Salsolinol-Containing Senna silvestris Exerts Antiviral Activity Against Hepatitis B Virus
Abstract
1. Introduction
- -
- Inhibition of HBV replication. In addition to PegIFN-α and nucleos(t)ide analogs, other antivirals, such as entry inhibitors, capsid assembly modulators (CAMs), short interfering RNAs (siRNAs), and antisense oligonucleotides (ASOs), are evaluated as potential candidates.
- -
- Inhibition of HBsAg production or secretion. PegIFN-α, siRNA, ASO, and second-generation CAMs can inhibit the production of this antigen, while nucleic acid polymers can inhibit secretion.
- -
- Finally, the functional cure could be achieved through the restoration or stimulation of the innate immune response (TLR7 or TLR8 agonists), and of the adaptive response (therapeutic vaccines), passive immunization (antibodies to HBsAg), and the removal of immune blockade (by attacking the immune checkpoint inhibitors). PegIFN-α, as an immune modulator, also plays a role in this critical aspect.
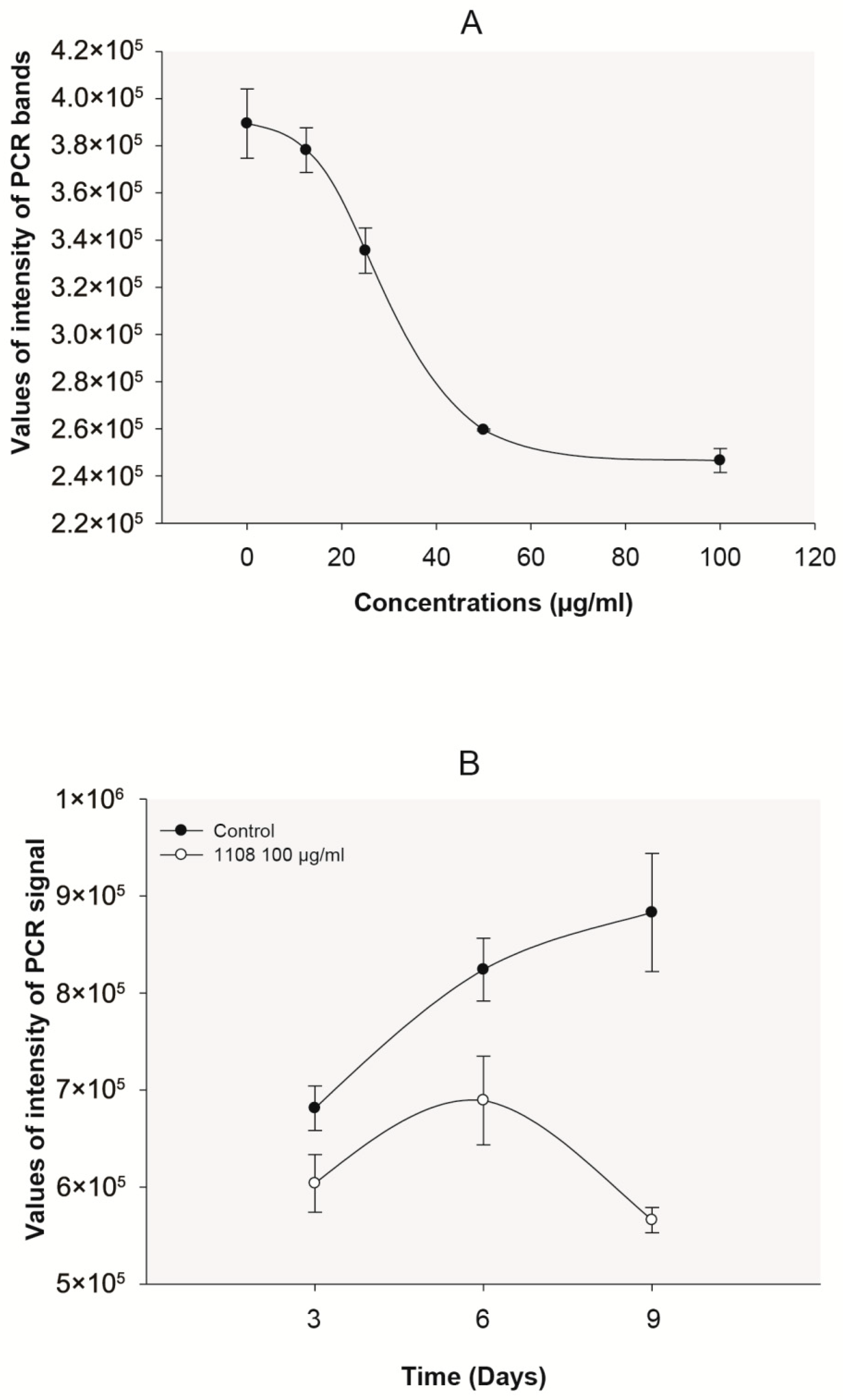
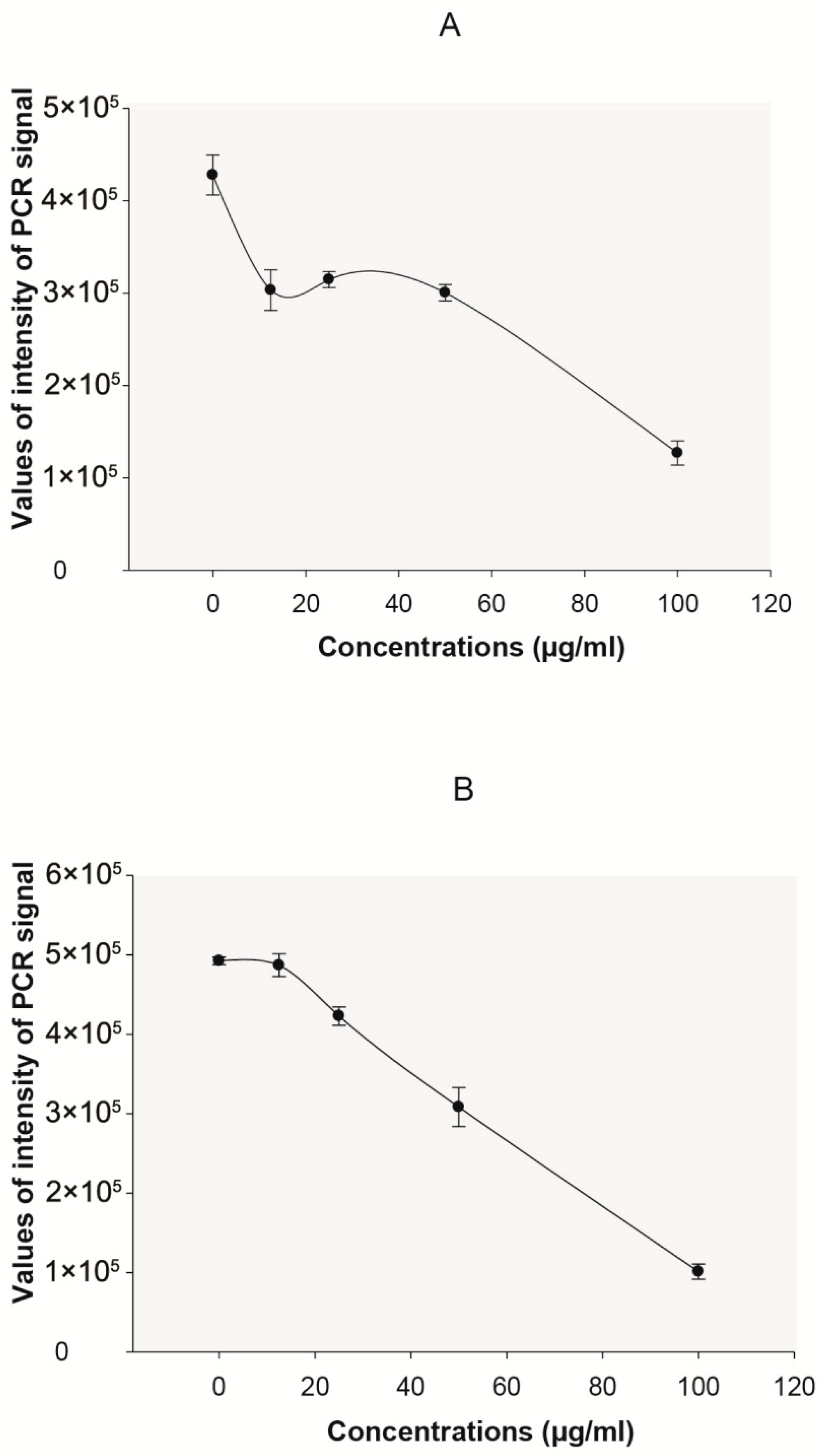
2. Results
3. Discussion
4. Materials and Methods
4.1. Isolation of Antiviral Compounds
4.2. Structural Analyses
4.3. Cells and Cytotoxicity Assays
4.4. Testing of Inhibitory Activity on HBV Replication
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GC-MS | Gas chromatography–mass spectrometry |
| HBV | Hepatitis B virus |
| HIV-1 | Human immunodeficiency virus type 1 |
| HPLC | High performance liquid chromatography |
| IC50 | Concentration exerting 50% inhibition |
| MIC | Minimal inhibitory concentration |
| NMR | Nuclear magnetic resonnance |
| SAL | Salsolinol |
References
