Via Air or Rhizosphere: The Phytotoxicity of Nepeta Essential Oils and Malus Dihydrochalcones
Abstract
1. Introduction
2. Phytotoxic Effects of Volatile Terpenes from the Lamiaceae Family
2.1. Phytotoxic Effects of Monoterpenes from the Genus Nepeta
2.2. The Biological Activity of Essential Oils Is Determined by the Stereochemistry of Nepetalactones
2.3. Advantages of Water Emulsions of Nepeta Essential Oils
2.4. Exploring the Potential of Nepeta rtanjensis Essential Oil as a Selective Bioherbicide and Crop Protector in Combination with a Broad-Spectrum Synthetic Herbicide—Phosphinothricin
3. Phytotoxic Effects of Phenolic Compounds
3.1. Phenolic Acids—Allelochemicals with a Promising Future
3.2. Flavonoids—A Diverse Group of Compounds with Phytotoxic Activity
3.3. Phytotoxic Effects of Chalcones and Their Potential as Future Bioherbicides
3.4. Dihydrochalcones—The Most Abundant Apple Phenolic Compounds
3.4.1. Can Dihydrochalcones Be Considered Allelochemicals?
3.4.2. Phytotoxic Potential of Dihydrochalcones
3.4.3. Phloretin as a Plant Growth Inhibitor
3.4.4. Phloretin Modulates Auxin Homeostasis in Roots
3.4.5. Phloretin’s Effects on Mesophyll Cell Ultrastructure and Antioxidative Status
3.4.6. The Prospects of Phloretin as a Bioherbicidal Agent
4. Limitations of Using Plant Products as Bioherbicides
4.1. Solution Stability and Soil Biodegradability of Nepetalactone and Phloretin
4.2. Enhancement of Bioactivity Using Nanotechnology
4.3. Effect of Nepetalactone and Phloretin on Non-Target Organisms
4.3.1. Effects on Pollinators and Non-Pollinating Insects
4.3.2. Effects on Soil Microbiota
4.3.3. Effects on Crops
4.4. Regulatory and Economic Challenges Associated with the Application of Nepetalactone and Phloretin in Commercial Agriculture
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABA | Abscisic acid |
| ABC | ATP-binding cassette |
| ARD | Apple replant disease |
| ATP | Adenosine triphosphate |
| CAT | Catalase |
| CDK | Cyclin-dependent kinase |
| Chl | Chlorophyll |
| CMPG | Carboxymethylation |
| CYC | Cyclin |
| DHC | Dihydrochalcones |
| DHNL | Dihydronepetalactone |
| DMBA | 7,12-dimethylbenz[a]anthracene |
| DMAPP | Dimethylallyl diphosphate |
| DPPH | 1,1-diphenyl-2-picryl-hydrazyl |
| EO | Essential oil |
| FDA | Food and Drug Administration |
| FGPP | Farnesyl geranyl pyrophosphate |
| FGPPS | Farnesyl geranyl pyrophosphate synthase |
| FPP | Farnesyl pyrophosphate |
| FPPS | Farnesyl pyrophosphate synthase |
| G8H | Geraniol 8-hydroxylase |
| GES | Geraniol synthase |
| GGPPS | Geranylgeranyl pyrophosphate synthase |
| GPP | Geranyl pyrophosphate |
| GPPS | Geranyl pyrophosphate synthase |
| GS | Glutamine synthetase |
| H2O2 | Hydrogen peroxide |
| HGO | Hydroxygeraniol oxidase |
| IAA | Indole-3-acetic acid |
| IPP | Isopentenyl diphosphate |
| ISY | Iridoid synthase |
| KEGG | Kyoto encyclopedia of genes and genomes |
| MDR/PGP | Multi-drug resistance/P-glycoprotein |
| MEP | 2-C-methyl-D-erythritol 4-phosphate |
| MLPL | Major-latex protein-like |
| MVA | Mevalonate |
| NA | Nepetalic acid |
| NADH | Nicotinamide adenine dinucleotide hydrogen |
| NE | Nanoemulsion |
| NL | Nepetalactone |
| NLC | Nanostructured lipid carriers |
| NcEO | Nepeta cataria EO |
| NrEO | Nepeta rtanjensis EO |
| 1O2 | Singlet oxygen |
| O2⁻ | Superoxide radicals |
| OH• | Hydroxyl radicals |
| OxIAA | Oxindole-3-acetic acid |
| PAT | Polar auxin transport |
| PEPC | Phosphoenolpyruvate carboxylase |
| PIN | Pin-formed |
| POX | Peroxidase |
| PPT | Phosphinothricin |
| ROS | Reactive oxygen species |
| SDR | Short-chain dehydrogenase |
| SLN | Solid lipid nanoparticle |
| SOD | Superoxide dismutase |
| UHPLC | Ultra-high-performance liquid chromatography |
| VOC | Volatile organic compound |
| WE | Water extract |
References
- Wang, Q.; Quan, S.; Xiao, H. Towards efficient terpenoid biosynthesis: Manipulating IPP and DMAPP supply. Bioresour. Bioprocess. 2019, 6, 6. [Google Scholar] [CrossRef]
- Molisch, H. Der Einfluss Einer Pflanze Auf Die Andere-Allelopathie; Fischer: Jena, Germany, 1937. [Google Scholar]
- Rice, E.L. Allelopathy, 2nd ed.; Academic: New York, NY, USA, 1984. [Google Scholar]
- Scavo, A.; Restuccia, A.; Mauromicale, G. Allelopathy: Principles and basic aspects for agroecosystem control. In Sustainable Agriculture Reviews 28: Ecology for Agriculture, 1st ed.; Gaba, S., Smith, B., Lichtfouse, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Macías, F.A.; Mejías, F.J.; Molinillo, J.M. Recent advances in allelopathy for weed control: From knowledge to applications. Pest Manag. Sci. 2019, 75, 2413–2436. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.H.; Li, Z.; Li, F.L.; Xia, X.X.; Wang, P. Chemically mediated plant–plant interactions: Allelopathy and allelobiosis. Plants 2024, 13, 626. [Google Scholar] [CrossRef] [PubMed]
- Hickman, D.; Rasmussen, A.; Ritz, K.; Birkett, M.; Neve, P. Review: Allelochemicals as multi-kingdom plant defence compounds: Towards an integrated approach. Pest Manag. Sci. 2021, 77, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Gniazdowska, A.; Bogatek, R. Allelopathic interactions between plants. Multi-site action of allelochemicals. Acta Physiol. Plant. 2005, 27, 395–407. [Google Scholar] [CrossRef]
- Cheng, F.; Cheng, Z. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front. Plant Sci. 2015, 6, 1020. [Google Scholar] [CrossRef] [PubMed]
- Macías, F.; Molinillo, J.; Varela, R.; Galindo, J. Allelopathy: A natural alternative for weed control. Pest Manag. Sci. 2007, 63, 327–348. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Jabran, K.; Cheema, Z.; Wahid, A.; Siddique, K. The role of allopathy in agricultural pest management. Pest Manag. Sci. 2011, 67, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.; Dayan, F.; Rimando, A.; Schrader, K.; Aliotta, G.; Oliva, A.; Romagn, J. Chemicals from nature for weed management. Weed Sci. 2002, 50, 138–151. [Google Scholar] [CrossRef]
- Bajwa, A.A.; Mahajan, G.; Chauhan, B.S. Nonconventional weed management strategies for modern agriculture. Weed Sci. 2015, 63, 723–747. [Google Scholar] [CrossRef]
- Hynes, R.K.; Boyetchko, S.M. Research initiatives in the art and science of biopesticide formulations. Soil Biol. Biochem. 2006, 38, 845–849. [Google Scholar] [CrossRef]
- Boyetchko, S.M.; Rosskopf, E.N.; Caesar, A.J.; Charudattan, R. Biological weed control with pathogens: From search for candidates to applications. In Applied Mycology and Biotechnology, Vol. II, Applications of Fungal Biotechnology to Food Production; Khachatourians, G.G., Arora, D.K., Eds.; Elsevier Science: New York, NY, USA, 2002; pp. 239–274. [Google Scholar] [CrossRef]
- Klaic, R.; Kuhn, R.C.; Foletto, E.L.; Dal Prá, V.; Jacques, R.J.; Guedes, J.V.; Treichel, H.; Mossi, A.J.; Oliveira, D.J.; Oliveira, V.; et al. An overview regarding bioherbicide and their production methods by fermentation. In Fungal Biomolecules: Sources, Applications and Recent Developments; Gupta, V.K., Mach, R.L., Sreenivasaprasad, S., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 183–199. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Z.; Zhuang, X.; Xu, L.; Chen, S.; Mingzhi, L. Research progress on microbial herbicides. Crop Prot. 2003, 22, 247–252. [Google Scholar] [CrossRef]
- Ash, G.J. The science, art and business of successful bioherbicides. Biocontrol 2010, 52, 230–240. [Google Scholar] [CrossRef]
- Cordeau, S.; Triolet, M.; Wayman, S.; Steinberg, C.; Guillemin, J.P. Bioherbicides: Dead in the water? A review of the existing products for integrated weed management. Crop Prot. 2016, 87, 44–49. [Google Scholar] [CrossRef]
- Charudattan, R. Use of plant viruses as bioherbicides: The first virus-based bioherbicide and future opportunities. Pest Manag. Sci. 2024, 80, 103–114. [Google Scholar] [CrossRef]
- Cantrell, C.L.; Dayan, F.E.; Duke, S.O. Natural products as sources for new pesticides. J. Nat. Prod. 2012, 75, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Kremer, R.J. Bioherbicides: Potential successful strategies for weed control. In Microbial Biopesticides; Koul, O., Dhaliwal, G.S., Eds.; Taylor & Francis: London, UK, 2002; pp. 307–323. [Google Scholar]
- Duke, S.O.; Dayan, F.E.; Romagni, J.G.; Rimando, A.M. Natural products as sources of herbicides: Current status and future trends. Weed Res. 2000, 40, 99–111. [Google Scholar] [CrossRef]
- Grayson, B.T.; Williams, K.S.; Freehauf, P.A.; Pease, R.R.; Ziesel, W.T.; Sereno, R.L.; Reinsfelder, R.E. The physical and chemical properties of the herbicide cinmethylin (SD 95481). Pestic. Sci. 1987, 21, 143–153. [Google Scholar] [CrossRef]
- Dayan, F.E.; Romagni, J.G.; Duke, S.O. Herbicides, cinmethylin. In Encyclopedia of Agrochemicals; Plimmer, J.R., Gammon, D.W., Ragsdale, N.A., Eds.; Wiley-Interscience: Hoboken, NJ, USA, 2003. [Google Scholar] [CrossRef]
- Campe, R.; Hollenbach, E.; Kämmerer, L.; Hendriks, J.; Höken, H.W.; Kraus, H.; Lerchl, J.; Mietzner, T.; Tresch, S.; Witschel, M.; et al. A new herbicidal site of action: Cinmethylin binds to acyl-ACP thioesterase and inhibits plant fatty acid synthase. Pestic. Bichem. Physiol. 2018, 148, 116–125. [Google Scholar] [CrossRef]
- Knudsen, C.G.; Lee, D.L.; Michaely, W.J.; Chin, H.-L.; Nguyen, N.H.; Rusay, R.J.; Cromartie, T.H.; Gray, R.; Lake, B.H.; Fraser, T.E.M.; et al. Discovery of the triketone class of HPPD inhibiting herbicides and their relationship to naturally occurring-triketones. In Allelopathy in Ecological Agriculture and Forestry; Narwal, S.S., Hoagland, R.E., Dilday, R.H., Reigosa, M.J., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 101–111. [Google Scholar]
- Dayan, F.E.; Duke, S.O.; Sauldubois, A.; Singh, N.; McCurdy, C.; Cantrell, C.L. p-Hydroxyphenylpyruvate dioxygenase is the herbicidal target site for-triketones from Leptospermum scoparium. Phytochemistry 2007, 68, 2004–2014. [Google Scholar] [CrossRef] [PubMed]
- Verdeguer, M.; Sánchez-Moreiras, A.M.; Araniti, F. Phytotoxic effects and mechanism of action of essential oils and terpenoids. Plants 2020, 9, 1571. [Google Scholar] [CrossRef] [PubMed]
- Kremer, R.J. Bioherbicides and nanotechnology: Current status and future trends. In Nano-Biopesticides Today and Future Perspectives; Koul, O., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 353–366. [Google Scholar] [CrossRef]
