Foliar Application of Amino Acids Increases Sweet Basil (Ocimum basilicum L.) Resistance to High-Temperature Stress
Abstract
1. Introduction
2. Results
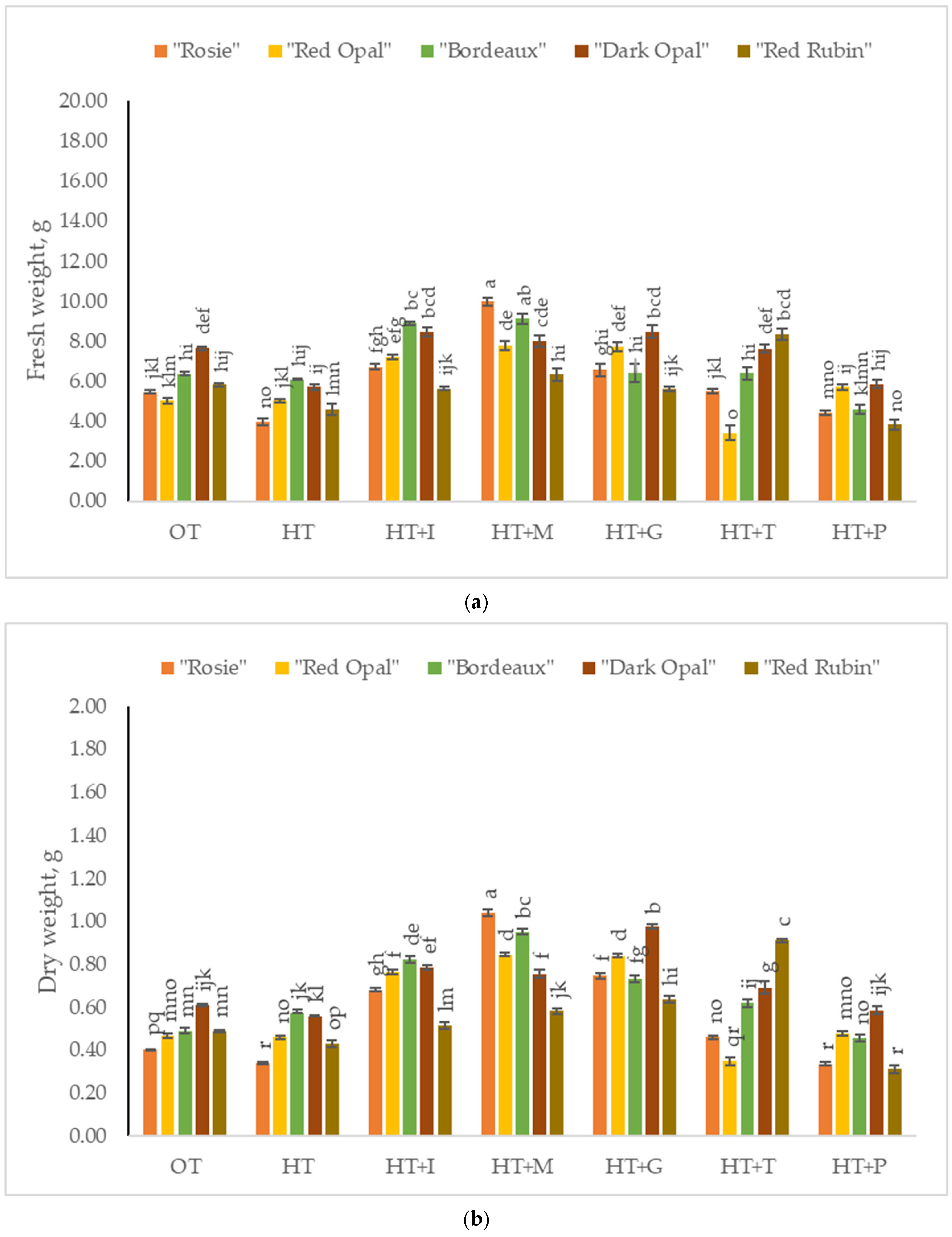
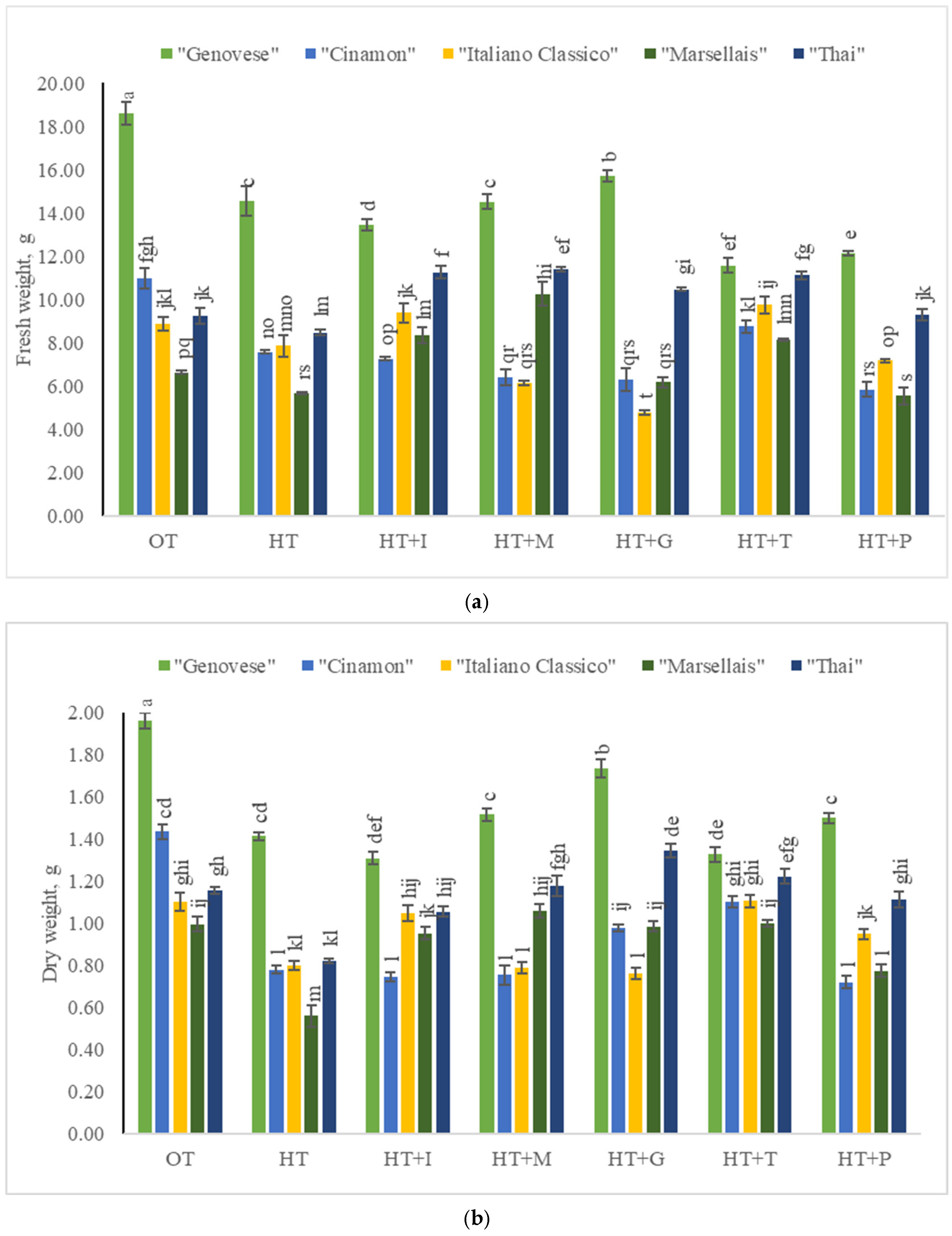
2.1. The Impact of High-Temperature Stress and Amino Acid Supplementation on the Fresh and Dry Weights
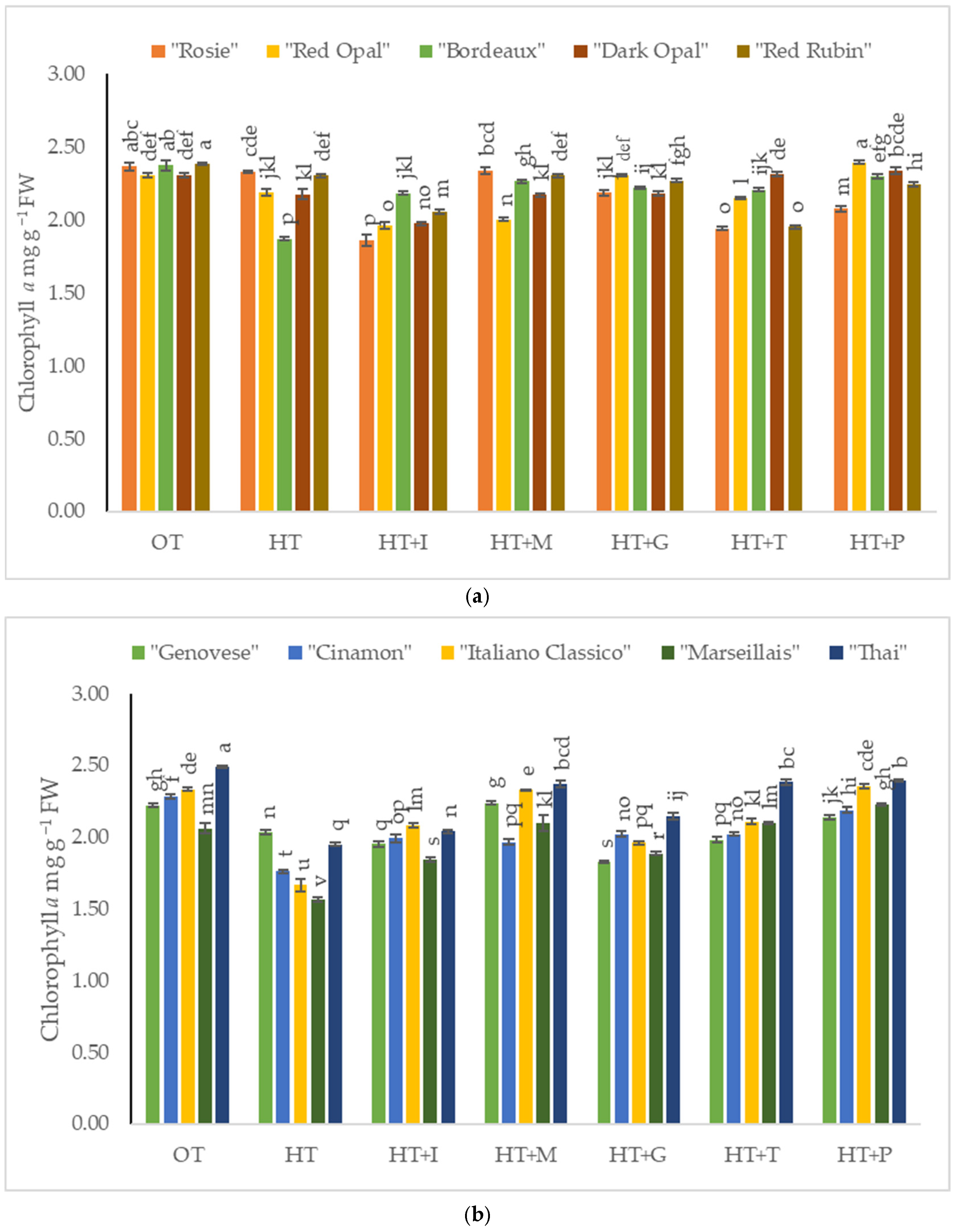
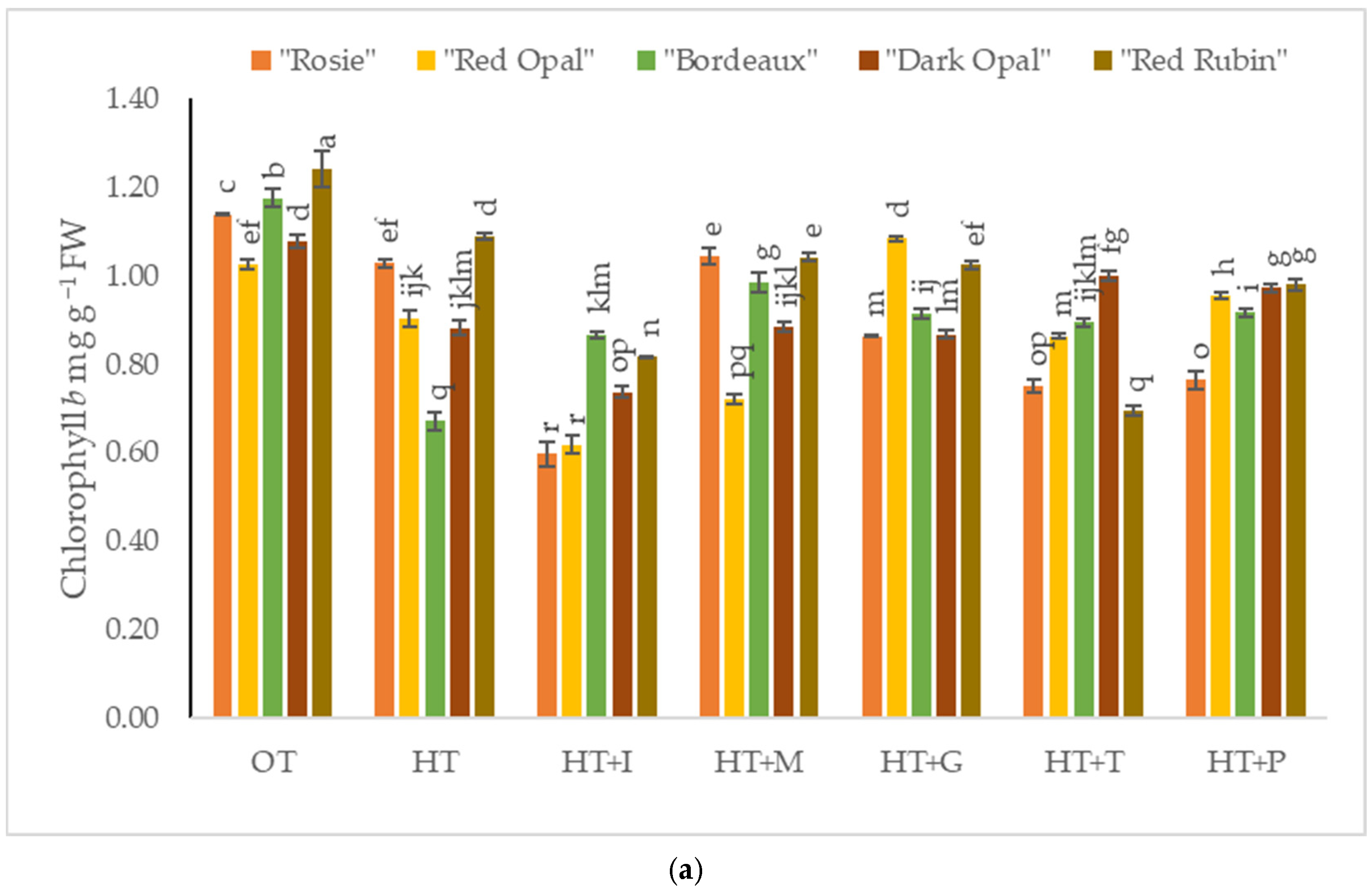
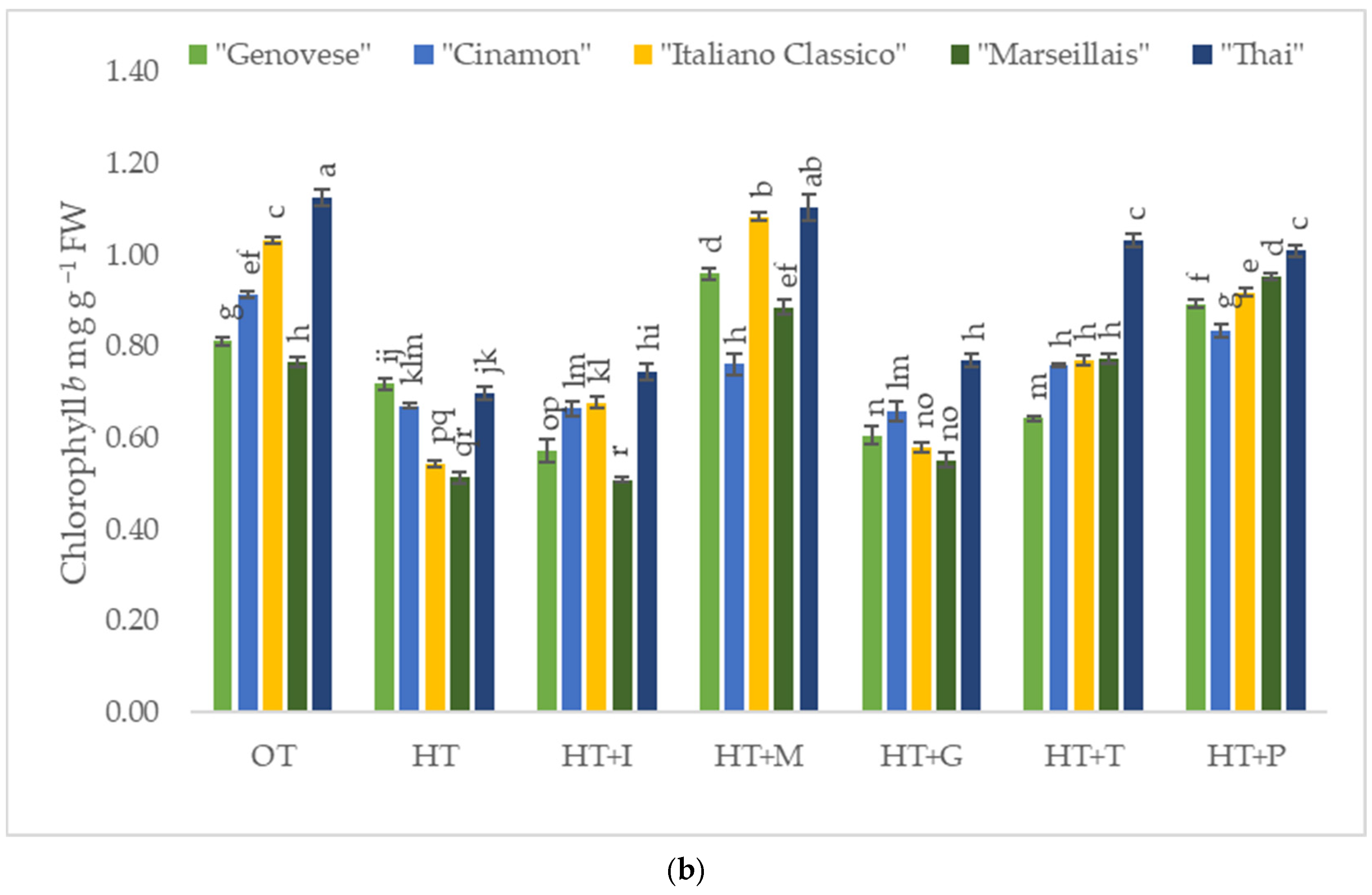
2.2. The Impact of High-Temperature Stress and Amino Acid Supplementation on Chlorophyll a and b Content
2.3. The Impact of High-Temperature Stress and Amino Acid Supplementation on the Total Phenolic Content
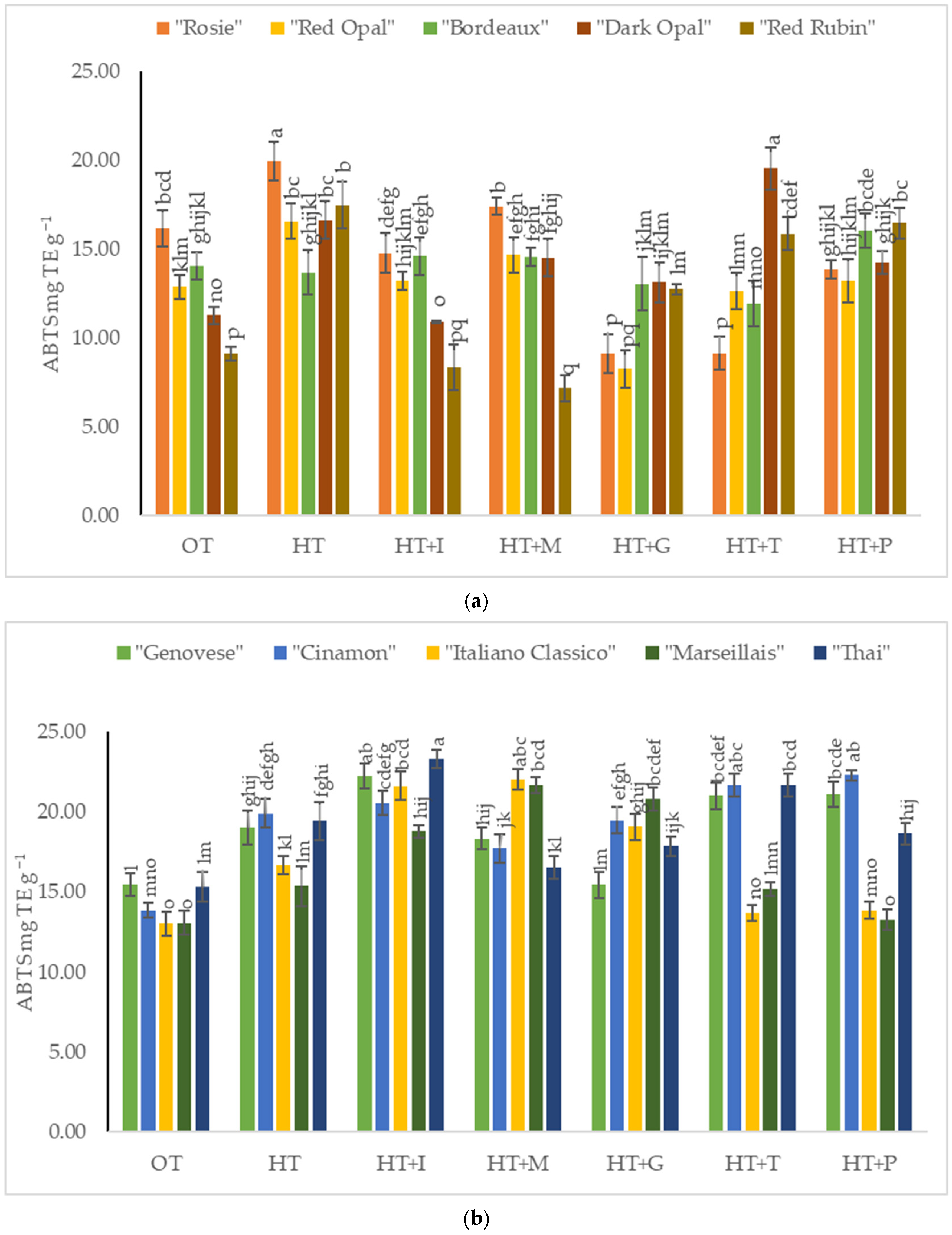
2.4. The Impact of High-Temperature Stress and Amino Acid Supplementation on the Antioxidant Activity
3. Discussion
4. Materials and Methods
4.1. Experiment Conditions
4.2. Determination of Chlorophyll Content
4.3. Determination of Fresh and Dry Weights
4.4. Determination of Phenolic Compound Contents and ABTS Radical Scavenging Capacity
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zheljazkov, V.D.; Callahan, A.; Cantrell, C.L. Yield and oil composition of 38 basil (Ocimum basilicum L.) accessions grown in Mississippi. J. Agric. Food Chem. 2008, 56, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Hikmawanti, N.P.E.; Nurhidayah, S. Chemical components of Ocimum basilicum L. and Ocimum tenuiflorum L. stem essential oils and evaluation of their antioxidant activities using DPPH method. Pharm. Sci. Res. 2019, 6, 3. [Google Scholar] [CrossRef]
- Lawrence, L.; Chika, A.; Abel, A. Extraction and characterization of the essential oil from the leaves of Ocimum basilicum and evaluation of its antioxidant properties. J. Biochem. Int. 2023, 10, 92–104. [Google Scholar] [CrossRef]
