Unveiling the Potential Role of Dhurrin in Sorghum During Infection by the Head Smut Pathogen Sporisorium reilianum f. sp. reilianum
Abstract
1. Introduction
2. Results
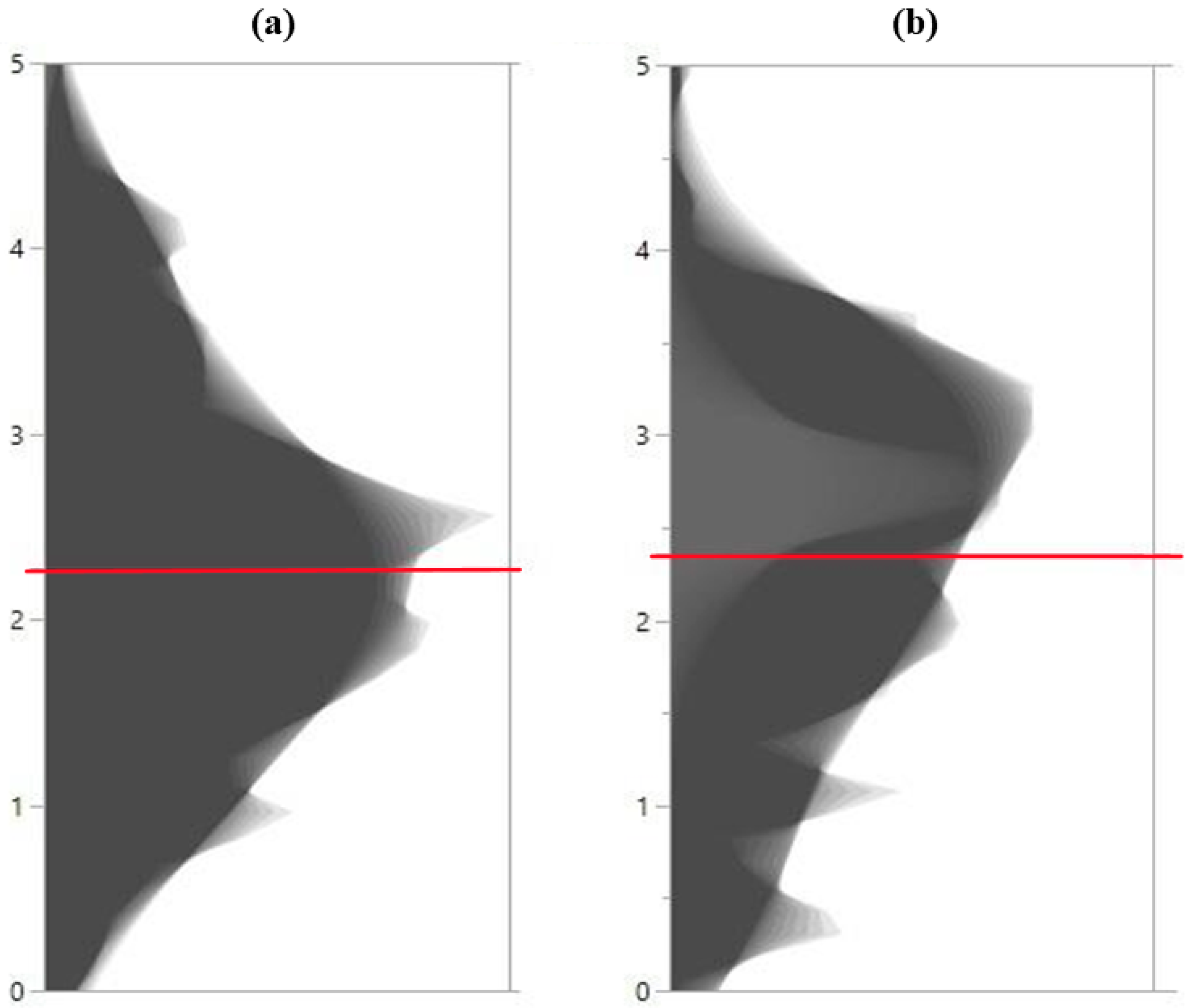
2.1. Distribution of HCNp Scores in Sorghum Accessions and Principal Component Analysis
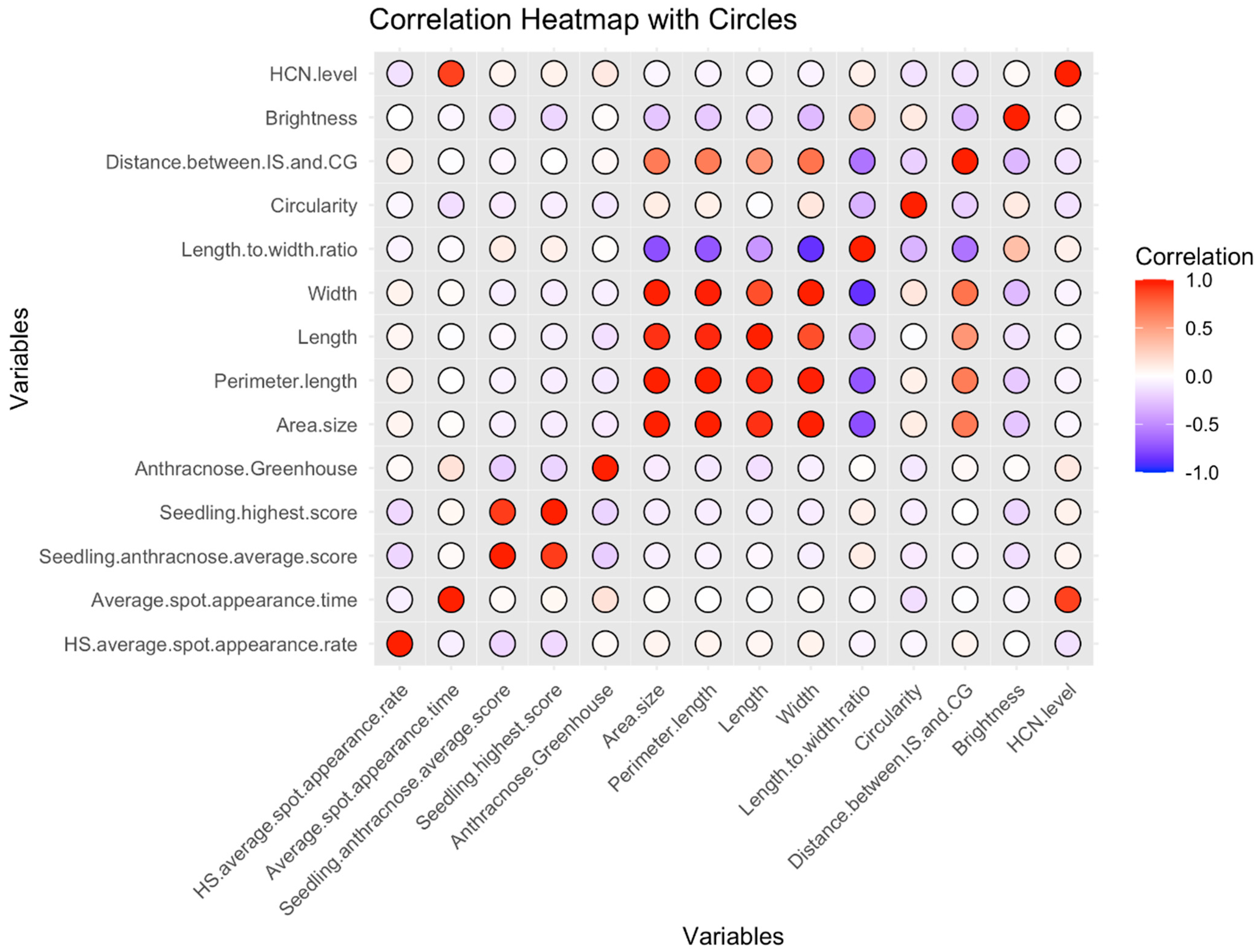
