A Spatial Structure of Key Tree Species Metrodorea nigra St. Hill. (Rutaceae) Is Associated with Historical Disturbance and Isolation in Southeastern Brazil
Abstract
1. Introduction
2. Results
2.1. Principal Coordinates Analysis and Analysis of Molecular Variance
2.2. Inbreeding and Fst
2.3. The Spatial Genetic Structure Between Generations
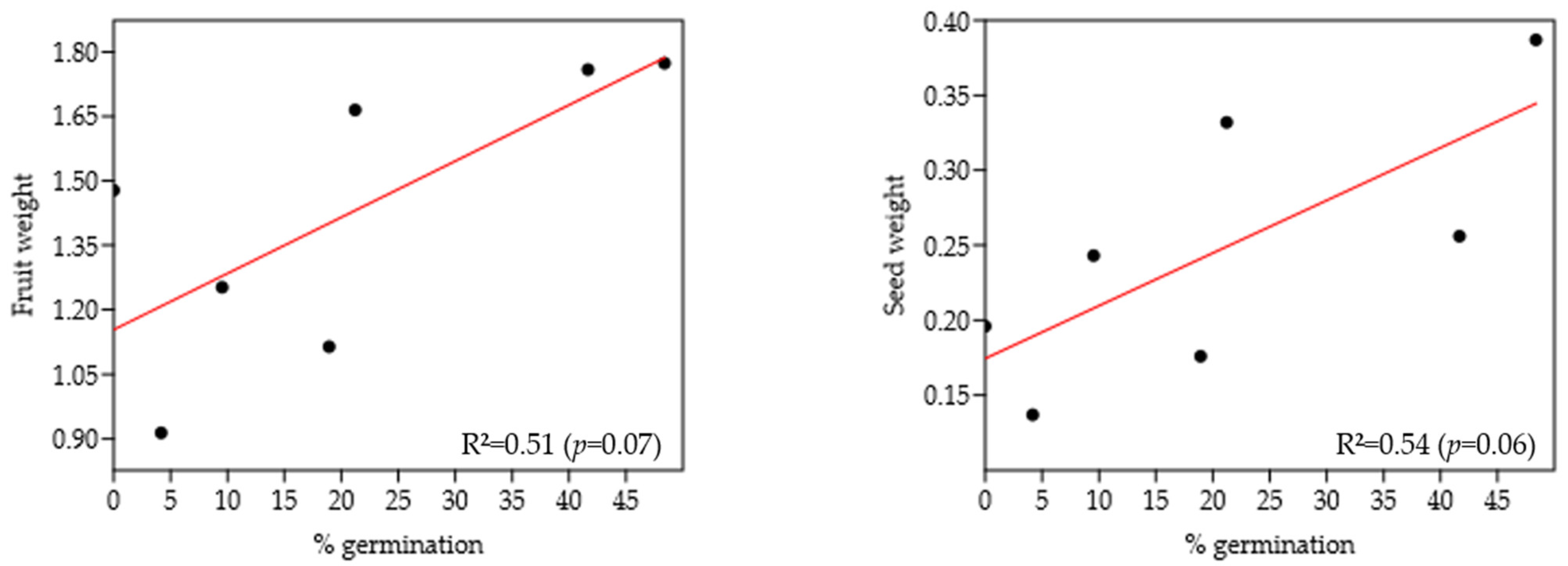
2.4. Phenology of Metrodorea nigra in BSQ-Rib
Seed Collection and Germination
3. Discussion
4. Materials and Methods
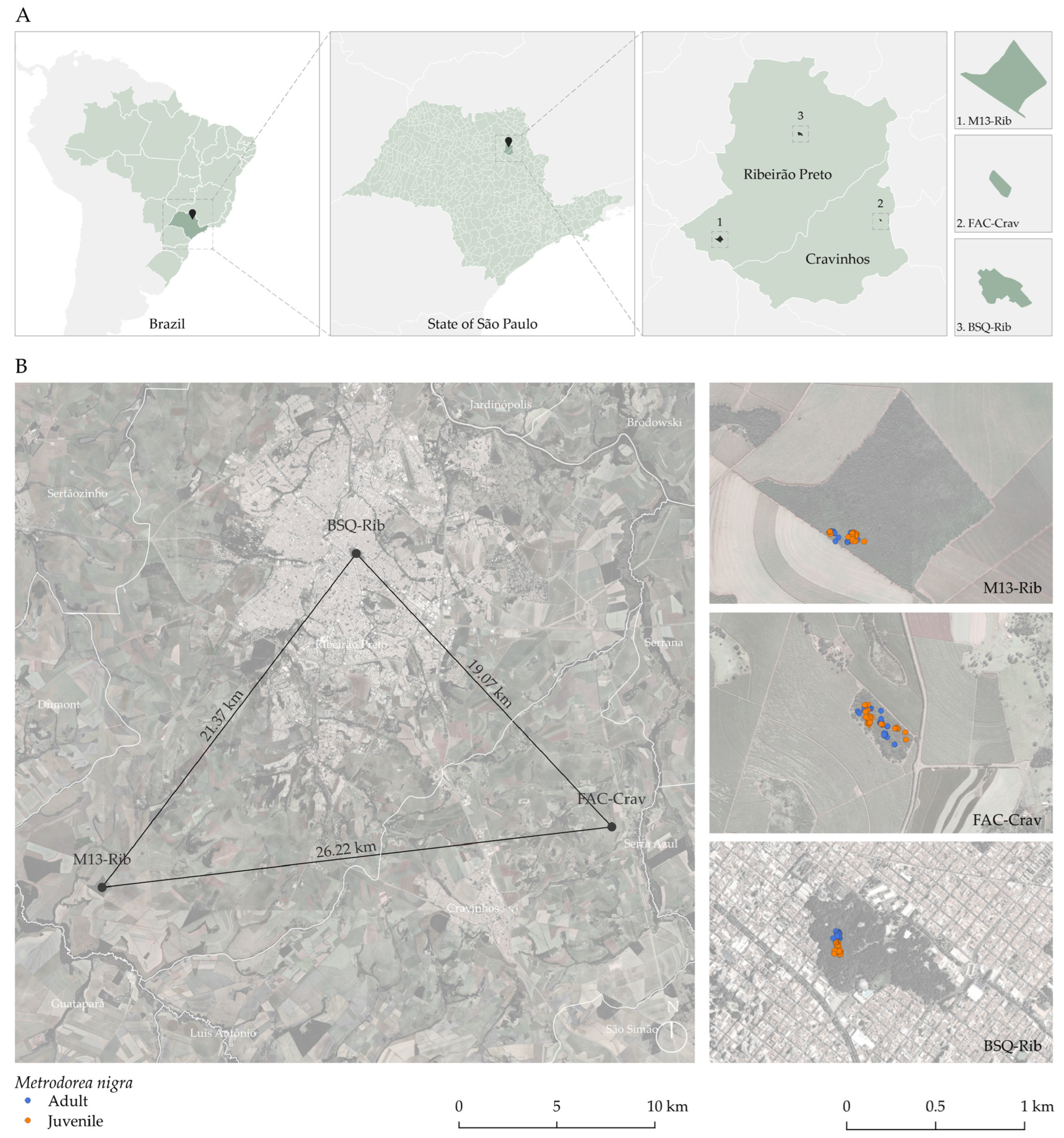
4.1. Selection of Remnants of the Semi-Deciduous Seasonal Forest for Spatial Analysis
4.2. Plant Material, DNA Extraction, and Molecular Markers
4.3. Phenology and Seed Germination of M. nigra in the BSQ-Rib Fragment
4.4. Statistical Analysis
4.4.1. Inbreeding and Genetic Structure
4.4.2. Spatial Genetic Structure
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMOVA | Analysis of Molecular Variance |
| ISSR | Inter Simple Sequence Repeat |
| SSR | Simple sequence repeats |
| PCoA | Principal Coordinates Analysis |
References
- Legendre, P.; Fortin, M.J. Spatial Pattern and Ecological Analysis. Vegetatio 1989, 80, 107–138. [Google Scholar] [CrossRef]
- Loveless, M.D.; Hamrick, J.L. Ecological Determinants of Genetic Structure in Plant Populations. Annu. Rev. Ecol. Syst. 1984, 15, 65–95. [Google Scholar] [CrossRef]
- Vekemans, X.; Hardy, O.J. New Insights from Fine-scale Spatial Genetic Structure Analyses in Plant Populations. Mol. Ecol. 2004, 13, 921–935. [Google Scholar] [CrossRef]
- Nazareno, A.G.; Jump, A.S. Species–Genetic Diversity Correlations in Habitat Fragmentation Can Be Biased by Small Sample Sizes. Mol. Ecol. 2012, 21, 2847–2849. [Google Scholar]
- Epperson, B.K. Spatial Structure of Genetic Variation within Populations of Forest Trees. In Proceedings of the Population Genetics of Forest Trees: Proceedings of the International Symposium on Population Genetics of Forest Trees, Corvallis, OR, USA, 31 July–2 August 1990; Springer: Berlin/Heidelberg, Germany, 1992; pp. 257–278. [Google Scholar]
