Xylooligosaccharides from Barley Malt Residue Produced by Microwave-Assisted Enzymatic Hydrolysis and Their Potential Uses as Prebiotics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Compositional Analysis of Barley Malt Residue
2.3. Statistical Optimization of Oligosaccharides Production Through Microwave-Assisted Enzymatic Hydrolysis of Barley Malt Residue
2.4. Analysis of Xylooligosaccharides
2.5. Microstructural Analysis of BMR Before and After Microwave Pretreatment
2.6. Purification of Xylooligosaccharide from Barley Malt Residue
2.6.1. Ethanol Precipitation Method
2.6.2. Activated Carbon Adsorption Method
2.6.3. Saccharomyces cerevisiae Treatment
2.7. Characterization of Xylooligosaccharide from Barley Malt Residue
2.7.1. Thin-Layer Chromatography
2.7.2. Fourier Transform Infrared Analysis
2.8. In Vitro Fermentation of Xylooligosaccharide from Barley Malt Residue by Probiotics
2.9. Analytical Methods
3. Results and Discussion
3.1. Composition of Barley Malt Residue
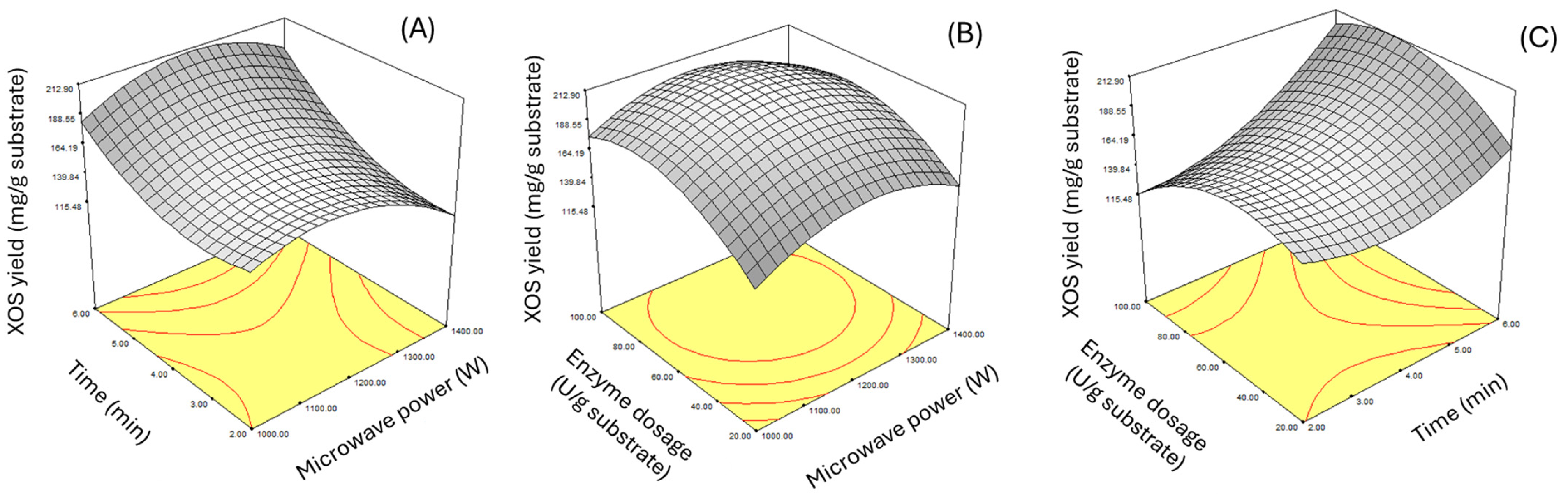
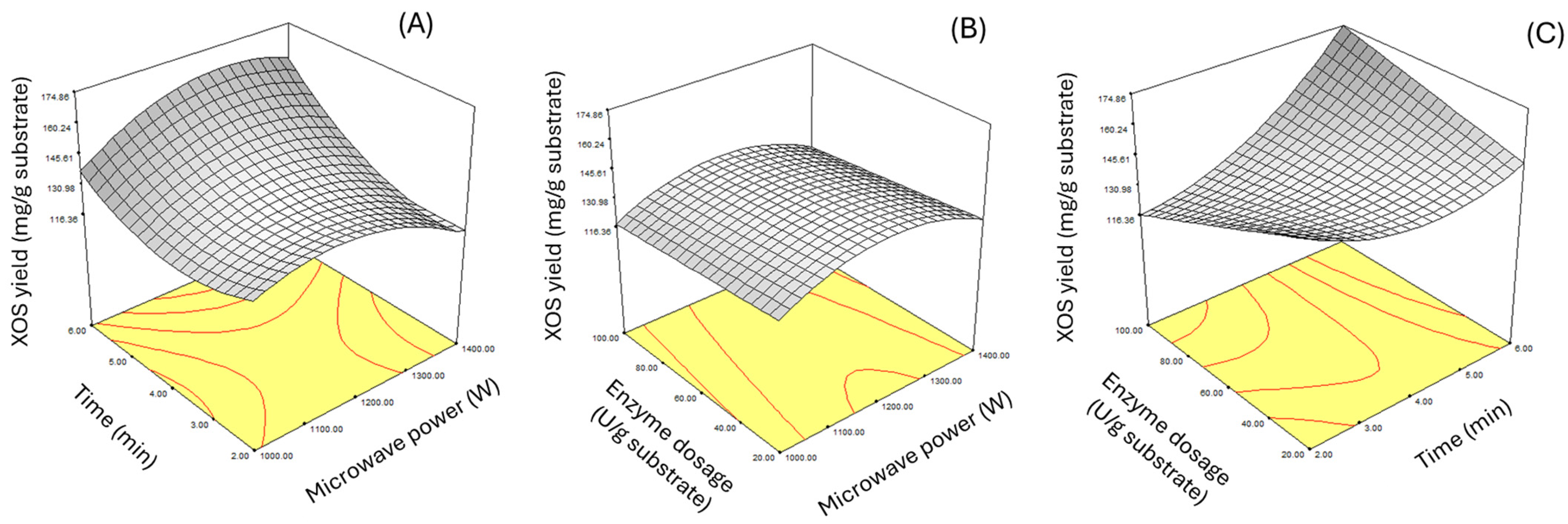
3.2. Optimization of Oligosaccharides Production Using Box-Behnken Design
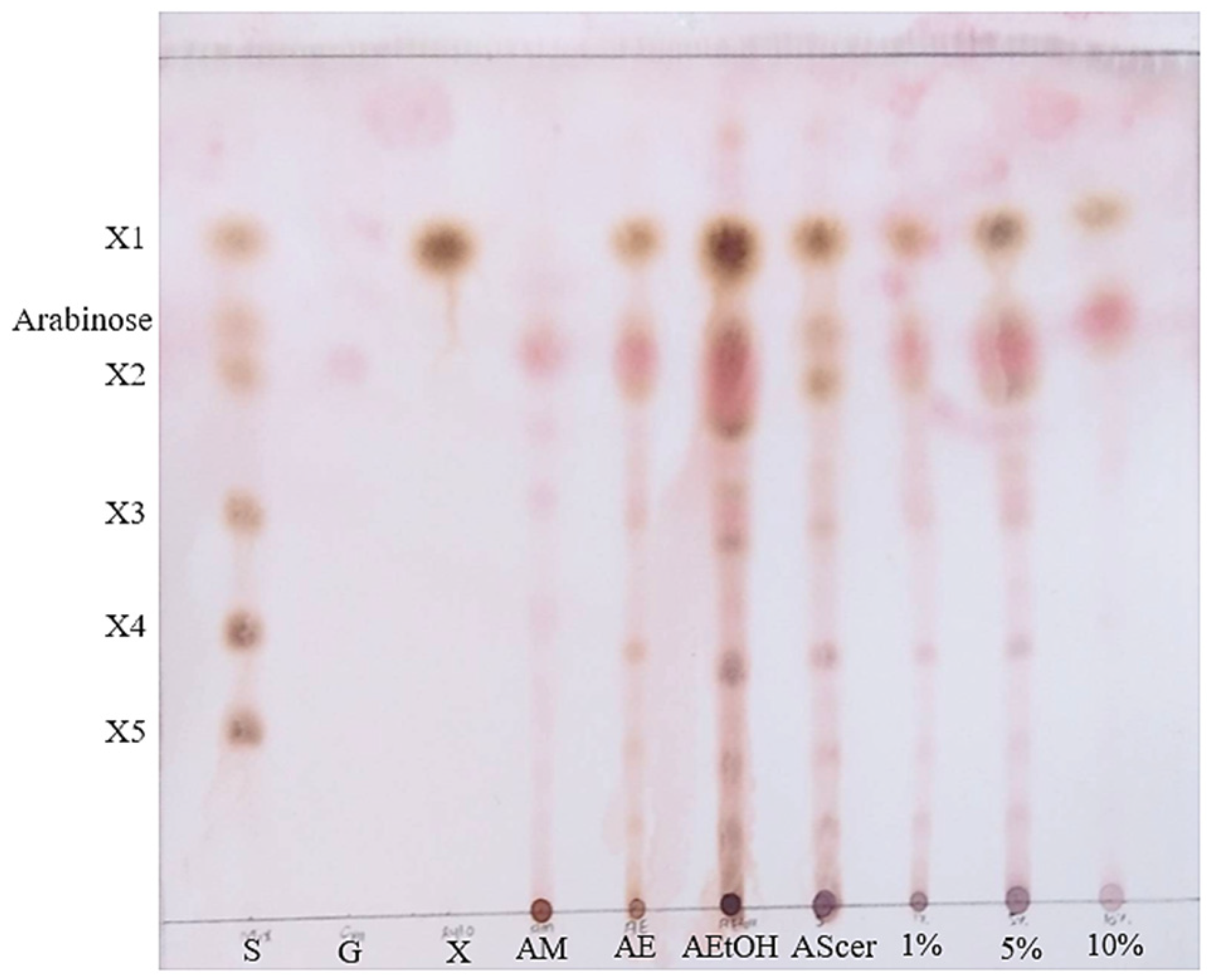
3.3. Purification of Xylooligosaccharides
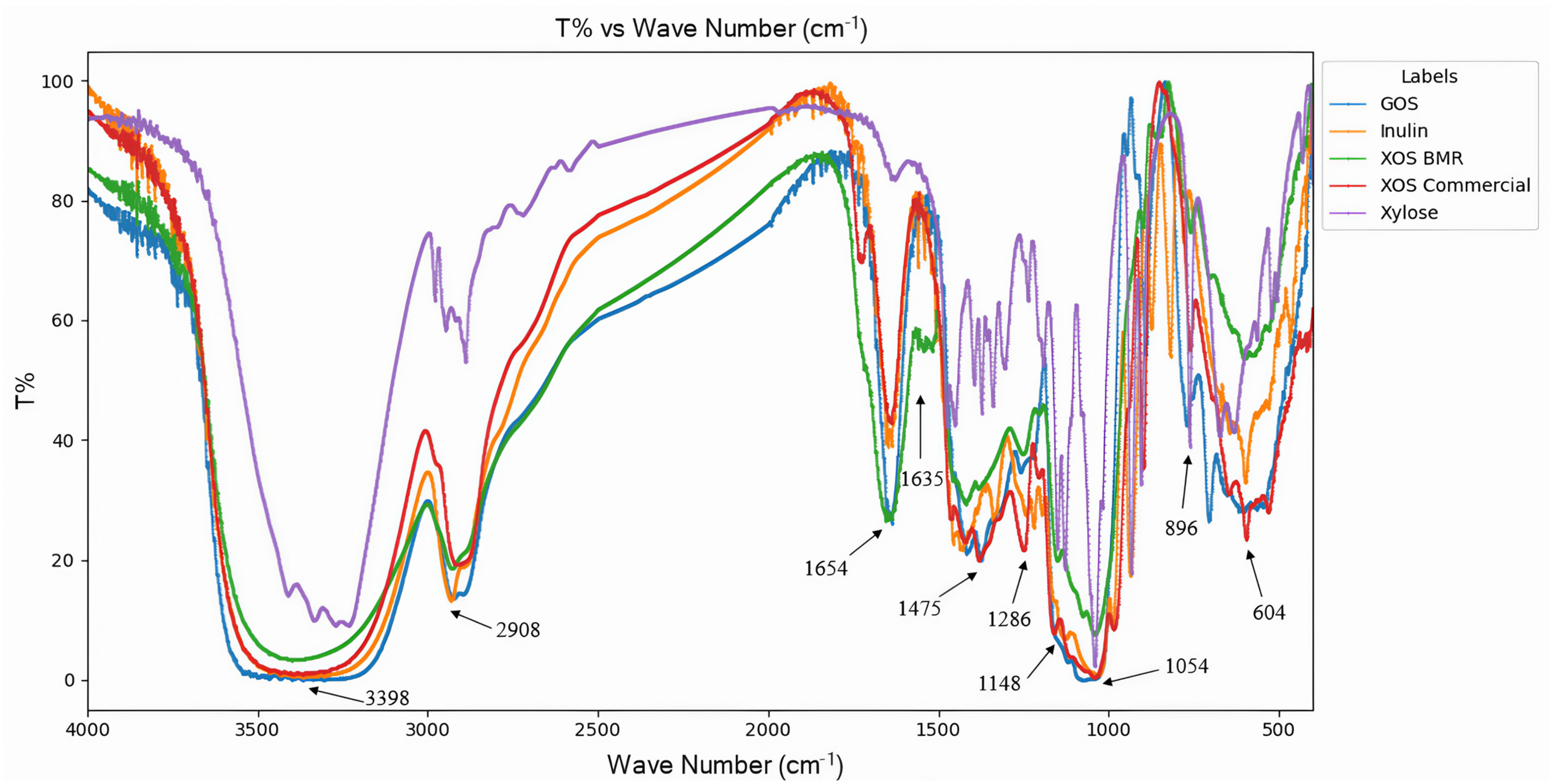
3.4. Structural Characterization of XOS
3.5. Growth Promotion of Probiotics by BMR-XOS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lynch, K.M.; Steffen, E.J.; Arendt, E.K. Brewers’ spent grain: A review with an emphasis on food and health. J. Inst. Brew. 2016, 122, 553–568. [Google Scholar] [CrossRef]
- Lebiocka, M.; Montusiewicz, A.; Szaja, A.; Rembisz, S.; Nowakowska, E. Thermophilic co-digestion of sewage sludge and brewery spent grain. J. Ecol. Eng. 2019, 20, 118–124. [Google Scholar] [CrossRef]
- Lalowski, P.; Zielińska, D. The most promising next-generation probiotic candidates—Impact on human health and potential application in food technology. Fermentation 2024, 10, 444. [Google Scholar] [CrossRef]
- Broekaert, W.F.; Courtin, C.M.; Verbeke, K.; Van de Wiele, T.; Verstraete, W.; Delcour, J.A. Prebiotic and other health-related effects of cereal-derived arabinoxylans, arabinoxylan-oligosaccharides, and xylooligosaccharides. Crit. Rev. Food Sci. Nutr. 2011, 51, 178–194. [Google Scholar] [CrossRef]
- Chen, M.; Liu, S.; Imam, K.M.S.U.; Sun, L.; Wang, Y.; Gu, T.; Wen, B.; Xin, F. The effect of xylooligosaccharide, xylan, and whole wheat bran on the human gut bacteria. Front. Microbiol. 2020, 11, 568457. [Google Scholar] [CrossRef]
