Comparison of Two Bacillus Strains Isolated from the Coastal Zone in Barley (Hordeum vulgare L.) Under Salt Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling of Rhizospheric Soils for Bacterial Isolation
2.2. Salt Resistance Screening of Isolates
2.3. Morphological and Biochemical Characterization of Bacteria
2.4. Determination of Plant Growth-Promoting Properties
2.5. Molecular Identification of Bacterial Isolates
2.6. Plant Inoculation and Growth
2.7. Plant Physiological and Biochemical Analysis
2.8. Statistical Analysis
3. Results
3.1. Morphological and Biochemical Characterization of Bacteria
3.2. Identifying Plant Growth-Promoting Properties of Bacteria
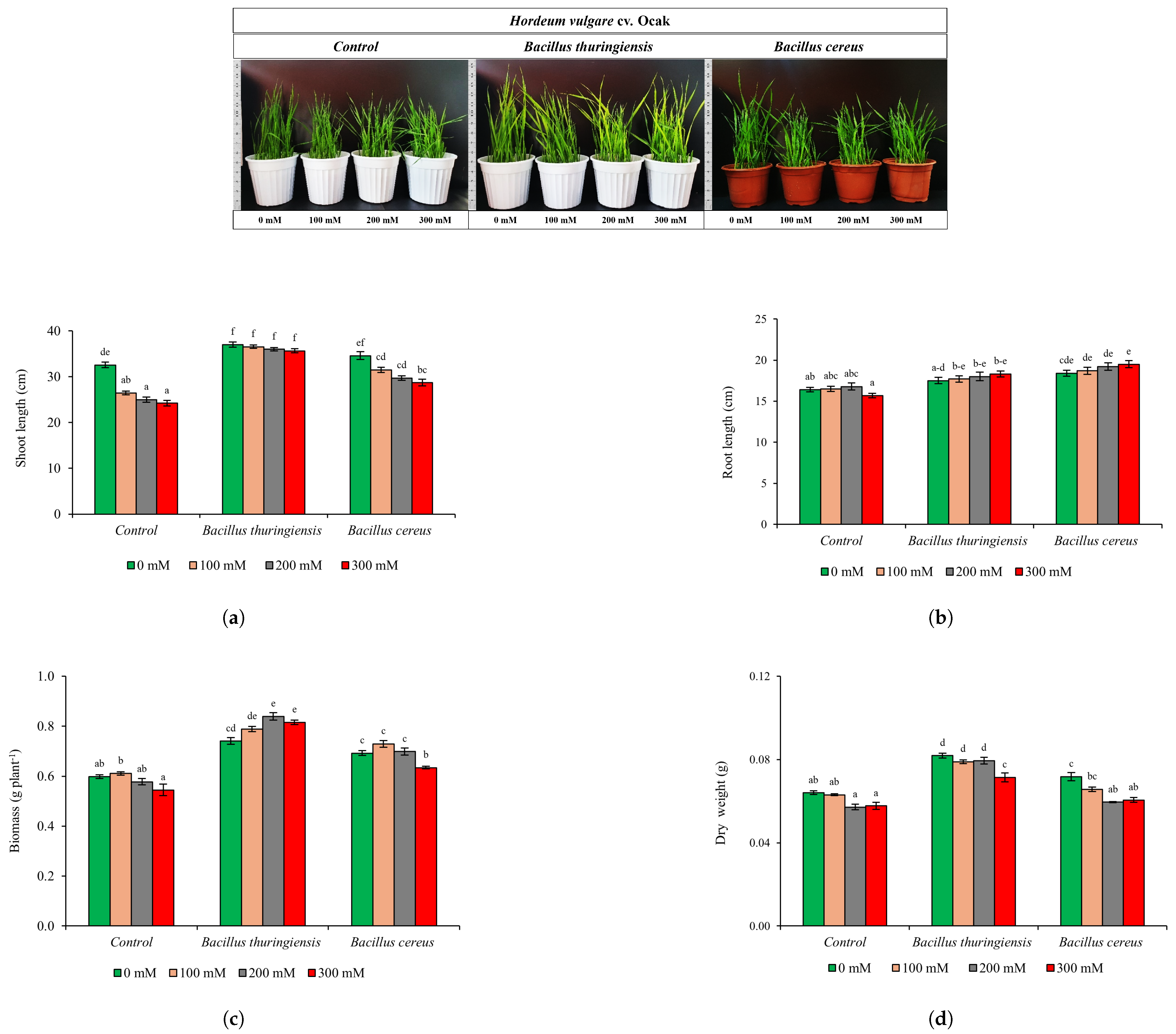
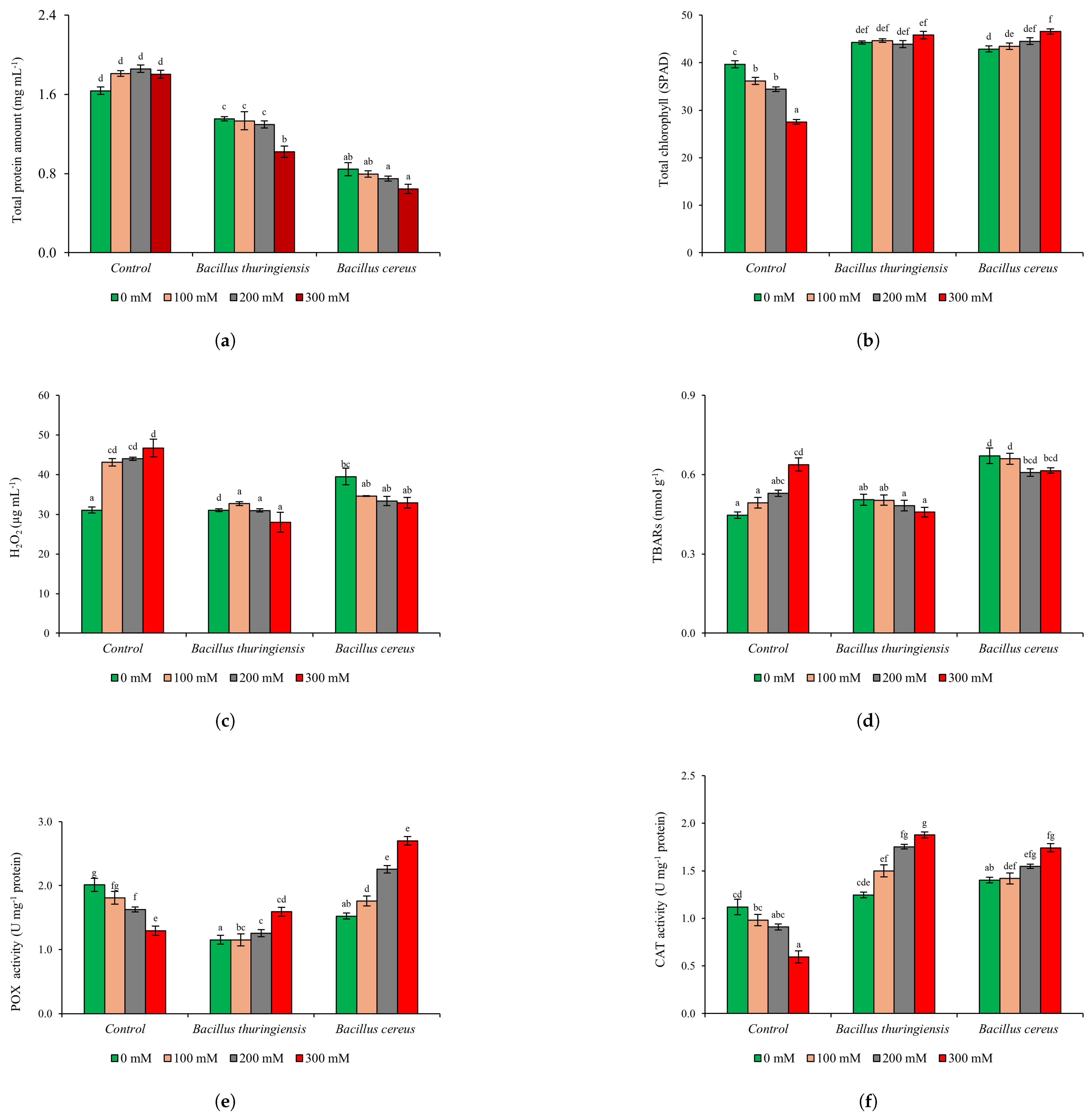
3.3. Physiological and Biochemical Analysis in Barley Under Salt Stress
3.3.1. Physiological Analysis
3.3.2. Biochemical Analysis
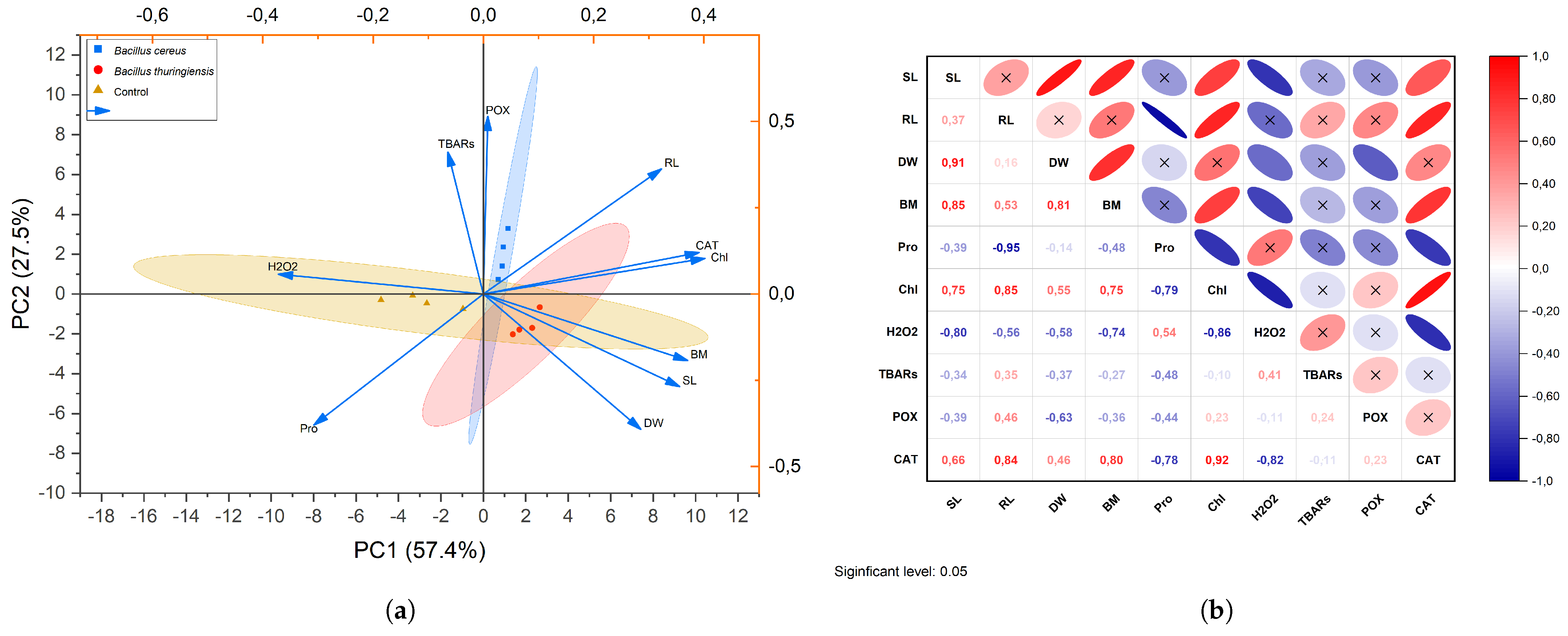
3.4. Correlations Between Physiological and Biochemical Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Zelm, E.; Zhang, Y.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef] [PubMed]
- Payandeh, Z.; Jahanbakhshi, A.; Mesri-Gundoshmian, T.; Clark, S. Improving energy efficiency of barley production using joint data envelopment analysis (DEA) and life cycle assessment (LCA): Evaluation of greenhouse gas emissions and optimization approach. Sustainability 2021, 13, 6082. [Google Scholar] [CrossRef]
- Giannelli, G.; Potestio, S.; Visioli, G. The contribution of PGPR in salt stress tolerance in crops: Unravelling the molecular mechanisms of cross-talk between plant and bacteria. Plants 2023, 12, 2197. [Google Scholar] [CrossRef] [PubMed]
- Nasrallah, A.K.; Atia, M.A.; Abd El-Maksoud, R.M.; Kord, M.A.; Fouad, A.S. Salt priming as a smart approach to mitigate salt stress in Faba bean (Vicia faba L.). Plants 2022, 11, 1610. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Mehta, S.; Yadav, S.; Nagar, G.; Ghosh, R.; Roy, A.; Chakraborty, A.; Singh, I.K. How to cope with the challenges of environmental stresses in the era of global climate change: An update on ROS stave off in plants. Int. J. Mol. Sci. 2022, 23, 1995. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Cakmakci, R.; Erturk, Y.; Varmazyari, A.; Atasever, A.; Kotan, R.; Haliloglu, K.; Erat, M.; Turkyilmaz, K.; Sekban, R.; Haznedar, A. The effect of bacteria-based formulations on tea (Camellia sinensis L.) growth, yield, and enzyme activities. Ann. Wars. Univ. Life-Sci.-Sggw Hortic. Landsc. Archit. 2017, 38, 5–18. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Al-Ghamdi, A.A.; Ali, H.M.; Alayafi, A.A. Azospirillum lipoferum FK1 confers improved salt tolerance in chickpea (Cicer arietinum L.) by modulating osmolytes, antioxidant machinery and stress-related genes expression. Environ. Exp. Bot. 2019, 159, 55–65. [Google Scholar] [CrossRef]
- Kumawat, K.C.; Nagpal, S.; Sharma, P. Potential of plant growth-promoting rhizobacteria-plant interactions in mitigating salt stress for sustainable agriculture: A review. Pedosphere 2022, 32, 223–245. [Google Scholar] [CrossRef]
- Lahsini, A.I.; Sallami, A.; Ait-Ouakrim, E.H.; El khedri, H.; Obtel, M.; Douira, A.; El Modafar, C.; Benkerroum, N.; Talbi, C.; Chakhchar, A.; et al. Isolation and molecular identification of an indigenous abiotic stress-tolerant plant growth-promoting rhizobacteria from the rhizosphere of the olive tree in southern Morocco. Rhizosphere 2022, 23, 100554. [Google Scholar] [CrossRef]
- Slama, H.B.; Chenari Bouket, A.; Alenezi, F.N.; Luptakova, L.; Baranov, O.; Ahadi, R.; Belbahri, L. Impacts of salt stress on the rhizosphere and endophytic bacterial role in plant salt alleviation. Int. J. Plant Biol. 2023, 14, 361–376. [Google Scholar] [CrossRef]