- Shapcott, A. The Spatial Genetic Structure in Natural Populations of the Australian Temperate Rainforest Tree Atherosperma moschatum (Labill.) (Monimiaceae). Heredity 1995, 74, 28–38. [Google Scholar] [CrossRef][Green Version]
- Young, A.; Boyle, T.; Brown, T. The Population Genetic Consequences of Habitat Fragmentation for Plants. Trends Ecol. Evol. 1996, 11, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Hardy, O.J.; Maggia, L.; Bandou, E.; Breyne, P.; Caron, H.; Chevallier, M.; Doligez, A.; Dutech, C.; Kremer, A.; Latouche-Hallé, C. Fine-scale Genetic Structure and Gene Dispersal Inferences in 10 Neotropical Tree Species. Mol. Ecol. 2006, 15, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Robledo-Arnuncio, J.J.; Klein, E.K.; Muller-Landau, H.C.; Santamaría, L. Space, Time and Complexity in Plant Dispersal Ecology. Mov. Ecol. 2014, 2, 1–17. [Google Scholar] [CrossRef]
- Fournier, L.A. El Dendrofenograma, Una Representación Gráfica Del Comportamiento Fenológico de Los Árboles. Turrialba 1976, 26, 96–97. [Google Scholar]
- Morellato, L.P.C. Estudo Da Fenologia de Arvores, Arbustose Lianas de Uma Floresta Semidecidua Do Sudeste Do Brasil. Ph.D. Thesis, Universidade Estadualde Campinas, Instituto de Biologia, Mexico City, Mexico, 1991. [Google Scholar]
- Morellato, L.P.C. Ecologia e Preservação de Uma Floresta Tropical Urbana: Reserva de Santa Genebra; Editora Da Unicamp: Campinas, Brazil, 1995; ISBN 8526803638. [Google Scholar]
- Morellato, L.P.C.; Altomare, M.; Gressler, E. A Review of Reproductive Plant Phenology in South and Central America: New Perspectives BT—Phenology: An Integrative Environmental Science; Schwartz, M.D., Ed.; Springer Nature: Cham, Switzerland, 2024; pp. 107–138. ISBN 978-3-031-75027-4. [Google Scholar]
- Ghazoul, J. Pollen and Seed Dispersal among Dispersed Plants. Biol. Rev. 2005, 80, 413–443. [Google Scholar] [CrossRef]
- Willi, Y.; Van Buskirk, J.; Schmid, B.; Fischer, M. Genetic Isolation of Fragmented Populations Is Exacerbated by Drift and Selection. J. Evol. Biol. 2007, 20, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Neaves, L.; Eales, J.; Whitlock, R.; Hollingsworth, P.M.; Burke, T.; Pullin, A. The fitness consequences of inbreeding in natural populations and their implications for species conservation—A systematic map. Environ. Evid. 2015, 4, 5. [Google Scholar] [CrossRef]