- Stanišić, M.; Ćosić, T.; Savić, J.; Krstić-Milošević, D.; Mišić, D.; Smigocki, A.; Ninković, S.; Banjac, N. Hairy root culture as a valuable tool for allelopathic studies in apple. Tree Physiolog. 2019, 39, 888–905. [Google Scholar] [CrossRef]
- Smailagić, D.; Banjac, N.; Ninković, S.; Savić, J.; Ćosić, T.; Pěnčík, A.; Ćalić, D.; Bogdanović, M.; Trajković, M.; Stanišić, M. New insights into the activity of apple dihydrochalcone phloretin: Disturbance of auxin homeostasis as physiological basis of phloretin phytotoxic action. Front. Plant Sci. 2022, 13, 875528. [Google Scholar] [CrossRef]
- Smailagić, D.; Maksimović, J.D.; Marin, M.; Stupar, S.; Ninković, S.; Banjac, N.; Stanišić, M. Phloretin inhibits the growth of Arabidopsis shoots by inducing chloroplast damage and programmed cell death. J. Plant Physiol. 2024, 303, 154354. [Google Scholar] [CrossRef]
- Đorđić, M.; Janošević, D.; Smailagić, D.; Banjac, N.; Ninković, S.; Stanišić, M.; Trajković, M. Effects of phloretin on seedling growth and histochemical distribution of phenols, polysaccharides and lipids in Capsella bursa-pastoris (L.) Medik. Plants 2024, 13, 1890. [Google Scholar] [CrossRef] [PubMed]
- Nestorović Živković, J. Antioxidative, Antimicrobial and Allelopathic Effects of Three Endemic Nepeta Species (Lamiaceae). Ph.D. Thesis, University of Belgrade, Belgrade, Serbia, 2013. [Google Scholar]
- Nestorović Živković, J.; Perišić, M.; Stojić, A.; Živković, S.; Dmitrović, S.; Šiler, B.; Mišić, D. Volatile compounds of three Nepeta species inhibit seed germination, reduce seedling growth and induce oxidative stress in garden cress (Lepidium sativum L.). In Book of Abstracts: 2nd International Conference on Plant Biology, 21st Symposium of the Serbian Plant Physiology Society, and COST Action FA1106 Quality Fruit Workshop, Petnica, Serbia, 17–20 June 2015; Serbian Plant Physiology Society: Belgrade, Serbia, 2015; p. 177. [Google Scholar]
- Dmitrović, S.; Perišić, M.; Stojić, A.; Živković, S.; Boljević, J.; Nestorović Živković, J.; Aničić, N.; Ristić, M.; Mišić, D. Essential oils of two Nepeta species inhibit growth and induce oxidative stress in ragweed (Ambrosia artemisiifolia L.) shoots in vitro. Acta Physiol. Plant. 2015, 37, 1–15. [Google Scholar] [CrossRef]
- Nestorović Živković, J.; Dmitrović, S.; Jovanović, V.; Živković, S.; Božić, D.; Aničić, N.; Mišić, D. Allelopathic potential of essential oil of Nepeta rtanjansis. Allelopathy J. 2016, 37, 207–219. [Google Scholar]
- Dmitrović, S.; Dragićević, M.; Savić, J.; Milutinović, M.; Živković, S.; Maksimović, V.; Matekalo, D.; Mišić, D. Nepetalactone-rich essential oil mitigates phosphinothricin-induced ammonium toxicity in Arabidopsis thaliana (L.) Heynh. J. Plant Physiol. 2019, 237, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Dmitrović, S.; Dragićević, M.; Savić, J.; Milutinović, M.; Živković, S.; Maksimović, V.; Matekalo, D.; Perišić, M.; Mišić, D. Antagonistic interaction between phosphinothricin and Nepeta rtanjensis essential oil affected ammonium metabolism and antioxidant defense of Arabidopsis grown in vitro. Plants 2021, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Prijović, M.; Nikolić, B.; Dragićević, I.; Nestorović Živković, J.; Dmitrović, S.; Giba, Z.; Jovanović, V. Water emulsion of the essential oil of Nepeta rtanjensis Diklić & Milojević: Potential use as a bioherbicide. Arch. Biol. Sci. 2024, 76, 5–14. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Lange, B.M.; Rujan, T.; Martin, W.; Croteau, R. Isoprenoid biosynthesis: The evolution of two ancient and distinct pathways across genomes. Proc. Natl. Acad. Sci. USA 2000, 97, 13172–13177. [Google Scholar] [CrossRef] [PubMed]
- Christianson, D.W. Unearthing the roots of the terpenome. Curr. Opin. Chem. Biol. 2008, 12, 141–150. [Google Scholar] [CrossRef] [PubMed]
- McGarvey, D.J.; Croteau, R. Terpenoid metabolism. Plant Cell 1995, 7, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Hemmerlin, A.; Harwood, J.L.; Bach, T.J. A raison d’etre for two distinct pathways in the early steps of plant isoprenoid biosynthesis? Prog. Lipid Res. 2012, 51, 95–148. [Google Scholar] [CrossRef]
- Tholl, D. Biosynthesis and biological functions of terpenoids in plants. In Biotechnology of Isoprenoids. Advances in Biochemical Engineering/Biotechnology; Schrader, J., Bohlmann, J., Eds.; Springer: Cham, Switzerland, 2015; p. 148. [Google Scholar] [CrossRef]
- Block, A.K.; Vaughan, M.M.; Schmelz, E.A.; Christensen, S.A. Biosynthesis and function of terpenoid defense compounds in maize (Zea mays). Planta 2019, 249, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Karunanithi, P.S.; Zerbe, P. Terpene synthases as metabolic gatekeepers in the evolution of plant terpenoid chemical diversity. Front. Plant Sci. 2019, 10, 1166. [Google Scholar] [CrossRef] [PubMed]
- Masyita, A.; Sari, R.M.; Astuti, A.D.; Yasir, B.; Rumata, N.R.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef] [PubMed]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Chang, W.-C.; Song, H.; Liu, H.-W.; Liu, P. Current development in isoprenoid precursor biosynthesis and regulation. Curr. Opin. Chem. Biol. 2013, 17, 571–579. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Bian, G.; Herbst-Gervasoni, C.J.; Mori, T.; Shinsky, S.A.; Hou, A.; Mu, X.; Huang, M.; Cheng, S.; Deng, Z.; et al. Discovery of the cryptic function of terpene cyclases as aromatic prenyltransferases. Nat. Commun. 2020, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Stanković, M. Lamiaceae Species: Biology, Ecology and Practical Uses; MDPI: Basel, Switzerland, 2020. [Google Scholar]
- Nestorović, J.; Mišić, D.; Šiler, B.; Soković, M.; Glamočlija, J.; Ćirić, A.; Maksimović, V.; Grubišić, D. Nepetalactone content in shoot cultures of three endemic Nepeta species and the evaluation of their antimicrobial activity. Fitoterapia 2010, 81, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Skorić, M.; Gligorijević, N.; Čavić, M.; Todorović, S.; Janković, R.; Ristić, M.; Mišić, D.; Radulović, S. Cytotoxic activity of Nepeta rtanjensis Diklić & Milojević essential oil and its mode of action. Ind. Crops Prod. 2017, 100, 163–170. [Google Scholar] [CrossRef]
- Nestorović Živković, J.; Živković, S.; Šiler, B.; Aničić, N.; Dmitrović, S.; Divac-Rankov, A.; Giba, Z.; Mišić, D. Differences in bioactivity of three endemic Nepeta species arising from main terpenoid and phenolic constituents. Arch. Biol. Sci. 2018, 70, 63–76. [Google Scholar] [CrossRef]
- Aničić, N.; Gašić, U.; Lu, F.; Ćirić, A.; Ivanov, M.; Jevtić, B.; Dimitrijević, M.; Anđelković, B.; Škorić, M.; Nestorović Živković, J.; et al. Antimicrobial and immunomodulating activities of two endemic Nepeta species and their major iridoids isolated from natural sources. Pharmaceuticals 2021, 14, 414. [Google Scholar] [CrossRef] [PubMed]
- Pavlović, M.O.; Lunić, T.; Graovac, S.; Mandić, M.; Repac, J.; Gašić, U.; Božić, N.B.; Božić, B. Extracts of selected Lamiaceae species as promising antidiabetics: Chemical profiling, in vitro and in silico approach combined with dynamical modeling. Ind. Crop. Prod. 2022, 186, 115200. [Google Scholar] [CrossRef]
- Islam, A.M.; Suttiyut, T.; Anwar, M.P.; Juraimi, A.S.; Kato-Noguchi, H. Allelopathic properties of Lamiaceae species: Prospects and challenges to use in agriculture. Plants 2022, 11, 1478. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, G.; Chaturvedi, T.; Kumar Gupta, A.; Kishori Lal, R.; Swaroop Verma, R.; Kumar Srivastava, R.; Singh, P.; Gangwar, B.; Darokar, M.P.; Singh Dhawan, S. Assessment of genetic diversity, micromorphology and antimicrobial activity in Nepeta cataria L. Chem. Biodivers. 2023, 20, e202200241. [Google Scholar] [CrossRef]
- Angelini, L.G.; Capranese, G.; Cioni, P.L.; Morelli, I.; Macchia, M.; Flamini, G. Essential oils from Mediterranean Lamiaceae as weed germination inhibitors. J. Agric. Food Chem. 2003, 51, 6158–6164. [Google Scholar] [CrossRef]
- Chen, F.; Peng, S.; Chen, B.; Ni, G.; Liao, H. Allelopathic potential and volatile compounds of Rosmarinus officinalis L. against weeds. Allelopathy J. 2013, 32, 57–66. [Google Scholar]
- Li, X.; He, T.; Wang, X.; Shen, M.; Yan, X.; Fan, S.; Wang, L.; Wang, X.; Xu, X.; Sui, H.; et al. Traditional uses, chemical constituents and biological activities of plants from the genus Thymus. Chem. Biodivers. 2019, 16, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Abd-El Gawad, A.M.; El Gendy, A.E.-N.G.; Assaeed, A.M.; Al-Rowaily, S.L.; Alharthi, A.S.; Mohamed, T.A.; Nassar, M.I.; Dewir, Y.H.; Elshamy, A.I. Phytotoxic effects of plant essential oils: A systematic review and structure-activity relationship based on chemometric analyses. Plants 2021, 10, 36. [Google Scholar] [CrossRef]
- De Almeida, L.F.R.; Frei, F.; Mancini, E.; De Martino, L.; De Feo, V. Phytotoxic activities of Mediterranean essential oils. Molecules 2010, 15, 4309–4323. [Google Scholar] [CrossRef] [PubMed]
- Mahdavikia, F.; Saharkhiz, M.J. Phytotoxic activity of essential oil and water extract of peppermint (Mentha x piperita L. CV. Mitcham). J. Appl. Res. Med. Aromat. Plants 2015, 2, 146–153. [Google Scholar] [CrossRef]
- Kordali, S.; Cakir, A.; Ozer, H.; Cakmakci, R.; Kesdek, M.; Mete, E. Antifungal, phytotoxic and insecticidal properties of essential oil isolated from Turkish Origanum acutidens and its three components, carvacrol, thymol and p-cymene. Bioresour. Technol. 2008, 99, 8788–8795. [Google Scholar] [CrossRef] [PubMed]
- Bozok, F.; Ulukanli, Z. Volatiles from the aerial parts of east Mediterranean clary sage: Phytotoxic activity. J. Essent. Oil Bear. Plants 2016, 19, 1192–1198. [Google Scholar] [CrossRef]
- Casella, F.; Vurro, M.; Valerio, M.; Perrino, E.V.; Mezzapesa, G.; Boari, A. Phytotoxic effects of essential oils from six Lamiaceae species. Agronomy 2023, 13, 257. [Google Scholar] [CrossRef]
- Tudora, C.; Muscalu, A.; Burnichi, F.; Gradila, M.; Jaloba, D.; Vladut, V.D. Allelopathic effect of the essential oil obtained from hyssop (Hyssopus officinalic L., fam. Lamiaceae). Sci. Pap. Ser. B Hortic. 2024, 68, 923–933. [Google Scholar]
- Singh, H.P.; Batish, D.R.; Kohli, R.K. Allelopathic effect of two volatile monoterpenes against bill goat weed (Ageratum conyzoides L.). Crop Protection 2004, 21, 347–350. [Google Scholar] [CrossRef]
