Phytochemical Study, Cytotoxicity, and Genotoxicity of the Methanolic Extract of Geranium diffusum Kunth
Abstract
1. Introduction
2. Results and Discussion
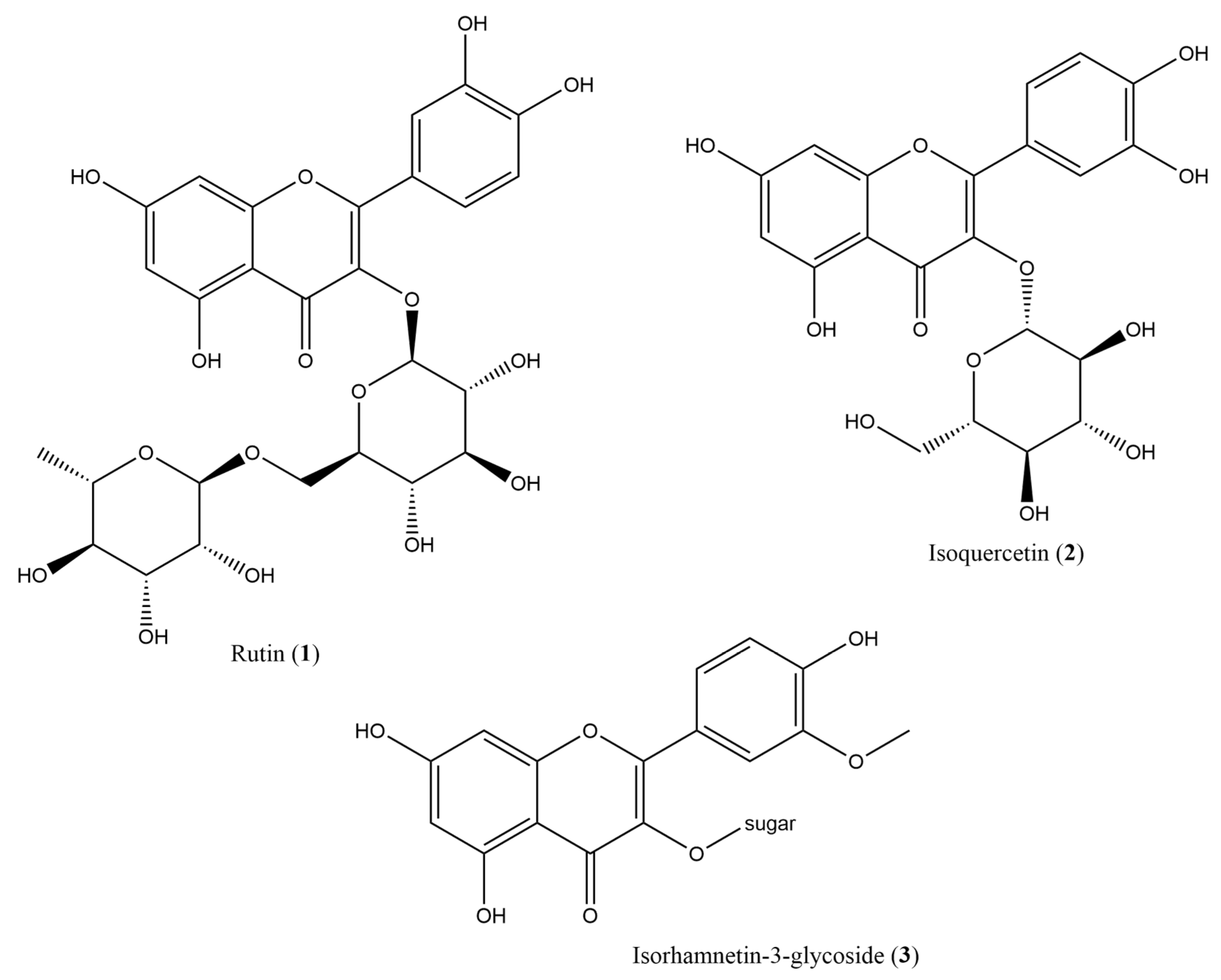
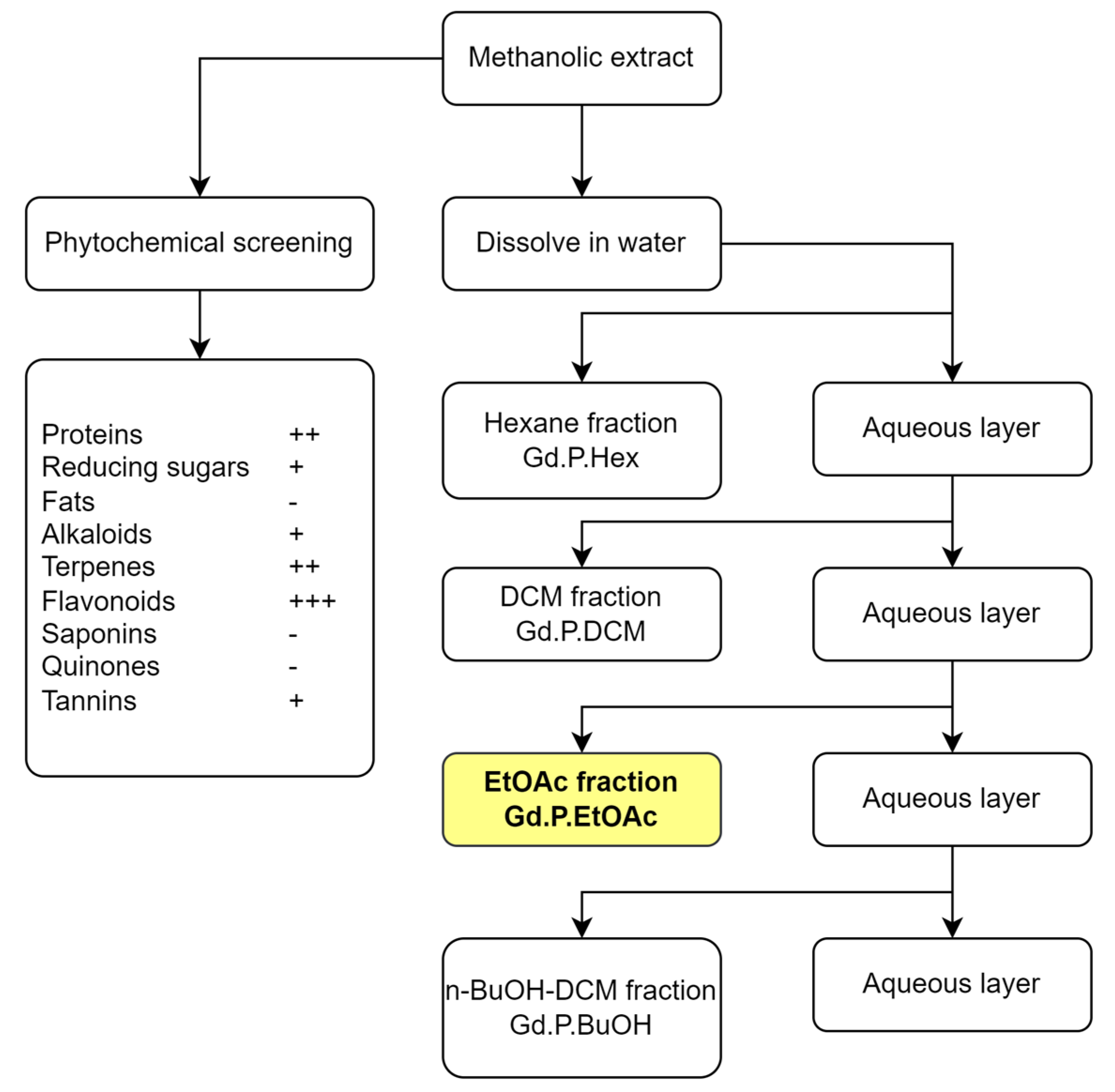
2.1. Phytochemical Composition
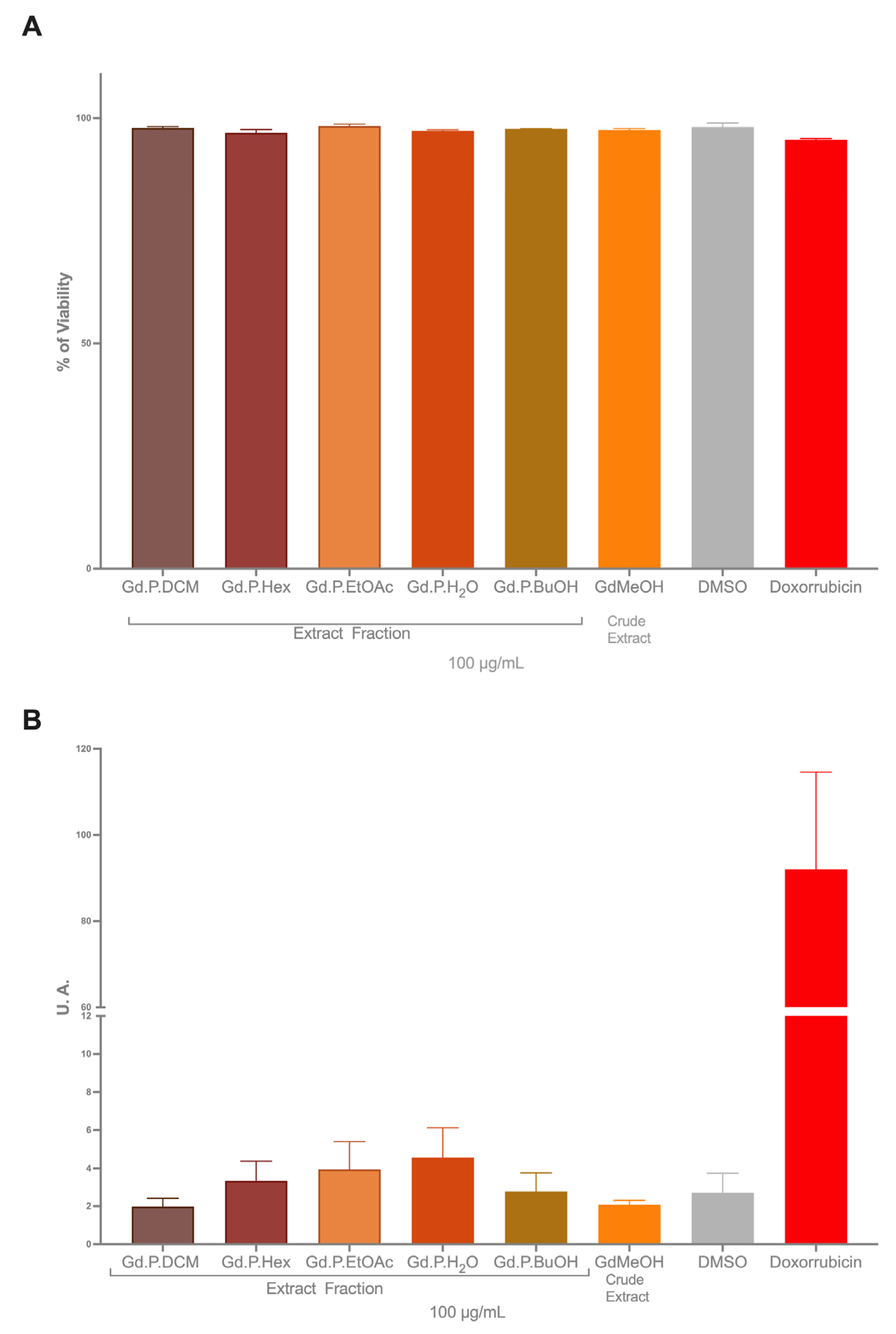
2.2. Citotoxicy on Colon Cancer Cells
2.3. Genotoxicity
3. Materials and Methods
3.1. Chemicals, Reagents, and Cell Lines
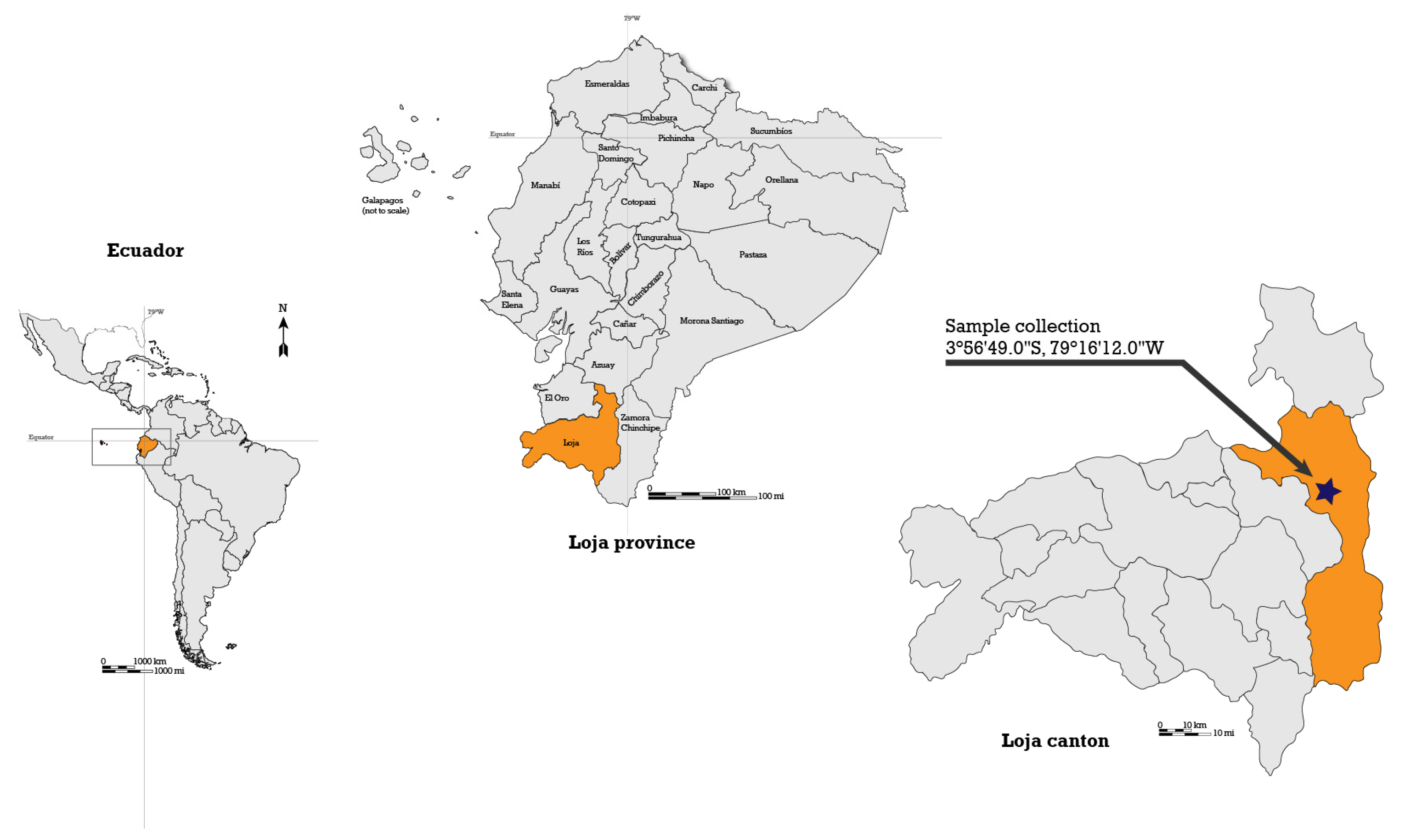
3.2. Plant Collection and Extracts
3.3. Phytochemical Screening
3.4. Fractioning of the Extract
3.5. Secondary Metabolite Isolation and Identification
3.6. Cell Viability
3.6.1. Cell Culture
3.6.2. Cell Viability Through MTS Testing
3.6.3. Inhibitory Concentration
3.6.4. Morphological Analysis
3.7. Genotoxicity
3.7.1. Cell Culture
3.7.2. Treatment and Viability FDA-Ethidium Bromide
3.7.3. Comet Assay