- Yamamoto, K.; Shiwa, Y.; Ishige, T.; Sakamoto, H.; Tanaka, K.; Uchino, M.; Tanaka, N.; Oguri, S.; Saitoh, H.; Tsushima, S. Bacterial diversity associated with the rhizosphere and endosphere of two halophytes: Glaux maritima and Salicornia europaea. Front. Microbiol. 2018, 9, 2878. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, Q.; Hou, J.; Tu, C.; Luo, Y.; Christie, P. Whole genome analysis of halotolerant and alkalotolerant plant growth-promoting rhizobacterium Klebsiella sp. D5A. Sci. Rep. 2016, 6, 26710. [Google Scholar] [CrossRef] [PubMed]
- Sorty, A.M.; Meena, K.K.; Choudhary, K.; Bitla, U.M.; Minhas, P.S.; Krishnani, K.K. Effect of plant growth promoting bacteria associated with halophytic weed (Psoralea corylifolia L) on germination and seedling growth of wheat under saline conditions. Appl. Biochem. Biotechnol. 2016, 180, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Sandhya, V.S.K.Z.; Ali, S.K.Z.; Grover, M.; Reddy, G.; Venkateswarlu, B. Effect of plant growth promoting Pseudomonas spp. on compatible solutes, antioxidant status and plant growth of maize under drought stress. Plant Growth Regul. 2010, 62, 21–30. [Google Scholar] [CrossRef]
- Mayak, S.; Tirosh, T.; Glick, B.R. Plant growth-promoting bacteria that confer resistance to water stress in tomatoes and peppers. Plant Sci. 2004, 2, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Sarma, R.K.; Saikia, R. Alleviation of drought stress in mung bean by strain Pseudomonas aeruginosa GGRJ21. Plant Soil 2014, 377, 111–126. [Google Scholar] [CrossRef]
- Han, Q.-Q.; Lü, X.-P.; Bai, J.-P.; Qiao, Y.; Paré, P.W.; Wang, S.-M.; Zhang, J.-L.; Wu, Y.N.; Pang, X.P.; Xu, W.-B.; et al. Beneficial soil bacterium Bacillus subtilis (GB03) augments salt tolerance of white clover. Front. Plant Sci. 2014, 5, 525. [Google Scholar] [CrossRef]
- Tiwari, S.; Singh, P.; Tiwari, R.; Meena, K.K.; Yandigeri, M.; Singh, D.; Arora, D.K. Salt-tolerant rhizobacteria-mediated induced tolerance in wheat (Triticum aestivum) and chemical diversity in rhizosphere enhance plant growth. Biol. Fertil. Soils 2011, 47, 907–916. [Google Scholar] [CrossRef]
- Dąbrowska, G.B.; Turkan, S.; Tylman-Mojżeszek, W.; Mierek-Adamska, A. In Silico Study of the RSH (RelA/SpoT Homologs) Gene Family and Expression Analysis in Response to PGPR Bacteria and Salinity in Brassica napus. Int. J. Mol. Sci. 2021, 22, 10666. [Google Scholar] [CrossRef] [PubMed]
- Azizoglu, U.; Yilmaz, N.; Simsek, O.; Ibal, J.C.; Tagele, S.B.; Shin, J.H. The fate of plant growth-promoting rhizobacteria in soilless agriculture: Future perspectives. 3 Biotech 2021, 11, 382. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.A.; Ruth, R.; Wolff, F.H.; Siggaard-Andersen, M.L.; Ruth, U.; Röthlisberger, R.; Wolff, E. Glacial/interglacial changes in mineral dust and sea-salt records in polar ice cores: Sources, transport, and deposition. Rev. Geophys. 2007, 45, 1. [Google Scholar] [CrossRef]
- Bozkurt, D. Determination of Prokaryotic Diversity in Boron-Containing Environments. Master’s Thesis, Eskişehir Osmangazi University, Eskişehir, Turkey, 2016. [Google Scholar]
- Bergey, D.H. Bergey’s Manual of Determinative Bacteriology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1994. [Google Scholar]
- Shahzad, S.M.; Arif, M.S.; Riaz, M.; Iqbal, Z.; Ashraf, M. PGPR with varied ACC-deaminase activity induced different growth and yield response in maize (Zea mays L.) under fertilized conditions. Eur. J. Soil Biol. 2013, 57, 27–34. [Google Scholar] [CrossRef]
- Han, K.W.; Hwan, R.Y.; Chun, S.R. Nitrogen balance and biological nitrogen fixation of soybean in soybean-barley cropping system. Korean J. Crop Sci. 2005, 50, 1–4. [Google Scholar]