- Araniti, F.; Landi, M.; Lupini, A.; Sunseri, F.; Guidi, L.; Abenavoli, M.R. Origanum vulgare essential oils inhibit glutamate and aspartate metabolism altering the photorespiratory pathway in Arabidopsis thaliana seedlings. J. Plant Physiol. 2018, 231, 297–309. [Google Scholar] [CrossRef]
- Romagni, J.G.; Allen, S.N.; Dayan, F.E. Allelopathic effects of volatile cineoles on two weedy plant species. J. Chem. Ecol. 2000, 26, 303–313. [Google Scholar] [CrossRef]
- Jamzad, Z.; Grayer, R.J.; Kite, G.C.; Simmonds, M.S.; Ingrouille, M.; Jalili, A. Leaf surface flavonoids in Iranian species of Nepeta (Lamiaceae) and some related genera. Biochem. Syst. Ecol. 2003, 31, 587–600. [Google Scholar] [CrossRef]
- Salehi, B.; Valussi, M.; Jugran, A.K.; Martorell, M.; Ramírez-Alarcón, K.; Stojanović-Radić, Z.Z.; Antolak, H.; Kregiel, P.; Mileski, K.; Sarifi-Rad, M.; et al. Nepeta species: From farm to food applications and phytotherapy. Trends Food Sci. Technol. 2018, 80, 104–122. [Google Scholar] [CrossRef]
- Dabiri, M.; Sefidkon, F. Chemical composition of the essential oil of Nepeta racemosa Lam. from Iran. Flavour Fragr. J. 2003, 18, 157–158. [Google Scholar] [CrossRef]
- Süntar, I.; Nabavi, S.M.; Barreca, D.; Fischer, N.; Efferth, T. Pharmacological and chemical features of Nepeta L. genus: Its importance as a therapeutic agent. Phytother. Res. 2018, 32, 185–198. [Google Scholar] [CrossRef]
- Sharma, A.; Cooper, R.; Bhardwaj, G.; Cannoo, D.S. The genus Nepeta: Traditional uses, phytochemicals and pharmacological properties. J. Ethnopharmacol. 2021, 268, 113679. [Google Scholar] [CrossRef]
- Formisano, C.; Rigano, D.; Senatore, F. Chemical constituens and biological activity of Nepeta species. Chem Biodivers. 2011, 8, 1783–1817. [Google Scholar] [CrossRef]
- Sparks, J.T.; Bohbot, J.D.; Ristić, M.; Mišić, D.; Skorić, M.; Mattoo, A.; Dickens, J.C. Chemosensory responses to the repellent Nepeta essential oil and its major component nepetalactone by Aedes aegypti (Diptera: Culicidae), a vector of Zika virus. J. Med. Ent. 2017, 54, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, S.; Atici, Ö. Allelopathic effect of Nepeta meyeri Benth. extracts on seed germination and seedling growth of some crop plants. Acta Phys. Plant. 2009, 31, 89–93. [Google Scholar] [CrossRef]
- Liblikas, I.; Santangelo, E.M.; Sandell, J.; Baeckström, P.; Svensson, M.; Jacobsson, U.; Unelius, C.R. Simplified isolation procedure and interconversion of the diastereomers of nepetalactone and nepetalactol. J. Nat. Prod. 2005, 68, 886–890. [Google Scholar] [CrossRef]
- Petrović, L.; Filipović, B.; Skorić, M.; Šiler, B.; Banjanac, T.; Matekalo, D.; Nestorović Živković, J.; Dmitrović, S.; Anicić, N.; Milutinović, M.; et al. Molecular background of the diverse metabolic profiles in leaves and inflorescences of naked catmint (Nepeta nuda L.). Front. Plant Sci. 2024, 15, 1452804. [Google Scholar] [CrossRef] [PubMed]
- Simkin, A.J.; Miettinen, K.; Claudel, P.; Burlat, V.; Gourmand, G.; Courdavault, V.; Papon, V.; Meyer, S.; Godet, S.; St-Pierre, B.; et al. Characterization of the plastidial geraniol synthase from Madagascar periwinkle which initiates the monoterpenoid branch of the alkaloid pathway in internal phloem associated parenchyma. Phytochemistry 2013, 85, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Lichman, B.R.; Godden, G.T.; Hamilton, J.P.; Palmer, L.; Kamileen, M.O.; Zhao, D.; Vaillancourt, B.; Wood, J.C.; Sun, M.; Kinser, T.J.; et al. The evolutionary origins of the cat attractant nepetalactone in catnip. Sci. Adv. 2020, 6, eaba0721. [Google Scholar] [CrossRef] [PubMed]
- Krithika, R.; Srivastava, P.L.; Rani, B.; Kolet, S.P.; Chopade, M.; Soniya, M.; Thulasiram, H.V. Dehydrogenase from Catharanthus roseus reveals cascaded enzymatic activity in iridoid biosynthesis. Sci. Rep. 2015, 5, 8258. [Google Scholar] [CrossRef]
- Nguyen, T.; O’Connor, S.E. The progesterone 5β-reductase/iridoid synthase family: A catalytic reservoir for specialized metabolism across land plants. ACS Chem. Bio. 2020, 15, 1780–1787. [Google Scholar] [CrossRef] [PubMed]
- Lichman, B.R.; Kamileen, M.O.; Titchiner, G.R.; Saalbach, G.; Stevenson, C.E.; Lawson, D.M.; O’Connor, S.E. Uncoupled activation and cyclization in catmint reductive terpenoid biosynthesis. Nat. Chem. Biol. 2019, 15, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Aničić, N.; Matekalo, D.; Skorić, M.; Nestorović Živković, J.; Petrović, L.; Dragićević, M.; Dmitrović, S.; Mišić, D. Alterations in nepetalactone metabolism during polyethylene glycol (PEG)-induced dehydration stress in two Nepeta species. Phytochemistry 2020, 174, 112340. [Google Scholar] [CrossRef] [PubMed]
- Aničić, N.; Matekalo, D.; Skorić, M.; Pećinar, I.; Brkusanin, M.; Nestorović Zčivković, J.; Dmitrović, S.; Dajić Stefanović, Z.; Schulz, H.; Mišić, D. Trichome-specific and developmentally regulated biosynthesis of nepetalactones in leaves of cultivated Nepeta rtanjensis plants. Ind. Crops Prod. 2018, 117, 347–358. [Google Scholar] [CrossRef]
- Aničić, N.; Matekalo, D.; Skorić, M.; Gašić, U.; Nestorović Živković, J.; Dmitrović, S.; Božunović, J.; Milutinović, M.; Petrović, L.; Dimitrijević, M.; et al. Functional iridoid synthases from iridoid producing and non-producing Nepeta species (subfam. Nepetoidae, fam. Lamiaceae). Front. Plant Sci. 2024, 14, 1211453. [Google Scholar] [CrossRef]
- Kordali, S.; Tazegul, A.; Cakir, A. Phytotoxic effects of Nepeta meyeri Benth. extracts and essential oil on seed germinations and seedling growths of four weed species. Rec. Nat. Prod. 2015, 9, 404–418. [Google Scholar]
- Saharkhiz, J.M.; Zadnour, P.; Kakouei, F. Essential oil analysis and phytotoxic activity of catnip (Nepeta cataria L.). Am. J. Essent. Oils Nat. Prod. 2016, 4, 40–45. [Google Scholar]
- Mutlu, S.; Atici, Ö.; Esim, N.; Mete, E. Essential oils of catmint (Nepeta meyeri Benth.) induce oxidative stress in early seedlings of various weed species. Acta Physiol. Plant. 2011, 33, 943–951. [Google Scholar] [CrossRef]
- Dyanat, M.; Asgari, F. Phytotoxic effects of essential oils from Nepeta glocephalata Rech.f. and N. ispahanica Boiss. on selected weed species. Acta Agric. Slov. 2021, 117, 1–12. [Google Scholar] [CrossRef]
- Diyanat, M.; Ghasemhkan-Ghajar, F. Herbicidal activity of essential oils from four Nepeta species against wild mustard (Sinapis arvensis L.) and winter wild oat (Avena ludoviciana Dur.). Iranian J. Weed Sci. 2020, 16, 1–15. [Google Scholar] [CrossRef]
- Mancini, E.; Apostolides Arnold, N.; De Feo, V.; Formisano, C.; Rigano, D.; Piozzi, F.; Senatore, F. Phytotoxic effects of essential oils of Nepeta curviflora Boiss. and Nepeta nuda L. subsp. albiflora growing wild in Lebanon. J. Plant Interact. 2009, 4, 253–259. [Google Scholar] [CrossRef]
- Eom, S.H.; Yang, H.S.; Weston, L.A. An evaluation of the allelopathic potential of selected perennial groundcovers: Foliar volatiles of catmint (Nepeta× faassenii) inhibit seedling growth. J. Chem. Ecol. 2006, 32, 1835–1848. [Google Scholar] [CrossRef]
- Bozok, F. Herbicidal activity of Nepeta flavida essential oil. J. Essent. Oil Bear Plants. 2018, 21, 1687–1693. [Google Scholar] [CrossRef]
- Bozok, F.; Cenet, M.; Sezer, G.; Ulukanli, Z. Essential oil and bioherbicidal potential of the aerial parts of Nepeta nuda subsp. albiflora (Lamiaceae). J. Essent. Oil Bear. Plants 2017, 20, 148–154. [Google Scholar] [CrossRef]
- Kobaisy, M.; Tellez, M.R.; Dayan, F.E.; Mamonov, L.K.; Mukanova, G.S.; Sitpaeva, G.T.; Gemejieva, N.G. Composition and phytotoxic activity of Nepeta pannonica L. essential oil. J. Essent. Oil Res. 2005, 17, 704–707. [Google Scholar] [CrossRef]
- Aryakia, E.; Naghavi, M.R.; Farahmand, Z.; Shahzadeh Fazeli, S.A.H. Evaluating allelopathic effects of some plant species in tissue culture media as an accurate method for selection of tolerant plant and screening of bioherbicides. J. Agric. Sci. Technol. 2015, 17, 1011–1023. [Google Scholar]
- Diklić, N. Nepeta rtanjensis Diklić & Milojević. In Red Data Book of Flora of Serbia 1. Extinct and Critically Endangered Taxa; Stevanović, V., Ed.; GC Etiketa: Beograd, Serbia, 1999. [Google Scholar]
- Mišić, D.; Ghalawenji, N.; Grubišić, D.; Konjević, R. Micropropagation and reintroduction of Nepeta rtanjensis, an endemic and critically endangered perennial of Serbia. Phyton-Ann. Rei Bot. 2005, 45, 1–20. [Google Scholar]
- Singh, H.P.; Batish, D.R.; Kaur, S.; Arora, K.; Kohli, R.K. α-pinene inhibits growth and induces oxidative stress in roots. Ann. Bot. 2006, 98, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Chowhan, N.; Singh, H.P.; Batish, D.R.; Kaur, S.; Ahuja, N.; Kohli, R.K. β-Pinene inhibited germination, and early growth involves membrane peroxidation. Protoplasma 2013, 250, 691–700. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Ahmad, P.; Sarwat, M.; Sharma, S. Reactive oxygen species, antioxidants and signaling in plants. J. Plant Biol. 2008, 51, 167–173. [Google Scholar] [CrossRef]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef]
- Orabi, S.A.; Abou-Hussein, S.D. Antioxidant defense mechanisms enhance oxidative stress tolerance in plants. A review. Curr. Sci. Int. 2019, 8, 565–576. [Google Scholar]
- Ul Islam, S.N.; Asgher, M.; Khan, N.A. Hydrogen peroxide and its role in abiotic stress tolerance in plants. In Gasotransmitters Signaling in Plant Abiotic Stress; Signaling and communication in plants; Fatma, M., Sehar, Z., Khan, N.A., Eds.; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Yap, P.S.X.; Yusoff, K.; Lim, S.H.E.; Chong, C.M.; Lai, K.S. Membrane disruption properties of essential oils—A double-edged sword? Processes 2021, 9, 595. [Google Scholar] [CrossRef]
- Abrahim, D.; Francischini, A.C.; Pergo, E.M.; Kelmer-Bracht, A.M.; Ishii-Iwamoto, E.L. Effects of α-pinene on the mitochondrial respiration of maize seedlings. Plant Physiol. Biochem. 2003, 41, 985–991. [Google Scholar] [CrossRef]
- Abrahim, D.; Takahashi, L.; Kelmer-Bracht, A.M.; Ishii-Iwamoto, E.L. Effects of phenolic acids and monoterpenes on the mitochondrial respiration of soybean hypocotyl axes. Allelopathy J. 2003, 11, 21–30. [Google Scholar]
- Ding, J.; Sun, Y.; Xiao, C.L.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Physiological basis of different allelopathic reactions of cucumber and fig leaf gourd plants to cinnamic acid. J. Exp. Bot. 2007, 58, 3765–3773. [Google Scholar] [CrossRef] [PubMed]