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shukla, S.S.; Jain, S.K.; Kalyani, G.; Gidwani, B.; Pandey, R.K.; Pandey, R.; Vyas, A. Zoopharmacognosy (Plant-Animal Interaction). In Evidence Based Validation of Traditional Medicines: A Comprehensive Approach; Mandal, S.C., Chakraborty, R., Sen, S., Eds.; Springer: Singapore, 2021; pp. 727–741. ISBN 978-981-15-8127-4. [Google Scholar]
- Salmerón-Manzano, E.; Garrido-Cardenas, J.A.; Manzano-Agugliaro, F. Worldwide Research Trends on Medicinal Plants. Int. J. Environ. Res. Public Health 2020, 17, 3376. [Google Scholar] [CrossRef]
- WHO. General Guidelines for Methodologies on Research and Evaluation of Traditional Medicine; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- VanPool, C. Ancient Medicinal Plants of South America. Proc. Natl. Acad. Sci. USA 2019, 116, 11087–11089. [Google Scholar] [CrossRef] [PubMed]
- Shahrajabian, M.H.; Cheng, Q.; Sun, W. The Importance of Neglected and Underutilized Medicinal Plants from South America in Modern Pharmaceutical Sciences. Lett. Drug Des. Discov. 2023, 20, 1688–1706. [Google Scholar] [CrossRef]
- Bailon-Moscoso, N.; Romero-Benavides, J.C.; Tinitana-Imaicela, F.; Ostrosky-Wegman, P. Medicinal Plants of Ecuador: A Review of Plants with Anticancer Potential and Their Chemical Composition. Med. Chem. Res. 2015, 24, 2283–2296. [Google Scholar] [CrossRef]
- Armijos, C.; Ramírez, J.; Vidari, G. Poorly Investigated Ecuadorian Medicinal Plants. Plants 2022, 11, 1590. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Turismo Ecuador y sus Cuatro Mundos, a Disposición de Todos los Ecuatorianos en el Feriado de Año Nuevo—Ministerio de Turismo. Available online: https://www.turismo.gob.ec/ecuador-y-sus-cuatro-mundos-a-disposicion-de-todos-los-ecuatorianos-en-el-feriado-de-ano-nuevo/ (accessed on 27 August 2019).