- Roye, C.; Henrion, M.; Chanvrier, H.; Loret, C.; King, R.; Lamothe, L.; Courtin, C.M. Changing wheat bran structural properties by extrusion-cooking on a pilot and industrial scale: A comparative study. Foods 2021, 10, 472. [Google Scholar] [CrossRef]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Oniszczuk, T.; Gancarz, M.; Szymańska, J. Role of gut microbiota, probiotics and prebiotics in the cardiovascular diseases. Molecules 2021, 26, 1172. [Google Scholar] [CrossRef]
- Samanta, A.; Jayapal, N.; Jayaram, C.; Roy, S.; Kolte, A.; Senani, S.; Sridhar, M. Xylooligosaccharides as prebiotics from agricultural by-products: Production and applications. Bioact. Carbohydr. Diet. Fibre 2015, 5, 62–71. [Google Scholar] [CrossRef]
- Valladares-Diestra, K.K.; de Souza Vandenberghe, L.P.; Vieira, S.; Goyzueta-Mamani, L.D.; de Mattos, P.B.G.; Manzoki, M.C.; Soccol, V.T.; Soccol, C.R. The potential of xylooligosaccharides as prebiotics and their sustainable production from agro-industrial by-products. Foods 2023, 12, 2681. [Google Scholar] [CrossRef]
- Vázquez, M.J.; Alonso, J.L.; Domínguez, H.; Parajó, J.C. Xylooligosaccharides: Manufacture and applications. Trends Food Sci. Technol. 2000, 11, 387–393. [Google Scholar] [CrossRef]
- Akpinar, O.; Erdogan, K.; Bakir, U.; Yilmaz, L. Comparison of acid and enzymatic hydrolysis of tobacco stalk xylan for preparation of xylooligosaccharides. LWT-Food Sci. Technol. 2010, 43, 119–125. [Google Scholar] [CrossRef]
- Álvarez, C.; González, A.; Ballesteros, I.; Gullón, B.; Negro, M.J. In vitro assessment of the prebiotic potential of xylooligosaccharides from barley straw. Foods 2022, 12, 83. [Google Scholar] [CrossRef]
- Immerzeel, P.; Falck, P.; Galbe, M.; Adlercreutz, P.; Karlsson, E.N.; Stålbrand, H. Extraction of water-soluble xylan from wheat bran and utilization of enzymatically produced xylooligosaccharides by Lactobacillus, Bifidobacterium and Weissella spp. LWT-Food Sci. Technol. 2014, 56, 321–327. [Google Scholar] [CrossRef]
- Wang, T.-H.; Lu, S. Production of xylooligosaccharide from wheat bran by microwave assisted enzymatic hydrolysis. Food Chem. 2013, 138, 1531–1535. [Google Scholar] [CrossRef]
- Klangpetch, W.; Pattarapisitporn, A.; Phongthai, S.; Utama-ang, N.; Laokuldilok, T.; Tangjaidee, P.; Wirjantoro, T.I.; Jaichakan, P. Microwave-assisted enzymatic hydrolysis to produce xylooligosaccharides from rice husk alkali-soluble arabinoxylan. Sci. Rep. 2022, 12, 11. [Google Scholar]
- Coelho, E.; Rocha, M.A.M.; Saraiva, J.A.; Coimbra, M.A. Microwave superheated water and dilute alkali extraction of brewers’ spent grain arabinoxylans and arabinoxylo-oligosaccharides. Carbohydr. Polym. 2014, 99, 415–422. [Google Scholar] [CrossRef]