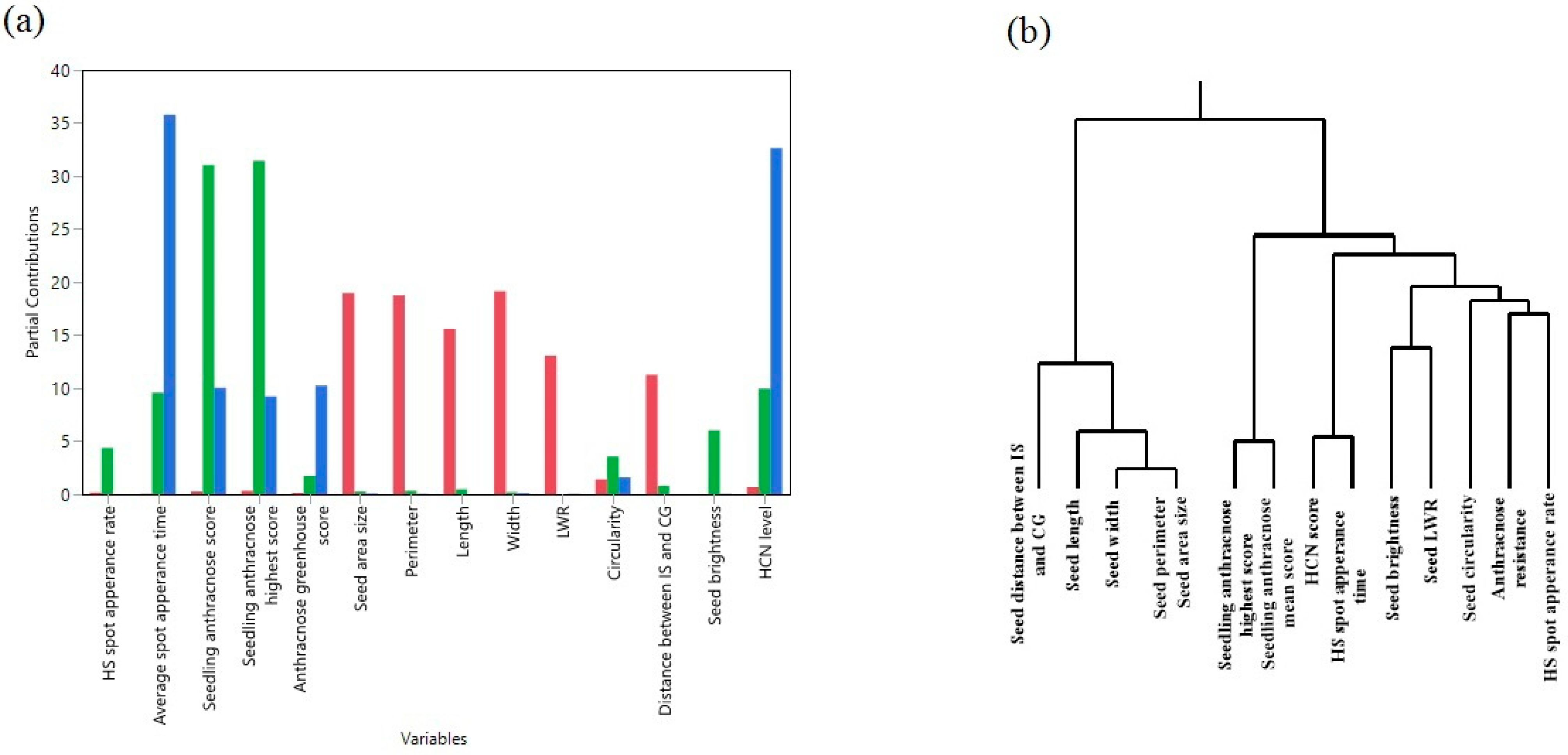
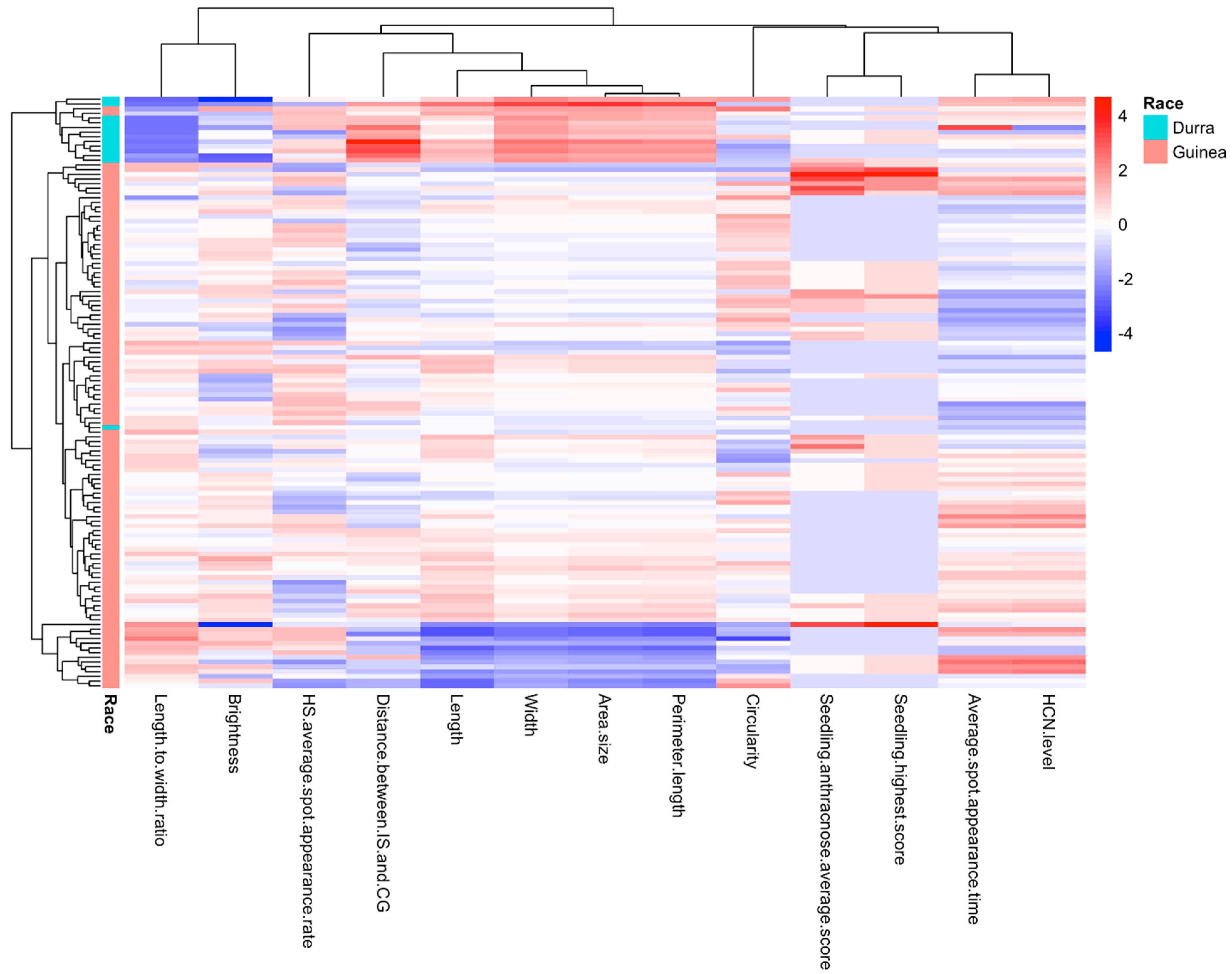
2.2. Correlation Between HCNp and Seed Morphology/Pathogen Inoculation Response Traits
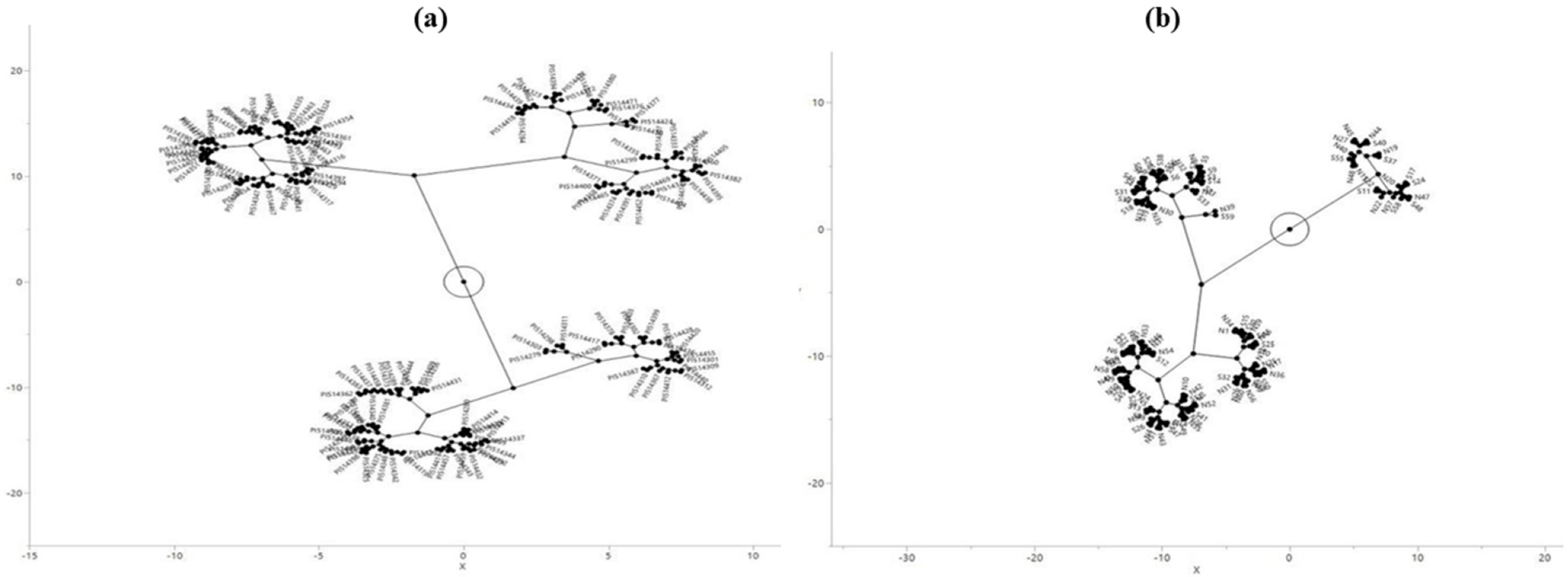
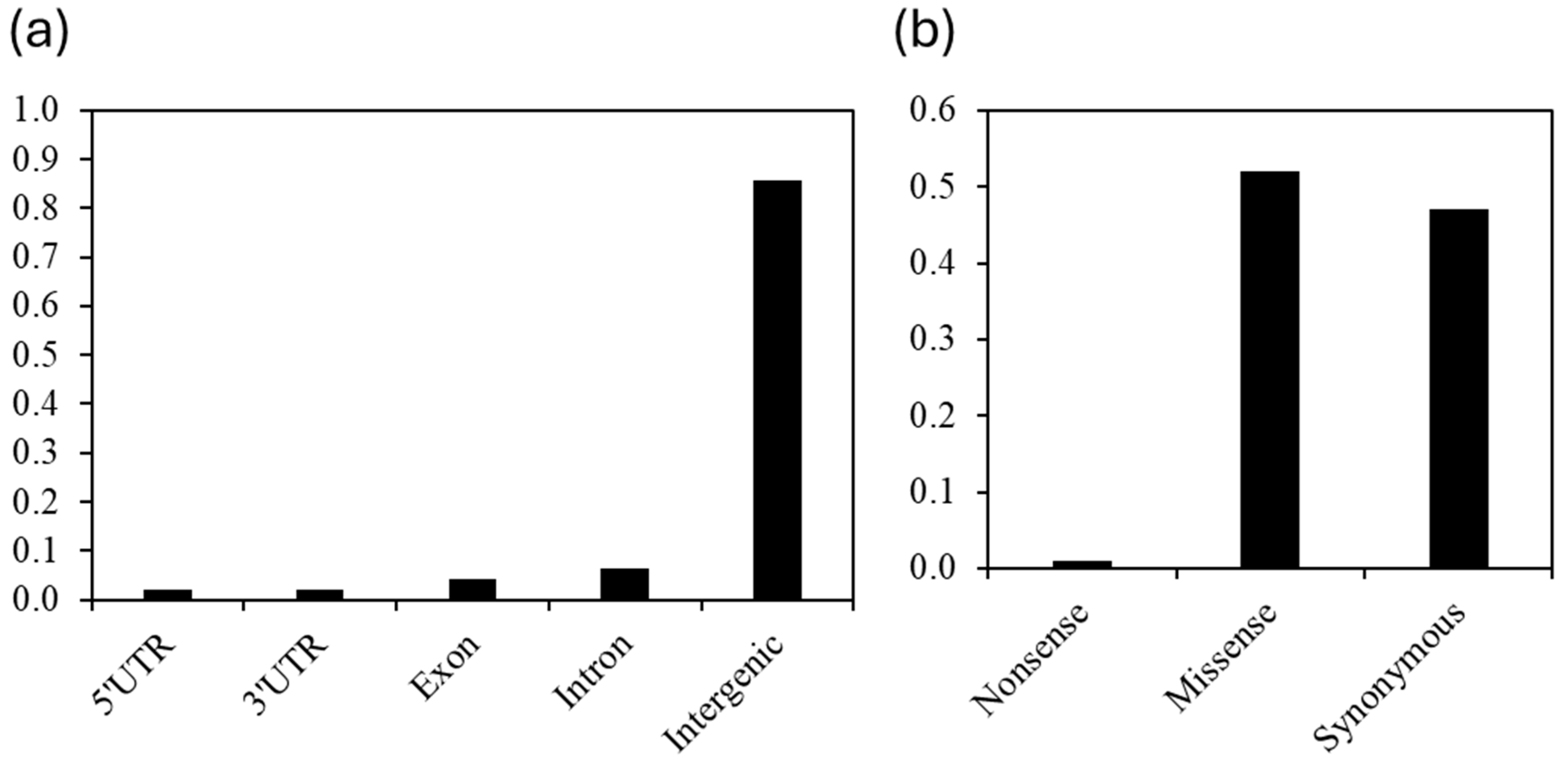
2.3. Distribution and Characteristics of SNPs in the C2 Accessions/Lines from Niger and Senegal
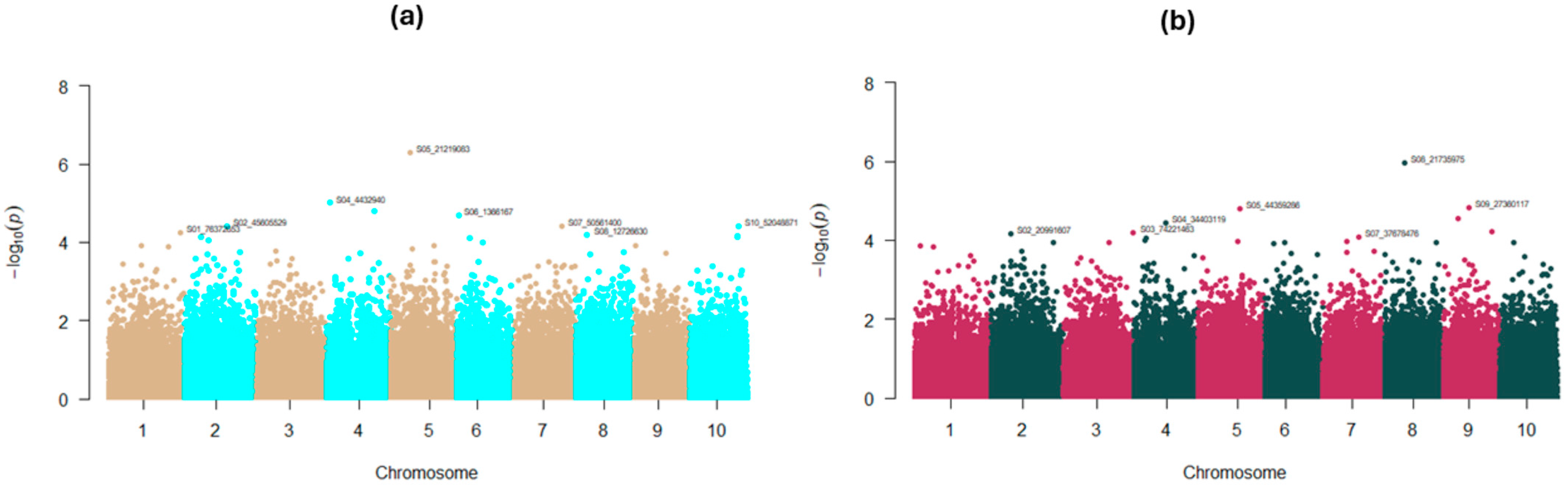