- Mehta, S.; Nautiyal, C.S. An efficient method for qualitative screening of phosphate-solubilizing bacteria. Curr. Microbiol. 2001, 43, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Sarker, A.; Al-Rashid, J. Analytical protocol for determination of Indole 3 acetic acid (IAA) production by Plant Growth Promoting Bacteria (PGPB). Tech. Rep. Quantif. IAA Microbes 2013, 3, 5. [Google Scholar]
- Gang, S.; Sharma, S.; Saraf, M.; Buck, M.; Schumacher, J. Analysis of indole-3-acetic acid (IAA) production in Klebsiella by LC-MS/MS and the Salkowski method. Bio-Protocol 2019, 9, e3230. [Google Scholar] [CrossRef] [PubMed]
- Schwyn, B.; Neilands, J. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; He, W.; Li, Z.; Ge, L.; Zhang, J.; Liu, T. Salt-tolerant endophytic bacterium Enterobacter ludwigii B30 enhance bermudagrass growth under salt stress by modulating plant physiology and changing rhizosphere and root bacterial community. Front. Plant Sci. 2022, 13, 959427. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Goyal, A. 16S rRNA-Based Identification of a Glucan-Hyperproducing Weissella confusa. Enzyme Res. 2011, 2011, 250842. [Google Scholar] [CrossRef]
- Dr, H. The water culture method for growing plants without soil. Calif. Agri. Exp. Stat. Circ. 1950, 347, 1–32. [Google Scholar]
- Peryea, F.; Kammereck, R. Use of Minolta SPAD-502 chlorophyll meter to quantify the effectiveness of mid-summer trunk injection of iron on chlorotic pear trees. J. Plant Nutr. 1997, 20, 1457–1463. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Cheeseman, J.M. Hydrogen peroxide concentrations in leaves under natural conditions. J. Exp. Bot. 2006, 57, 2435–2444. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.M.; Sresty, T. Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci. 2000, 157, 113–128. [Google Scholar] [PubMed]
- Bergmeyer, H.U. Methoden der Enzymatischen Analyse: Band 1; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 1970. [Google Scholar]
- Kanner, J.; Kinsella, J.E. Lipid deterioration initiated by phagocytic cells in muscle foods:. beta.-carotene destruction of a myeloperoxidase-hydrogen peroxide-halide system. J. Agric. Food Chem. 1983, 31, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Gunn, L.H.; Martin Avila, E.; Birch, R.; Whitney, S.M. The dependency of red Rubisco on its cognate activase for enhancing plant photosynthesis and growth. Proc. Natl. Acad. Sci. USA 2020, 117, 25890–25896. [Google Scholar] [CrossRef] [PubMed]
- Dere, S. Mitigating the Adverse Effects of Salt Stress on Pepper Plants Through Arbuscular Mycorrhizal Fungi (AMF) and Beneficial Bacterial (PGPR) Inoculation. Horticulturae 2024, 10, 1150. [Google Scholar] [CrossRef]
- Agunbiade, V.F.; Fadiji, A.E.; Agbodjato, N.A.; Babalola, O.O. Isolation and Characterization of Plant-Growth-Promoting, Drought-Tolerant Rhizobacteria for Improved Maize Productivity. Plants 2024, 13, 1298. [Google Scholar] [CrossRef] [PubMed]
- Bedini, A.; Mercy, L.; Schneider, C.; Franken, P.; Lucic-Mercy, E. Unraveling the initial plant hormone signaling, metabolic mechanisms and plant defense triggering the endomycorrhizal symbiosis behavior. Front. Plant Sci. 2018, 9, 1800. [Google Scholar] [CrossRef] [PubMed]
- Boiero, L.; Perrig, D.; Masciarelli, O.; Penna, C.; Cassán, F.; Luna, V. Phytohormone production by three strains of Bradyrhizobium japonicum and possible physiological and technological implications. Appl. Microbiol. Biotechnol. 2007, 74, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Yanni, Y.G.; Rizk, R.Y.; Abd El-Fattah, F.K.; Squartini, A.; Corich, V.; Giacomini, A.; Bruijn, F.; Rademaker, J.; Maya-Flores, J.; Ostrom, P. The beneficial plant growth-promoting association of Rhizobium leguminosarum bv. trifolii with rice roots. Funct. Plant Biol. 2001, 28, 845–870. [Google Scholar] [CrossRef]