- Shiwakoti, S.; Saleh, O.; Poudyal, S.; Barka, A.; Qian, Y.; Zheljazkov, V.D. Yield, composition and antioxidant capacity of the essential oil of sweet basil and holy basil as influenced by distillation methods. Chem. Biodivers. 2017, 14, e1600417. [Google Scholar] [CrossRef]
- Bora, K.S.; Arora, S.; Shri, R. Role of Ocimum basilicum L. in prevention of ischemia and reperfusion-induced cerebral damage, and motor dysfunctions in mice brain. J. Ethnopharmacol. 2011, 137, 1360–1365. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Abdel Fattah, M.M.; El Mallah, G.M.; Elallawy, H.M. Effect of some herbs as feed additives on performance, digestibility, carcass characteristics and blood parameters of broilers. Egypt. J. Nutr. Feed. 2019, 22, 191–199. [Google Scholar] [CrossRef][Green Version]
- Barátová, S.; Mezeyová, I.; Hegedúsová, A.; Andrejiová, A. Impact of biofortification, variety and cutting on chosen qualitative characteristic of basil (Ocimum basilicum L.). Acta Fytotech. Zootech. 2015, 18, 71–75. [Google Scholar] [CrossRef]
- Viyoch, J.; Pisutthanan, N.; Faikreua, A.; Nupangta, K.; Wangtorpol, K.; Ngokkuen, J. Evaluation of in vitro antimicrobial activity of Thai basil oils and their micro-emulsion formulas against Propionibacterium acnes. Int. J. Cosmetic Sci. 2006, 28, 125–133. [Google Scholar] [CrossRef]
- Carocho, M.; Barros, L.; Barreira, J.C.; Calhelha, R.C.; Soković, M.; Fernandez-Ruiz, V.; Buelga, C.S.; Morales, P.; Ferreira, I.C. Basil as functional and preserving ingredient in “Serra da Estrela” cheese. Food Chem. 2016, 207, 51–59. [Google Scholar] [CrossRef]
- Maurer, D.; Sadeh, A.; Chalupowicz, D.; Barel, S.; Shimshoni, J.A.; Kenigsbuch, D. Hydroponic versus soil-based cultivation of sweet basil: Impact on plants’ susceptibility to downy mildew and heat stress, storability and total antioxidant capacity. J. Sci. Food Agric. 2023, 103, 7809–7815. [Google Scholar] [CrossRef]