- Ministerio del Ambiente del Ecuador Estrategia Nacional de Biodiversidad 2015–2030 2016. Available online: http://maetransparente.ambiente.gob.ec/documentacion/WebAPs/Estrategia%20Nacional%20de%20Biodiversidad%202015-2030%20-%20CALIDAD%20WEB.pdf (accessed on 17 December 2024).
- Tinitana, F.; Rios, M.; Romero-Benavides, J.C.; de la Cruz Rot, M.; Pardo-de-Santayana, M. Medicinal Plants Sold at Traditional Markets in Southern Ecuador. J. Ethnobiol. Ethnomed. 2016, 12, 29. [Google Scholar] [CrossRef]
- Armijos, C.; Ramírez, J.; Salinas, M.; Vidari, G.; Suárez, A.I. Pharmacology and Phytochemistry of Ecuadorian Medicinal Plants: An Update and Perspectives. Pharmaceuticals 2021, 14, 1145. [Google Scholar] [CrossRef]
- Duarte-Casar, R.; González-Jaramillo, N.; Bailon-Moscoso, N.; Rojas-Le-Fort, M.; Romero-Benavides, J.C. Five Underutilized Ecuadorian Fruits and Their Bioactive Potential as Functional Foods and in Metabolic Syndrome: A Review. Molecules 2024, 29, 2904. [Google Scholar] [CrossRef]
- Aguirre-Rodríguez, A.; Duarte-Casar, R.; Rojas-Le-Fort, M.; Romero-Benavides, J.C. Food Uses, Functional Activities, and Bioactive Compounds of Three Ecuadorian Vasconcellea Fruits: Bibliometric Analysis and Review. J. Agric. Food Res. 2024, 17, 101244. [Google Scholar] [CrossRef]
- Duarte-Casar, R. Antimicrobial Activity and Potential of the Ingredients of Horchata—A Traditional Southern Ecuadorian Highlands Herbal Drink. In Antimicrobials for Sustainable Food Storage; Maddela, N.R., Campos García, G.A., Jaskiran, K., Eds.; CRC Press: Boca Raton, FL, USA, 2024; ISBN 978-1-032-26485-1. [Google Scholar]
- Armijos, C.; Valarezo, E.; Cartuche, L.; Zaragoza, T.; Finzi, P.V.; Mellerio, G.G.; Vidari, G. Chemical Composition and Antimicrobial Activity of Myrcianthes Fragrans Essential Oil, a Natural Aromatizer of the Traditional Ecuadorian Beverage Colada Morada. J. Ethnopharmacol. 2018, 225, 319–326. [Google Scholar] [CrossRef]
- Andrade, J.M.; Lucero Mosquera, H.; Armijos, C. Ethnobotany of Indigenous Saraguros: Medicinal Plants Used by Community Healers “Hampiyachakkuna” in the San Lucas Parish, Southern Ecuador. Biomed. Res. Int. 2017, 2017, 9343724. [Google Scholar] [CrossRef] [PubMed]
- Ogburn, D.E. Becoming Saraguro: Ethnogenesis in the Context of Inca and Spanish Colonialism. Ethnohistory 2008, 55, 287–319. [Google Scholar] [CrossRef]
- Bussmann, R.W.; Sharon, D. Traditional Medicinal Plant Use in Loja Province, Southern Ecuador. J. Ethnobiol. Ethnomed. 2006, 2, 44. [Google Scholar] [CrossRef] [PubMed]
- Armijos, C.; Cota, I.; González, S. Traditional Medicine Applied by the Saraguro Yachakkuna: A Preliminary Approach to the Use of Sacred and Psychoactive Plant Species in the Southern Region of Ecuador. J. Ethnobiol. Ethnomed. 2014, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Sucholas, J.; Greinwald, A.; Ukhanova, M.; Luick, R. Achieving Health Sovereignty with Medicinal Plants on an Agroecological Farm—From Theory to Practice. In Medicinal Agroecology; CRC Press: New York, NY, USA, 2023; pp. 17–41. ISBN 978-1-003-14690-2. [Google Scholar]
- Marcussen, T.; Meseguer, A.S. Species-Level Phylogeny, Fruit Evolution and Diversification History of Geranium (Geraniaceae). Mol. Phylogenetics Evol. 2017, 110, 134–149. [Google Scholar] [CrossRef]
- Aedo, C. Geranium pseudodiffusum (Geraniaceae), a New Species from Ecuador and Peru. Syst. Bot. 2010, 35, 168–171. [Google Scholar] [CrossRef]
- Aedo, C.; Pando, F. A Distribution and Taxonomic Reference Dataset of Geranium in the New World. Sci. Data 2017, 4, 170049. [Google Scholar] [CrossRef]
- Léon-Yánez, S.; Valencia, R.; Pitman, N. (Eds.) Libro rojo de las Plantas Endémicas del Ecuador, 2nd ed.; Pontificia Universidad Católica del Ecuador: Quito, Ecuador, 2011; ISBN 978-9942-03-393-2. [Google Scholar]
- de la Torre, L.; Navarrete, H.; Muriel, P.; Macía, M.; Balslev, H. Enciclopedia de Las Plantas Útiles Del Ecuador; Herbario QCA de la Escuela de Ciencias Biológicas de la Pontificia Universidad Católica del Ecuador: Quito, Ecuador, 2008; ISBN 978-9978-77-135-8. [Google Scholar]
- Ansaloni, R.; Izco, J.; Amigo, J.; Minga, D. Analysis of the Vascular Flora in the Cajas National Park (Central Andes, Ecuador). Mediterr. Bot. 2022, 43, e76491. [Google Scholar] [CrossRef]
- 2004 IUCN Red List of Threatened Species: A Global Species Assessment; IUCN: Cambridge, UK, 2004. Available online: https://portals.iucn.org/library/node/9830 (accessed on 17 December 2024).