- de Jong, T.J.; Waser, N.M.; Klinkhamer, P.G. Geitonogamy: The neglected side of selfing. Trends Ecol. Evol. 1993, 8, 321–325. [Google Scholar] [CrossRef]
- Morellato, L.P.C.; Haddad, C.F.B. Introduction: The Brazilian Atlantic Forest 1. Biotropica 2000, 32, 786–792. [Google Scholar] [CrossRef]
- Ribeiro, M.C.; Metzger, J.P.; Martensen, A.C.; Ponzoni, F.J.; Hirota, M.M. The Brazilian Atlantic Forest: How Much Is Left, and How Is the Remaining Forest Distributed? Implications for Conservation. Biol. Conserv. 2009, 142, 1141–1153. [Google Scholar] [CrossRef]
- Kotchetkoff-Henriques, O. Characterization of the Natural Vegetation in Ribeirão Preto, SP: Bases for Conservation. DSc thesis, Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto, Universidade de São Paulo, São Paulo, Brazil, 2003. [Google Scholar]
- Carvalho, S.; Varanda, E.M. Biodiversidade e Pioneirismo Na Floresta Da USP Ribeirão Preto. Fecunda. 2008. Available online: https://biofecunda.wordpress.com/2008/10/23/biodiversidade-e-pioneirismo-na-floresta-da-usp-ribeirao-preto/ (accessed on 14 February 2025).
- Eckert, C.G.; Kalisz, S.; Geber, M.A.; Sargent, R.; Elle, E.; Cheptou, P.-O.; Goodwillie, C.; Johnston, M.O.; Kelly, J.K.; Moeller, D.A. Plant Mating Systems in a Changing World. Trends Ecol. Evol. 2010, 25, 35–43. [Google Scholar] [CrossRef]
- Durigan, G.; Franco, G.A.D.C.; Saito, M.; Baitello, J.B. Estrutura e Diversidade Do Componente Arbóreo Da Floresta Na Estação Ecológica Dos Caetetus, Gália, SP. Braz. J. Bot. 2000, 23, 371–383. [Google Scholar] [CrossRef]
- Metzger, J.P. Tree Functional Group Richness and Landscape Structure in a Brazilian Tropical Fragmented Landscape. Ecol. Appl. 2000, 10, 1147–1161. [Google Scholar] [CrossRef]
- Bertoncini, A.P. Estrutura e Dinamica de Uma Area Perturbada Na Terra Indigena Arariba (Avai, SP): Implicações Para o Manejo e a Restauração Florestal. 2003. Available online: https://ssbr.oa.mg/work/10.47749/t/unicamp.2003.290969 (accessed on 14 February 2025).