- Ilić, A.S.; Antić, M.P.; Jelačić, S.C.; Knudsen, T.M.Š. Chemical composition of the essential oils of three Ocimum basilicum L. cultivars from Serbia. Not. Bot. Horti Agrobot. 2019, 47, 347–351. [Google Scholar] [CrossRef]
- Wiset, L.; Poomsa-ad, N.; Jindamol, H.; Thongtip, A.; Mosaleeyanon, K.; Toojinda, T.; Darwell, C.T.; Saputro, T.B.; Chutimanukul, P. Quality and bioactive compound accumulation in two holy basil cultivars as affected by microwave-assisted hot air drying at an industrial scale. Front. Sustain. Food Syst. 2023, 7, 1219540. [Google Scholar] [CrossRef]
- Kumar, B.; Gupta, E.; Yadav, R.; Singh, S.C.; Lal, R.K. Temperature effects on seed germination potential of holy basil (Ocimum tenuiflorum). Seed Technol. 2014, 36, 75–79. [Google Scholar]
- Barickman, T.C.; Olorunwa, O.J.; Sehgal, A.; Walne, C.H.; Reddy, K.R.; Gao, W. Yield, physiological performance, and phytochemistry of basil (Ocimum basilicum L.) under temperature stress and elevated CO2 concentrations. Plants 2021, 10, 1072. [Google Scholar] [CrossRef]
- Quint, M.; Delker, C.; Franklin, K.A.; Wigge, P.A.; Halliday, K.J.; Van Zanten, M. Molecular and genetic control of plant thermomorphogenesis. Nat. Plants 2016, 2, 15190. [Google Scholar] [CrossRef]
- Li, B.; Gao, K.; Ren, H.; Tang, W. Molecular mechanisms governing plant responses to high temperatures. J. Integr. Plant Biol. 2018, 60, 757–779. [Google Scholar] [CrossRef]
- Xu, Y.; Chu, C.; Yao, S. The impact of high-temperature stress on rice: Challenges and solutions. Crop J. 2021, 9, 963–976. [Google Scholar] [CrossRef]