- Nishida, N.; Tamotsu, S.; Nagata, N.; Saito, C.; Sakai, A. Allelopathic effects of volatile monoterpenoids produced by Salvia leucophylla: Inhibition of cell proliferation and DNA synthesis in the root apical meristem of Brassica campestris seedlings. J. Chem. Ecol. 2005, 31, 1187–1203. [Google Scholar] [CrossRef] [PubMed]
- Abrahim, D.; Braguini, W.L.; Kelmer-Bracht, A.M.; Ishii-Iwamoto, E.L. Effects of four monoterpenes on germination, primary root growth and mitochondrial respiration of maize. J. Chem. Ecol. 2000, 26, 611–624. [Google Scholar] [CrossRef]
- Jones, C.D.; Woods, K.E.; Setze, W.N. A chemical ecological investigation of the allelopathic potential of Lamium amplexicaule and Lamium purpureum. Open J. Ecol. 2012, 2, 167–177. [Google Scholar] [CrossRef]
- Dayan, F.E.; Duke, S.O. Natural products for weed management in organic farming in the USA. Outlooks Pest Manag. 2010, 21, 156–160. [Google Scholar] [CrossRef]
- Shekari, F.; Shekari, F.; Najafi, J.; Abassi, A.; Radmanesh, Z.; Bones, A.M. Phytotoxic effects of catnip (Nepeta meyeri Benth.) on early growth stages development and infection potential of field dodder (Cuscuta campestris Yunck). Plants 2022, 11, 2629. [Google Scholar] [CrossRef]
- Dragoeva, A.; Stoyanova, Z.; Koleva, V.; Dragolova, D. Allelopathic activity of Nepeta nuda L. subsp. nuda water extracts. Acta Sci. Natur. 2017, 4, 46–51. [Google Scholar] [CrossRef]
- De Nux, C.; Hou, A.; Fultz, L. Evaluation of organic and synthetic herbicide applications on weed suppression in a conventional cropping system in Louisiana. Sustainability 2024, 16, 3019. [Google Scholar] [CrossRef]
- Chen, H.; Singh, H.; Bhardwaj, N.; Bhardwaj, S.K.; Khatri, M.; Kim, K.H.; Peng, W. An exploration on the toxicity mechanisms of phytotoxins and their potential utilities. Crit. Rev. Environ. Sci. Technol. 2022, 52, 395–435. [Google Scholar] [CrossRef]
- Hanke, W.; Jurewicz, J. The risk of adverse reproductive and developmental disorders due to occupational pesticide exposure: An overview of current epidemiological evidence. Int. J. Occup. Med. Environ. Health 2004, 17, 223–243. [Google Scholar]
- Parrón, T.; Requena, M.; Hernández, A.F.; Alarcón, R. Association between environmental exposure to pesticides and neurodegenerative diseases. Toxicol. Appl. Pharmacol. 2011, 256, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Synowiec, A.; Kalemba, D.; Drozdek, E.; Bocianowski, J. Phytotoxic potential of essential oils from temperate climate plants against the germination of selected weeds and crops. J. Pest Sci. 2017, 90, 407–419. [Google Scholar] [CrossRef]
- Beckie, H.J.; Reboud, X. Selecting for weed resistance: Herbicide rotation and mixture. Weed Technol. 2009, 23, 363–370. [Google Scholar] [CrossRef]
- Ganie, Z.A.; Jhala, A.J. Interaction of 2,4-D or dicamba with glufosinate for control of glyphosate-resistant giant ragweed (Ambrosia trifida L.) in glufosinate-resistant maize (Zea mays L.). Front. Plant Sci. 2017, 8, 1207. [Google Scholar] [CrossRef]
- Bethke, R.K.; Molin, W.T.; Sprague, C.; Penner, D. Evaluation of the interaction between glyphosate and glufosinate. Weed Sci. 2013, 61, 41–47. [Google Scholar] [CrossRef]
- Merchant, R.M.; Sosnoskie, L.M.; Culpepper, A.S.; Steckel, L.E.; York, A.C.; Braxton, L.B.; Ford, J.C. Weed response to 2,4-D, 2,4-DB, and dicamba applied alone or with glufosinate. J. Cotton Sci. 2013, 17, 212–218. [Google Scholar]
- Takano, H.K.; Beffa, R.; Preston, C.; Westra, P.; Dayan, F.E. Glufosinate enhances the activity of protoporphyrinogen oxidase inhibitors. Weed Sci. 2020, 68, 324–332. [Google Scholar] [CrossRef]
- Koger, C.H.; Burke, I.C.; Miller, D.K.; Kendig, J.A.; Reddy, K.N.; Wilcut, J.W. MSMA antagonizes glyphosate and glufosinate efficacy on broadleaf and grass weeds. Weed Technol. 2007, 21, 159–165. [Google Scholar] [CrossRef]
- Dassanayake, M.K.; Chong, C.H.; Khoo, T.J.; Figiel, A.; Szumny, A.; Choo, C.M. Synergistic field crop pest management properties of plant-derived essential oils in combination with synthetic pesticides and bioactive molecules: A review. Foods 2021, 10, 2016. [Google Scholar] [CrossRef] [PubMed]
- Faraone, N.; Hillier, N.K.; Cutler, G.C. Plant essential oils synergize and antagonize toxicity of different conventional insecticides against Myzus persicae (Hemiptera: Aphididae). PLoS ONE 2015, 10, e0127774. [Google Scholar] [CrossRef] [PubMed]
- Dayan, F.E.; Cantrell, C.L.; Duke, S.O. Natural products in crop protection. Bioorg. Med. Chem. 2009, 17, 4022–4034. [Google Scholar] [CrossRef] [PubMed]
- Soltys, D.; Krasuska, U.; Bogatek, R.; Gniazdowska, A. Allelochemicals as bioherbicides—Present and perspectives. In Herbicides—Current Research and Case Studies in Use; Price, A., Ed.; InTech: Houston, TX, USA, 2013. [Google Scholar] [CrossRef]
- Manderscheid, R.; Wild, A. Studies on the mechanism of inhibition by phosphinothricin of glutamine synthetase isolated from Triticum aestivum L. J. Plant Physiol. 1986, 123, 135–142. [Google Scholar] [CrossRef]
- Nikolić, R.; Zdravković-Korać, S.; Ninković, S.; Dragićević, M.; Miljuš-Ð, J.; Banović, B.; Bohanec, B.; Savić, J.; Mitić, N. Fertile transgenic Lotus corniculatus resistant to the non-selective herbicide phosphinothricin. Ann. Appl. Biol. 2013, 163, 475–493. [Google Scholar] [CrossRef]
- Takano, H.K.; Dayan, F.E. Glufosinate-ammonium: A review of the current state of knowledge. Pest Manag. Sci. 2020, 76, 3911–3925. [Google Scholar] [CrossRef] [PubMed]
- Britto, D.T.; Kronzucker, H.J. NH4+ toxicity in higher plants: A critical review. J. Plant. Physiol. 2002, 159, 567–584. [Google Scholar] [CrossRef]
- Bernard, S.M.; Habash, D.Z. The importance of cytosolic glutamine synthetase in nitrogen assimilation and recycling. New Phytol. 2009, 182, 608–620. [Google Scholar] [CrossRef]
- Li, B.; Li, G.; Kronzucker, H.J.; Baluška, F.; Shi, W. Ammonium stress in Arabidopsis: Signaling, genetic loci, and physiological targets. Trends Plant. Sci. 2014, 19, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Bittsánszky, A.; Pilinszky, K.; Gyulai, G.; Komives, T. Overcoming ammonium toxicity. Plant. Sci. 2015, 231, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Dragićević, M.; Todorović, S.; Bogdanović, M.; Filipović, B.; Mišić, D.; Simonović, A. Knockout mutants as a tool to identify the subunit composition of Arabidopsis glutamine synthetase isoforms. Plant Physiol. Biochem. 2014, 79, 1–9. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Ghasemzadeh, N. Flavonoids and phenolic acids: Role and biochemical activity in plants and human. J. Med. Plants Res. 2011, 5, 6697–6703. [Google Scholar] [CrossRef]
- Afnan; Saleem, A.; Akhtar, M.F.; Sharif, A.; Akhtar, B.; Siddique, R.; Ashraf, G.M.; Alghamdi, B.S.; Alharthy, S.A. Anticancer, cardio protective and anti-inflammatory potential of natural-sources-derived phenolic acids. Molecules 2022, 27, 7286. [Google Scholar] [CrossRef] [PubMed]
- Hoang Anh, L.; Van Quan, N.; Tuan Nghia, L.; Dang Xuan, T. Phenolic allelochemicals: Achievements, limitations, and prospective approaches in weed management. Weed Biol. Manag. 2021, 21, 37–67. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Neilson, A.P.; Ferruzzi, M.G. Influence of formulation and processing on absorption and metabolism of flavan-3-ols from tea and cocoa. Annu. Rev. Food Sci. Technol. 2011, 2, 125–151. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Miyazawa, M. Tyrosinase inhibitory activities of cinnamic acid analogues. Pharmazie 2010, 65, 913–918. [Google Scholar]
- Taylor, D.C.; Wightman, F.; Kazakoff, C.W. Metabolism of chlorophenylalanines in crop and weed plants in relation to the formation of potential herbicidal end products. Phytochemistry 1988, 27, 51–71. [Google Scholar] [CrossRef]
- Manuja, R.; Sachdeva, S.; Jain, A.; Chaudhary, J. A comprehensive review on biological activities of p-hydroxy benzoic acid and its derivatives. Int. J. Pharm. Sci. Rev. Res. 2013, 22, 109–115. [Google Scholar]
- Li, X.Z.; Yan, Z.Q.; Pan, L.; Jin, H.; Yang, X.Y.; Liu, J.D.; He, X.F.; Ren, X.; Xie, M.; Guo, K.; et al. Caffeic acid derivatives as growth inhibitors of Setaria viridis: Structure-activity relationships and mechanisms. Phytochem. Lett. 2017, 20, 208–213. [Google Scholar] [CrossRef]
- Inderjit; Streibig, J.C.; Olofsdotter, M. Joint action of phenolic acid mixtures and its significance in allelopathy research. Phys. Plant. 2002, 114, 422–428. [Google Scholar] [CrossRef]
- Pinho, I.A.; Lopes, D.V.; Martins, R.C.; Quina, M.J. Phytotoxicity assessment of olive mill solid wastes and the influence of phenolic compounds. Chemosphere 2017, 185, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Bravetti, M.M.D.M.; Carpinella, M.C.; Palacios, S.M. Phytotoxicity of Cortaderia speciosa extract, active principles, degradation in soil and effectiveness in field tests. Chemoecology 2020, 30, 15–24. [Google Scholar] [CrossRef]
- Batish, D.R.; Singh, H.P.; Kaur, S.; Kohli, R.K.; Yadav, S.S. Caffeic acid affects early growth, and morphogenetic response of hypocotyl cuttings of mung bean (Phaseolus aureus). J. Plant Physiol. 2008, 165, 297–305. [Google Scholar] [CrossRef]
- Mitić, N.; Dmitrović, S.; Djordjević, M.; Zdravković-Korać, S.; Nikolić, R.; Raspor, M.; Djordjević, T.; Maksimović, V.; Živković, S.; Krstić-Milošević, D.; et al. Use of Chenopodium murale L. transgenic hairy root in vitro culture system as a new tool for allelopathic assays. J. Plant Physiol. 2012, 169, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Kaushik, S. Cellular evidence of allelopathic interference of benzoic acid to mustard (Brassica juncea L.) seedling growth. Plant Physiol. Biochem. 2005, 43, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Reigosa, M.J.; Pazos-Malvido, E. Phytotoxic effects of 21 plant secondary metabolites on Arabidopsis thaliana germination and root growth. J. Chem. Ecol. 2007, 33, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Bravo, H.R.; Copaja, S.V.; Lamborot, M. Phytotoxicity of phenolic acids from cereals. In Herbicides—Advances in Research; Price, A., Kelton, J., Eds.; InTech: Houston, TX, USA, 2013; p. 37. [Google Scholar] [CrossRef]
- Al Harun, M.A.Y.; Johnson, J.; Uddin, M.N.; Robinson, R.W. Identification and phytotoxicity assessment of phenolic compounds in Chrysanthemoides monilifera subsp. monilifera (Boneseed). PLoS ONE 2015, 10, e0139992. [Google Scholar] [CrossRef]