- El Sayed, K.A. Natural Products as Antiviral Agents. Stud. Nat. Prod. Chem. 2007, 24, 473–572. [Google Scholar] [CrossRef]
- Jeng, W.J.; Papatheodoridis, G.V.; Lok, A.S.F. Hepatitis B. Lancet 2023, 401, 1039–1052. [Google Scholar] [CrossRef]
- Boonstra, A.; Sari, G. HBV cccDNA: The Molecular Reservoir of Hepatitis B Persistence and Challenges to Achieve Viral Eradication. Biomolecules 2025, 15, 62. [Google Scholar] [CrossRef]
- Lok, A.S.F. Toward a Functional Cure for Hepatitis B. Gut Liver 2024, 18, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.G.; Baumert, T.F.; Boni, C.; Gane, E.; Levrero, M.; Lok, A.S.; Maini, M.K.; Terrault, N.A.; Zoulim, F. The scientific basis of combination therapy for chronic hepatitis B functional cure. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 238–253. [Google Scholar] [CrossRef]
- Chattopadhyay, D.; Naik, T.N. Antivirals of ethnomedicinal origin: Structure-activity relationship and scope. Mini Rev. Med. Chem. 2007, 7, 275–301. [Google Scholar] [CrossRef]
- Quintero, A.; Fabbro, R.; Maillo, M.; Barrios, M.; Milano, M.B.; Fernández, A.; Williams, B.; Michelangeli, F.; Rangel, H.R.; Pujol, F.H. Inhibition of hepatitis B virus and human immunodeficiency virus (HIV-1) replication by Warscewiczia coccinea (Vahl) Kl. (Rubiaceae) ethanol extract. Nat. Prod. Res. 2011, 25, 1565–1569. [Google Scholar] [CrossRef]
- Soloway, S.; Wilen, S.H. Improved Ferric Chloride Test for Phenols. Anal. Chem. 1952, 24, 979–983. [Google Scholar] [CrossRef]
- Yamamura, S.; Nishiyama, S. Reduction of CX to CH2 by dissolving metals and related methods. In Comprehensive Organic Synthesis; Elsevier: Amsterdam, The Netherlands, 1991; pp. 7–325. [Google Scholar] [CrossRef]