- Al-Huqail, A.; El-Dakak, R.M.; Sanad, M.N.; Badr, R.H.; Ibrahim, M.M.; Soliman, D.; Khan, F. Effects of climate temperature and water stress on plant growth and accumulation of antioxidant compounds in sweet basil (Ocimum basilicum L.) leafy vegetable. Scientifica 2020, 2020, 3808909. [Google Scholar] [CrossRef]
- Jakovljević, D.; Momčilović, J.; Bojović, B.; Stanković, M. The short-term metabolic modulation of basil (Ocimum basilicum L. cv.‘Genovese’) after exposure to cold or heat. Plants 2021, 10, 590. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, Y.; Huang, M.; Yao, Y.; Bassu, S.; Ciais, P. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331. [Google Scholar] [CrossRef]
- Dong, J.; Gruda, N.; Li, X.; Tang, Y.; Zhang, P.; Duan, Z. Sustainable vegetable production under changing climate: The impact of elevated CO2 on yield of vegetables and the interactions with environments—A review. J. Clean. Prod. 2020, 253, 119920. [Google Scholar] [CrossRef]
- Copolovici, L.; Kännaste, A.; Pazouki, L.; Niinemets, Ü. Emissions of green leaf volatiles and terpenoids from Solanum lycopersicum are quantitatively related to the severity of cold and heat shock treatments. J. Plant Physiol. 2012, 169, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Finka, A.; Goloubinoff, P. How do plants feel the heat? Trends Biochem. Sci. 2012, 37, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef]
- Erb, M.; Kliebenstein, D.J. Plant secondary metabolites as defenses, regulators, and primary metabolites: The blurred functional trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef]
- Qin, L.; Li, C.; Li, D.; Wang, J.; Yang, L.; Qu, A.; Wu, Q. Physiological, metabolic and transcriptional responses of Basil (Ocimum basilicum Linn. var. pilosum (Willd.) Benth.) to heat stress. Agronomy 2022, 12, 1434. [Google Scholar] [CrossRef]