2.4. GWAS
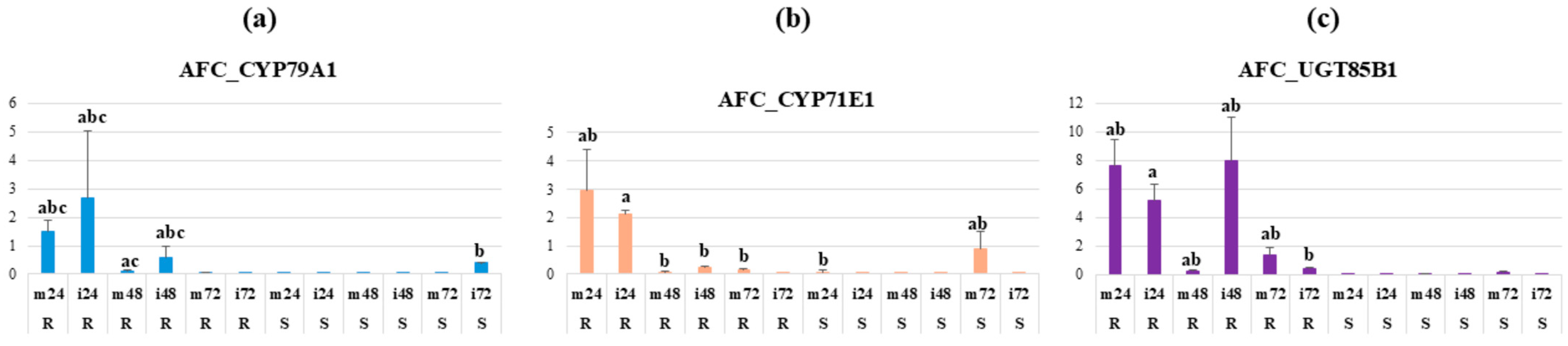
2.5. Expression of the Dhurrin Biosynthetic Genes upon Inoculation with SRS
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Fungal Material
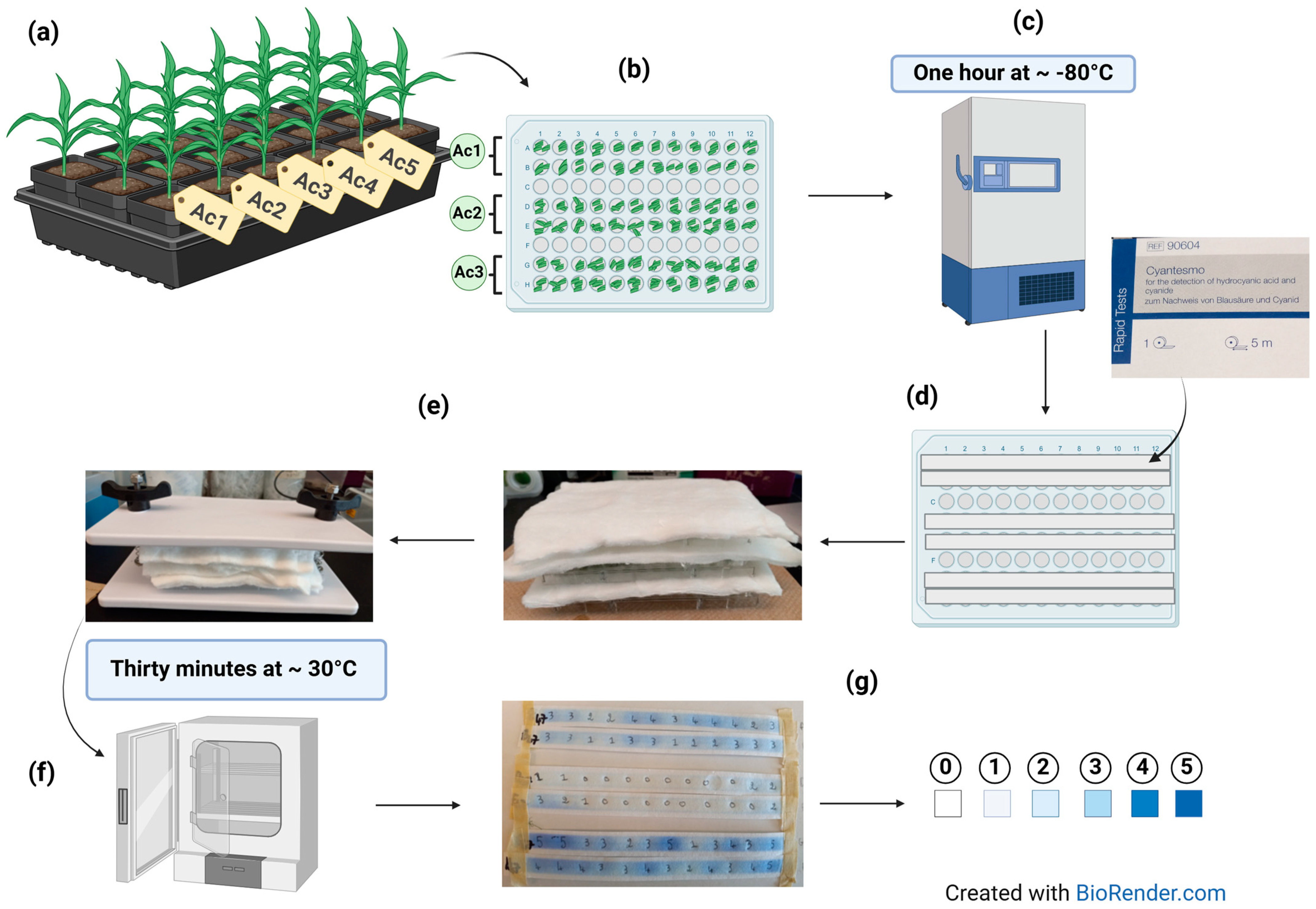
4.3. HCNp Rating
4.4. Gene Expression Study
4.4.1. Head Smut Pathogen Isolation and Identification
4.4.2. Oligonucleotides