- Hegab, M.M.; Erdei, L.; Abd Elgawad, H. Isolation and phytotoxicity of an active fraction and its pure compound (gallic acid) from sun spurge (Euphorbia helioscopia L.) against harmful weeds. Acta Biol. Szeged. 2016, 60, 17–25. [Google Scholar]
- Gulzar, A.; Siddiqui, M.B.; Bi, S. Phenolic acid allelochemicals induced morphological, ultrastructural, and cytological modification on Cassia sophera L. and Allium cepa L. Protoplasma 2016, 253, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Q.; Zhang, L.L.; Jiang, X.W.; Liu, Q.Z. Allelopathic effects of phenolic acids on the growth and physiological characteristics of strawberry plants. Allelopathy J. 2015, 35, 61. [Google Scholar]
- Politycka, B.; Kozłowska, M.; Mielcarz, B. Cell wall peroxidases in cucumber roots induced by phenolic allelochemicals. Allelopathy J. 2004, 13, 29–36. [Google Scholar]
- Patterson, D.T. Effects of allelopathic chemicals on growth and physiological response of soybean (Glycine max). Weed Sci. 1981, 29, 53–58. [Google Scholar] [CrossRef]
- Yu, J.Q.; Ye, S.F.; Zhang, M.F.; Hu, W.H. Effects of root exudates and aqueous root extracts of cucumber (Cucumis sativus) and allelochemicals, on photosynthesis and antioxidant enzymes in cucumber. Biochem. Syst. Ecol. 2003, 31, 129–139. [Google Scholar] [CrossRef]
- Lee, T.T.; Skoog, F. Effects of hydroxybenzoic acids on indoleacetic acid inactivation by tobacco callus extracts. Physiol. Plant. 1965, 18, 577–585. [Google Scholar] [CrossRef]
- He, H.Q.; Lin, W.X. Studies on allelopathic physiobiochemical characteristics of rice. Chin. J. Eco Agric. Res. 2001, 9, 56–57. [Google Scholar]
- Garg, N.; Garg, O.P. Effect of exogenous treatment with some phenolic compounds on nitrogen fixation, growth and yield in Cicer arietinum L. (chickpea). Curr. Sci. 1989, 58, 31–32. [Google Scholar]
- Liu, X.F.; Hu, X.J. Effects of allelochemical ferulic acid on endogenous hormone level of wheat seedling. Chin. J. Eco-Agric. 2001, 1, 31. [Google Scholar]
- Mucciarelli, M.; Gallino, M.; Maffei, M.; Scannerini, S. Effects of 3, 4-dihydroxybenzoic acid on tobacco (Nicotiana tabacum L.) cultured in vitro. Growth regulation in callus and organ cultures. Plant Biosys. 2000, 134, 185–192. [Google Scholar] [CrossRef]
- Anwar, S.; Naseem, S.; Karimi, S.; Asi, M.R.; Akrem, A.; Ali, Z. Bioherbicidal activity and metabolic profiling of potent allelopathic plant fractions against major weeds of wheat—Way forward to lower the risk of synthetic herbicides. Front. Plant Sci. 2021, 12, 632390. [Google Scholar] [CrossRef] [PubMed]
- Weston, L.A.; Mathesius, U. Flavonoids: Their structure, biosynthesis and role in the rhizosphere, including allelopathy. J. Chem. Ecol. 2013, 39, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Karak, P. Biological activities of flavonoids: An overview. Int. J. Pharm. Sci. Res. 2019, 10, 1567–1574. [Google Scholar] [CrossRef]
- González-Sarrías, A.; Tomás-Barberán, F.A.; García-Villalba, R. Structural diversity of polyphenols and distribution in foods. In Dietary Polyphenols: Their Metabolism and Health Effects; Tomás-Barberán, F.A., González-Sarrías, A., Rocío Gar-cía-Villalba, R., Eds.; Wiley: Chichester, UK, 2020; pp. 1–29. [Google Scholar] [CrossRef]
- Samanta, A.; Das, G.; Das, S.K. Roles of flavonoids in plants. Carbon 2011, 100, 12–35. [Google Scholar]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef] [PubMed]
- D’Amelia, V.; Aversano, R.; Chiaiese, P.; Carputo, D. The antioxidant properties of plant flavonoids: Their exploitation by molecular plant breeding. Phytochem. Rev. 2018, 17, 611–625. [Google Scholar] [CrossRef]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The role of polyphenols in abiotic stress response: The influence of molecular structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Ahmad, P.; Jaleel, C.A.; Azooz, M.M.; Nabi, G. Generation of ROS and non-enzymatic antioxidants during abiotic stress in plants. Bot. Res. Int. 2009, 2, 11–20. [Google Scholar]
- Bais, H.P.; Walker, T.S.; Kennan, A.J.; Stermitz, F.R.; Vivanco, J.M. Structure-dependent phytotoxicity of catechins and other flavonoids: Flavonoid conversions by cell-free protein extracts of Centaurea maculosa (spotted knapweed) roots. J. Agric. Food Chem. 2003, 51, 897–901. [Google Scholar] [CrossRef] [PubMed]
- De Martino, L.; Mencherini, T.; Mancini, E.; Aquino, R.P.; De Almeida, L.F.R.; De Feo, V. In vitro phytotoxicity and antioxidant activity of selected flavonoids. Int. J. Mol. Sci. 2012, 13, 5406–5419. [Google Scholar] [CrossRef]
- Peer, W.A.; Murphy, A.S. Flavonoids and auxin transport: Modulators or regulators? Trends Plant Sci. 2007, 12, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Weir, T.L.; Park, S.W.; Vivanco, J.M. Biochemical and physiological mechanisms mediated by allelochemicals. Cur. Opin. Plant Biol. 2004, 7, 472–479. [Google Scholar] [CrossRef]
- Rolim del Almeida, L.F.; Delachiave, M.E.; Sannomiya, M.; Vilegas, W.; Santos, L.C.; Mancini, E.; de Feo, V. In vitro potential allelopathic of Leonurus sibiricus leaves. J. Plant Interact. 2008, 3, 39–48. [Google Scholar] [CrossRef]
- Dziągwa-Becker, M.; Oleszek, M.; Zielińska, S.; Oleszek, W. Chalcones—Features, identification techniques, attributes, and application in agriculture. Molecules 2024, 29, 2247. [Google Scholar] [CrossRef]
- Iwashina, T. The structure and distribution of the flavonoids in plants. J. Plant Res. 2000, 113, 287. [Google Scholar] [CrossRef]
- Giannasi, D.E. The Flavonoid Systematics of the Genus Dahlia (Compositae); The University of Iowa: Iowa City, IA, USA, 1972. [Google Scholar]
- Crawford, D.J.; Stuessy, T.F. The taxonomic significance of anthochlors in the subtribe Coreopsidinae (Compositae, Heliantheae). Am. J. Bot. 1981, 68, 107–117. [Google Scholar] [CrossRef]
- Harborne, J.B. Comparative biochemistry of the flavonoids-I. Distribution of chalcone and alurone pigments in plants. Phytochemistry 1966, 5, 111–115. [Google Scholar] [CrossRef]
- Fenwick, G.R.; Lutomski, J.; Nieman, C. Liquorice, Glycyrrhiza glabra L.—Composition, uses and analysis. FoodChem 1990, 38, 119–143. [Google Scholar] [CrossRef]
- Abu, N.; Ho, W.Y.; Yeap, S.K.; Akhtar, M.N.; Abdullah, M.P.; Omar, A.R.; Alitheen, N.B. The flavokawains: Uprising medicinal chalcones. Cancer Cell Int. 2013, 13, 102. [Google Scholar] [CrossRef] [PubMed]
- Isa, N.M.; Abdelwahab, S.I.; Mohan, S.; Abdul, A.B.; Sukari, M.A.; Taha, M.M.E.; Syam, S.; Narrima, P.; Cheah, S.C.; Ahmad, S.; et al. In vitro anti-inflammatory, cytotoxic and antioxidant activities of boesenbergin A, a chalcone isolated from Boesenbergia rotunda (L.) (fingerroot). Braz. J. Med. Biol. Res. 2012, 45, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Ajiboye, T.O.; Yakubu, M.T.; Oladiji, A.T. Cytotoxic, antimutagenic, and antioxidant activities of methanolic extract and chalcone dimers (Lophirone B and C) derived from Lophira alata (Van Tiegh. Ex Keay) stem bark. Evid. Based Complement. Alternat. Med. 2014, 19, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.J.; Yun, M.S.; Yogo, Y. Effects of root-applied naringenin and chalcone on the growth of annual plants. Weed Biol. Manag. 2004, 4, 235–238. [Google Scholar] [CrossRef]
- Chen, W.J.; Yun, M.S.; Yogo, Y. The rates of maize growth and lignin biosynthesis change after root-applied chalcone. Weed Biol. Manag. 2005, 5, 118–122. [Google Scholar] [CrossRef]
- Yun, M.S.; Chen, W.; Deng, F.; Yogo, Y. Selective growth suppression of five annual plant species by chalcone and naringenin correlates with the total amount of 4-coumarate: Coenzyme A ligase. Weed Biol. Manag. 2009, 9, 27–37. [Google Scholar] [CrossRef]
- Chen, W.J.; Yun, M.S.; Deng, F.; Yogo, Y. Chalcone suppresses lignin biosynthesis in illuminated soybean cells. Weed Biol. Manag. 2011, 11, 49–56. [Google Scholar] [CrossRef]
- Díaz-Tielas, C.; Graña, E.; Sotelo, T.; Reigosa, M.J.; Sánchez-Moreiras, A.M. The natural compound trans-chalcone induces programmed cell death in Arabidopsis thaliana roots. Plant Cell Environ. 2012, 35, 1500–1517. [Google Scholar] [CrossRef]
- Díaz-Tielas, C.; Sotelo, T.; Graña, E.; Reigosa, M.J.; Sánchez-Moreiras, A.M. Phytotoxic potential of trans-chalcone on crop plants and model species. J. Plant Growth Reg. 2014, 33, 181–194. [Google Scholar] [CrossRef]
- Díaz-Tielas, C.; Graña, E.; Maffei, M.E.; Reigosa, M.J.; Sánchez-Moreiras, A.M. Plasma membrane depolarization precedes photosynthesis damage and long-term leaf bleaching in (E)-chalcone-treated Arabidopsis shoots. J. Plant Phys. 2017, 218, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Tielas, C.; Graña, E.; Sánchez-Moreiras, A.M.; Reigosa, M.J.; Vaughn, J.N.; Pan, Z.; Duke, S.O. Transcriptome responses to the natural phytotoxin t-chalcone in Arabidopsis thaliana L. Pest Manag. Sci. 2019, 75, 2490–2504. [Google Scholar] [CrossRef]
- Chotsaeng, N.; Laosinwattana, C.; Charoenying, P. Herbicidal activity of flavokawains and related trans-chalcones against Amaranthus tricolor L. and Echinochloa crus-galli (L.) Beauv. ACS Omega 2019, 4, 20748–20755. [Google Scholar] [CrossRef] [PubMed]
- Perera, W.H.; Meepagala, K.M.; Fronczek, F.R.; Cook, D.D.; Wedge, D.E.; Duke, S.O. Bioassay-guided isolation and structure elucidation of fungicidal and herbicidal compounds from Ambrosia salsola (Asteraceae). Molecules 2019, 24, 835. [Google Scholar] [CrossRef]
- Nguyen, G.T.T.; Erlenkamp, G.; Jäck, O.; Küberl, A.; Bott, M.; Fiorani, F.; Gohlke, H.; Groth, G. Chalcone-based selective inhibitors of a C4 plant key enzyme as novel potential herbicides. Sci. Rep. 2016, 6, 27333. [Google Scholar] [CrossRef]
- Behzad, S.; Sureda, A.; Barreca, D.; Nabavi, S.F.; Rastrelli, L.; Nabavi, S.M. Health effects of phloretin: From chemistry to medicine. Phytochem. Rev. 2017, 16, 527–533. [Google Scholar] [CrossRef]
- Choi, B.Y. Biochemical basis of anti-cancer-effects of phloretin—A natural dihydrochalcone. Molecules 2019, 24, 278. [Google Scholar] [CrossRef] [PubMed]
- Tuli, H.S.; Rath, P.; Chauhan, A.; Ramniwas, S.; Vashishth, K.; Varol, M.; Jaswal, V.S.; Haque, S.; Sak, K. Phloretin, as a potent anticancer compound: From chemistry to cellular interactions. Molecules 2022, 27, 8819. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. The molecular pharmacology of phloretin: Anti-inflammatory mechanisms of action. Biomedicines 2023, 11, 143. [Google Scholar] [CrossRef]