- Bashir, A.; Rizwan, M.; Ali, S.; Zia ur Rehman, M.; Ishaque, W.; Atif Riaz, M.; Maqbool, A. Effect of foliar-applied iron complexed with lysine on growth and cadmium (Cd) uptake in rice under Cd stress. Environ. Sci. Pollut. Res. 2018, 25, 20691–20699. [Google Scholar] [CrossRef]
- Saudy, H.S.; Hamed, M.F.; Abd El-Momen, W.R.; Hussein, H. Nitrogen use rationalization and boosting wheat productivity by applying packages of humic, amino acids, and microorganisms. Commun. Soil Sci. Plant Anal. 2020, 51, 1036–1047. [Google Scholar] [CrossRef]
- Zemanová, V.; Pavlík, M.; Pavlíková, D.; Tlustoš, P. The significance of methionine, histidine and tryptophan in plant responses and adaptation to cadmium stress. Plant Soil Environ. 2014, 60, 426–432. [Google Scholar] [CrossRef]
- Duan, L.; Liu, H.; Li, X.; Xiao, J.; Wang, S. Multiple phytohormones and phytoalexins are involved in disease resistance to Magnaporthe oryzae invaded from roots in rice. Physiol. Plantarum 2014, 152, 486–500. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Li, Q.; Zhou, D.; Jia, M.; Liu, Z.; Hou, Z.; Ren, Q.; Ji, S.; Sang, S.; Lu, S.B. subtilis CNBG-PGPR-1 induces methionine to regulate ethylene pathway and ROS scavenging for improving salt tolerance of tomato. Plant J. 2024, 117, 193–211. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kim, Y.; Alizadeh, H.; Leung, D.W. Protection of Italian ryegrass (Lolium multiflorum L.) seedlings from salinity stress following seed priming with L-methionine and casein hydrolysate. Seed Sci. Res. 2021, 31, 51–59. [Google Scholar] [CrossRef]
- Khan, S.; Yu, H.; Li, Q.; Gao, Y.; Sallam, B.N.; Wang, H.; Liu, P.; Jiang, W. Exogenous application of amino acids improves the growth and yield of lettuce by enhancing photosynthetic assimilation and nutrient availability. Agronomy 2019, 9, 266. [Google Scholar] [CrossRef]