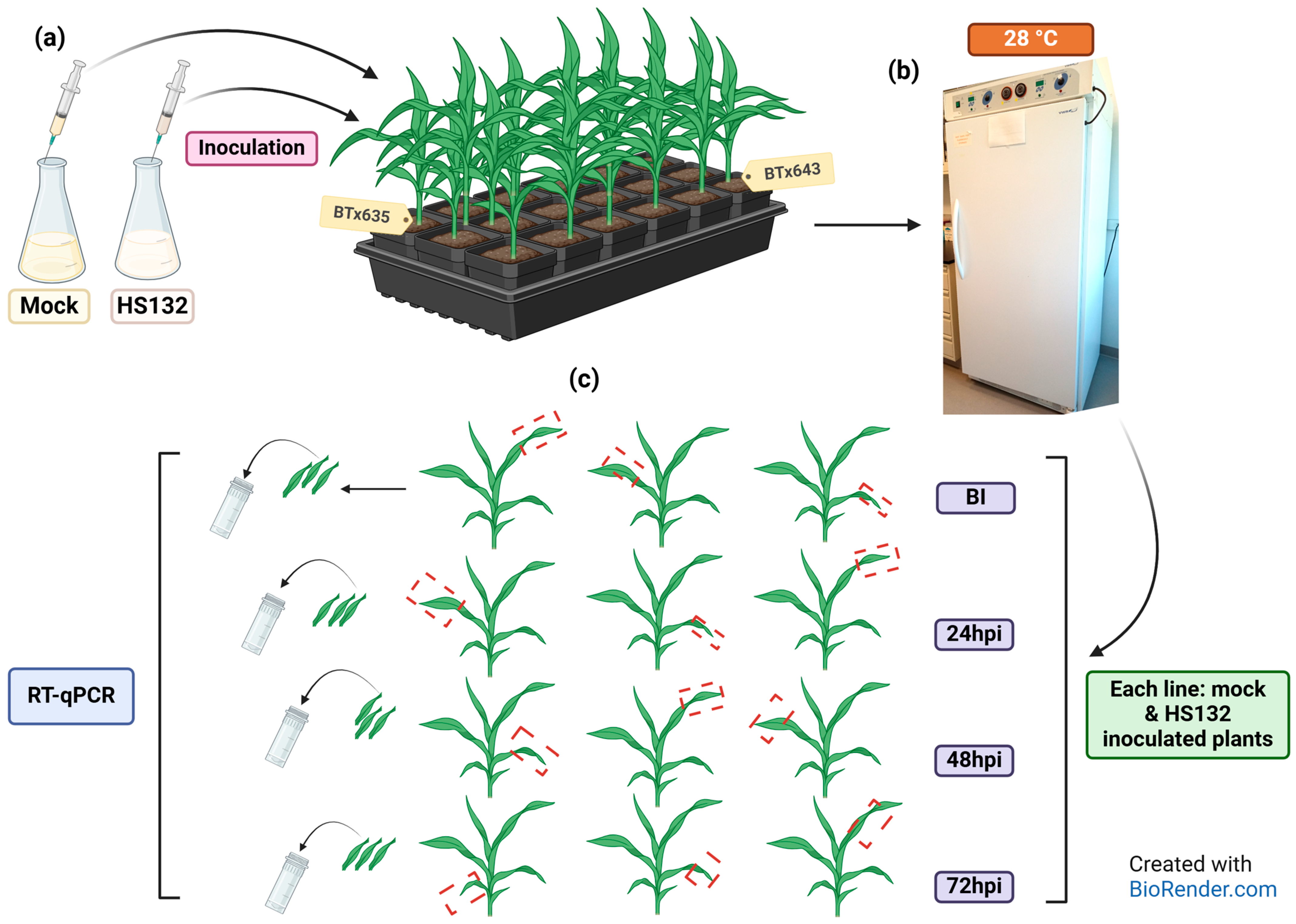
4.4.3. Plants
4.4.4. Inoculation and Sampling
4.4.5. RT-qPCR
4.5. SNP Dataset
4.6. Data Analysis
4.6.1. Distribution of HCNp Scores and Principal Component Analysis (PCA)
4.6.2. Correlation Between HCNp and Seed Morphology/Pathogen Inoculation Response Traits
4.6.3. Distribution and Properties of the SNPs in the Sorghum from C2
4.6.4. GWAS
4.6.5. RT-qPCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO; US Department of Agriculture. Worldwide Production of Grain in 2023/24, by Type (in Million Metric Tons)*. Statista. 12 January. 2024. Available online: https://www.statista.com/statistics/263977/world-grain-production-by-type/ (accessed on 7 May 2024).