- Kormin, F.; Ahmed, I.; Yunus, R.; Yusof, Z.A.M. The potential of modified microwave extraction system (MMES) to extract bioactive components from ferns. Int. J. Eng. Technol. 2010, 10, 7–21. [Google Scholar]
- Aachary, A.A.; Prapulla, S.G. Xylooligosaccharides (XOS) as an emerging prebiotic: Microbial synthesis, utilization, structural characterization, bioactive properties, and applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 2–16. [Google Scholar] [CrossRef]
- Azwanida, N. A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med. Aromat. Plants 2015, 4, 1000196. [Google Scholar]
- Handa, S.; Khanuja, S.; Longo, G.; Rakesh, D. Extraction technologies for medicinal and aromatic plants: Earth. Environ. Marine Sci. Technol. 2008, 1, 21–25. [Google Scholar]
- Aguilar-Reynosa, A.; Romaní, A.; Rodriguez-Jasso, R.M.; Aguilar, C.N.; Garrote, G.; Ruiz, H.A. Microwave heating processing as alternative of pretreatment in second-generation biorefinery: An overview. Energy Convers. Manag. 2017, 136, 50–65. [Google Scholar] [CrossRef]
- Mobarec, H.; Villagomez, R.; Nordberg Karlsson, E.; Linares-Pastén, J.A. Microwave-assisted xylanase reaction: Impact in the production of prebiotic xylooligosaccharides. RSC Adv. 2021, 11, 11882–11888. [Google Scholar] [CrossRef] [PubMed]
- Gissibl, A.; Care, A.; Parker, L.M.; Iqbal, S.; Hobba, G.; Nevalainen, H.; Sunna, A. Microwave pretreatment of paramylon enhances the enzymatic production of soluble β-1, 3-glucans with immunostimulatory activity. Carbohydr. Polym. 2018, 196, 339–347. [Google Scholar] [CrossRef]
- Palm, M.; Zacchi, G. Extraction of hemicellulosic oligosaccharides from spruce using microwave oven or steam treatment. Biomacromolecules 2003, 4, 617–623. [Google Scholar] [CrossRef]
- Kumar, V.; Satyanarayana, T. Secretion of recombinant thermo-alkali-stable endoxylanase of polyextremophilic Bacillus halodurans TSEV1 and its utility in generating xylooligosaccharides from renewable agro-residues. Process Biochem. 2014, 49, 1875–1883. [Google Scholar] [CrossRef]
- Bonifácio-Lopes, T.; Catarino, M.D.; Vilas-Boas, A.A.; Ribeiro, T.B.; Campos, D.A.; Teixeira, J.A.; Pintado, M. Impact of circular brewer’s spent grain flour after in vitro gastrointestinal digestion on human gut microbiota. Foods 2022, 11, 2279. [Google Scholar] [CrossRef]
- Koirala, P.; Costantini, A.; Maina, H.N.; Rizzello, C.G.; Verni, M.; Beni, V.D.; Polo, A.; Katina, K.; Cagno, R.D.; Coda, R. Fermented brewers’ spent grain containing dextran and oligosaccharides as ingredient for composite wheat bread and its impact on gut metabolome in vitro. Fermentation 2022, 8, 487. [Google Scholar] [CrossRef]