- Inclan, D. Long-Stalked Cranesbill (Geranium diffusum). Available online: https://www.inaturalist.org/observations/19996937 (accessed on 27 November 2023).
- Tintaya Sullca, B. Evaluación Fitoquímica y su Actividad Tintoria del Geranium diffusum HBK (Ajotillo); Universidad Nacional de San Antonio Abad del Cusco: Cusco, Peru, 2002. [Google Scholar]
- Geranium Diffusum Kunth. Available online: http://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:372978-1 (accessed on 22 November 2023).
- Armijos, C. Medicinal and Dye Plants Used by the Saraguro Ethnic Group in Southern Ecuador; Acta Ethnica; Pavia University Press: Pavia, Italy, 2016; ISBN 978-88-6952-039-6. [Google Scholar]
- Dimensions Definitions of Publications Data Fields in Dimensions (Details Page and Export). Available online: https://dimensions.freshdesk.com/support/solutions/articles/23000018864-definitions-of-publications-data-fields-in-dimensions-details-page-and-export- (accessed on 18 September 2023).
- Armijos, C.; Suárez, A. Chemical Constituents of Medicinal Plants Used in the Community Saraguro, Ecuador; Sociedad Colombiana de Ciencias Químicas: Cartagena de Indias, Colombia, 2017; p. 149. Available online: https://inbio.uca.es/wp-content/uploads/2017/09/MEMORIAS-SILAE-2017-VF2.pdf? (accessed on 17 December 2024).
- Jácome, E.A.M.; Cárdenas, P.V.V.; Vásconez, D.F.L.; Reinoso, F.M.R. Estudio etnobotánico de la flora nativa de la ruta turística hieleros del Chimborazo en la parroquia San Andrés del cantón Guano en la provincia de Chimborazo. Polo Conoc. 2023, 8, 1219–1235. [Google Scholar] [CrossRef]
- Alshehri, B. The Geranium Genus: A Comprehensive Study on Ethnomedicinal Uses, Phytochemical Compounds, and Pharmacological Importance. Saudi J. Biol. Sci. 2024, 31, 103940. [Google Scholar] [CrossRef]
- Renda, G.; Celik, G.; Korkmaz, B.; Karaoglu, S.A.; Yayli, N. Antimicrobial Activity and Analyses of Six Geranium L. Species with Headspace SPME and Hydrodistillation. J. Essent. Oil Bear. Plants 2016, 19, 2003–2016. [Google Scholar] [CrossRef]
- Mahase, E. Cancer Overtakes CVD to Become Leading Cause of Death in High Income Countries. BMJ Br. Med. J. (Online) 2019, 366, l5368. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Hashim, D.; Boffetta, P.; La Vecchia, C.; Rota, M.; Bertuccio, P.; Malvezzi, M.; Negri, E. The Global Decrease in Cancer Mortality: Trends and Disparities. Ann. Oncol. 2016, 27, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2020. CA A Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Huang, M.; Lu, J.-J.; Ding, J. Natural Products in Cancer Therapy: Past, Present and Future. Nat. Prod. Bioprospect. 2021, 11, 5–13. [Google Scholar] [CrossRef]
- Ordoñez, A.K.R.; Francis, A.A.P.; Holguín, L.S.S.; Jalca, A.D.C. Cáncer de Mama: Prevalencia, Factores de Riesgo y Métodos Diagnósticos. Rev. Científica Higía Salud 2022, 7. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Kshirsagar, P.; Gaikwad, S.; Pai, S.; Desai, N.; Bapat, V. Evaluation of Antioxidant Capacity and Phytochemical Investigation of Eleven Clusiaceae Members from Western Ghats, India. Biocatal. Agric. Biotechnol. 2022, 44, 102476. [Google Scholar] [CrossRef]
- Adeonipekun, P.A.; Adeniyi, T.A.; Chidinma, O.Q.; Omolayo, R.O. Proximate, Phytochemical, and Antimicrobial Evaluation of Flowers of Mangifera indica L., Stamens of Terminalia catappa L., and Anther of Delonix regia (Bojer Ex Hook.) Raf. S. Afr. J. Bot. 2023, 155, 223–229. [Google Scholar] [CrossRef]
- Patel, K.; Patel, D.K. Chapter 26—The Beneficial Role of Rutin, a Naturally Occurring Flavonoid in Health Promotion and Disease Prevention: A Systematic Review and Update. In Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases (Second Edition); Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 457–479. ISBN 978-0-12-813820-5. [Google Scholar]
- Ganbaatar, C.; Gruner, M.; Mishig, D.; Duger, R.; Schmidt, A.W.; Knölker, H.-J. Flavonoid Glycosides from the Aerial Parts of Polygonatum Odoratum (Mill.) Druce Growing in Mongolia. Open Nat. Prod. J. 2015, 8, 1–7. [Google Scholar] [CrossRef]
- Atay, İ.; Kirmizibekmez, H.; Gören, A.; Yeşilada, E. Secondary Metabolites from Sambucus Ebulus. Turk. J. Chem. 2015, 39, 34–41. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Yang, L.; Zhou, D.; Zhang, J. Purification and Characterization of Flavonoids from the Leaves of Zanthoxylum bungeanum and Correlation between Their Structure and Antioxidant Activity. PLoS ONE 2014, 9, e105725. [Google Scholar] [CrossRef]