- Khalifa, M.; Eltahir, E.A.B. Assessment of Global Sorghum Production, Tolerance, and Climate Risk. Front. Sustain. Food Syst. 2023, 7, 1184373. [Google Scholar] [CrossRef]
- Yulvianti, M.; Zidorn, C. Chemical Diversity of Plant Cyanogenic Glycosides: An Overview of Reported Natural Products. Molecules 2021, 26, 719. [Google Scholar] [CrossRef]
- Gleadow, R.M.; Møller, B.L. Cyanogenic glycosides: Synthesis, physiology, and phenotypic plasticity. Annu. Rev. Plant. Biol. 2014, 65, 155–185. [Google Scholar] [CrossRef] [PubMed]
- Emendack, Y.; Burke, J.; Laza, H.; Sanchez, J.; Hayes, C. Abiotic Stress Effects on Sorghum Leaf Dhurrin and Soluble Sugar Contents Throughout Plant Development. Crop Sci. 2018, 58, 1706–1716. [Google Scholar] [CrossRef]
- Wang, B.; Xiong, W.; Guo, Y. Dhurrin in Sorghum: Biosynthesis, Regulation, Biological Function and Challenges for Animal Production. Plants 2024, 13, 2291. [Google Scholar] [CrossRef]
- Hansen, C.C.; Sørensen, M.; Veiga, T.A.M.; Zibrandtsen, J.F.S.; Heskes, A.M.; Olsen, C.E.; Boughton, B.A.; Møller, B.L.; Neilson, E.H.J. Reconfigured Cyanogenic Glucoside Biosynthesis in Eucalyptus cladocalyx Involves a Cytochrome P450 CYP706C55. Plant Physiol. 2018, 178, 1081–1095. [Google Scholar] [CrossRef] [PubMed]
- Darbani, B.; Motawia, M.S.; Olsen, C.E.; Nour-Eldin, H.H.; Møller, B.L.; Rook, F. The Biosynthetic Gene Cluster for the Cyanogenic Glucoside Dhurrin in Sorghum bicolor Contains Its Co-expressed Vacuolar MATE Transporter. Sci. Rep. 2016, 6, 37079. [Google Scholar] [CrossRef]
- Sibbesen, O.; Koch, B.; Halkier, B.A.; Møller, B.L. Isolation of the Heme-thiolate Enzyme Cytochrome P-450TYR, Which Catalyzes the Committed Step in the Biosynthesis of the Cyanogenic Glucoside Dhurrin in Sorghum bicolor (L.) Moench. Proc. Natl. Acad. Sci. USA 1994, 91, 9740–9744. [Google Scholar] [CrossRef] [PubMed]
- Sibbesen, O.; Koch, B.; Halkier, B.A.; Møller, B.L. Cytochrome P-450TYR Is a Multifunctional Heme-thiolate Enzyme Catalyzing the Conversion of L-tyrosine to P-hydroxyphenylacetaldehyde Oxime in the Biosynthesis of the Cyanogenic Glucoside Dhurrin in Sorghum bicolor (L.) Moench. J. Biol. Chem. 1995, 270, 3506–3511. [Google Scholar] [CrossRef]
- Bak, S.; Kahn, R.A.; Nielsen, H.L.; Møller, B.L.; Halkier, B.A. Cloning of three A-type cytochromes P450, CYP71E1, CYP98, and CYP99 from Sorghum bicolor (L.) Moench by a PCR approach and identification by expression in Escherichia coli of CYP71E1 as a multifunctional cytochrome P450 in the biosynthesis of the cyanogenic glucoside dhurrin. Plant Mol. Biol. 1998, 36, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.R.; Møller, B.L.; Høj, P.B. The Udp-glucose:p-hydroxymandelonitrile-o-glucosyltransferase That Catalyzes the Last Step in Synthesis of the Cyanogenic Glucoside Dhurrin in Sorghum bicolor. J. Biol. Chem. 1999, 274, 35483–35491. [Google Scholar] [CrossRef] [PubMed]
- Morant, A.V.; Jørgensen, K.; Jørgensen, C.; Paquette, S.M.; Sánchez-Pérez, R.; Møller, B.L.; Bak, S. β-glucosidases as detonators of plant chemical defense. Phytochemistry 2008, 69, 1795–1813. [Google Scholar] [CrossRef]
- Kojima, M.; Poulton, J.E.; Thayer, S.S.; Conn, E.E. Tissue Distributions of Dhurrin and of Enzymes Involved in Its Metabolism in Leaves of Sorghum bicolor. Plant Physiol. 1979, 63, 1022–1028. [Google Scholar] [CrossRef]
- Cicek, M.; Esen, A. Structure and Expression of a Dhurrinase (β-glucosidase) from Sorghum1. Plant Physiol. 1998, 116, 1469–1478. [Google Scholar] [CrossRef]
- Rosati, V.C.; Blomstedt, C.K.; Møller, B.L.; Garnett, T.; Gleadow, R. The Interplay Between Water Limitation, Dhurrin, and Nitrate in the Low-cyanogenic Sorghum Mutant Adult Cyanide Deficient Class 1. Front. Plant Sci. 2019, 10, 1458. [Google Scholar] [CrossRef] [PubMed]
- Cowan, M.; Møller, B.L.; Norton, S.; Knudsen, C.; Crocoll, C.; Furtado, A.; Henry, R.; Blomstedt, C.; Gleadow, R.M. Cyanogenesis in the Sorghum Genus: From Genotype to Phenotype. Genes 2022, 13, 140. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.H.; Karthikeyan, B.J.; Agasinayi, S.; Raja, R.B.; Thiruvengadan, V.; Ram, S.G. Rapid screening assay for precise and reliable estimation of cyanide content in sorghum. Aust. J. Crop. Sci. 2016, 10, 1388–1392. [Google Scholar] [CrossRef]
- Johnson, K. Phenotyping Tools and Genetic Knowledge to Facilitate Breeding of Dhurrin Content and Cyanogenic Potential in Sorghum. Master’s Thesis, Colorado State University, Fort Collins, CO, USA, 2023. Available online: https://mountainscholar.org/items/8fbd25ae-8cd5-4639-9a5d-89be0c07d648 (accessed on 8 August 2024).