- Naidu, V.S.G.R.; Panwar, J.D.S.; Annapurna, K. Effect of synthetic auxins and Azorhizobium caulinodans on growth and yield of rice. Indian J. Microbiol. 2004, 44, 211–213. [Google Scholar]
- Senthilkumar, M.; Madhaiyan, M.; Sundaram, S.P.; Kannaiyan, S. Intercellular colonization and growth promoting effects of Methylobacterium sp. with plant-growth regulators on rice (Oryza sativa L. Cv CO-43). Microbiol. Res. 2009, 164, 92–104. [Google Scholar] [CrossRef]
- Chi, F.; Yang, P.; Han, F.; Jing, Y.; Shen, S. Proteomic analysis of rice seedlings infected by Sinorhizobium meliloti 1021. Proteomics 2010, 10, 1861–1874. [Google Scholar] [CrossRef] [PubMed]
- Derhab, N.; Mabrouk, M.E.M.; El-Metwally, M.M.; Mohammed, Y.M.M. Thermostable keratinase from Bacillus cereus L10: Optimization and some potential biotechnological applications. Biomass Convers. Biorefinery 2013, 14, 1–17. [Google Scholar] [CrossRef]
- Wagi, S.; Ahmed, A. Bacillus spp.: Potent microfactories of bacterial IAA. PeerJ 2019, 7, e7258. [Google Scholar] [CrossRef] [PubMed]
- Kouas, S.; Djedidi, S.; Debez, I.B.S.; Sbissi, I.; Alyami, N.M.; Hirsch, A.M. Halotolerant phosphate solubilizing bacteria isolated from arid area in Tunisia improve P status and photosynthetic activity of cultivated barley under P shortage. Heliyon 2024, 10, e38653. [Google Scholar] [CrossRef] [PubMed]
- Marulanda, A.; Azcón, R.; Chaumont, F.; Ruiz-Lozano, J.M.; Aroca, R. Regulation of plasma membrane aquaporins by inoculation with a Bacillus megaterium strain in maize (Zea mays L.) plants under unstressed and salt-stressed conditions. Planta 2010, 232, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Poveda, J.; González-Andrés, F. Bacillus as a source of phytohormones for use in agriculture. Appl. Microbiol. Biotechnol. 2021, 105, 8629–8645. [Google Scholar] [CrossRef] [PubMed]
- Jalmi, S.K.; Sinha, A.K. Ambiguities of PGPR-induced plant signaling and stress management. Front. Microbiol 2022, 13, 899563. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Müller, K.M.; Charles, T.C.; Vesely, S.; Glick, B.R. 1-aminocyclopropane-1-carboxylate (ACC) deaminase genes in rhizobia from southern Saskatchewan. Microb. Ecol. 2009, 57, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Onofre-Lemus, J.; Hernández-Lucas, I.; Girard, L.; Caballero-Mellado, J. ACC (1-aminocyclopropane-1-carboxylate) deaminase activity, a widespread trait in Burkholderia species, and its growth-promoting effect on tomato plants. Appl. Environ. Microbiol 2009, 75, 6581–6590. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Bano, A.; Rai, S.; Dubey, P.; Khan, F.; Pathak, N.; Sharma, S. Plant Growth Promoting Rhizobacteria (PGPR): A sustainable agriculture to rescue the vegetation from the effect of biotic stress: A Review. Lett. Appl. NanoBiosci. 2021, 10, 2459–2465. [Google Scholar]
- Bessadok, K.; Navarro-Torre, S.; Pajuelo, E.; Mateos-Naranjo, E.; Redondo-Gómez, S.; Caviedes, M.A.; Fterich, A.; Mars, M.; Rodríguez-Llorente, I.D. The ACC-deaminase producing bacterium Variovorax sp. CT7. 15 as a tool for improving Calicotome villosa nodulation and growth in arid regions of Tunisia. Microorganisms 2020, 8, 541. [Google Scholar] [CrossRef]
- Ferreira, M.J.; Silva, H.; Cunha, A. Siderophore-producing rhizobacteria as a promising tool for empowering plants to cope with iron limitation in saline soils: A review. Pedosphere 2019, 29, 409–420. [Google Scholar] [CrossRef]
- Saha, M.; Sarkar, S.; Sarkar, B.; Sharma, B.K.; Bhattacharjee, S.; Tribedi, P. Microbial siderophores and their potential applications: A review. Environ. Res. 2019, 23, 3984–3999. [Google Scholar] [CrossRef] [PubMed]