- Maukonen, J.; Aura, A.-M.; Niemi, P.; Raza, G.S.; Niemelä, K.; Walkowiak, J.; Mattila, I.; Poutanen, K.; Buchert, J.; Herzig, K.-H. Interactions of Insoluble residue from enzymatic hydrolysis of brewer’s spent grain with intestinal microbiota in mice. J. Agric. Food Chem. 2017, 65, 3748–3756. [Google Scholar] [CrossRef]
- AOAC. Official Method of Analysis, 18th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2012. [Google Scholar]
- Tappi, T. 222 om-02: Acid-Insoluble Lignin in Wood and Pulp. 2002–2003 TAPPI Test Methods. 2002. Available online: https://www.tappi.org/content/sarg/t222.pdf (accessed on 15 December 2024).
- Tappi, T. Alpha-, Beta-and Gamma-cellulose in Pulp. TAPPI Stand. Test Methods 1999, 203, 5–9. [Google Scholar]
- Browning, B.L. Methods of Wood Chemistry. Volumes I & II; John Wiley & Sons: Hoboken, NJ, USA, 1967. [Google Scholar]
- Thipchai, P.; Jantanasakulwong, K.; Sawangrat, C.; Suhr, J.; Khotchapong, K.; Wattanachai, P.; Rachtanapun, P. Microstructural characterization of cellulose nanocrystals and microcellulose from bamboo (Bambusa longispatha) for reinforcing ordinary portland cement matrix. Polymers 2024, 16, 3558. [Google Scholar] [CrossRef]
- Zhu, Y.; Kim, T.H.; Lee, Y.; Chen, R.; Elander, R.T. Enzymatic production of xylooligosaccharides from corn stover and corn cobs treated with aqueous ammonia. App. Biochem. Biotechnol. 2006, 130, 586–598. [Google Scholar] [CrossRef]
- Cunha, J.T.; Romaní, A.; Inokuma, K.; Johansson, B.; Hasunuma, T.; Kondo, A.; Domingues, L. Consolidated bioprocessing of corn cob-derived hemicellulose: Engineered industrial Saccharomyces cerevisiae as efficient whole cell biocatalysts. Biotechnol. Biofuels 2020, 13, 138. [Google Scholar] [CrossRef]
- Kanpiengjai, A.; Nuntikaew, P.; Wongsanittayarak, J.; Leangnim, N.; Khanongnuch, C. Isolation of efficient xylooligosaccharides-fermenting probiotic lactic acid bacteria from ethnic pickled bamboo shoot products. Biology 2022, 11, 638. [Google Scholar] [CrossRef]
- Adapa, P.; Schonenau, L.; Canam, T.; Dumonceaux, T. Quantitative analysis of lignocellulosic components of non-treated and steam exploded barley, canola, oat and wheat straw using Fourier transform infrared spectroscopy. J. Agric. Sci. Technol. 2011, 1, 177–188. [Google Scholar]
- Trevizan, J.A.C.; Bido, G.d.S.; Ferrari, A.; Felipe, D.F. Nutritional composition of malted barley residue from brewery. J. Mgmt. Sustain. 2021, 11, 27. [Google Scholar] [CrossRef]
- Muthusamy, N. Chemical composition of brewers spent grain. Int. J. Sci. Environ. Technol 2014, 3, 2109–2112. [Google Scholar]
- Meneses, N.G.T.; Martins, S.; Teixeira, J.A.; Mussatto, S.I. Influence of extraction solvents on the recovery of antioxidant phenolic compounds from brewer’s spent grains. Sep. Purif. Technol. 2013, 108, 152–158. [Google Scholar] [CrossRef]