- Feigl, F.; Anger, V. Replacement of Benzidine by Copper Ethylacetoacetate and Tetra Base as Spot-test Reagent for Hydrogen Cyanide and Cyanogen. Analyst 1966, 91, 282. [Google Scholar] [CrossRef]
- Kakes, P. A rapid and sensitive method to detect cyanogenesis using microtiter plates. Biochem. Syst. Ecol. 1991, 19, 519–522. [Google Scholar] [CrossRef]
- Gleadow, R.M.; Mckinley, B.A.; Blomstedt, C.K.; Lamb, A.C.; Møller, B.L.; Mullet, J.E. Regulation of Dhurrin Pathway Gene Expression During Sorghum bicolor Development. Planta 2021, 254, 119. [Google Scholar] [CrossRef] [PubMed]
- Hayes, C.M.; Burow, G.B.; Brown, P.J.; Thurber, C.; Xin, Z.; Burke, J.J. Natural Variation in Synthesis and Catabolism Genes Influences Dhurrin Content in Sorghum. Plant Genome 2015, 8, plantgenome2014. [Google Scholar] [CrossRef] [PubMed]
- Prom, L.K.; Ahn, E.J.S.; Perumal, R.; Isakeit, T.S.; Odvody, G.N.; Magill, C.W. Genetic and Pathogenic Variability Among Isolates of Sporisorium reilianum Causing Sorghum Head Smut. J. Fungi 2024, 10, 62. [Google Scholar] [CrossRef]
- Ramasamy, P.; Frederiksen, R.A.; Prom, L.K.; Magill, C.W. Head Smut in 2007. In Screening Techniques for Sorghum Diseases. Information Bulletin No. 76; Thakur, R.P., Reddy, B.V.S., Mathur, K., Eds.; Patancheru 502 324; International Crops Research Institute for the Semi-Arid Tropics: Andhra Pradesh, India, 2007; p. 92. ISBN 978-92-9066-504-5. [Google Scholar]
- Poloni, A.; Schirawski, J. Host Specificity in Sporisorium reilianum Is Determined by Distinct Mechanisms in Maize and Sorghum. Mol. Plant Pathol. 2016, 17, 741–754. [Google Scholar] [CrossRef]
- Abreha, K.B.; Ortiz, R.; Carlsson, A.S.; Geleta, M. Understanding the Sorghum–Colletotrichum sublineola Interactions for Enhanced Host Resistance. Front. Plant Sci. 2021, 12, 641969. [Google Scholar] [CrossRef]
- Ahn, E.; Prom, L.K.; Hu, Z.; Odvody, G.; Magill, C. Genome-wide Association Analysis for Response of Senegalese Sorghum Accessions to Texas Isolates of Anthracnose. Plant Genome 2021, 14, e20097. [Google Scholar] [CrossRef] [PubMed]
- Ahn, E.; Fall, C.; Prom, L.K.; Magill, C. A Genome-wide Association Study of Senegalese Sorghum Seedlings Responding to Pathotype 5 of Sporisorium reilianum. Plants 2022, 11, 2999. [Google Scholar] [CrossRef]
- Ahn, E.; Fall, C.; Prom, L.K.; Magill, C. Genome-wide Association Study of Senegalese Sorghum Seedlings Responding to a Texas Isolate of Colletotrichum sublineola. Sci. Rep. 2022, 12, 13025. [Google Scholar] [CrossRef] [PubMed]
- Ahn, E.; Botkin, J.; Ellur, V.; Lee, Y.; Poudel, K.; Prom, L.K.; Magill, C. Genome-wide Association Study of Seed Morphology Traits in Senegalese Sorghum Cultivars. Plants 2023, 12, 2344. [Google Scholar] [CrossRef]
- Prom, L.K.; Botkin, J.R.; Ahn, E.J.S.; Sarr, M.P.; Diatta, C.; Fall, C.; Magill, C.W. A Genome-wide Association Study of Nigerien and Senegalese Sorghum Germplasm of Exserohilum turcicum, the Causal Agent of Leaf Blight. Plants 2023, 12, 4010. [Google Scholar] [CrossRef]
- Paterson, A.H.; Bowers, J.E.; Bruggmann, R.; Dubchak, I.; Grimwood, J.; Gundlach, H.; Haberer, G.; Hellsten, U.; Mitros, T.; Poliakov, A. The Sorghum bicolor Genome and the Diversification of Grasses. Nature 2009, 457, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Cingolani, P.; Platts, A.; Wang, L.L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A Program for Annotating and Predicting the Effects of Single Nucleotide Polymorphisms, Snpeff. Fly 2012, 6, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Wilke, C.O. The Speed of Adaptation in Large Asexual Populations. Genetics 2004, 167, 2045–2053. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.T.; Salas Fernandez, M.G.; Casa, A.M.; Mitchell, S.E.; Paterson, A.H.; Kresovich, S. Equilibrium Processes Cannot Explain High Levels of Short- and Medium-range Linkage Disequilibrium in the Domesticated Grass Sorghum bicolor. Genetics 2005, 171, 1247–1256. [Google Scholar] [CrossRef]
- Manils, J.; Marruecos, L.; Soler, C. Exonucleases: Degrading DNA to Deal with Genome Damage, Cell Death, Inflammation and Cancer. Cells 2022, 11, 2157. [Google Scholar] [CrossRef] [PubMed]
- Kayukawa, K.; Kitajima, Y.; Tamura, T. TBP-interacting protein TIP120A is a new global transcription activator with bipartite functional domains. Genes Cells 2001, 6, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Rai, A.K.; Kanwar, S.S.; Sharma, T.R. Comparative Analysis of Zinc Finger Proteins Involved in Plant Disease Resistance. PLoS ONE 2012, 7, e42578. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lian, L.; Qi, J.; Fang, Y.; Nyporko, A.; Yu, Q.; Bai, L.; Pan, L. Metabolic Resistance to Acetolactate Synthase Inhibitors in Beckmannia syzigachne: Identification of CYP81Q32 and Its Transcription Regulation. Plant J. 2023, 115, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Pandian, B.A.; Sathishraj, R.; Djanaguiraman, M.; Prasad, P.V.V.; Jugulam, M. Role of Cytochrome P450 Enzymes in Plant Stress Response. Antioxidants 2020, 9, 454. [Google Scholar] [CrossRef]
- Dos Santos, C.; Franco, O.L. Pathogenesis-related Proteins (prs) with Enzyme Activity Activating Plant Defense Responses. Plants 2023, 12, 2226. [Google Scholar] [CrossRef]
- Zan, X.; Zhou, Z.; Wan, J.; Chen, H.; Zhu, J.; Xu, H.; Zhang, J.; Li, X.; Gao, X.; Chen, R. Overexpression of Oshad3, a Member of HAD Superfamily, Decreases Drought Tolerance of Rice. Rice 2023, 16, 31. [Google Scholar] [CrossRef]
- Kahn, R.A.; Fahrendorf, T.; Halkier, B.A.; Møller, B.M. Substrate specificity of the cytochrome P450 enzymes CYP79A1 and CYP71E1 involved in the biosynthesis of the cyanogenic glucoside dhurrin in Sorghum bicolor (L.) Moench. Arch Biochem Biophys. 1999, 363, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, L.J.; Stuart, P.; Pičmanová, M.; Rasmussen, S.; Olsen, C.E.; Harholt, J.; Møller, B.L.; Bjarnholt, N. Dhurrin Metabolism in the Developing Grain of Sorghum bicolor (L.) Moench Investigated by Metabolite Profiling and Novel Clustering Analyses of Time-resolved Transcriptomic Data. BMC Genom. 2016, 17, 1021. [Google Scholar] [CrossRef]