- Sansinenea, E. Intellectual Property Issues in Microbiology. In Applications and Patents of Bacillus spp. in Agriculture; Singh, H.B., Keswani, C., Singh, S.P., Eds.; Springer: Singapore, 2019. [Google Scholar]
- Kumar, P.; Pahal, V.; Gupta, A.; Vadhan, R.; Chandra, H.; Dubey, R. Effect of silver nanoparticles and Bacillus cereus LPR2 on the growth of Zea mays. Sci. Rep. 2020, 10, 20409. [Google Scholar] [CrossRef]
- Sherpa, M.T.; Bag, N.; Das, S.; Haokip, P.; Sharma, L. Isolation and characterization of plant growth promoting rhizobacteria isolated from organically grown high yielding pole type native pea (Pisum sativum L.) variety Dentami of Sikkim, India. Curr. Res. Microb. Sci. 2021, 2, 100068. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.S.; Ikhajiagbe, B. The growth response of rice (Oryza sativa L. var. FARO 44) in vitro after inoculation with bacterial isolates from a typical ferruginous ultisol. Sci. Bull. 2021, 45, 1–20. [Google Scholar] [CrossRef]
- Ali, S.; Khan, N. Delineation of mechanistic approaches employed by plant growth promoting microorganisms for improving drought stress tolerance in plants. Microbiol. Res. 2021, 249, 126771. [Google Scholar] [CrossRef] [PubMed]
- Praca, L.B.; Gomes, A.C.M.M.; Cabral, G.; Martins, E.S.; Sujii, E.H.; Monnerat, R.G. Endophytic colonization by Brazilian strains of Bacillus thuringiensis on cabbage seedlings grown in vitro. Bt Res. 2012, 3, 11–19. [Google Scholar]
- Abdeljalil, N.B.; Vallance, J.; Gerbore, J.; Bruez, E.; Martins, G.; Rey, P.; Daami-Remadi, M. Characterization of tomato-associated rhizobacteria recovered from various tomato-growing sites in Tunisia. J. Plant Pathol. Microbiol. 2016, 7, 351. [Google Scholar] [CrossRef]
- Hyder, S.; Gondal, A.S.; Rizvi, Z.F.; Ahmad, R.; Alam, M.M.; Hannan, A.; Ahmed, W.; Fatima, N.; Inam-ul-Haq, M. Characterization of native plant growth promoting rhizobacteria and their anti-oomycete potential against Phytophthora capsici affecting chilli pepper (Capsicum annum L.). Sci. Rep. 2020, 10, 13859. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.; Tagele, S.B.; Pham, H.Q.; Kim, M.-C.; Choi, S.D.; Kim, M.J.; Park, Y.J.; Ibal, J.C.; Park, G.S.; Shin, J.H. Response of soil bacterial community and pepper plant growth to application of Bacillus thuringiensis KNU-07. Agronomy 2020, 10, 551. [Google Scholar] [CrossRef]
- Shafi, J.; Tian, H.; Ji, M. Bacillus species as versatile weapons for plant pathogens: A review. Biotechnol. Biotechnol. Equip. 2017, 31, 446–459. [Google Scholar] [CrossRef]
- Borges, N.O.; Silva, S.; Antônio, J.; Franscischini, R.; Campos, H.D.; Olivera, J.S.B.; Schwan-Estrada, K.R.F. Induction of soybean resistance mechanisms to anthracnose by biocontrol agents. Rev. Caatinga 2022, 35, 265–275. [Google Scholar] [CrossRef]
- Samaras, A.; Roumeliotis, E.; Ntasiou, P.; Karaoglanidis, G. Bacillus subtilis MBI600 promotes growth of tomato plants and induces systemic resistance contributing to the control of soilborne pathogens. Plants 2021, 10, 1113. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Ren, Z.-H.; Zu, X.; Yu, X.-Y.; Zhu, H.-J.; Li, X.-J.; Zhong, J.; Liu, E.-M. Efficacy of plant growth-promoting bacteria Bacillus cereus YN917 for biocontrol of rice blast. Front. Microbiol. 2021, 12, 684888. [Google Scholar] [CrossRef] [PubMed]
- El-Wakil, D.A.; Essa, A.M.M. Antagonistic potential of some bacterial strains against Xanthomonas campestris, the cause of bacterial blight in Hordeum vulgare. BioResources 2020, 15, 4205. [Google Scholar] [CrossRef]
- Yang, J.W.; Yu, S.H.; Ryu, C.-M. Priming of defense-related genes confers root-colonizing bacilli-elicited induced systemic resistance in pepper. Plant Pathol. J. 2009, 25, 389–399. [Google Scholar] [CrossRef]