- Hacham, Y.; Shitrit, O.; Nisimi, O.; Friebach, M.; Amir, R. Elucidating the importance of the catabolic enzyme, methionine-gamma-lyase, in stresses during Arabidopsis seed development and germination. Front. Plant Sci. 2023, 14, 1143021. [Google Scholar] [CrossRef]
- Stavridou, E.; Voulgari, G.; Michailidis, M.; Kostas, S.; Chronopoulou, E.G.; Labrou, N.E.; Madesis, P.; Nianiou-Obeidat, I. Overexpression of a biotic stress-inducible Pvgstu gene activates early protective responses in tobacco under combined heat and drought. Int. J. Mol. Sci. 2021, 22, 2352. [Google Scholar] [CrossRef]
- Walters, K.J.; Currey, C.J. Hydroponic greenhouse basil production: Comparing systems and cultivars. HortTechnology 2015, 25, 645–650. [Google Scholar] [CrossRef]
- Prinsi, B.; Negrini, N.; Morgutti, S.; Espen, L. Nitrogen starvation and nitrate or ammonium availability differently affect phenolic composition in green and purple basil. Agronomy 2020, 10, 498. [Google Scholar] [CrossRef]
- Helaly, A.A.; Ibrahim, F.R. Improving growth and productivity of Safflower (Carthamus tinctorius L.) Plant by Utilizing L-Tryptophan and Phenylalanine Acids under different Potassium fertilizer rate. J. Plant Prod. 2024, 15, 169–174. [Google Scholar] [CrossRef]
- Rafique, M.; Ali, A.; Naveed, M.; Abbas, T.; Al-Huqail, A.A.; Siddiqui, M.H.; Nawaz, A.; Brtnicky, M.; Holatko, J.; Kintl, A. Deciphering the potential role of symbiotic plant microbiome and amino acid application on growth performance of chickpea under field conditions. Front. Plant Sci. 2022, 13, 852851. [Google Scholar] [CrossRef]
- Noroozlo, Y.A.; Souri, M.K.; Delshad, M. Stimulation effects of foliar applied glycine and glutamine amino acids on lettuce growth. Open Agric. 2019, 4, 164–172. [Google Scholar] [CrossRef]