- Thomas, A.W.; Frieden, A. The Gelatin-Tannin Reaction. Ind. Eng. Chem. 1923, 15, 839–841. [Google Scholar] [CrossRef]
- Calderon, P.; Van Buren, J.; Robinson, W.B. Factors influencing the formation of precipitates and hazes by gelatin and condensed and hydrolysable tannins. J. Agric. Food Chem. 1968, 16, 479–482. [Google Scholar] [CrossRef]
- Dragendorff, G. Ueber einige neue Reagentien auf Alkaloide. Pharm. Zschr Russl. 1866, 5, 82–85. [Google Scholar] [CrossRef]
- Koziol, M.J. Afrosimetric estimation of threshold saponin concentration for bitterness in quinoa (Chenopodium quinoa Willd). J. Sci. Food Agric. 1991, 54, 211–219. [Google Scholar] [CrossRef]
- Nath, M.C.; Chakravorty, M.K.; Chowdhury, S.R. Liebermann-burchard reaction for steroids. Nature 1946, 157, 103–104. [Google Scholar] [CrossRef]
- Francisco, M.C.; Nasser, A.L.; Lopes, L.M. Tetrahydroisoquinoline alkaloids and 2-deoxyribonolactones from Aristolochia arcuata. Phytochemistry 2003, 62, 1265–1270. [Google Scholar] [CrossRef]
- Nagasawa, Y.; Ueoka, R.; Yamanokuchi, R.; Horiuchi, N.; Ikeda, T.; Rotinsulu, H.; Mangindaan, R.E.; Ukai, K.; Kobayashi, H.; Namikoshi, M.; et al. Isolation of salsolinol, a tetrahydroisoquinoline alkaloid, from the marine sponge Xestospongia cf. vansoesti as a proteasome inhibitor. Chem. Pharm. Bull. 2011, 59, 287–290. [Google Scholar] [CrossRef]
- Oladeji, O.S.; Adelowo, F.E.; Oluyori, P. The genus Senna (Fabaceae): A review on its traditional uses, botany, phytochemistry, pharmacology and toxicology. S. Afr. J. Bot. 2021, 138, 1–32. [Google Scholar] [CrossRef]