- Akhter, M.S.; Noreen, S.; Mahmood, S.; Aqeel, M.; Zafar, Z.U.; Rashid, M.; Arshad, M.N.; Owais, M.; Ahmad, J.; Shah, K.H. Silicon supplement improves growth and yield under salt stress by modulating ionic homeostasis and some physiological indices in Hordeum vulgare L. J. Soil Sci. Plant Nutr. 2023, 23, 1694–1712. [Google Scholar] [CrossRef]
- Sreesaeng, J.; Qiu, C.W.; Zhang, S.; Shi, S.H.; Luo, L.; Holford, P.; Wu, F. Identification and characterization of hull-less barley (Hordeum vulgare L.) germplasms for salt tolerance. Plant Growth Regul. 2024, 104, 975–989. [Google Scholar] [CrossRef]
- Khan, I.; Lubna; Asaf, S.; Bilal, S.; Alamri, S.S.; Jan, R.; Asif, S.; Kim, K.-M.; Al-Harrasi, A. Enhanced growth and stress tolerance in Barley (Hordeum vulgare) through biopriming with Aspergillus niger CSR3: A promising approach for sustainable agriculture in saline environments. Cereal Res. Commun. 2024, 52, 1201–1213. [Google Scholar] [CrossRef]
- Ferioun, M.; Bouhraoua, S.; Belahcen, D.; Zouitane, I.; Srhiouar, N.; Louahlia, S.; El Ghachtouli, N. PGPR Consortia Enhance Growth and Yield in Barley Cultivars Subjected to Severe Drought Stress and Subsequent Recovery. Rhizosphere 2024, 31, 100926. [Google Scholar] [CrossRef]
- Al-Shammari, W.B.; Al-Huquil, A.A.; Alshammery, K.; Lotfi, S.; Altamimi, H.; Alshammari, A.; Al-Harbi, N.A.; Rashed, A.A.; Abdelaal, K. Alleviation of drought stress damages by melatonin and Bacillus thuringiensis associated with adjusting photosynthetic efficiency, antioxidative system, and anatomical structure of Glycine max (L.). Heliyon 2024, 10, e34754. [Google Scholar] [CrossRef]
- Çam, S.; Küçük, Ç.; Almaca, A. Bacillus strains exhibit various plant growth promoting traits and their biofilm-forming capability correlates to their salt stress alleviation effect on maize seedlings. J. Biotech. 2023, 369, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Zai, X.M.; Fan, J.J.; Hao, Z.P.; Liu, X.M.; Zhang, W.X. Effect of co-inoculation with arbuscular mycorrhizal fungi and phosphate solubilizing fungi on nutrient uptake and photosynthesis of beach palm under salt stress environment. Sci. Rep. 2021, 11, 5761. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, R. Modulation of ethylene and ascorbic acid on reactive oxygen species scavenging in plant salt response. Front. Plant Sci. 2019, 10, 319. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Abdel Latef, A.A.; Hashem, A.; Abd_Allah, E.F.; Gucel, S.; Tran, L.S.P. Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front. Plant Sci. 2016, 7, 347. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, L.; Ma, J.; Li, Y.; Chen, F.; Peijnenburg, W. Comparative physiological and metabolomics analyses using Ag NPs and HAS31 (PGPR) to alleviate Cr stress in barley (Hordeum vulgare L.). Environ. Pollut. 2023, 333, 122010. [Google Scholar] [CrossRef] [PubMed]
- Bharti, N.; Barnawal, D. Amelioration of salinity stress by PGPR: ACC deaminase and ROS scavenging enzymes activity. In PGPR Amelioration in Sustainable Agriculture; Singh, A.K., Kumar, A., Singh, P.K., Eds.; Woodhead Publishing: Sawston, UK; Elsevier: Amsterdam, The Netherlands, 2019; pp. 85–106. [Google Scholar]
- Slimani, A.; Raklami, A.; Oufdou, K.; Meddich, A. Isolation and characterization of PGPR and their potenzial for drought alleviation in barley plants. Gesunde Pflanz. 2023, 75, 377–391. [Google Scholar] [CrossRef]