- Villela, D.M.; Nascimento, M.T.; de Aragao, L.E.O.C.; Da Gama, D.M. Effect of Selective Logging on Forest Structure and Nutrient Cycling in a Seasonally Dry Brazilian Atlantic Forest. J. Biogeogr. 2006, 33, 506–516. [Google Scholar] [CrossRef]
- Cassola, H. Aspectos Da Estrutura Fitossociológica e Silvigenética Em Fragmentos de Floresta Estacional Semidecídua Com Diferentes Histórias de Perturbação Em Botucatu. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2008. [Google Scholar]
- Marcondelli, A.C.B. Estrutura de Uma Comunidade Arbórea de Floresta Estacional Semidecídua Não Pertubada No Noroeste Paulista Em Relação à Outra Comunidade Com Indicadores de Perturbação. Master’s Thesis, Universidade Estadual Paulista, São Paulo, Brazil, 2010. [Google Scholar]
- Schwarcz, K.D.; Pataca, C.L.; Abreu, A.G.; Bariani, J.M.; Macrini, C.M.T.; Solferini, V.N. Genetic Diversity in Atlantic Forest Trees: Fragmentation Effects on Astronium Graveolens (Anacardiaceae) and Metrodorea Nigra (Rutaceae), Species with Distinct Seed Dispersal Strategies. Bot. J. Linn. Soc. 2010, 164, 326–336. [Google Scholar] [CrossRef][Green Version]
- Dias, P.; Udulutsch, R.G.; Pirani, J.R. Molecular Phylogeny and Biogeography of the South American Genus Metrodorea (Rutaceae). Turk. J. Bot. 2015, 39, 825–834. [Google Scholar] [CrossRef]
- Tanaka, G.K.; Groppo, M. Estrutura e Florística Do Estrado Arbóreo de Um Fragmento de Floresta Estacional Semidecidual: Estação Ecológica de Ribeirão Preto. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2009. [Google Scholar]
- Capretz, R.L.; Batista, J.L.F.; Sotomayor, J.F.M.; da Cunha, C.R.; Nicoletti, M.F.; Rodrigues, R.R. Padrão Espacial de Quatro Formações Florestais Do Estado de São Paulo, Através Da Função K de Ripley. Ciência Florest. 2012, 22, 551–565. [Google Scholar] [CrossRef]
- Lorenzi, H. Árvores Brasileiras: Manual de Identificação e Cultivo de Espécies Arbóreas Nativas Do Brasil; Instituto Plantarum: Nova Odessa, Brazil, 2002; 384p. [Google Scholar]
- Pombal, E.C.P.; Patrícia, L.; Morellato, C. Differentiation of Floral Color and Odor in Two Fly Pollinated Species of Metrodorea (Rutaceae) from Brazil. Plant Syst. Evol. 2000, 221, 141–156. [Google Scholar] [CrossRef]
- de Souza, L.A.; Moscheta, I.S.; Mourão, K.S.M.; Rosa, S.M. da Morphology and Anatomy of the Flower and Anthesis of Metrodorea Nigra St. Hill.(Rutaceae). Braz. Arch. Biol. Technol. 2004, 47, 107–112. [Google Scholar] [CrossRef]
- Barbosa; Luiz, M.; Regina, T.S.; Fernando, C.D.L.; Paulo, R.T.O.; Karina, C.B.; Tiago, C.B. Lista de espécies indicadas para restauração ecológica para diversas regiões do Estado de São Paulo/Luiz Mauro Barbosa; Instituto de Botânica: São Paulo, Brazil, 2017; 344p. [Google Scholar]
- de Souza, L.A.; da Rosa, S.M.; Moscheta, I.S. Anatomy of the Developing Fruit of Metrodorea Nigra A. St.-Hil.(Rutaceae). Braz. Arch. Biol. Technol. 2008, 51, 1171–1179. [Google Scholar] [CrossRef]
- Guidugli, M.C.; Ferreira-Ramos, R.; de Sousa, A.C.B.; Cidade, F.W.; Marconi, T.G.; Mestriner, M.A.; Groppo, M.; Alzate-Marin, A.L. Genetic Diversity of Metrodorea Nigra (Rutaceae) from a Small Forest Remnant in Brazil Assessed with Microsatellite Markers. Genet. Mol. Res. 2012, 11, 10–16. [Google Scholar] [CrossRef]
- Moraes Filho, R.M.; Bonifácio-Anacleto, F.; Alzate-Marin, A.L. Fragmentation Effects and Genetic Diversity of the Key Semidecidual Forest Species Metrodorea Nigra in Southwestern Brazil. Genet. Mol. Res. 2015, 14, 3509–3524. [Google Scholar] [CrossRef] [PubMed]
- CIIAGRO—Portal Agrometeorológico e Hidrológico do Estado de São Paulo. 2025. Available online: http://www.ciiagro.org.br/mensal/cmensal (accessed on 14 February 2025).