- Hennebelle, T.; Weniger, B.; Joseph, H.; Sahpaz, S.; Bailleul, F. Senna alata. Fitoterapia 2009, 80, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Islam, M.S. Anti-diabetic effects of the acetone fraction of Senna singueana stem bark in a type 2 diabetes rat model. J. Ethnopharmacol. 2014, 153, 392–399. [Google Scholar] [CrossRef] [PubMed]
- McFadden, W.M.; Sarafianos, S.G. Biology of the hepatitis B virus (HBV) core and capsid assembly modulators (CAMs) for chronic hepatitis B (CHB) cure. Glob. Health Med. 2023, 5, 199–207. [Google Scholar] [CrossRef]
- Jamal, M.; Ameno, K.; Kubota, T.; Ameno, S.; Zhang, X.; Kumihashi, M.; Ijiri, I. In vitro formation of salsolinol induced by high acetaldehyde concentration in rat striatum employing microdialysis. Alcohol. Alcohol. 2003, 38, 197–201. [Google Scholar] [CrossRef][Green Version]
- Storch, A.; Kaftan, A.; Burkhardt, K.; Schwarz, J. 1-methyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline (salsolinol) is toxic to dopaminergic neuroblastoma SH-SY5Y cells via impairment of cellular energy metabolism. Brain Res. 2000, 855, 67–75. [Google Scholar] [CrossRef]
- Mosca, L.; Blarzino, C.; Coccia, R.; Foppoli, C.; Rosei, M. Melanins from tetrahydroisoquinolines: Spectroscopic characteristics, scavenging activity and redox transfer properties. Free. Radic. Biol. Med. 1998, 24, 161–167. [Google Scholar] [CrossRef]
- Melzig, M.; Putscher, I.; Henklein, P.; Haber, H. In vitro pharmacological activity of the tetrahydroisoquinoline salsolinol present in products from Theobroma cacao L. like cocoa and chocolate. J. Ethnopharmacol. 2000, 73, 153–159. [Google Scholar] [CrossRef]