- Kanauchi, O.; Mitsuyama, K.; Araki, Y. Development of a functional germinated barley foodstuff from brewer’s spent grain for the treatment of ulcerative colitis. J. Am. Soc. Brew. Chem. 2001, 59, 59–62. [Google Scholar] [CrossRef]
- Pérocheau Arnaud, S. Valorisation of brewer’s spent grain: Lignocellulosic fractionation and its potential for polymer and composite material applications. Chem. Afr. 2024, 7, 2989–3010. [Google Scholar] [CrossRef]
- Santos, M.; Jiménez, J.; Bartolomé, B.; Gómez-Cordovés, C.; Del Nozal, M. Variability of brewer’s spent grain within a brewery. Food Chem. 2003, 80, 17–21. [Google Scholar] [CrossRef]
- Hughes, S.A.; Shewry, P.R.; Li, L.; Gibson, G.R.; Sanz, M.L.; Rastall, R.A. In vitro fermentation by human fecal microflora of wheat arabinoxylans. J. Agric. Food Chem. 2007, 55, 4589–4595. [Google Scholar] [CrossRef]
- Faria, N.T.; Marques, S.; Ferreira, F.C.; Fonseca, C. Production of xylanolytic enzymes by Moesziomyces spp. using xylose, xylan and brewery’s spent grain as substrates. New Biotechol. 2019, 49, 137–143. [Google Scholar] [CrossRef]
- Akpinar, O.; Erdogan, K.; Bostanci, S. Production of xylooligosaccharides by controlled acid hydrolysis of lignocellulosic materials. Carbohydr. Res. 2009, 344, 660–666. [Google Scholar] [CrossRef]
- Akpinar, O.; Ak, O.; Kavas, A.; Bakir, U.; Yilmaz, L. Enzymatic production of xylooligosaccharides from cotton stalks. J. Aric. Food Chem. 2007, 55, 5544–5551. [Google Scholar] [CrossRef]
- Bian, J.; Peng, F.; Peng, X.-P.; Peng, P.; Xu, F.; Sun, R.-C. Structural features and antioxidant activity of xylooligosaccharides enzymatically produced from sugarcane bagasse. Bioresour. Technol. 2013, 127, 236–241. [Google Scholar] [CrossRef]
- Rohman, A.; Dijkstra, B.W.; Puspaningsih, N.N.T. β-Xylosidases: Structural diversity, catalytic mechanism, and inhibition by monosaccharides. Int. J. Mol. Sci. 2019, 20, 5524. [Google Scholar] [CrossRef]
- Bastos, R.; Coelho, E.; Coimbra, M.A. Arabinoxylans from cereal by-products: Insights into structural features, recovery, and applications. In Sustainable Recovery and Reutilization of Cereal Processing by-Products; Elsevier: Amsterdam, The Netherlands, 2018; pp. 227–251. [Google Scholar]
- Chen, W.-H.; Tu, Y.-J.; Sheen, H.-K. Disruption of sugarcane bagasse lignocellulosic structure by means of dilute sulfuric acid pretreatment with microwave-assisted heating. Appl. Energy 2011, 88, 2726–2734. [Google Scholar] [CrossRef]
- Ethaib, S.; Omar, R.; Kamal, S.M.; Biak, D.A. Microwave-assisted pretreatment of lignocellulosic biomass: A review. J. Eng. Sci. Technol. 2015, 10, 97–109. [Google Scholar]