- Shelke, V.; Kale, A.; Kulkarni, Y.A.; Gaikwad, A.B. Phloretin: A comprehensive review of its potential against diabetes and associated complications. J. Pharm. Pharmacol. 2024, 76, 201–212. [Google Scholar] [CrossRef]
- Pontais, I.; Treutter, D.; Paulin, J.P.; Brisset, M.N. Erwinia amylovora modifies phenolic profiles of susceptible and resistant apple through its type III secretion system. Physiol. Plant. 2008, 132, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Dugé de Bernoville, T.; Gaucher, M.; Guyot, S.; Durel, C.E.; Dat, J.F.; Brisset, M.N. The constitutive phenolic composition of two Malus × domestica genotypes is not responsible for their contrasted susceptibilities to fire blight. Environ. Exp. Bot. 2011, 74, 65–73. [Google Scholar] [CrossRef]
- Mikulic Petkovšek, M.; Stampar, F.; Veberic, R. Seasonal changes in phenolic compounds in the leaves of scab-resistant and susceptible apple cultivars. Can. J. Plant Sci. 2009, 89, 745–753. [Google Scholar] [CrossRef]
- De Koninck, L. Ueber das phloridzin (phlorrhizin). Eur. J. Org. Chem. 1835, 15, 75–77. [Google Scholar] [CrossRef]
- Zhou, K.; Hu, L.; Li, P.; Gong, X.; Ma, F. Genome-wide identification of glycosyltransferases converting phloretin to phloridzin in Malus species. Plant Sci. 2017, 265, 131–145. [Google Scholar] [CrossRef]
- Aldasoro, J.J.; Aedo, C.; Navarro, C. Phylogenetic and phytogeographical relationships in Maloideae (Rosaceae) based on morphological and anatomical characters. Blumea Biodiv. Evol. Biogeogr. Plants 2005, 50, 3–32. [Google Scholar] [CrossRef]
- Fernández de Simón, B.; Pérez-Ilzarbe, J.; Hernández, T.; Gómez-Cordovés, C.; Estrella, I. Importance of phenolic compounds for the characterization of fruit juices. J. Agric. Food Chem. 1992, 40, 1531–1535. [Google Scholar] [CrossRef]
- Hilt, P.; Schieber, A.; Yildirim, C.; Arnold, G.; Klaiber, I.; Conrad, J.; Beifuss, U.; Carle, R. Detection of phloridzin in strawberries (Fragaria x ananassa Duch.) by HPLC-PDA-MS/MS and NMR spectroscopy. J. Agric. Food Chem. 2003, 51, 2896–2899. [Google Scholar] [CrossRef]
- Hvattum, E. Determination of phenolic compounds in rose hip (Rosa canina) using liquid chromatography coupled to electrospray ionisation tandem mass spectrometry and diode-array detection. Rapid Commun. Mass Spectrom. 2002, 16, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.; Chen, S.N.; Joike, M.K.; Pendland, S.L.; Pauli, G.F.; Farnsworth, N.R. Inhibition of uropathogenic Escherichia coli by cranberry juice: A new antiadherence assay. J. Agricult. Food Chem. 2005, 53, 8940–8947. [Google Scholar] [CrossRef]
- Dong, H.; Ning, Z.; Yu, L.; Li, L.; Lin, L.; Huang, J. Preparative separation and identification of the flavonoid phlorhizin from the crude extract of Lithocarpus polystachyus Rehd. Molecules 2007, 12, 552–562. [Google Scholar] [CrossRef]
- Ciarmiello, L.F.; Mazzeo, M.F.; Minasi, P.; Peluso, A.; De Luca, A.; Piccirillo, P.; Siciliano, R.A.; Carbone, V. Analysis of different European hazelnut (Corylus avellana L.) cultivars: Authentication, phenotypic features, and phenolic profiles. J. Agric. Food Chem. 2014, 62, 6236–6246. [Google Scholar] [CrossRef]
- Börner, H. The apple replant problem. I. The excretion of phlorizin from apple root residues. Contrib. Boyce Thompson Inst. 1959, 20, 39–56. [Google Scholar]
- Wittenmayer, L.; Szabó, K. The role of root exudates in specific apple (Malus x domestica Borkh.) replant disease (SARD). J. Plant Nutr. Soil Sci. 2000, 163, 399–404. [Google Scholar] [CrossRef]
- Savory, B.M. Studies on the Occurrence and Aetiology of Specific Replant Diseases of Perennial Fruit Crops. Ph.D. Thesis, University of London, London, UK, 1966. [Google Scholar]
- Hoestra, H. Replant Diseases of Apple in The Netherlands. Ph.D. Thesis, Mededeelingen van de Landbouwhoge School, Wageningen, The Netherlands, 1968. [Google Scholar]
- Caruso, F.L.; Neubauer, B.F.; Begin, M.D. A histological study of apple roots affected by replant disease. Canad. J. Bot. 1989, 67, 742–749. [Google Scholar] [CrossRef]
- Somera, T.S.; Mazzola, M. Toward a holistic view of orchard ecosystem dynamics: A comprehensive review of the multiple factors governing development or suppression of apple replant disease. Front. Microbiol. 2022, 13, 949404. [Google Scholar] [CrossRef] [PubMed]
- Börner, H. Liberation of organic substances from higher plants and their role in the soil sickness problem. Bot. Rev. 1960, 26, 393–424. [Google Scholar] [CrossRef]
- Zhang, J.H.; Mao, Z.Q.; Wang, L.Q.; Shu, H.R. Bioassay and identification of root exudates of three fruit tree species. J. Integr. Plant Biol. 2007, 49, 257–261. [Google Scholar] [CrossRef]
- Bai, R.; Zhao, X.; Ma, F.; Li, C. Identification and bioassay of allelopathic substances from the root exudates of Malus prunifolia. Allelopathy J. 2009, 23, 477–484. [Google Scholar]
- Yin, C.; Xiang, L.; Wang, G.; Wang, Y.; Shen, X.; Chen, X.; Mao, Z. How to plant apple trees to reduce replant disease in apple orchard: A study on the phenolic acid of the replanted apple orchard. PLoS ONE 2016, 11, e0167347. [Google Scholar] [CrossRef] [PubMed]
- Nicola, L.; Vrhovsek, U.; Soini, E.; Insam, H.; Pertot, I. Phlorizin released by apple root debris is related to apple replant disease. Phytopathol. Mediterr. 2016, 55, 432–437. [Google Scholar] [CrossRef]
- Jianghong, Z.; Zhiquan, M.; Liqin, W. Effect of phloridzin on physiological characteristics of Malus hupehensis Rehd. seedlings. Sci. Agric. Sin. 2007, 40, 492–498. [Google Scholar]
- Cui, X.; Wang, Y.; Zhen, W. The effects of phlorizin stress on the protective enzyme and metabolic regulation substances in the root of M. micromalus. Front. Agric. China 2010, 4, 323–327. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Hu, Y.L.; Zhou, H.; Zhan, X.; Mao, Z.Q.; Zhu, S.H. Effects of phloridzin on the tri-carboxylic acid cycle enzymes of roots of Malus hupehensis Rehd. Sci. Agric. Sin. 2012, 45, 3108–3114. [Google Scholar]
- Yin, C.; Duan, Y.; Xiang, L.; Wang, G.; Zhang, X.; Shen, X.; Chen, X.; Zhang, M.; Mao, Z. Effects of phloridzin, phloretin and benzoic acid at the concentrations measured in soil on the root proteome of Malus hupehensis Rehd seedlings. Sci. Hortic. 2018, 228, 10–17. [Google Scholar] [CrossRef]
- Xiang, L.; Wang, M.; Jiang, W.; Wang, Y.; Chen, X.; Yin, C.; Mao, Z. Key indicators for renewal and reconstruction of perennial trees soil: Microorganisms and phloridzin. Ecotoxicol. Environ. Saf. 2021, 225, 112723. [Google Scholar] [CrossRef]
- Berckmans, B.; De Veylder, L. Transcriptional control of the cell cycle. Curr. Opin. Plant Biol. 2009, 12, 599–605. [Google Scholar] [CrossRef]
- Ishii, T. Isolation and characterization of a diferuloyl arabinoxylan hexasaccharide from bamboo shoot cell-walls. Carbohyd. Res. 1991, 219, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Renard, C.M.G.C.; Baron, A.; Guyot, S.; Drilleau, J.F. Interactions between apple cell walls and native apple polyphenols: Quantification and some consequences. Int. J. Biol. Macromol. 2001, 29, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Bindon, K.A.; Bacic, A.; Kennedy, J.A. Tissue-specific and developmental modifications of grape cell walls influence the adsorption of proanthocyanidins. J. Agric. Food Chem. 2012, 60, 9249–9260. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P.A.; Le Bourvellec, C.; Renard, C.M.; Wessel, D.F.; Cardoso, S.M.; Coimbra, M.A. Interactions of arabinan-rich pectic polysaccharides with polyphenols. Carbohydr. Polym. 2020, 230, 115644. [Google Scholar] [CrossRef] [PubMed]
- Saura-Calixto, F. Dietary fiber as a carrier of dietary antioxidants: An essential physiological function. J. Agric. Food Chem. 2011, 59, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Zažímalová, E.; Murphy, A.S.; Yang, H.; Hoyerová, K.; Hošek, P. Auxin transporters—Why so many? Cold Spring Harb. Perspect. Biol. 2010, 2, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Geisler, M.; Aryal, B.; Di Donato, M.; Hao, P. A critical view on ABC transporters and their interacting partners in auxin transport. Plant Cell Physiol. 2017, 58, 1601–1614. [Google Scholar] [CrossRef] [PubMed]
- Friml, J.; Justyna, W.; Eva, B.; Kurt, M.; Klaus, P. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 2002, 415, 806–809. [Google Scholar] [CrossRef] [PubMed]
- Geisler, M.; Blakeslee, J.J.; Bouchard, R.; Lee, O.R.; Vincenzetti, V.; Bandyopadhyay, A.; Titapiwatanakun, B.; Peer, W.A.; Bailly, A.; Richards, E.L.; et al. Cellular efflux of auxin catalyzed by the Arabidopsis MDR/PGP transporter AtPGP1. Plant J. 2005, 44, 179–194. [Google Scholar] [CrossRef]
- Kleine-Vehn, J.; Ding, Z.; Jones, A.R.; Tasaka, M.; Morita, M.T.; Friml, J. Gravity-induced PIN transcytosis for polarization of auxin fluxes in gravity-sensing root cells. Proc. Natl. Acad. Sci. USA 2010, 107, 22344–22349. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Ahmad-Hamdani, M.S.; Rosli, A.M.; Hamdan, H. Bioherbicides: An Eco-friendly tool for sustainable weed management. Plants 2021, 10, 1212. [Google Scholar] [CrossRef]
- Moghaddam, H.H.; Jafari, A.A.; Sefidkon, F.; Jari, S.K. Influence of climatic factors on essential oil content and composition of 20 populations of Nepeta binaludensis Jamzad from Iran. Appl. Biol. Chem. 2023, 66, 2. [Google Scholar] [CrossRef]
- Petrović, L.; Skorić, M.; Šiler, B.; Banjanac, T.; Gašić, U.; Matekalo, D.; Lukić, T.; Nestorović Živković, J.; Dmitrović, S.; Aničić, N.; et al. Patterns of genetic variation of Nepeta nuda L. from the Central Balkans: Understanding drivers of chemical diversity. Plants 2024, 13, 1483. [Google Scholar] [CrossRef] [PubMed]
- De Mastro, G.; El Mahdi, J.; Ruta, C. Bioherbicidal potential of the essential oils from Mediterranean Lamiaceae for weed control in organic farming. Plants 2021, 10, 818. [Google Scholar] [CrossRef]
- Islam, A.K.M.M.; Karim, S.M.R.; Kheya, S.A.; Yeasmin, S. Unlocking the potential of bioherbicides for sustainable and environment friendly weed management. Heliyon 2024, 10, e36088. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, A.; Simon, J.E.; Wu, Q. Stability study of Nepeta cataria iridoids analyzed by LC/MS. Phytochem. Anal. 2024, 35, 1674. [Google Scholar] [CrossRef] [PubMed]
- FDA. Guidance for Industry: Q1A(R2) Stability Testing of New Drug Substances and Products; U.S. Department of Health and Human Services, Food and Drug Administration: Silver Spring, MD, USA, 2003; pp. 1–22.