- Imani, A.; Maleki, N.; Bohlouli, S.; Kouhsoltani, M.; Sharifi, S.; Maleki Dizaj, S. Molecular Mechanisms of Anticancer Effect of Rutin. Phytother. Res. 2021, 35, 2500–2513. [Google Scholar] [CrossRef]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Ali Shah, S.A.; Khatib, A.; Mukhtar, S.; Alsharif, M.A.; Parveen, H.; Zakaria, Z.A. Antibacterial Effects of Flavonoids and Their Structure-Activity Relationship Study: A Comparative Interpretation. Molecules 2022, 27, 1149. [Google Scholar] [CrossRef] [PubMed]
- Won, Y.-S.; Kim, J.-H.; Lizardo, R.C.M.; Min, H.-J.; Cho, H.-D.; Hong, S.-M.; Seo, K.-I. The Flavonol Isoquercitrin Promotes Mitochondrial-Dependent Apoptosis in SK-Mel-2 Melanoma Cell via the PI3K/AKT/mTOR Pathway. Nutrients 2020, 12, 3683. [Google Scholar] [CrossRef]
- Guilherme di Camillo Orfali, A.C.D.; Jo, F.B.M.P.; Priolli, A. Review of Anticancer Mechanisms of Isoquercitin. World J. Clin. Oncol. 2016, 7, 189–199. [Google Scholar] [CrossRef]
- Gong, G.; Guan, Y.-Y.; Zhang, Z.-L.; Rahman, K.; Wang, S.-J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A Review of Pharmacological Effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef]
- Biswas, P.; Kaium, M.A.; Tareq, M.M.I.; Tauhida, S.J.; Hossain, M.R.; Siam, L.S.; Parvez, A.; Bibi, S.; Hasan, M.H.; Rahman, M.M.; et al. The Experimental Significance of Isorhamnetin as an Effective Therapeutic Option for Cancer: A Comprehensive Analysis. Biomed. Pharmacother. 2024, 176, 116860. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, Q.; Zhang, C.; Yang, W.; Liu, H.; Lv, Z.; Liu, J.; Jiao, Z. Inhibition of Dipeptidyl Peptidase-4 by Flavonoids: Structure–Activity Relationship, Kinetics and Interaction Mechanism. Front. Nutr. 2022, 9, 892426. [Google Scholar] [CrossRef]
- Magozwi, D.K.; Dinala, M.; Mokwana, N.; Siwe-Noundou, X.; Krause, R.W.M.; Sonopo, M.; McGaw, L.J.; Augustyn, W.A.; Tembu, V.J. Flavonoids from the Genus Euphorbia: Isolation, Structure, Pharmacological Activities and Structure–Activity Relationships. Pharmaceuticals 2021, 14, 428. [Google Scholar] [CrossRef] [PubMed]
- Bailon-Moscoso, N.; Coronel-Hidalgo, J.; Duarte-Casar, R.; Guamán-Ortiz, L.M.; Figueroa, J.G.; Romero-Benavides, J.C. Exploring the Antioxidant Potential of Tragia volubilis L.: Mitigating Chemotherapeutic Effects of Doxorubicin on Tumor Cells. Antioxidants 2023, 12, 2003. [Google Scholar] [CrossRef]
- Graça, V.C.; Ferreira, I.C.F.R.; Santos, P.F. Phytochemical Composition and Biological Activities of Geranium robertianum L.: A Review. Ind. Crops Prod. 2016, 87, 363–378. [Google Scholar] [CrossRef]
- Sergazy, S.; Vetrova, A.; Orhan, I.E.; Senol Deniz, F.S.; Kahraman, A.; Zhang, J.-Y.; Aljofan, M. Antiproliferative and Cytotoxic Activity of Geraniaceae Plant Extracts Against Five Tumor Cell Lines. Future Sci. OA 2022, 8, FSO775. [Google Scholar] [CrossRef] [PubMed]
- Sulikovska, I.; Ivanova, E.; Ivanov, I.; Tasheva, D.; Dimitrova, M.; Nikolova, B.; Iliev, I. Study on the Phototoxicity and Antitumor Activity of Plant Extracts from Tanacetum vulgare L., Epilobium parviflorum Schreb., and Geranium sanguineum L. Int. J. Bioautom. 2023, 27, 39–50. [Google Scholar] [CrossRef]
- Świątek, Ł.; Wasilewska, I.; Boguszewska, A.; Grzegorczyk, A.; Rezmer, J.; Rajtar, B.; Polz-Dacewicz, M.; Sieniawska, E. Herb Robert’s Gift against Human Diseases: Anticancer and Antimicrobial Activity of Geranium robertianum L. Pharmaceutics 2023, 15, 1561. [Google Scholar] [CrossRef]
- Jaramillo, S.; Lopez, S.; Varela, L.M.; Rodriguez-Arcos, R.; Jimenez, A.; Abia, R.; Guillen, R.; Muriana, F.J.G. The Flavonol Isorhamnetin Exhibits Cytotoxic Effects on Human Colon Cancer Cells. J. Agric. Food Chem. 2010, 58, 10869–10875. [Google Scholar] [CrossRef] [PubMed]
- Saud, S.M.; Young, M.R.; Jones-Hall, Y.L.; Ileva, L.; Evbuomwan, M.O.; Wise, J.; Colburn, N.H.; Kim, Y.S.; Bobe, G. Chemopreventive Activity of Plant Flavonoid Isorhamnetin in Colorectal Cancer Is Mediated by Oncogenic Src and β-Catenin. Cancer Res. 2013, 73, 5473–5484. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; El-Biyally, E.; Sakran, W. An Innovative Approach for Formulation of Rutin Tablets Targeted for Colon Cancer Treatment. AAPS PharmSciTech 2023, 24, 68. [Google Scholar] [CrossRef]