- Sarkar, A.; Pramanik, K.; Mitra, S.; Soren, T.; Maiti, T.K. Enhancement of growth and salt tolerance of rice seedlings by ACC deaminase-producing Burkholderia sp. MTCC 12259. J. Plant Physiol. 2018, 231, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Ikram, M.; Sarfaraz, S.; ul Haq, I.; Khan, A.; Murad, Z.; Munsif, F. Influence of Plant Growth Promoting Rhizobacteria (PGPR) on the Growth and Yield of Sunflower (Helianthus annus L.) Under Salt Stress. J. Crop Health 2024, 76, 1221–1234. [Google Scholar] [CrossRef]
- Silambarasan, S.; Logeswari, P.; Cornejo, P.; Abraham, J.; Valentine, A. Simultaneous mitigation of aluminum, salinity and drought stress in Lactuca sativa growth via formulated plant growth promoting Rhodotorula mucilaginosa CAM4. Ecotoxicol. Environ. Saf. 2019, 180, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Hafeez, A.; Ahmad, S.; Javed, M.A.; Sumaira; Afridi, M.S.; Dawoud, T.M.; Almaary, K.S.; Muresan, C.C.; Marc, R.A.; et al. Bacillus thuringiensis PM25 ameliorates oxidative damage of salinity stress in maize via regulating growth, leaf pigments, antioxidant defense system, and stress responsive gene expression. Front. Plant Sci. 2022, 13, 921668. [Google Scholar] [CrossRef] [PubMed]
- Janků, M.; Luhová, L.; Petřivalský, M. On the Origin and Fate of Reactive Oxygen Species in Plant Cell Compartments. Antioxidants 2019, 8, 105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tian, Z.; Xi, Y.; Wang, X.; Chen, S.; He, M.; Chen, Y.; Guo, Y. Improvement of salt tolerance of Arabidopsis thaliana seedlings inoculated with endophytic Bacillus cereus KP120. J. Plant Interact. 2022, 17, 884–893. [Google Scholar] [CrossRef]
- Azeem, M.; Haider, M.Z.; Javed, S.; Saleem, M.H.; Alatawi, A. Drought stress amelioration in maize (Zea mays L.) by inoculation of Bacillus spp. strains under sterile soil conditions. Agriculture 2022, 12, 50. [Google Scholar] [CrossRef]
- Andy, A.K.; Rajput, V.D.; Burachevskaya, M.; Gour, V.S. Exploring the identity and properties of two Bacillis strains and their potential to alleviate drought and heavy metal stress. Horticulturae 2023, 9, 46. [Google Scholar] [CrossRef]
- Meenakshi; Annapurna, K.; Govindasamy, V.; Ajit, V.; Choudhary, D.K. Mitigation of drought stress in wheat crop by drought tolerant endophytic bacterial isolates. Vegetos 2019, 4, 486–493. [Google Scholar] [CrossRef]
- Hernández-Huerta, J.; Tamez-Guerra, P.; Gomez-Flores, R.; Delgado-Gardea, M.C.E.; Robles-Hernández, L.; Gonzalez-Franco, A.C.; Infante-Ramirez, R. Pepper growth promotion and biocontrol against Xanthomonas euvesicatoria by Bacillus cereus and Bacillus thuringiensis formulations. Int. J. Plant Biol. 2023, 11, e14633. [Google Scholar] [PubMed]
- Akhtyamova, Z.; Arkhipova, T.; Martynenko, E.; Nuzhnaya, T.; Kuzmina, L.; Kudoyarova, G.; Veselov, D. Growth-promoting effect of rhizobacterium (Bacillus subtilis IB22) in salt-stressed barley depends on abscisic acid accumulation in the roots. Int. J. Mol. Sci. 2021, 22, 10680. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, K.; Rashwan, E.; Mohamed, H.H.; Awadalla, A.; Omara, A.E.D.; Hafez, E.M.; Alshaal, T. Application of silica nanoparticles in combination with two bacterial strains improves the growth, antioxidant capacity and production of barley irrigated with saline water in salt-affected soil. Plants 2022, 11, 2026. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Peng, X.; Lang, D.; Ma, X.; Zhang, X. Physio-biochemical and transcriptomic analysis reveals that the mechanism of Bacillus cereus G2 alleviated oxidative stress of salt-stressed Glycyrrhiza uralensis Fisch. seedlings. Ecotoxicol. Environ. Saf. 2022, 247, 114264. [Google Scholar] [CrossRef] [PubMed]
- Mashabela, M.D.; Piater, L.A.; Dubery, I.A.; Tugizimana, F.; Mhlongo, M.I. Rhizosphere tripartite interactions and PGPR-mediated metabolic reprogramming towards ISR and plant priming: A metabolomics review. Biology 2022, 11, 346. [Google Scholar] [CrossRef] [PubMed]