- Nayaka, A.; Bhushan, B. An overview of the recent trends on the waste valorization techniques for food waste. J. Environ. Manag. 2019, 233, 352–370. [Google Scholar] [CrossRef]
- Puligundla, P.; Oh, S.-E.; Mok, C. Microwave-assisted pretreatment technologies for the conversion of lignocellulosic biomass to sugars and ethanol: A review. Carbon Lett. 2016, 17, 1–10. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef]
- Binod, P.; Satyanagalakshmi, K.; Sindhu, R.; Janu, K.U.; Sukumaran, R.K.; Pandey, A. Short duration microwave assisted pretreatment enhances the enzymatic saccharification and fermentable sugar yield from sugarcane bagasse. Renew. Energy 2012, 37, 109–116. [Google Scholar] [CrossRef]
- Zhong, Y.; Liang, W.; Pu, H.; Blennow, A.; Liu, X.; Guo, D. Short-time microwave treatment affects the multi-scale structure and digestive properties of high-amylose maize starch. Int. J. Biol. Macromol. 2019, 137, 870–877. [Google Scholar] [CrossRef]
- Aisara, J.; Wongsanittayarak, J.; Leangnim, N.; Utama, K.; Sangthong, P.; Sriyotai, W.; Mahatheeranont, S.; Phongthai, S.; Unban, K.; Lumyong, S.; et al. Purification and characterization of crude fructooligosaccharides extracted from red onion (Allium cepa var. viviparum) by yeast treatment. Microb. Cell Fact. 2024, 23, 17. [Google Scholar] [CrossRef]
- Ku, Y.; Jansen, O.; Oles, C.J.; Lazar, E.Z.; Rader, J.I. Precipitation of inulins and oligoglucoses by ethanol and other solvents. Food Chem. 2003, 81, 125–132. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.; Zhang, W.; Wang, Y.; Ai, E.; Liu, Z.; Wei, L.; Li, Q. Synthesis of an eco-friendly xylooligosaccharides and its mechanistic evaluation in water-based drilling fluids. Sustainability 2023, 15, 15993. [Google Scholar] [CrossRef]
- Kathiresan, N.; Karuppiah, V.; Gopal, L.; Abraham, D.R.; Thangavel, K. Production and characterization of xylooligosaccharides from sugarcane bagasse using response surface methodology and its prebiotic properties. Biomass Convers. Biorefinery 2024, 1–15. [Google Scholar] [CrossRef]
- Hesam, F.; Tarzi, B.G.; Honarvar, M.; Jahadi, M. Pistachio (Pistacia vera) shell as a new candidate for enzymatic production of xylooligosaccharides. J. Food Meas. Charact. 2021, 15, 33–45. [Google Scholar] [CrossRef]
- Kačuráková, M.; Belton, P.S.; Wilson, R.H.; Hirsch, J.; Ebringerová, A. Hydration properties of xylan-type structures: An FTIR study of xylooligosaccharides. J. Sci. Food Agric. 1998, 77, 38–44. [Google Scholar] [CrossRef]