- Pedrinho, A.; Karas, P.A.; Kanellopoulos, A.; Feray, E.; Korman, I.; Wittenberg, G.; Ramot, O.; Karpouzas, D.G. The effect of natural products used as pesticides on the soil microbiota: OECD 216 nitrogen transformation test fails to identify effects that were detected via q-PCR microbial abundance measurement. Pest Manag. Sci. 2024, 80, 2563–2576. [Google Scholar] [CrossRef]
- Dziągwa-Becker, M.D.; Oleszek, M.; Ukalska-Jaruga, A.; Kucharski, M.; Kozłowska, W.; Nowak, B.; Zielińska, S. Evaluation of pinocembrin dihydrochalcone properties for potential use as a biopesticide. Preprints 2024, 2024121463. [Google Scholar] [CrossRef]
- Zhang, R.T.; Xu, Y.; Wang, Q.W. Study on the stability of phloretin by HPLC. Northwest Pharmac. J. 2018, 33, 80–83. [Google Scholar]
- Jinshui, C.; Qun, X.; Lina, L.; Rohrer, J. Determination of phenolic compounds in apple orchard soil. Thermo Fish. Sci. 2008, 88, 1–4. [Google Scholar]
- Hofmann, A.; Wittenmayer, L.; Arnold, G.; Schieber, A.; Merbach, W. Root exudation of phloridzin by apple seedlings (Malus x domestica Borkh.) with symptoms of apple replant disease. J. Appl. Bot. Food Qual. 2009, 82, 193–198. [Google Scholar]
- Jiang, W.; Chen, R.; Zhao, L.; Duan, Y.; Wang, H.; Yan, Z.; Shen, X.; Chen, X.; Yin, C.; Mao, Z. Isolation of phloridzin-degrading, IAA-producing bacterium Ochrobactrum haematophilum and its effects on the apple replant soil environment. Hortic. Plant J. 2023, 9, 199–208. [Google Scholar] [CrossRef]
- Mondéjar-López, M.; García-Simarro, M.P.; Navarro-Simarro, P.; Gómez-Gómez, L.; Ahrazem, O.; Niza, E. A review on the encapsulation of "eco-friendly" compounds in natural polymer-based nanoparticles as next generation nano-agrochemicals for sustainable agriculture and crop management. Int. J. Biol. Macromol. 2024, 280, 136030. [Google Scholar] [CrossRef] [PubMed]
- Amighi, M.; Zahedifar, M.; Alizadeh, H.; Payandeh, M. Encapsulation of Nepeta hormozganica and Nepeta dschuprensis essential oils in shrimp chitosan NPs: Enhanced antifungal activity. Int. J. Biol. Macromol. 2023, 238, 124112. [Google Scholar] [CrossRef] [PubMed]
- Cheraghipour, K.; Zivdari, M.; Beiranvand, M.; Shakib, P.; Kheirandish, F.; Zebardast Pour, M.; Ghafarypour, M.; Marzban, A.; Alhameedawi, A.K. Encapsulation of Nepeta cataria essential oils in a chitosan nanocomposite with lethality potential against Toxoplasma gondii. Emerg. Mater. 2023, 5, 653–663. [Google Scholar] [CrossRef]
- Mahalleh, A.A.; Sharayei, P.; Azarpazhooh, E. Investigating the characteristics of the Nepeta binaludensis encapsulated extract and its release kinetics in laboratory conditions. Food Bioprocess Tech. 2021, 14, 164–176. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, X.; Pei, Y.; Han, X.; Yan, B.; Qian, Y.; Dong, H.; Yu, X.; Cheng, Z. Mosquito repellent microcapsules of Nepeta essential oil were prepared by electro-atomization complex coacervation. Polym. Int. 2023, 72, 671–676. [Google Scholar] [CrossRef]
- Hogenbom, J.; Istanbouli, M.; Faraone, N. Novel β-cyclodextrin and catnip essential oil inclusion complex and its tick repellent properties. Molecules 2021, 26, 7391. [Google Scholar] [CrossRef]
- Sari, B.R.; Yesilot, S.; Ozmen, O.; Acar, C.A. Superior in vivo wound-healing activity of biosynthesized silver nanoparticles with Nepeta cataria (catnip) on excision wound model in rat. Biol. Trace. Elem. Res. 2024, 1–16. [Google Scholar] [CrossRef]
- Mani, M.; Sundararaj, A.S.; Al-Ghanim, K.A.; John, S.P.; Elumalai, K.; Nicoletti, M.; Govindarajan, M. Rapid synthesis of copper nanoparticles using Nepeta cataria leaves: An eco-friendly management of disease-causing vectors and bacterial pathogens. Green Process. Synth. 2023, 12, 20230022. [Google Scholar] [CrossRef]
- Nakhate, K.T.; Badwaik, H.; Choudhary, R.; Sakure, K.; Agrawal, Y.O.; Sharma, C.; Ojha, S.; Goyal, S.N. Therapeutic potential and pharmaceutical development of a multitargeted flavonoid phloretin. Nutrients 2022, 14, 3638. [Google Scholar] [CrossRef] [PubMed]
- Pouton, C.W.; Porter, C.J. Formulation of lipid-based delivery systems for oral administration: Materials, methods and strategies. Adv. Drug Deliv. Rev. 2008, 60, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Sun, R.; Wang, W.; Xia, Q. Nanostructured lipid carriers for the encapsulation of phloretin: Preparation and in vitro characterization studies. Chem. Phys. Lipids. 2022, 242, 105150. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Fourmentin, S.; Fenyvesi, É.; Torri, G.; Fourmentin, M.; Morin-Crini, N. Cyclodextrins, from molecules to applications. Environ. Chem. Lett. 2018, 16, 1361–1375. [Google Scholar] [CrossRef]
- Ishizuka, Y.; Fujiwara, M.; Kanazawa, K.; Nemoto, T.; Fujita, K.I.; Nakanishi, H. Three-dimensional structure of the inclusion complex between phloridzin and β-cyclodextrin. Carbohydr. Res. 2002, 337, 1737–1743. [Google Scholar] [CrossRef] [PubMed]
- Aree, T. How cyclodextrin encapsulation improves molecular stability of apple polyphenols phloretin, phlorizin, and ferulic acid: Atomistic insights through structural chemistry. Food Chem. 2023, 409, 135326. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhou, Z.; Han, L.; Li, S.; Zhou, W. Preparation and characterization of phloretin by complexation with cyclodextrins. New J. Chem. 2020, 44, 5218–5223. [Google Scholar] [CrossRef]
- Sharma, P.; Bal, T.; Singh, S.K.; Sharma, N. Biodegradable polymeric nanocomposite containing phloretin for enhanced oral bioavailability and improved myocardial ischaemic recovery. J. Microencapsul. 2024, 41, 754–769. [Google Scholar] [CrossRef]
- Cassano, R.; Curcio, F.; Sole, R.; Trombino, S. Transdermal delivery of phloretin by gallic acid microparticles. Gels 2023, 9, 226. [Google Scholar] [CrossRef] [PubMed]
- Mariadoss, A.V.A.; Vinayagam, R.; Xu, B.; Venkatachalam, K.; Sankaran, V.; Vijayakumar, S.; Bakthavatsalam, S.R.; Mohamed, S.A.; David, E. Phloretin loaded chitosan nanoparticles enhance the antioxidants and apoptotic mechanisms in DMBA induced experimental carcinogenesis. Chem. Biol. Interact. 2019, 308, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xue, J.; Luo, Y. Encapsulation of phloretin in a ternary nanocomplex prepared with phytoglycogen–caseinate–pectin via electrostatic interactions and chemical cross-linking. J. Agric. Food Chem. 2020, 68, 13221–13230. [Google Scholar] [CrossRef]
- Peterson, C.J.; Nemetz, L.T.; Jones, L.M.; Coats, J.R. Behavioral activity of catnip (Lamiaceae) essential oil components to the German cockroach (Blattodea: Blattellidae). Entomol. Soc. Am. 2002, 95, 377–380. [Google Scholar] [CrossRef]
- Reichert, W.; Ejercito, J.; Guda, T.; Dong, X.; Wu, Q.; Ray, A.; Simon, J.E. Repellency assessment of Nepeta cataria essential oils and isolated nepetalactones on Aedes aegypti. Sci. Rep. 2019, 9, 1524. [Google Scholar] [CrossRef]
- Da Silva, I.M.; Zanuncio, J.C.; Brügger, B.P.; Soares, M.A.; Zanuncio, A.J.V.; Wilcken, C.F.; Tavares, W.D.S.; Serrão, J.E.; Sediyama, C.S. Selectivity of the botanical compounds to the pollinators Apis mellifera and Trigona hyalinata (Hymenoptera: Apidae). Sci. Rep. 2020, 10, 4820. [Google Scholar] [CrossRef]
- Fotirić Akšić, M.M.; Pešić, M.B.; Pećinar, I.; Dramićanin, A.; Milinčić, D.D.; Kostić, A.Ž.; Gašić, U.; Jakanovski, M.; Kitanović, M.; Meland, M. Diversity and chemical characterization of apple (Malus sp.) pollen: High antioxidant and nutritional values for both humans and insects. Antioxidants 2024, 13, 1374. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, Q.; Zheng, Y.; Zhang, Y.; Liu, B.; Shi, W.; Zeng, Z. Kaempferitrin: A Flavonoid marker to distinguish Camellia oleifera honey. Nutrients 2023, 15, 435. [Google Scholar] [CrossRef] [PubMed]
- Pardo, A.; Borges, P.A.V. Worldwide importance of insect pollination in apple orchards: A review. Agric. Ecosyst. Environ. 2020, 293, 106839. [Google Scholar] [CrossRef]