- Murata, M.; Midorikawa, M.; Kawanishi, S. Molecular Link Between Alcohol and Breast Cancer: The Role of Salsolinol. In Molecular Aspects of Alcohol and Nutrition; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Chapter 25; pp. 315–324. [Google Scholar]
- Iwasa, K.; Moriyasu, M.; Tachibana, Y.; Kim, H.; Wataya, Y.; Wiegrebe, W.; Bastow, K.; Consentido, M.; Kozuka, M.; Lee, K. Simple Isoquinoline and Benzylisoquinoline alkaloids as potential antimicrobial, antimalarial, cytotoxic, and anti-HIV agents. Bioorg. Med. Chem. 2001, 9, 2871–2884. [Google Scholar] [CrossRef]
- Kurnik-Łucka, M.; Panula, P.; Bugajski, A.; Gil, K. Salsolinol: An Unintelligible and Double-Faced Molecule-Lessons Learned from In Vivo and In Vitro Experiments. Neurotox. Res. 2018, 33, 485–514. [Google Scholar] [CrossRef] [PubMed]
- Moser, A.; Kömpf, D. Presence of methyl-6, 7-dihydroxy-1,2,3,4-tetrahydroisoquinolines, derivatives of the neurotoxin isoquinoline, in parkinsonian lumbar CSF. Life Sci. 1992, 50, 1885–1891. [Google Scholar] [CrossRef]
- Wang, J.; Ran, Y.; Li, Z.; Zhao, T.; Zhang, F.; Wang, J.; Liu, Z.; Chen, X. Salsolinol as an RNA m6A methylation inducer mediates dopaminergic neuronal death by regulating YAP1 and autophagy. Neural Regen. Res. 2025, 20, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Midorikawa, K.; Kawanishi, S. Oxidative DNA damage and mammary cell proliferation by alcohol-derived salsolinol. Chem. Res. Toxicol. 2013, 26, 1455–1463. [Google Scholar] [CrossRef]
- Zhang, X.; Shao, Z.; Ni, Y.; Chen, F.; Yu, X.; Wen, J. Salsolinol improves angiotensin II-induced myocardial fibrosis in vitro via inhibition of LSD1 through regulation of the STAT3/Notch-1 signaling pathway. Exp. Ther. Med. 2023, 26, 527. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Block, T.M.; Gerlich, W.H. Protease-induced infectivity of hepatitis B virus for a human hepatoblastoma cell line. J. Virol. 1996, 70, 2277–2285. [Google Scholar] [CrossRef]