- Stutts, L.R.; Vermerris, W. Elucidating Anthracnose Resistance Mechanisms in Sorghum-a Review. Phytopathology® 2020, 110, 1863–1876. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.L.; Heslop-Harrison, P.; Spillane, C.; Mckeown, P.C.; Edwards, D.; Goldman, I.; Ortiz, R. Evolutionary Dynamics and Adaptive Benefits of Deleterious Mutations in Crop Gene Pools. Trends Plant Sci. 2023, 28, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Valluru, R.; Gazave, E.E.; Fernandes, S.B.; Ferguson, J.N.; Lozano, R.; Hirannaiah, P.; Zuo, T.; Brown, P.J.; Leakey, A.D.B.; Gore, M.A. Deleterious Mutation Burden and Its Association with Complex Traits in Sorghum (Sorghum bicolor). Genetics 2019, 211, 1075–1087. [Google Scholar] [CrossRef]
- Rosati, V.C.; Quinn, A.A.; Gleadow, R.M.; Blomstedt, C.K. The Putative GATA Transcription Factor Sbgata22 as a Novel Regulator of Dhurrin Biosynthesis. Life 2024, 14, 470. [Google Scholar] [CrossRef] [PubMed]
- Blomstedt, C.K.; O’Donnell, N.H.; Bjarnholt, N.; Neale, A.D.; Hamill, J.D.; Møller, B.L.; Gleadow, R.M. Metabolic Consequences of Knocking Out UGT85B1, the Gene Encoding the Glucosyltransferase Required for Synthesis of Dhurrin In Sorghum bicolor (L. Moench). Plant Cell Physiol. 2016, 57, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Magill, J.; Frederiksen, R.; Magill, C. Chalcone synthase and phenylalanine ammonia-lyase mRNA levels following exposure of sorghum seedlings to three fungal pathogens. Physiol. Mol. Plant. Pathol. 1996, 49, 187–199. [Google Scholar] [CrossRef]
- Wang, P.; Sandrock, R.W.; VanEtten, H.D. Disruption of the cyanide hydratase gene in Gloeocercospora sorghi increases its sensitivity to the phytoanticipin cyanide but does not affect its pathogenicity on the cyanogenic plant Sorghum. Fungal Genet. Biol. 1999, 28, 126–134. [Google Scholar] [CrossRef]
- Orczyk, W.; Hipskind, J.; De Neegaard, E.; Goldsbrough, P. Stimulation of phenylalanine ammonia-lyase in sorghum in response to inoculation with Bipolaris maydis. Physiol. Mol. Plant. Pathol. 1996, 48, 55–64. [Google Scholar] [CrossRef]
- Lo, C.; Coolbaugh, R.C.; Nicholson, R.L. Molecular Characterization and in Silico Expression Analysis of a Chalcone Synthase Gene Family in Sorghum bicolor. Physiol. Mol. Plant. Pathol. 2002, 61, 179–188. [Google Scholar] [CrossRef]
- Irmisch, S.; Zeltner, P.; Handrick, V.; Gershenzon, J.; Köllner, T.G. The Maize Cytochrome P450 CYP79A61 Produces Phenylacetaldoxime and Indole-3-acetaldoxime in Heterologous Systems and Might Contribute to Plant Defense and Auxin Formation. BMC Plant Biol. 2015, 15, 128. [Google Scholar] [CrossRef]
- Osorio, J.A.; Frederiksen, R.A. Development of an Infection Assay for Sporisorium reilianum, the Head Smut Pathogen on Sorghum. Plant Dis. 1998, 82, 1232–1236. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Radwan, G.L.; Prom, L.K.; Odvody, G.; Magill, C.W. Mating Type a Locus Alleles and Genomic Polymorphism in Sporisorium reilianum: Comparison of Sorghum Isolates to Those from Maize. Australas. Plant Pathol. 2019, 48, 119–129. [Google Scholar] [CrossRef]
- Conlon, B.H.; Schmidtt, S.; Poulsen, M.; Shik, J.Z. Orthogonal protocols for DNA extraction from filamentous fungi. STAR Protoc. 2022, 3, 101126. [Google Scholar] [CrossRef] [PubMed]
- Das, I.K.; Fakrudin, B.; Arora, D.K. RAPD cluster analysis and chlorate sensitivity of some Indian isolates of Macrophomina phaseolina from sorghum and their relationship with pathogenicity. Microbiol. Res. 2008, 163, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Koch, B.M.; Sibbesen, O.; Halkier, B.A.; Svendsen, I.; Møller, B.L. The Primary Sequence of Cytochrome P450tyr, the multi-functional N-hydroxylase catalyzing the conversion of L-Tyrosine to –Hydroxyphenylacetaldehyde Oxime in the Biosynthesis of the Cyanogenic Glucoside Dhurrin in Sorghum bicolor (L.) Moench. Arch. Biochem. Biophys. 1995, 323, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-∆∆CT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Kolde, R. Pheatmap: Pretty Heatmaps (Version 1.0.12) [R Core Team (2019)]. 2019. Available online: https://CRAN.R-project.org/package=pheatmap (accessed on 25 February 2025).
- Deraad, D.A. Snpfiltr: An R Package for Interactive and Reproducible SNP Filtering. Mol. Ecol. Resour. 2022, 22, 2443–2453. [Google Scholar] [CrossRef] [PubMed]
- Knaus, B.J.; Grünwald, N.J. Vcfr: A Package to Manipulate and Visualize Variant Call Format Data in R. Mol. Ecol. Resour. 2017, 17, 44–53. [Google Scholar] [CrossRef]
- McCaw, Z. RNOmni: Rank Normal Transformation Omnibus Test (Version 1.0.1.2). 2023. Available online: https://CRAN.R-project.org/package=RNOmni (accessed on 25 February 2025).
- Turner, S.D. Qqman: An R Package for Visualizing GWAS Results Using Q-Q and Manhattan Plots. J. Open Source Softw. 2018, 3, 731. [Google Scholar] [CrossRef]
- Phytozome 13, Joint Genome Institute (JGI). Sorghum bicolor (v5.1). Available online: https://phytozome-next.jgi.doe.gov/info/Sbicolor_v5_1 (accessed on 22 October 2024).
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests (Version 0.7.2). 2023. Available online: https://CRAN.R-project.org/package=rstatix (accessed on 25 February 2025).
- Mangiafico, S.S. Rcompanion: Functions to Support Extension Education Program Evaluation (Version 2.4.36). Rutgers Cooperative Extension. New Brunswick, New Jersey. 2024. Available online: https://CRAN.R-project.org/package=rcompanion (accessed on 25 February 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fall, C.; Lim, S.; Ahn, E.; Park, S.; Prom, L.K.; Magill, C.W. Unveiling the Potential Role of Dhurrin in Sorghum During Infection by the Head Smut Pathogen Sporisorium reilianum f. sp. reilianum. Plants 2025, 14, 740. https://doi.org/10.3390/plants14050740
Fall C, Lim S, Ahn E, Park S, Prom LK, Magill CW. Unveiling the Potential Role of Dhurrin in Sorghum During Infection by the Head Smut Pathogen Sporisorium reilianum f. sp. reilianum. Plants. 2025; 14(5):740. https://doi.org/10.3390/plants14050740
Chicago/Turabian StyleFall, Coumba, Seunghyun Lim, Ezekiel Ahn, Sunchung Park, Louis K. Prom, and Clint W. Magill. 2025. "Unveiling the Potential Role of Dhurrin in Sorghum During Infection by the Head Smut Pathogen Sporisorium reilianum f. sp. reilianum" Plants 14, no. 5: 740. https://doi.org/10.3390/plants14050740
APA StyleFall, C., Lim, S., Ahn, E., Park, S., Prom, L. K., & Magill, C. W. (2025). Unveiling the Potential Role of Dhurrin in Sorghum During Infection by the Head Smut Pathogen Sporisorium reilianum f. sp. reilianum. Plants, 14(5), 740. https://doi.org/10.3390/plants14050740