- Hendrickson, T.; Dunn, B.L.; Goad, C.; Hu, B.; Singh, H. Effects of elevated water temperature on growth of basil using nutrient film technique. HortScience 2022, 57, 925–932. [Google Scholar] [CrossRef]
- Eskandarzade, P.; Zare Mehrjerdi, M.; Didaran, F.; Gruda, N.S.; Aliniaeifard, S. Shading level and harvest time affect the photosynthetic and physiological properties of basil varieties. Agronomy 2023, 13, 2478. [Google Scholar] [CrossRef]
- Li, Z.; Peng, Y.; Huang, B. Physiological effects of γ-aminobutyric acid application on improving heat and drought tolerance in creeping bentgrass. J. Am. Soc. Hort. Sci. 2016, 141, 76–84. [Google Scholar] [CrossRef]
- Hirakawa, T.; Ohara, K. N-Acetylglutamic acid enhances tolerance to oxidative and heat stress in Humulus lupulus. Horticulturae 2024, 10, 484. [Google Scholar] [CrossRef]
- Pascual, M.B.; El-Azaz, J.; de la Torre, F.N.; Cañas, R.A.; Avila, C.; Cánovas, F.M. Biosynthesis and metabolic fate of phenylalanine in conifers. Front. Plant Sci. 2016, 7, 1030. [Google Scholar] [CrossRef]
- Hassan, A.; Margay, A.R.; Din, S. Effect of foliar sprays of Phenylalanine, Nano-potash and Potassium Sulphate on fruit quality attributes of apple (Malus × domestica Borkh) cv. ambri. Int. J. Plant Soil Sci. 2024, 36, 317–325. [Google Scholar] [CrossRef]
- Hęś, M.; Golcz, A.; Gramza-Michałowska, A.; Jędrusek-Golińska, A.; Dziedzic, K.; Mildner-Szkudlarz, S. Influence of Nitrogen Fertilizer on the Antioxidative Potential of basil varieties (Ocimum basilicum L.). Molecules 2022, 27, 5636. [Google Scholar] [CrossRef]
- Hussein, H.A.; Alshammari, S.O.; Kenawy, S.K.; Elkady, F.M.; Badawy, A.A. Grain-priming with L-arginine improves the growth performance of wheat (Triticum aestivum L.) plants under drought stress. Plants 2022, 11, 1219. [Google Scholar] [CrossRef]