- Guiller, A.; Decocq, G.; Kichey, T.; Poli, P.; Vandepitte, K.; Dubois, F.; Honnay, O.; Closset-Kopp, D. Spatial Genetic Structure of Two Forest Plant Metapopulations in Dynamic Agricultural Landscapes. Landsc. Urban Plan. 2023, 231, 104648. [Google Scholar] [CrossRef]
- Alzate-Marin, A.L.; Guidugli, M.C.; Soriani, H.H.; Martinez, C.A.; Mestriner, M.A. An Efficient and Rapid DNA Minipreparation Procedure Suitable for PCR/SSR and RAPD Analyses in Tropical Forest Tree Species. Braz. Arch. Biol. Technol. 2009, 52, 1217–1224. [Google Scholar] [CrossRef]
- Moraes Filho, R.M.; Jimenez, H.J.; Montarroyos, A.V.V.; Musser, R.S.; Silva, M.M.; Silva, E.F.; Martins, L.S.S. Variabilidade genética em genótipos da coleção de germoplasma de Citrus, do Instituto Agronômico de Pernambuco Brejão-PE, por meio de marcadores moleculares ISSR. Citrus Res. Technol. 2011, 32, 67–76. [Google Scholar] [CrossRef]
- Alzate-Marin, A.L.; Bonifacio-Anacleto, F.; de Moraes Filho, R.M.; Machado, G.P.; Nazareno, A.G. Genetic Analysis across Life Stages of Metrodorea nigra (Rutaceae) in a Population Located in an Urban Landscape of Southeastern Brazil Using a New Set of Microsatellite Markers. Braz. J. Bot. 2016, 39, 795–799. [Google Scholar] [CrossRef]
- Peakall, R.O.D.; Smouse, P.E. GenAlEx 6.5: Genetic Analysis in Excel. Population Genetic Software for Teaching and Research—an Update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1. [Google Scholar]
- Segelbacher, G.; Cushman, S.A.; Epperson, B.K.; Fortin, M.J.; Francois, O.; Hardy, O.J.; Holderegger, R.; Taberlet, P.; Waits, L.P.; Manel, S. Applications of landscape genetics in conservation biology: Concepts and challenges. Conserv. Genet. 2010, 11, 375–385. [Google Scholar] [CrossRef]
- Smouse, P.E.; Peakall, R.O.D. Spatial Autocorrelation Analysis of Individual Multiallele and Multilocus Genetic Structure. Heredity 1999, 82, 561–573. [Google Scholar] [CrossRef]
- Peakall, R.; Ruibal, M.; Lindenmayer, D.B. Spatial Autocorrelation Analysis Offers New Insights into Gene Flow in the Australian Bush Rat, Rattus Fuscipes. Evolution 2003, 57, 1182–1195. [Google Scholar]
- Ferreira-Ramos, R.; Monteiro, M.; Zucchi, M.I.; Pinheiro, J.B.; Martinez, C.A.; Mestriner, M.A.; Alzate-Marin, A.L. Microsatellite markers for Aspidosperma polyneuron (Apocynaceae), an endangered tropical tree species. Am. J. Bot. 2011, 98, e300–e302. [Google Scholar] [CrossRef] [PubMed]