- Darband, S.G.; Kaviani, M.; Yousefi, B.; Sadighparvar, S.; Pakdel, F.G.; Attari, J.A.; Mohebbi, I.; Naderi, S.; Majidinia, M. Quercetin: A Functional Dietary Flavonoid with Potential Chemo-preventive Properties in Colorectal Cancer. J. Cell. Physiol. 2018, 233, 6544–6560. [Google Scholar] [CrossRef] [PubMed]
- Al-Naqeb, G.; Kalmpourtzidou, A.; Giampieri, F.; De Giuseppe, R.; Cena, H. Genotoxic and Antigenotoxic Medicinal Plant Extracts and Their Main Phytochemicals: “A Review”. Front. Pharmacol. 2024, 15, 1448731. [Google Scholar] [CrossRef] [PubMed]
- Bailon-Moscoso, N.; Tinitana, F.; Martínez-Espinosa, R.; Jaramillo-Velez, A.; Palacio-Arpi, A.; Aguilar-Hernandez, J.; Romero-Benavides, J.C. Cytotoxic, Antioxidative, Genotoxic and Antigenotoxic Effects of Horchata, Beverage of South Ecuador. BMC Complement. Altern. Med. 2017, 17, 539. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.A.; Lima, C.F.; Pereira, M.L.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Antigenotoxic Effects of Quercetin, Rutin and Ursolic Acid on HepG2 Cells: Evaluation by the Comet Assay. Toxicol. Lett. 2008, 177, 66–73. [Google Scholar] [CrossRef]
- Benkovic, V.; Horvat Knezevic, A.; Dikic, D.; Lisicic, D.; Orsolic, N.; Basic, I.; Kosalec, I.; Kopjar, N. Radioprotective Effects of Propolis and Quercetin in γ-Irradiated Mice Evaluated by the Alkaline Comet Assay. Phytomedicine 2008, 15, 851–858. [Google Scholar] [CrossRef]
- Johnson, M.K.; Loo, G. Effects of Epigallocatechin Gallate and Quercetin on Oxidative Damage to Cellular DNA. Mutat. Res./DNA Repair. 2000, 459, 211–218. [Google Scholar] [CrossRef]
- Panchal, H.; Bhardwaj, J.K. Quercetin Supplementation Alleviates Cadmium Induced Genotoxicity-Mediated Apoptosis in Caprine Testicular Cells. Biol. Trace Elem. Res. 2024, 202, 1–14. [Google Scholar] [CrossRef]
- Oršolić, N.; Car, N. Quercetin and Hyperthermia Modulate Cisplatin-Induced DNA Damage in Tumor and Normal Tissues in Vivo. Tumor Biol. 2014, 35, 6445–6454. [Google Scholar] [CrossRef]
- El-Alfy, N.; Mahmoud, M.; Elashry, S. The Possible Protective Effects of Quercetin and/or Resveratrol against Acrolein–Induced Sister Chromatid Exchanges and DNA Damage in Male Albino Mice. Res. Sq. 2022. preprint. [Google Scholar]
- Ibrahim, N.; Kebede, A. In Vitro Antibacterial Activities of Methanol and Aqueous Leave Extracts of Selected Medicinal Plants against Human Pathogenic Bacteria. Saudi J. Biol. Sci. 2020, 27, 2261–2268. [Google Scholar] [CrossRef]
- Romero-Benavides, J.C.; Atiencie-Valarezo, N.C.; Duarte-Casar, R. Flavonoid Composition and Antioxidant Activity of Tragia volubilis L. Methanolic Extract. Plants 2023, 12, 3139. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.C.; Mandal, V.; Das, A.K. Qualitative Phytochemical Screening. In Essentials of Botanical Extraction; Elsevier: Amsterdam, The Netherlands, 2015; pp. 173–185. ISBN 978-0-12-802325-9. [Google Scholar]
- Miranda-Martínez, M. Farmacognosia y Productos Naturales; Editorial Félix Varela: Havana, Cuba, 2012; ISBN 978-959-07-1794-9. [Google Scholar]
- Monteiro, F.; Shetty, S.S.; Ranjitha, K.; Shetty, V.V.; Shetty, D.P.; Patil, P.; Suchetha, K.N. Phytochemical Profiling, Total Flavonoid, Total Phenolic Content and in-Vitro Antioxidant Evaluation of Citrus Maxima Extract. Biomedicine 2022, 42, 912–919. [Google Scholar] [CrossRef]
- Kongkham, B.; Duraivadivel, P.; Hariprasad, P. Acorus calamus L. Rhizome Extract and Its Bioactive Fraction Exhibits Antibacterial Effect by Modulating Membrane Permeability and Fatty Acid Composition. J. Ethnopharmacol. 2024, 331, 118323. [Google Scholar] [CrossRef] [PubMed]
- Machinski, I.; Andrade, E.A.; Schaffka, V.M.; de Almeida, V.P.; Santos, A.; Bueno, D.; Perera, W.H.; Pereira, R.P.; Manfron, J.; Miyoshi, E.; et al. Exploring the Pharmacognostical and Phytochemical Profiles of Aqueous Extracts of Kalanchoe. Chem. Biodivers. 2024, 21, e202400660. [Google Scholar] [CrossRef]
- Habibi, E.; Sepehrara, A.; Arabnozari, H.; Sharifianjazi, F.; Enderami, S.E.; Sarker, S.D.; Hassannia, H.; Nahar, L. Comparative Evaluation of the Anti-Proliferative Effects of Alkaloid-Rich Extract of Jujube Seed and Paclitaxel on MDA-MB-231 Breast Cancer Cell Line. J. Agric. Food Res. 2024, 18, 101438. [Google Scholar] [CrossRef]
- Guevara-Vásquez, A.M.; Marín-Tello, C.L. Wound Healing Activity of Allium cepa L. Bulbs in a Second-Degree Burn Wound Model in Holtzman Rats. Vitae 2021, 28. [Google Scholar] [CrossRef]