| Chemical Analysis | Compound Detected | Result |
|---|---|---|
| FeCl3 | Polyphenols | Positive |
| Shinoda | Flavonoids | Negative |
| Jelly | Tannins | Negative |
| Dragendorff | Alkaloids | Positive |
| Afrosymetric index | Saponins | Negative |
| Libermann-Bouchard | Triterpenes | Negative |
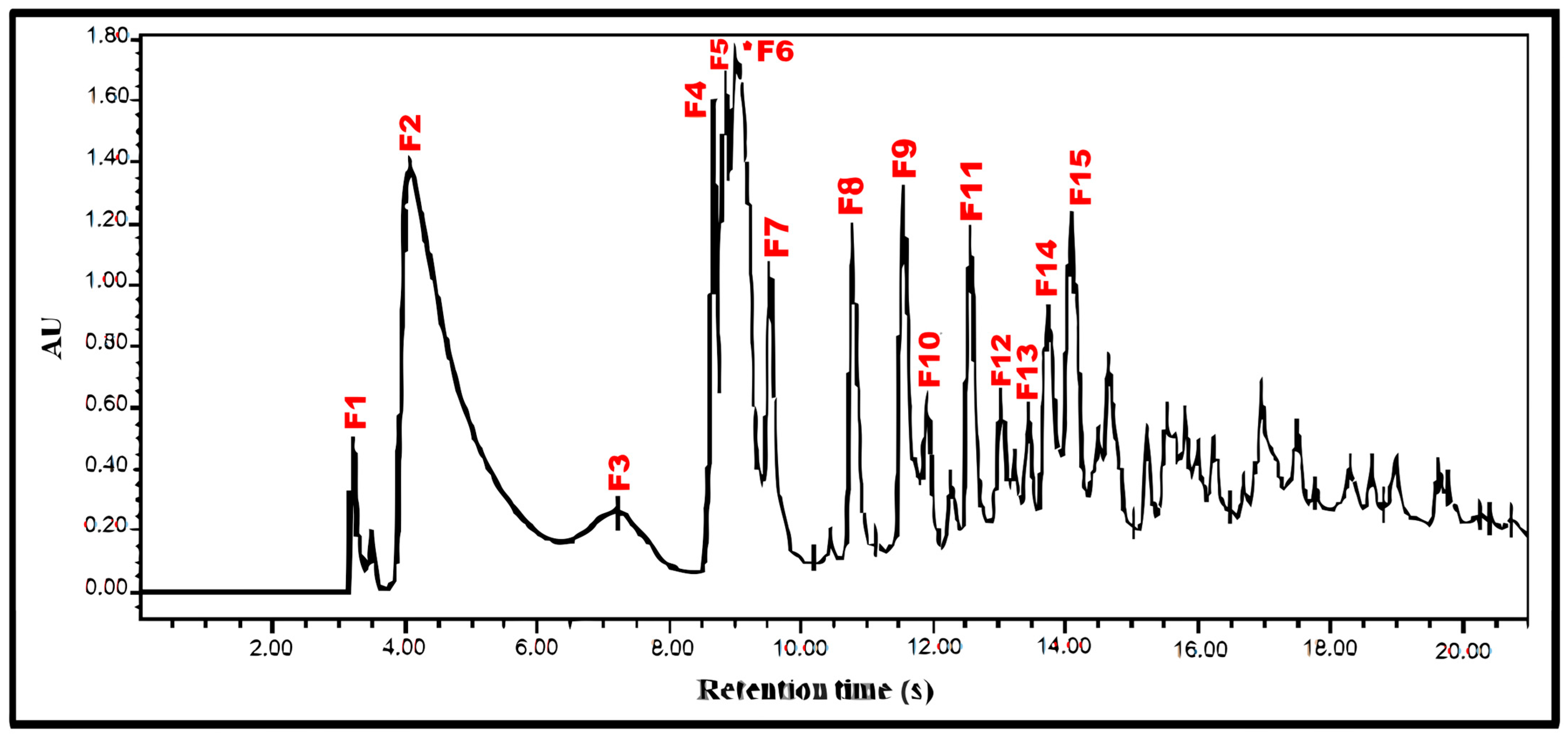
| Fractions | Retention Time | Mass | % RS |
|---|---|---|---|
| (100 μg/mL) | (min) | (mg) | |
| F1 | 3.486 | 2.3 | 113 ± 4.22 |
| F2 | 4.074 | 32.6 | 103 ± 3.0 |
| F3 | 7.21 | 3.4 | 140 ± 5.7 |
| F4 | 8.666 | 3.4 | 100 ± 3.4 |
| F5 | 8.878 | 2 | 65.8 ± 2.9 |
| F6 1 | 9.002 | 34.3 | 64.1 ± 3.1 |
| F7 | 9.539 | 14.5 | 82.9 ± 4.1 |
| F8 | 10.799 | 4.4 | 114 ± 2.8 |
| F9 | 11.583 | 5.2 | 127 ± 4.0 |
| F10 | 11.92 | 3 | 111 ± 3.9 |
| F11 | 12.569 | 1 | 120 ± 2.5 |
| F12 | 13.039 | 12 | 108 ± 2.0 |
| F13 | 13.452 | 6.2 | 142 ± 3.8 |
| F14 | 13.753 | 1 | 121 ± 4.5 |
| F15 | 14.102 | 7.3 | 117 ± 2.6 |
| Fractions | Retention Time | Mass | %RS |
|---|---|---|---|
| 100 µg/mL | (min) | (mg) | |
| G1 | 4.509 | 0 | 0 |
| G2 | 5.93 | 3.2 | 96.9 ± 3.2 |
| G3 1 | 7.053 | 8.6 | 28.42 ± 4.6 |
| G4 | 7.852 | 20.4 | 96.31 ± 3.5 |
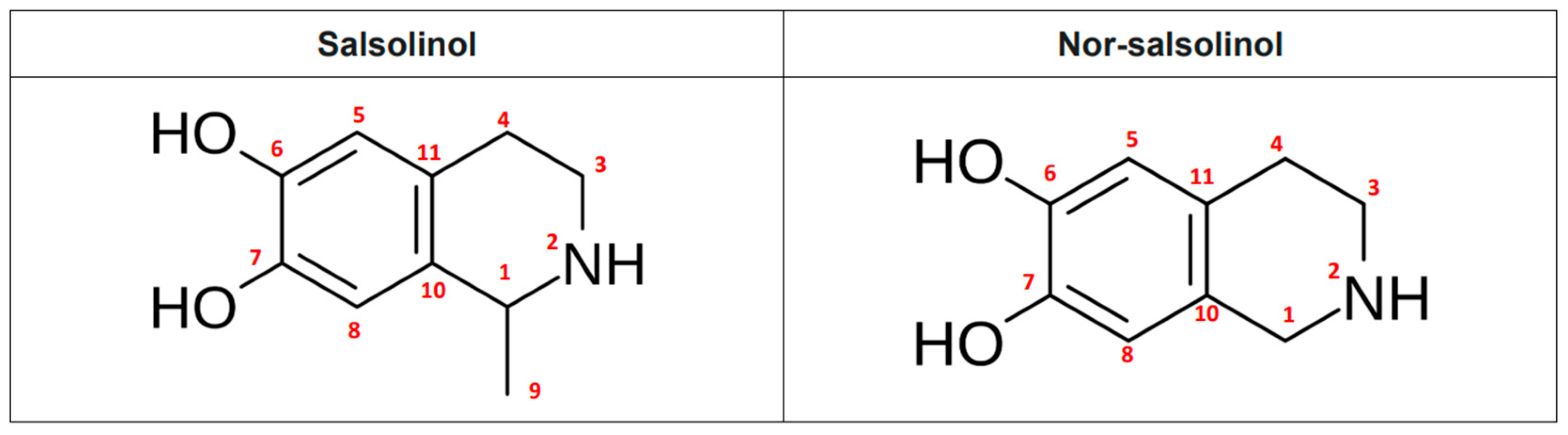
| N | Type of Proton | δ (ppm) | Salsolinol | Nor-Salsolinol |
|---|---|---|---|---|
| H1 | CH | 4.46 (q, J ≈ 6.8 Hz) | ✔ | ✘ (CH2) |
| H9 | CH3 | 1.54 (d, J ≈ 6.8 Hz) | ✔ | ✘ |
| H3 | CH2 | 3.49–3.28 (m) | ✔ | ✔ |
| H4 | CH2 | 2.94–2.88 (m) | ✔ | ✔ |