- Guidugli, M.C.; Nazareno, A.G.; Feres, J.M.; Contel, E.P.B.; Mestriner, M.A.; Alzate-Marin, A.L. Small but not isolated: A population genetic survey of the tropical tree Cariniana estrellensis (Lecythidaceae) in a highly fragmented habitat. Heredity 2016, 116, 339–347. [Google Scholar] [CrossRef]
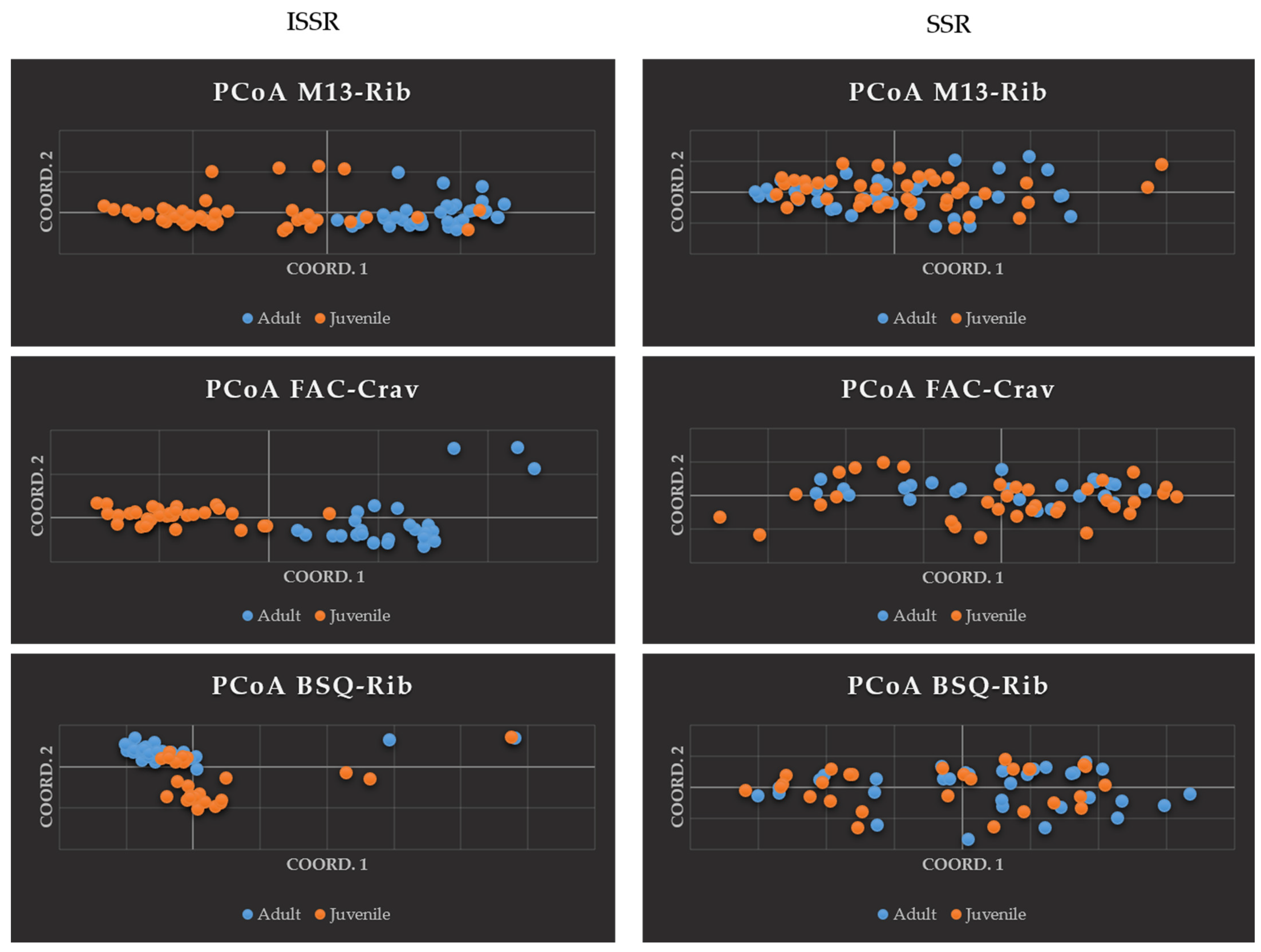
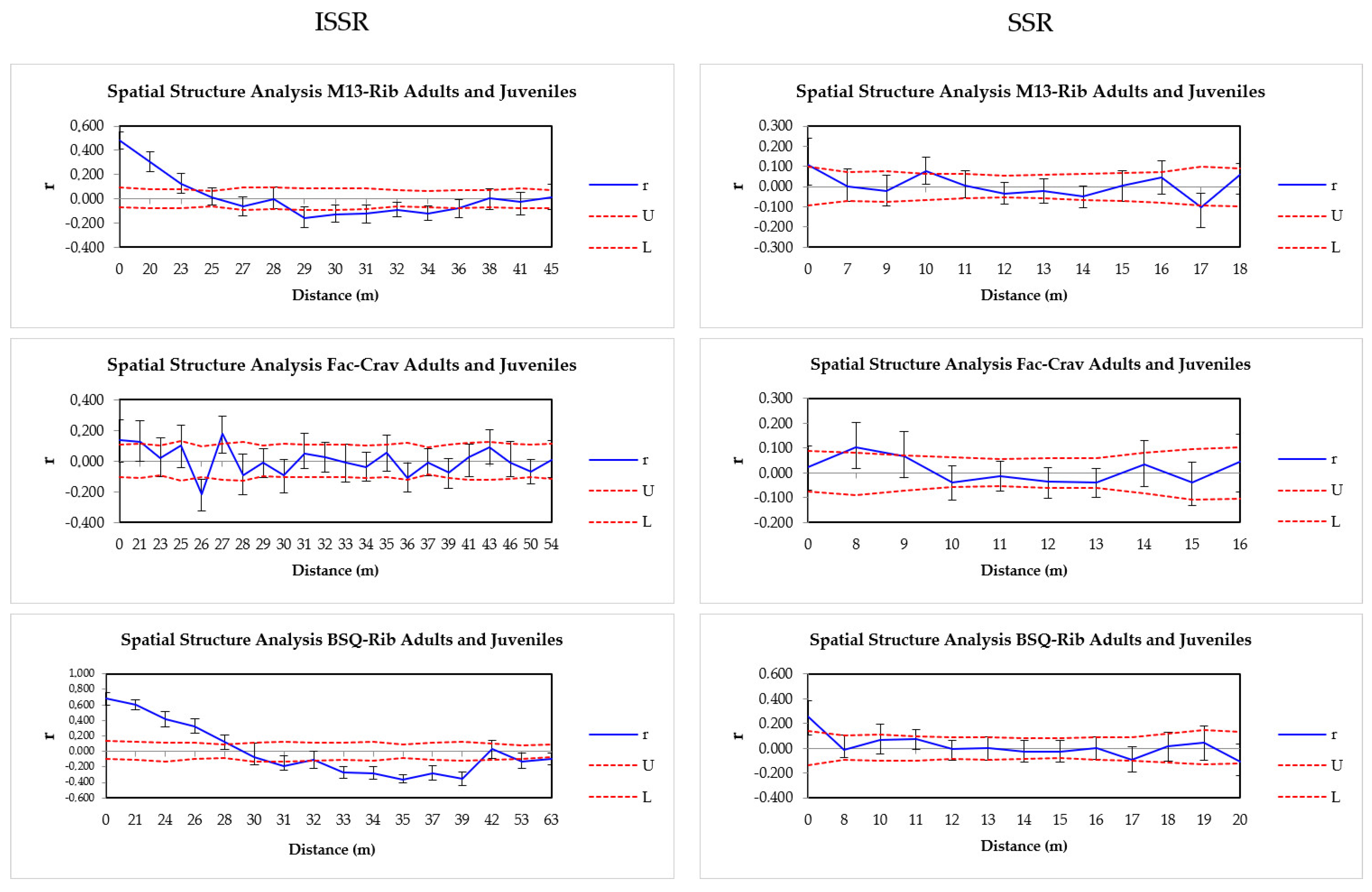

| AMOVA | M13-Rib ISSR | M13-Rib SSR | FAC-Crav ISSR | FAC-Crav SSR | BSQ-Rib ISSR | BSQ-Rib SSR |
|---|---|---|---|---|---|---|
| Among generations | 13% | 0% | 19% | 1% | 12% | 1% |
| Within generations | 87% | 100% | 81% | 99% | 88% | 99% |
| Total | 100% | 100% | 100% | 100% | 100% | 100% |
| p-values | 0.001 * | 0.712 | 0.001 * | 0.210 | 0.001 * | 0.131 |
| M13-Rib | FAC-Crav | BSQ-Rib SSR | ||||
|---|---|---|---|---|---|---|
| Value | p-Values | Value | p-Values | Value | p-Values | |
| Fst | −0.003 | 0.806 | 0.004 | 0.155 | 0.007 | 0.074 |
| Fis | 0.317 | 0.001 * | 0.256 | 0.001 * | 0.356 | 0.001 * |
| Fit | 0.315 | 0.001 * | 0.259 | 0.001 * | 0.360 | 0.001 * |
| Nm | - | - | 59 | - | 35 | - |
| Marcador | População | N | Int | r | p-Values |
|---|---|---|---|---|---|
| ISSR all generations | M13-Rib | 80 | 23 | 0.13 | 0.002 * |
| FAC-Crav | 60 | 21 | 0.13 | 0.016 * | |
| BSQ-Rib | 60 | 28 | 0.12 | 0.005 * | |
| SSR all generations | M13-Rib | 80 | 0 | 0.11 | 0.022 * |
| FAC-Crav | 60 | 8 & | 0.10 | 0.011 * | |
| BSQ-Rib | 60 | 0 | 0.23 | 0.001 * | |
| ISSR Adult/juvenile | M13-Rib (adult) | 37 | 24 | 0.24 | 0.006 * |
| M13-Rib (Juvenile) | 43 | 19 | 0.13 | 0.020 * | |
| FAC-Crav (adult) | 27 | - | - | - | |
| FAC-Crav (Juvenile) | 33 | 27 & | 0.28 | 0.005 * | |
| BSQ-Rib (adult) | 32 | 24 | 0.34 | 0.003 * | |
| BSQ-Rib (Juvenile) | 28 | 26 | 0.21 | 0.026 * | |
| SSR Adult/juvenile | M13-Rib (adult) | 37 | 10 | 0.16 | 0.024 * |
| M13-Rib (Juvenile) | 43 | 0 | 0.34 | 0.001 * | |
| FAC-Crav (adult) | 27 | - | - | - | |
| FAC-Crav (Juvenile) | 33 | 8 & | 0.15 | 0.031 * | |
| BSQ-Rib (adult) | 32 | 9 | 0.18 | 0.012 * | |
| BSQ-Rib (Juvenile) | 28 | 7 | 0.42 | 0.001 * |
| 2014 | 2015 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nov | Dec | Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov | |
| Leaf sprouting | A-J | A-J | |||||||||||