- Ohara, H.; Owaki, M.; Sonomoto, K. Xylooligosaccharide fermentation with Leuconostoc lactis. J. Biosci. Bioeng. 2006, 101, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Holt, S.M.; Miller-Fosmore, C.M.; Côté, G.L. Growth of various intestinal bacteria on alternansucrase-derived oligosaccharides. Lett. Appl. Microbiol. 2005, 40, 385–390. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Microwave power (Watts), A | 1000 | 1200 | 1400 |
| Exposure time (min), B | 2 | 4 | 6 |
| Enzyme dosage (U/g substrate), C | 20 | 60 | 100 |
| Run | Microwave Power (Watts), A | Exposure Time (min), B | Enzyme Dosage (U/g Substrate), C |
|---|---|---|---|
| 1 | 1000 | 2 | 60 |
| 2 | 1400 | 2 | 60 |
| 3 | 1000 | 6 | 60 |
| 4 | 1400 | 6 | 60 |
| 5 | 1000 | 4 | 20 |
| 6 | 1400 | 4 | 20 |
| 7 | 1000 | 4 | 100 |
| 8 | 1400 | 4 | 100 |
| 9 | 1200 | 2 | 20 |
| 10 | 1200 | 6 | 20 |
| 11 | 1200 | 2 | 100 |
| 12 | 1200 | 6 | 100 |
| 13 | 1200 | 4 | 60 |
| 14 | 1200 | 4 | 60 |
| 15 | 1200 | 4 | 60 |
| 16 | 1200 | 4 | 60 |
| 17 | 1200 | 4 | 60 |
| Composition | Content (%, w/w) | Composition | Content (%, w/w) |
|---|---|---|---|
| Crude fiber | 32.05 ± 0.15 | Holocellulose | 38.17 ± 1.12 |
| Carbohydrate | 37.04 ± 0.13 | Cellulose | 20.77 ± 0.31 |
| Protein | 20.64 ± 0.14 | Hemicellulose | 17.40 ± 0.37 |
| Fat | 4.00 ± 0.16 | Lignin | 14.50 ± 1.05 |
| Moisture content | 3.74 ± 0.24 | ||
| Total ash | 2.50 ± 0.18 |
| Run | A Microwave Power (Watts) | B Exposure Time (min) | C Enzyme Dosage (U/g Substrate) | Yield of XOS (mg/g Substrate) | |
|---|---|---|---|---|---|
| 4 h | 12 h | ||||
| 1 | 1000 | 2 | 60 | 154.4 ± 0.6 | 142.33 ± 0.8 |
| 2 | 1400 | 2 | 60 | 118.56 ± 0.7 | 107.09 ± 0.1 |
| 3 | 1000 | 6 | 60 | 180.8 ± 0.1 | 145.13 ± 0.2 |
| 4 | 1400 | 6 | 60 | 180.8 ± 0.9 | 145.13 ± 0.3 |
| 5 | 1000 | 4 | 20 | 121.48 ± 0.1 | 113.84 ± 0.9 |
| 6 | 1400 | 4 | 20 | 117.88 ± 0.5 | 137.91 ± 0.7 |
| 7 | 1000 | 4 | 100 | 111.17 ± 0.2 | 108.18 ± 0.6 |
| 8 | 1400 | 4 | 100 | 99.32 ± 0.3 | 130.43 ± 0.8 |
| 9 | 1200 | 2 | 20 | 137.67 ± 0.3 | 155.2 ± 0.4 |
| 10 | 1200 | 6 | 20 | 163.87 ± 0.9 | 152.58 ± 0.3 |
| 11 | 1200 | 2 | 100 | 118.94 ± 0.3 | 113.28 ± 0.1 |
| 12 | 1200 | 6 | 100 | 212.14 ± 0.3 | 176.8 ± 0.2 |
| 13 | 1200 | 4 | 60 | 164.47 ± 0.2 | 135.09 ± 0.4 |
| 14 | 1200 | 4 | 60 | 167.02 ± 0.1 | 139.22 ± 0.2 |
| 15 | 1200 | 4 | 60 | 164.81 ± 0.7 | 135.55 ± 0.6 |
| 16 | 1200 | 4 | 60 | 160.42 ± 0.3 | 135.15 ± 0.9 |
| 17 | 1200 | 4 | 60 | 163.54 ± 0.7 | 138.76 ± 0.9 |
| Variable | 4 h | 12 h | ||
|---|---|---|---|---|
| Coefficient Estimate | p-Value | Coefficient Estimate | p-Value | |
| Model/Intercept | 164.05 | 0.0005 sig | 136.75 | 0.0493 sig |
| A—Microwave power | −6.41 | 0.0953 | 1.39 | 0.7401 |
| B—Exposure time | 26.01 | 0.0001 | 12.72 | 0.0157 |
| C—Enzyme dosage | 0.084 | 0.9806 | −3.85 | 0.3686 |
| A2 | −25.55 | 0.0008 | −14.35 | 0.0356 |