| H5 | Aromatic CH | 6.69 (s) | ✔ | ✔ |
| H8 | Aromatic CH | 6.72 (s) | ✔ | ✔ |
| N | Type of Carbon | δ (ppm) | Salsolinol | Nor-Salsolinol |
|---|---|---|---|---|
| C1 | CH (chiral) | 50.4 | ✔ | ✘ (CH2 ≈ 45 ppm) |
| C9 | CH3 | 19.6 | ✔ | ✘ |
| C3 | CH2 | 40.1 | ✔ | ✔ |
| C4 | CH2 | 24.8 | ✔ | ✔ |
| C5 | Aromatic CH | 115.5 | ✔ | ✔ |
| C6 | Aromatic C-OH | 145.4 | ✔ | ✔ |
| C7 | Aromatic C-OH | 144.74 | ✔ | ✔ |
| C8 | Aromatic CH | 113.2 | ✔ | ✔ |
| C10 | Aromatic CH | 124.7 | ✔ | ✔ |
| C11 | Aromatic CH | 122.3 | ✔ | ✔ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintero, A.; Maillo, M.; Gomes, N.; Fernández, A.; Rangel, H.R.; Michelangeli, F.; Pujol, F.H. Salsolinol-Containing Senna silvestris Exerts Antiviral Activity Against Hepatitis B Virus. Plants 2025, 14, 2372. https://doi.org/10.3390/plants14152372
Quintero A, Maillo M, Gomes N, Fernández A, Rangel HR, Michelangeli F, Pujol FH. Salsolinol-Containing Senna silvestris Exerts Antiviral Activity Against Hepatitis B Virus. Plants. 2025; 14(15):2372. https://doi.org/10.3390/plants14152372
Chicago/Turabian StyleQuintero, Alberto, Maria Maillo, Nelson Gomes, Angel Fernández, Hector R. Rangel, Fabian Michelangeli, and Flor H. Pujol. 2025. "Salsolinol-Containing Senna silvestris Exerts Antiviral Activity Against Hepatitis B Virus" Plants 14, no. 15: 2372. https://doi.org/10.3390/plants14152372
APA StyleQuintero, A., Maillo, M., Gomes, N., Fernández, A., Rangel, H. R., Michelangeli, F., & Pujol, F. H. (2025). Salsolinol-Containing Senna silvestris Exerts Antiviral Activity Against Hepatitis B Virus. Plants, 14(15), 2372. https://doi.org/10.3390/plants14152372