- Anee, T.I.; Islam, M.N.N.; Hassan, M.; Masud, A.A.C.; Alam, M.M.; Hasanuzzaman, M. Organic amendments improve plant morpho-physiology and antioxidant metabolism in mitigating drought stress in bread wheat (Triticum aestivum L.). Phyton 2022, 91, 9. [Google Scholar]
- Benaffari, W.; Boutasknit, A.; Anli, M.; Ait-El-Mokhtar, M.; Ait-Rahou, Y.; Ben-Laouane, R.; Ben Ahmed, H.; Mitsui, T.; Baslam, M.; Meddich, A. The native arbuscular mycorrhizal fungi and vermicompost-based organic amendments enhance soil fertility, growth performance, and the drought stress tolerance of quinoa. Plants 2022, 11, 393. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Naseer, I.; Hussain, A.; Zahid Mumtaz, M.; Mustafa, A.; Hilger, T.; Ahmad Zahir, Z.; Xu, M. Appraising endophyte–plant symbiosis for improved growth, nodulation, nitrogen fixation and abiotic stress tolerance: An experimental investigation with chickpea (Cicer arietinum L.). Agronomy 2019, 9, 621. [Google Scholar] [CrossRef]
- Ibáñez, A.; Diez-Galán, A.; Cobos, R.; Calvo-Peña, C.; Barreiro, C.; Medina-Turienzo, J.; Sánchez-García, M.; Coque, J.J.R. Using rhizosphere phosphate solubilizing bacteria to improve barley (Hordeum vulgare) plant productivity. Microorganisms 2021, 9, 1619. [Google Scholar] [CrossRef] [PubMed]
- Talaat, N.B. Drought stress alleviator melatonin reconfigures water-stressed barley (Hordeum vulgare L.) plants’ photosynthetic efficiency, antioxidant capacity, and endogenous phytohormone profile. Int. J. Mol. Sci. 2023, 24, 16228. [Google Scholar] [CrossRef] [PubMed]
- Yanti, Y.; Nasution, C.R. Effectivity of Bacillus cereus to control Ralstonia syzygii subsp. indonesiensis and growth promoting of chili pepper. J. Crop Prot. 2017, 10, 2. [Google Scholar] [CrossRef]
- Tunsagool, P.; Jutidamrongphan, W.; Phaonakrop, N.; Jaresitthikunchai, J.; Roytrakul, S.; Leelasuphakul, W. Insights into stress responses in mandarins triggered by Bacillus subtilis cyclic lipopeptides and exogenous plant hormones upon Penicillium digitatum infection. Plant Cell Rep. 2019, 38, 559–575. [Google Scholar] [CrossRef] [PubMed]

| Bacillus cereus CUN6 | Bacillus thuringiensis SIRB2 | |
|---|---|---|
| Pigment | Orange | Orange |
| Gram Stain | + | + |
| Morphology | Bacil | Bacil |
| Oxidase Test | + | − |
| Catalase Test | − | + |
| Indole Test | + | + |
| Citrate Test | + | − |
| Phenol Test | + | − |
| VP Test | + | − |
| H2S Test | Y SC | R SC |
| Y DC | R DC |
| Isolate | NBRIP (mm) | CAS (mm) | ACC | Nitrogen Fixation | IAA | ||
|---|---|---|---|---|---|---|---|
| Zone Diameter | Colony Diameter | Phosphorus Dissolution | Zone Diameter | Reproductive | Reproductive | 530 nm | |
| Bacillus cereus CUN6 | 12 | 7 | 1.71 | 24 | + | + | 75.0 |
| Bacillus thuringiensis SIRB2 | 18 | 16 | 1.12 | 19 | + | + | 65.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teker Yıldız, M.; Acar, O. Comparison of Two Bacillus Strains Isolated from the Coastal Zone in Barley (Hordeum vulgare L.) Under Salt Stress. Plants 2025, 14, 723. https://doi.org/10.3390/plants14050723
Teker Yıldız M, Acar O. Comparison of Two Bacillus Strains Isolated from the Coastal Zone in Barley (Hordeum vulgare L.) Under Salt Stress. Plants. 2025; 14(5):723. https://doi.org/10.3390/plants14050723
Chicago/Turabian StyleTeker Yıldız, Müge, and Okan Acar. 2025. "Comparison of Two Bacillus Strains Isolated from the Coastal Zone in Barley (Hordeum vulgare L.) Under Salt Stress" Plants 14, no. 5: 723. https://doi.org/10.3390/plants14050723
APA StyleTeker Yıldız, M., & Acar, O. (2025). Comparison of Two Bacillus Strains Isolated from the Coastal Zone in Barley (Hordeum vulgare L.) Under Salt Stress. Plants, 14(5), 723. https://doi.org/10.3390/plants14050723