| B2 | 20.14 | 0.0032 | 12.52 | 0.0580 |
| C2 | −26.04 | 0.0008 | 0.19 | 0.9735 |
| AB | 8.96 | 0.0985 | 8.81 | 0.1644 |
| AC | −2.06 | 0.6743 | −0.45 | 0.9383 |
| BC | 16.75 | 0.0092 | 16.54 | 0.0225 |
| R-Squared (R2) | 0.9587 | 0.8262 | ||
| Adjusted R-Squared | 0.9056 | 0.6028 | ||
| Carbon Sources | L. lactis TISTR 1401 | L. brevis FS 2.1 | L. casei TISTR 1463 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Max. Viable Cell (logCFU/mL) | μmax (h−1) | Final pH at 48 h | Max. Viable Cell (logCFU/mL) | μmax (h−1) | Final pH at 48 h | Max. Viable Cell (logCFU/mL) | μmax (h−1) | Final pH at 48 h | |
| Glucose | 9.26 ± 0.17 a | 1.006 | 4.19 | 8.88 ± 0.05 b | 0.731 | 4.08 | 9.13 ± 0.01 ab | 0.368 | 4.15 |
| Xylose | 10.43 ± 0.01 a | 1.222 | 4.37 | 10.39 ± 0.01 b | 0.870 | 4.41 | 9.39 ± 0.02 c | 0.145 | 4.38 |
| Commercial XOS | 10.36 ± 0.03 a | 0.993 | 4.39 | 10.18 ± 0.01 b | 0.791 | 4.43 | 9.42 ± 0.01 c | 0.403 | 4.79 |
| Commercial GOS | 9.39 ± 0.01 b | 0.955 | 5.24 | 8.37 ± 0.01 c | 0.551 | 4.75 | 10.31 ± 0.02 a | 0.510 | 4.74 |
| Commercial Inulin | 9.40 ± 0.01 a | 0.406 | 5.31 | 9.37 ± 0.01 b | 0.813 | 3.79 | 9.41 ± 0.01 a | 1.114 | 3.72 |
| BMR-XOS | 10.38 ± 0.01 a | 1.085 | 4.72 | 10.31 ± 0.01 b | 0.891 | 4.82 | 10.36 ± 0.01 a | 0.532 | 4.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fareed, S.Z.; Tangjaidee, P.; Khumsap, T.; Klangpetch, W.; Phongthai, S.; Kanpiengjai, A.; Khanongnuch, C.; Unban, K. Xylooligosaccharides from Barley Malt Residue Produced by Microwave-Assisted Enzymatic Hydrolysis and Their Potential Uses as Prebiotics. Plants 2025, 14, 769. https://doi.org/10.3390/plants14050769
Fareed SZ, Tangjaidee P, Khumsap T, Klangpetch W, Phongthai S, Kanpiengjai A, Khanongnuch C, Unban K. Xylooligosaccharides from Barley Malt Residue Produced by Microwave-Assisted Enzymatic Hydrolysis and Their Potential Uses as Prebiotics. Plants. 2025; 14(5):769. https://doi.org/10.3390/plants14050769
Chicago/Turabian StyleFareed, Shah Zaib, Pipat Tangjaidee, Tabkrich Khumsap, Wannaporn Klangpetch, Suphat Phongthai, Apinun Kanpiengjai, Chartchai Khanongnuch, and Kridsada Unban. 2025. "Xylooligosaccharides from Barley Malt Residue Produced by Microwave-Assisted Enzymatic Hydrolysis and Their Potential Uses as Prebiotics" Plants 14, no. 5: 769. https://doi.org/10.3390/plants14050769
APA StyleFareed, S. Z., Tangjaidee, P., Khumsap, T., Klangpetch, W., Phongthai, S., Kanpiengjai, A., Khanongnuch, C., & Unban, K. (2025). Xylooligosaccharides from Barley Malt Residue Produced by Microwave-Assisted Enzymatic Hydrolysis and Their Potential Uses as Prebiotics. Plants, 14(5), 769. https://doi.org/10.3390/plants14050769