- Sitohang, N.A.; Putra, E.D.L.; Kamil, H.; Musman, M. Phytochemical Screening of Putat Air [Barringtonia racemosa (L.)] Spreng Herbal Plants Found in Bireun, Aceh, Indonesia. Res. J. Pharm. Technol. 2022, 15, 2727–2732. [Google Scholar] [CrossRef]
- Izzuddin, A.; Ridwan, M.; Iskandar, C.D.; Purnawarman, A.; Husna, F.; Nurkhalis, N.; Yafi, D.A.; Fajri, F.; Fitriani, F. Anti-Atherosclerotic Effect of Nigella sativa L. in High-Fat Diet Fed Wistar Rats. Trop. J. Nat. Prod. Res. 2024, 8, 9632–9636. [Google Scholar] [CrossRef]
- Yandev, D.; Ngbede, J.I.; Abongaby, G.C.; Idowu, O.A.; Kyernum, T.T.; Adikwu, P. Trypanosocidal Activity of Methanolic Extract of Lemon Grass (Cymbopogon citratus). Niger. J. Parasitol. 2024, 45, 64–76. [Google Scholar] [CrossRef]
- Zahi, A.; Driouech, M.; Hakkou, Z.; Mansouri, F.; El Hajji, F.; Ziyyat, A.; Mekhfi, H.; Bnouham, M.; Legssyer, A. Vasorelaxant Effect of Fennel Seeds (Foeniculum vulgare Mill) Extracts on Rat Mesenteric Arteries: Assessment of Phytochemical Profiling and Antioxidant Potential. Fitoterapia 2025, 181, 106359. [Google Scholar] [CrossRef]
- Silva-Rivas, R.; Bailon-Moscoso, N.; Cartuche, L.; Romero-Benavides, J. The Antioxidant and Hypoglycemic Properties and Phytochemical Profile of Clusia Latipes Extracts. Pharmacogn. J. 2020, 12, 144–149. [Google Scholar] [CrossRef]
- Bayir, A.G.; Kiziltan, H.S.; Kocyigit, A. Chapter 1—Plant Family, Carvacrol, and Putative Protection in Gastric Cancer. In Dietary Interventions in Gastrointestinal Diseases; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 3–18. ISBN 978-0-12-814468-8. [Google Scholar]
- Coskun, O. Separation Techniques: Chromatography. North. Clin. Istanb. 2016, 3, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Maia, S.D.S.; Smiderle, O.J.; Souza, A.D.G.; Torres, S.B. Adaptation of Tetrazolium Test Methodology to Estimate the Viability of Eugenia stipitata McVaugh ssp. Sororia McVaugh Seeds. Hoehnea 2023, 50, e142023. [Google Scholar] [CrossRef]
- Bailon-Moscoso, N.; Romero Benavides, J.C.; Ramirez Orellana, M.I.; Ojeda, K.; Granda, G.; Ratoviski, E.A.; Ostrosky-Wegman, P. Cytotoxic and Genotoxic Effects of Extracts from Annona montana M. Fruit. Food Agric. Immunol. 2016, 27, 559–569. [Google Scholar] [CrossRef]
- Bailon-Moscoso, N.; González-Arévalo, G.; Velásquez-Rojas, G.; Malagon, O.; Vidari, G.; Zentella-Dehesa, A.; Ratovitski, E.A.; Ostrosky-Wegman, P. Phytometabolite Dehydroleucodine Induces Cell Cycle Arrest, Apoptosis, and DNA Damage in Human Astrocytoma Cells through P73/P53 Regulation. PLoS ONE 2015, 10, e0136527. [Google Scholar] [CrossRef]




| Use | Plant Organ | Country | Preparation/ Administration | Ref. |
|---|---|---|---|---|
| Stomach pains | NS | Ecuador | Leaf infusion | [34] |
| Postpartum infections, gangrene | Whole plant | Ecuador | Crushed plant/Oral | [16] |
| Wound-healing, analgesic | NS | Peru | NS | [29] |
| Fraction | Weight (g) | Yield (%) |
|---|---|---|
| Hexane | 7.45 | 29.8 |
| Dichloromethane | 0.078 | 0.31 |
| Ethyl acetate | 4.18 | 16.7 |
| Butanol | 4.45 | 17.8 |
| Aqueous | 2.35 | 9.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Benavides, J.C.; Añazco-Loayza, T.; Correa-Sinche, A.; Alvarez-Ruiz, A.; Guamán-Ortiz, L.M.; Duarte-Casar, R.; Bailon-Moscoso, N. Phytochemical Study, Cytotoxicity, and Genotoxicity of the Methanolic Extract of Geranium diffusum Kunth. Plants 2025, 14, 777. https://doi.org/10.3390/plants14050777
Romero-Benavides JC, Añazco-Loayza T, Correa-Sinche A, Alvarez-Ruiz A, Guamán-Ortiz LM, Duarte-Casar R, Bailon-Moscoso N. Phytochemical Study, Cytotoxicity, and Genotoxicity of the Methanolic Extract of Geranium diffusum Kunth. Plants. 2025; 14(5):777. https://doi.org/10.3390/plants14050777
Chicago/Turabian StyleRomero-Benavides, Juan Carlos, Tatiana Añazco-Loayza, Anabel Correa-Sinche, Andrea Alvarez-Ruiz, Luis Miguel Guamán-Ortiz, Rodrigo Duarte-Casar, and Natalia Bailon-Moscoso. 2025. "Phytochemical Study, Cytotoxicity, and Genotoxicity of the Methanolic Extract of Geranium diffusum Kunth" Plants 14, no. 5: 777. https://doi.org/10.3390/plants14050777
APA StyleRomero-Benavides, J. C., Añazco-Loayza, T., Correa-Sinche, A., Alvarez-Ruiz, A., Guamán-Ortiz, L. M., Duarte-Casar, R., & Bailon-Moscoso, N. (2025). Phytochemical Study, Cytotoxicity, and Genotoxicity of the Methanolic Extract of Geranium diffusum Kunth. Plants, 14(5), 777. https://doi.org/10.3390/plants14050777