- Prinsi, B.; Morgutti, S.; Negrini, N.; Faoro, F.; Espen, L. Insight into composition of bioactive phenolic compounds in leaves and flowers of green and purple basil. Plants 2019, 9, 22. [Google Scholar] [CrossRef]
- Pirbalouti, A.G.; Malekpoor, F.; Salimi, A.; Golparvar, A.; Hamedi, B. Effects of foliar of the application chitosan and reduced irrigation on essential oil yield, total phenol content and antioxidant activity of extracts from green and purple basil. Acta Sci. Polonorum. Hortorum Cultus 2017, 16, 177–186. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Yim, S.; Nam, S. Physiochemical, nutritional and functional characterization of 10 different pear cultivars (Pyrus spp.). J. Appl. Bot. Food Qual. 2016, 89, 73–81. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deveikytė, J.; Blinstrubienė, A.; Burbulis, N.; Baltušnikienė, A. Foliar Application of Amino Acids Increases Sweet Basil (Ocimum basilicum L.) Resistance to High-Temperature Stress. Plants 2025, 14, 739. https://doi.org/10.3390/plants14050739
Deveikytė J, Blinstrubienė A, Burbulis N, Baltušnikienė A. Foliar Application of Amino Acids Increases Sweet Basil (Ocimum basilicum L.) Resistance to High-Temperature Stress. Plants. 2025; 14(5):739. https://doi.org/10.3390/plants14050739
Chicago/Turabian StyleDeveikytė, Justina, Aušra Blinstrubienė, Natalija Burbulis, and Aldona Baltušnikienė. 2025. "Foliar Application of Amino Acids Increases Sweet Basil (Ocimum basilicum L.) Resistance to High-Temperature Stress" Plants 14, no. 5: 739. https://doi.org/10.3390/plants14050739
APA StyleDeveikytė, J., Blinstrubienė, A., Burbulis, N., & Baltušnikienė, A. (2025). Foliar Application of Amino Acids Increases Sweet Basil (Ocimum basilicum L.) Resistance to High-Temperature Stress. Plants, 14(5), 739. https://doi.org/10.3390/plants14050739