| Floral buds | A-J * | ||||||||||||
| Flower opening | A-J * | A | A | ||||||||||
| Leaf falling | L | L | L | L | H | H | L | L | L | L | L | L | L |
| Beginning of fruiting | A-J * | A | |||||||||||
| Fruit ripening | A-J * | A | |||||||||||
| AT (°C) 1 | 25 | 25 | 26 | 25 | 24 | 23 | 20 | 20 | 20 | 22 | 24 | 27 | 25 |
| ARH (%) 2 | 64 | 68 | 62 | 69 | 75 | 69 | 70 | 65 | 65 | 49 | 58 | 54 | 70 |
| AP (mm) 3 | 111 | 240 | 148 | 239 | 178 | 29 | 94 | 26 | 14 | 3 | 93 | 53 | 210 |
| Indivídual | GPS | FN | SN | WDF (g) | WDS (g) | NGS | NUS | Germination (%) |
|---|---|---|---|---|---|---|---|---|
| JUV09 | S 21°10′20.0″ W 047°48′05.6″ (Ψ 7 m) | 5 | 31 | 1.77 | 0.39 | 15 | 16 | 48.38 |
| JUV10 | S 21°10′20.1″ W 047°48′05.7″ (Ψ 5 m) | 3 | 21 | 1.25 | 0.24 | 2 | 19 | 9.52 |
| MTN11 | S 21°10′18.3″ W 047°48′07.7″ (Ψ 5 m) | 5 | 33 | 1.48 | 0.20 | 0 | 33 | 0.00 |
| MTN12 | S 21°10′18.8″ W 047°48′08.2″ (Ψ 7 m) | 5 | 33 | 1.67 | 0.33 | 7 | 27 | 21.21 |
| MTN13 | S 21°10′19.8″ W 047°48′05.6″ (Ψ 8 m) | 6 | 36 | 1.76 | 0.26 | 15 | 21 | 41.66 |
| MTN14 | S 21°10′20.2″ W 047°48′05.6″ (Ψ 7 m) | 6 | 37 | 1.11 | 0.18 | 7 | 30 | 18.92 |
| MTN16 | S 21°10′21.0″ W 047°48′05.6″ (Ψ 6 m) | 6 | 24 | 0.91 | 0.14 | 1 | 23 | 04.17 |
| Total | 36 | 215 | 9.95 | 1.74 | 47 | 169 | 144 | |
| Average | 5.14 | 30.71 | 1.42 | 0.25 | 6.71 | 24.14 | 20.55 | |
| SE | 0.40 | 2.28 | 0.13 | 0.03 | 2.38 | 2.31 | 6.96 |
| Fragment | Area (ha) | Altitude/GPS | Characteristics | Access | Matrix |
|---|---|---|---|---|---|
| M13-Rib | 84 | 601 m (21°19′45.90″ S; 47°55′30.38″ W) | Located at “Bomba” Farm (Ribeirão Preto, SP), M13 is in better condition than other nearby large fragments, hosting a population of M. nigra in a denser region of the forest, especially in the shade of larger trees. Part of the forest is regenerating after a process of fire and selective logging. Large portions of this fragment exhibit edge effects and the presence of lianas. We sampled all adult individuals in this population. | Open/private property | Extensive sugarcane plantations |
