Acta Physiol. Plant. 2012, 34, 1789–1799. [Google Scholar] [CrossRef]
- Santin, M.; Sciampagna, M.C.; Mannucci, A.; Puccinelli, M.; Angelini, L.G.; Tavarini, S.; Accorsi, M.; Incrocci, L.; Ranieri, A.; Castagna, A. Supplemental UV-B Exposure Influences the Biomass and the Content of bioactive Compounds in Linum usitatissimum L. Sprouts and Microgreens. Horticulturae 2022, 8, 213. [Google Scholar] [CrossRef]



| NaCl | Lutein | β-Carotene | Chlorophyll a | Chlorophyll b | |
|---|---|---|---|---|---|
| 0 mM | Control | 121.93 ± 4.17 d | 16.42 ± 0.97 f | 196.64 ± 22.33 e | 143.77 ± 0.93 e |
| Chlorella | 64.19 ± 8.5 e | 0 g | 94.01 ± 13.9 f | 66.99 ± 9.75 f | |
| Ettlia | 71.38 ± 3.36 e | 0 g | 119.19 ± 0.93 ef | 75.5 ± 0.79 f | |
| 100 mM | Control | 320.39 ± 1.6 b | 49.61 ± 2 c | 544.53 ± 15.65 c | 381.8 ± 16.21 b |
| Chlorella | 151.43 ± 4.29 cd | 23.09 ± 0.76 e | 318.7 ± 2.94 d | 187.2 ± 3.15 de | |
| Ettlia | 189.75 ± 10.15 c | 28.93 ± 1.04 d | 338.33 ± 13.68 d | 201.32 ± 11.65 d | |
| 200 mM | Control | 470.43 ± 5.46 a | 78.81 ± 0.82 a | 930.51 ± 28.2 a | 552.08 ± 10.09 a |
| Chlorella | 341.03 ± 16.84 b | 58.39 ± 2.07 b | 690.88 ± 33.19 b | 387.21 ± 20.43 b | |
| Ettlia | 313.53 ± 17.01 b | 50.84 ± 2.09 c | 568.94 ± 33.84 c | 310.97 ± 18.52 c | |
| ANOVA | |||||
| Microalgae (A) | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | |
| NaCl (B) | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | |
| A × B | p = 0.0002 | p = 0.0002 | p < 0.0001 | p < 0.0001 |
| NaCl | Lutein | β-Carotene | Chlorophyll a | Chlorophyll b | |
|---|---|---|---|---|---|
| 0 mM | Control | 389.44 ± 0.83 d | 63.85 ± 1.96 c | 889.76 ± 1.35 d | 479.57 ± 6.57 e |
| Chlorella | 265.31 ± 11.52 e | 36.57 ± 0.3 d | 593.16 ± 34.5 e | 363.73 ± 16.96 cd | |
| Ettlia | 253.49 ± 31.98 e | 25.58 ± 1.64 d | 494.47 ± 115.43 e | 282.06 ± 58.55 f | |
| 100 mM | Control | 687.81 ± 35.7 b | 118.3 ± 2.1 a | 1693.59 ± 78.4 a | 910.56 ± 43.35 a |
| Chlorella | 514.22 ± 28.98 c | 78.63 ± 5.18 c | 1186.23 ± 8.81 c | 675.39 ± 8.54 cd | |
| Ettlia | 502.39 ± 21.52 c | 86.41 ± 1.61 bc | 1179.25 ± 58.18 c | 607.65 ± 28.62 d | |
| 200 mM | Control | 792.03 ± 16.8 a | 122 ± 1.61 a | 1685.9 ± 31.38 a | 827.92 ± 17.92 ab |
| Chlorella | 752.23 ± 15.46 ab | 121.03 ± 0.49 a | 1598.41 ± 5.5 ab | 786.75 ± 2.96 bc | |
| Ettlia | 716.89 ± 30.5 ab | 105.77 ± 2.87 ab | 1439.16 ± 63.05 b | 725.74 ± 34.02 bc | |
| ANOVA | |||||
| Microalgae (A) | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | |
| NaCl (B) | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | |
| A × B | p = 0.0247 | p = 0.0106 | p = 0.0078 | p = 0.0063 |
| Pearson’s Coefficient | ||
|---|---|---|
| Can 1 | Can 2 | |
| Seedling length | −0.53 * | −0.21 |
| Germination % | 0.15 | −0.10 |
| Total phenolics | −0.14 | −0.13 |
| Total flavonoids | 0.45 | 0.46 |
| DPPH | 0.75 * | −0.35 |
| FRAP | 0.67 * | 0.56 * |
| ROS | −0.61 * | −0.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiorentino, S.; Bellani, L.; Santin, M.; Castagna, A.; Echeverria, M.C.; Giorgetti, L. Effects of Microalgae as Biostimulants on Plant Growth, Content of Antioxidant Molecules and Total Antioxidant Capacity in Chenopodium quinoa Exposed to Salt Stress. Plants 2025, 14, 781. https://doi.org/10.3390/plants14050781
Fiorentino S, Bellani L, Santin M, Castagna A, Echeverria MC, Giorgetti L. Effects of Microalgae as Biostimulants on Plant Growth, Content of Antioxidant Molecules and Total Antioxidant Capacity in Chenopodium quinoa Exposed to Salt Stress. Plants. 2025; 14(5):781. https://doi.org/10.3390/plants14050781
Chicago/Turabian StyleFiorentino, Sofia, Lorenza Bellani, Marco Santin, Antonella Castagna, Maria Cristina Echeverria, and Lucia Giorgetti. 2025. "Effects of Microalgae as Biostimulants on Plant Growth, Content of Antioxidant Molecules and Total Antioxidant Capacity in Chenopodium quinoa Exposed to Salt Stress" Plants 14, no. 5: 781. https://doi.org/10.3390/plants14050781
APA StyleFiorentino, S., Bellani, L., Santin, M., Castagna, A., Echeverria, M. C., & Giorgetti, L. (2025). Effects of Microalgae as Biostimulants on Plant Growth, Content of Antioxidant Molecules and Total Antioxidant Capacity in Chenopodium quinoa Exposed to Salt Stress. Plants, 14(5), 781. https://doi.org/10.3390/plants14050781