| FAC-Crav | 8 | 610 m (21°17′54.22″ S; 47°40′28.10″ W) | This forest fragment at “Águas Claras” farm (Cravinhos—SP) appears to be in well-preserved conservation condition due to being fenced and having less contact with human populations. We observed a few clearings, and we did not find signs of burning. The collected individuals occur throughout the fragment, in the shade of large trees, especially ancient Aspidosperma polyneuron and Cariniana estrellensis trees. | Closed /private property | Extensive sugarcane plantations |
| BSQ-Rib | 3 | 570 m (21°10′26.37″ S; 47°48′1.49″ W) | The semi-deciduous forest fragment, where the M. nigra population is found, is located along a trail and on the hillside within the area of the Municipal Park of Morro de São Bento. The park covers a total of 18 hectares and is situated in the middle of Ribeirão Preto city, SP. The fragment exhibits several clearings and is visited by people for educational activities, having a history of selective logging up until the 1960s. The collected individuals occur throughout the fragment, especially in the shade of tall trees. | Closed /public | Intensely urbanized |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moraes Filho, R.M.d.; Bonifácio-Anacleto, F.; Alzate-Martinez, F.A.; Martinez, C.A.; Alzate-Marin, A.L. A Spatial Structure of Key Tree Species Metrodorea nigra St. Hill. (Rutaceae) Is Associated with Historical Disturbance and Isolation in Southeastern Brazil. Plants 2025, 14, 702. https://doi.org/10.3390/plants14050702
Moraes Filho RMd, Bonifácio-Anacleto F, Alzate-Martinez FA, Martinez CA, Alzate-Marin AL. A Spatial Structure of Key Tree Species Metrodorea nigra St. Hill. (Rutaceae) Is Associated with Historical Disturbance and Isolation in Southeastern Brazil. Plants. 2025; 14(5):702. https://doi.org/10.3390/plants14050702
Chicago/Turabian StyleMoraes Filho, Rômulo Maciel de, Fernando Bonifácio-Anacleto, Fabio Alberto Alzate-Martinez, Carlos Alberto Martinez, and Ana Lilia Alzate-Marin. 2025. "A Spatial Structure of Key Tree Species Metrodorea nigra St. Hill. (Rutaceae) Is Associated with Historical Disturbance and Isolation in Southeastern Brazil" Plants 14, no. 5: 702. https://doi.org/10.3390/plants14050702
APA StyleMoraes Filho, R. M. d., Bonifácio-Anacleto, F., Alzate-Martinez, F. A., Martinez, C. A., & Alzate-Marin, A. L. (2025). A Spatial Structure of Key Tree Species Metrodorea nigra St. Hill. (Rutaceae) Is Associated with Historical Disturbance and Isolation in Southeastern Brazil. Plants, 14(5), 702. https://doi.org/10.3390/plants14050702