- Sharma, H.K.; Devi, M.; Thakur, P.; Sharma, R.; Rana, K.; Thakur, M. Apis mellifera L. stock varied in apple pollen foraging preference. Indian J. Entomol. 2023, 85, 84–87. [Google Scholar] [CrossRef]
- Terra, W.R.; Ferreira, C.; Jordao, B.P.; Dillon, R.J. Digestive enzymes. In Biology of the Insect Midgut; Lehane, M.J., Billingsley, P.F., Eds.; Springer: Dordrecht, The Netherlands, 1996; pp. 153–194. [Google Scholar] [CrossRef]
- Klingauf, F. Die wirkung des glucosids phlorizin auf das wirtswhalverhalten von Rhopalosiphum insertum und Aphis pomi de geer (Homoptera: Aphididae). Z. Fuer Angew. Entomol. 1971, 68, 41–55. [Google Scholar] [CrossRef]
- Gutbrodt, B.; Mody, K.; Wittwer, R.; Dorn, S. Within-plant distribution of induced resistance in apple seedlings: Rapid acropetal and delayed basipetal responses. Planta 2011, 133, 1199–1207. [Google Scholar] [CrossRef]
- Fulcher, A.F.; Ranney, T.G.; Burton, J.D.; Walgenbach, J.F.; Danehower, D.A. Role of foliar phenolics in host plant resistance of Malus taxa to adult Japanese beetles. Hort. Sci. 1998, 33, 862–865. [Google Scholar] [CrossRef]
- Patton, C.A.; Ranney, T.G.; Burton, J.D.; Walgenbach, J.F. Feeding responses of Japanese beetle to naturally occurring metabolites found in rosaceous plants. J. Environ. Hortic. 1997, 15, 222–227. [Google Scholar] [CrossRef]
- Winkelmann, T.; Smalla, K.; Amelung, W.; Baab, G.; Grunewaldt-Stöcker, G.; Kanfra, X.; Meyhöfer, R.; Reim, S.; Vetterlein, D.; Wrede, A.; et al. Apple replant disease: Causes and mitigation strategies. Curr. Issues Mol. Biol. 2019, 30, 89–106. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.N. Trehalose—The insect “blood” sugar. Adv. Insect Physiol. 2003, 31, 205–285. [Google Scholar] [CrossRef]
- Kono, Y.; Takahashi, M.; Mihara, M.; Matsushita, K.; Nishina, M.; Kameda, Y. Effect of a trehalase inhibitor, validoxylamine A, on oocyte development and ootheca formation in Periplaneta americana (Blattodea, Blattidae). Appl. Entomol. Zool. 1997, 32, 293–301. [Google Scholar] [CrossRef][Green Version]
- Takahashi, M.; Kono, Y.; Kurahashi, H.; Matsushita, K.; Nishina, M.; Kameda, Y. Effect of a trehalase inhibitor, validoxylamine A, on three species of flies. Appl. Entomol. Zool. 1995, 30, 231–239. [Google Scholar] [CrossRef]
- Wegener, G.; Tschiedel, V.; Schlöder, P.; Ando, O. The toxic and lethal effects of the trehalase inhibitor trehazolin in locusts are caused by hypoglycaemia. J. Exp. Biol. 2003, 206, 1233–1240. [Google Scholar] [CrossRef]
- Silva, M.C.; Terra, W.R.; Ferreira, C. Absorption of toxic β-glucosides produced by plants and their effect on tissue trehalases from insects. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2006, 143, 367–373. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Keogh, D.P.; Crooks, J.R.; Smith, S.L. Effects of plant flavonoids and other allelochemicals on insect cytochrome P-450 dependent steroid hydroxylase activity. Insect Biochem. Mol. Biol. 1993, 23, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.L.; Bollenbacher, W.E.; Cooper, D.Y.; Schleyer, H.; Wielgus, J.J.; Gilbert, L.I. Ecdysone 20-monooxygenase: Characterization of an insect cytochrome P-450 dependent steroid hydroxylase. Mol. Cell. Endocrinol. 1979, 15, 111–133. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, L.I. Halloween genes encode P450 enzymes that mediate steroid hormone biosynthesis in Drosophila melanogaster. Mol. Cell. Endocrinol. 2004, 215, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Joußen, N.; Agnolet, S.; Lorenz, S.; Schöne, S.E.; Ellinger, R.; Schneider, B.; Heckel, D.G. Resistance of Australian Helicoverpa armigera to fenvalerate is due to the chimeric P450 enzyme CYP337B3. Proc. Natl. Acad. Sci. USA 2012, 109, 15206–15211. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Tittiger, C.; Wicker-Thomas, C.; Le Goff, G.; Young, S.; Wajnberg, E.; Fricaux, T.; Taquet, N.; Blomquist, G.J.; Feyereisen, R. An insect-specific P450 oxidative decarbonylase for cuticular hydrocarbon biosynthesis. Proc. Natl. Acad. Sci. USA 2012, 109, 14858–14863. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef]
- Shakeri, A.; Khakdan, F.; Soheili, V.; Sahebkar, A.; Rassam, G.; Asili, J. Chemical composition, antibacterial activity, and cytotoxicity of essential oil from Nepeta ucrainica L. spp. kopetdaghensis. Ind. Crops Prod. 2014, 58, 315. [Google Scholar] [CrossRef]
- Vokou, D.; Liotiri, S. Stimulation of soil microbial activity by essential oils. Chemoecology 1999, 9, 41–45. [Google Scholar] [CrossRef]
- Vokou, D.; Chalkos, D.; Karamanlidou, G.; Yiangou, M. Activation of soil respiration and shift of the microbial population balance in soil as a response to Lavandula stoechas essential oil. J. Chem. Ecol. 2002, 28, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Khare, P.; Srivastava, S.; Nigam, N.; Singh, A.K.; Singh, S. Impact of essential oils of E. citriodora, O. basilicum and M. arvensis on three different weeds and soil microbial activities. Environ. Technol. Innov. 2019, 14, 100343. [Google Scholar] [CrossRef]
- Jouini, A.; Verdeguer, M.; Pinton, S.; Araniti, F.; Palazzolo, E.; Badalucco, L.; Laudicina, V.A. Potential effects of essential oils extracted from Mediterranean aromatic plants on target weeds and soil microorganisms. Plants 2020, 9, 1289. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, M.; Manici, L.M. Apple replant disease: Role of microbial ecology in cause and control. Annu. Rev. Phytopathol. 2012, 50, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Jaffee, B.A.; Abawi, G.S.; Mai, W.F. Role of soil microflora and Pratylenchus penetrans in an apple replant disease. Phytopathology 1982, 72, 247–251. [Google Scholar] [CrossRef]
- Otto, G.; Winkler, H.; Szabo, K. Proof of actinomycetes in rootlets of species of Rosaceae from a SARD soil—A contribution to the specificity of replant diseases. Acta Hortic. 1994, 363, 43–48. [Google Scholar] [CrossRef]
- Utkhede, R.S. Soil sickness, replant problem or replant disease and its integrated control. Allelopathy J. 2006, 18, 23–38. [Google Scholar]
- Radl, V.; Winkler, J.B.; Kublik, S.; Yang, L.; Winkelmann, T.; Vestergaard, G.; Schröder, P.; Schloter, M. Reduced microbial potential for the degradation of phenolic compounds in the rhizosphere of apple plantlets grown in soils affected by replant disease. Environ. Microbiome 2019, 14, 1–12. [Google Scholar] [CrossRef]
- Peruzzi, E.; Franke-Whittle, I.H.; Kelderer, M.; Ciavatta, C.; Insam, H. Microbial indication of soil health in apple orchards affected by replant disease. Appl. Soil Ecol. 2017, 119, 115–127. [Google Scholar] [CrossRef]
- Jansen, D.J.; Shenvi, R.A. Synthesis of medicinally relevant terpenes: Reducing the cost and time of drug discovery. Future Med. Chem. 2014, 6, 1127–1146. [Google Scholar] [CrossRef] [PubMed]
- González-Hernández, R.A.; Valdez-Cruz, N.A.; Trujillo-Roldán, M.A. Factors that influence the extraction methods of terpenes from natural sources. Chem. Pap. 2024, 78, 2783–2810. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Lei, D.; Zhao, G.R. Modular metabolic engineering for production of phloretic acid, phloretin and phlorizin in Escherichia coli. Chem. Eng. Sci. 2022, 247, 116931. [Google Scholar] [CrossRef]
- Reigosa, M.J.; Souto, X.C.; Gonzalez, L. Effect of phenolic compounds on the germination of six weeds species. Plant Growth Regul. 1999, 28, 83–88. [Google Scholar] [CrossRef]
- Kocacë Aliskan, I.; Terzi, I. Allelopathic effects of walnut leaf extracts and juglone on seed germination and seedling growth. J. Hortic. Sci. Biotech. 2001, 76, 436–440. [Google Scholar] [CrossRef]
- Biopesticides Registration Action. Refined Oil of Nepeta cataria. Document U.S. Environmental Protection Agency. Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/registration/decision_PC-004801_25-Aug-10.pdf (accessed on 4 January 2025).
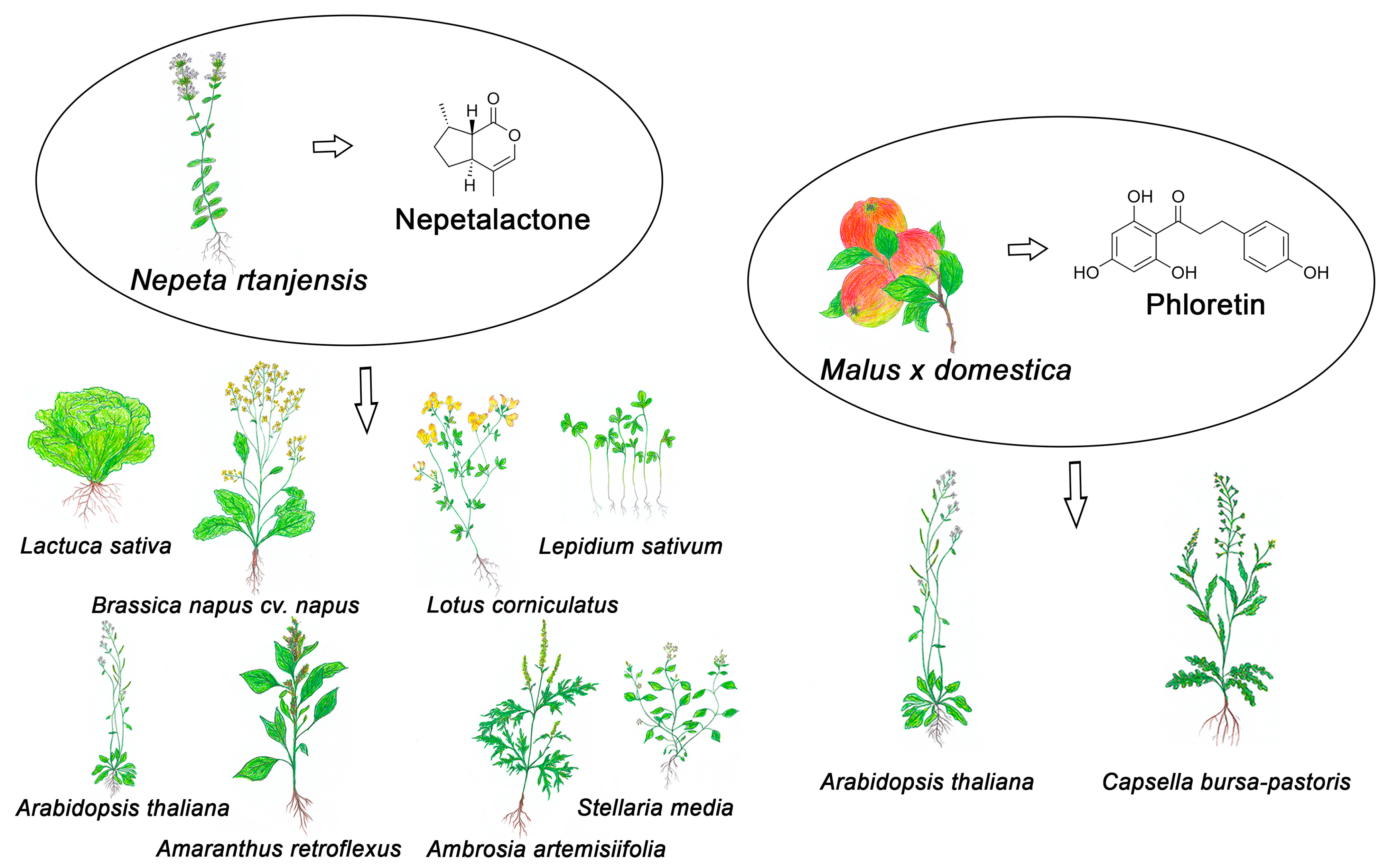


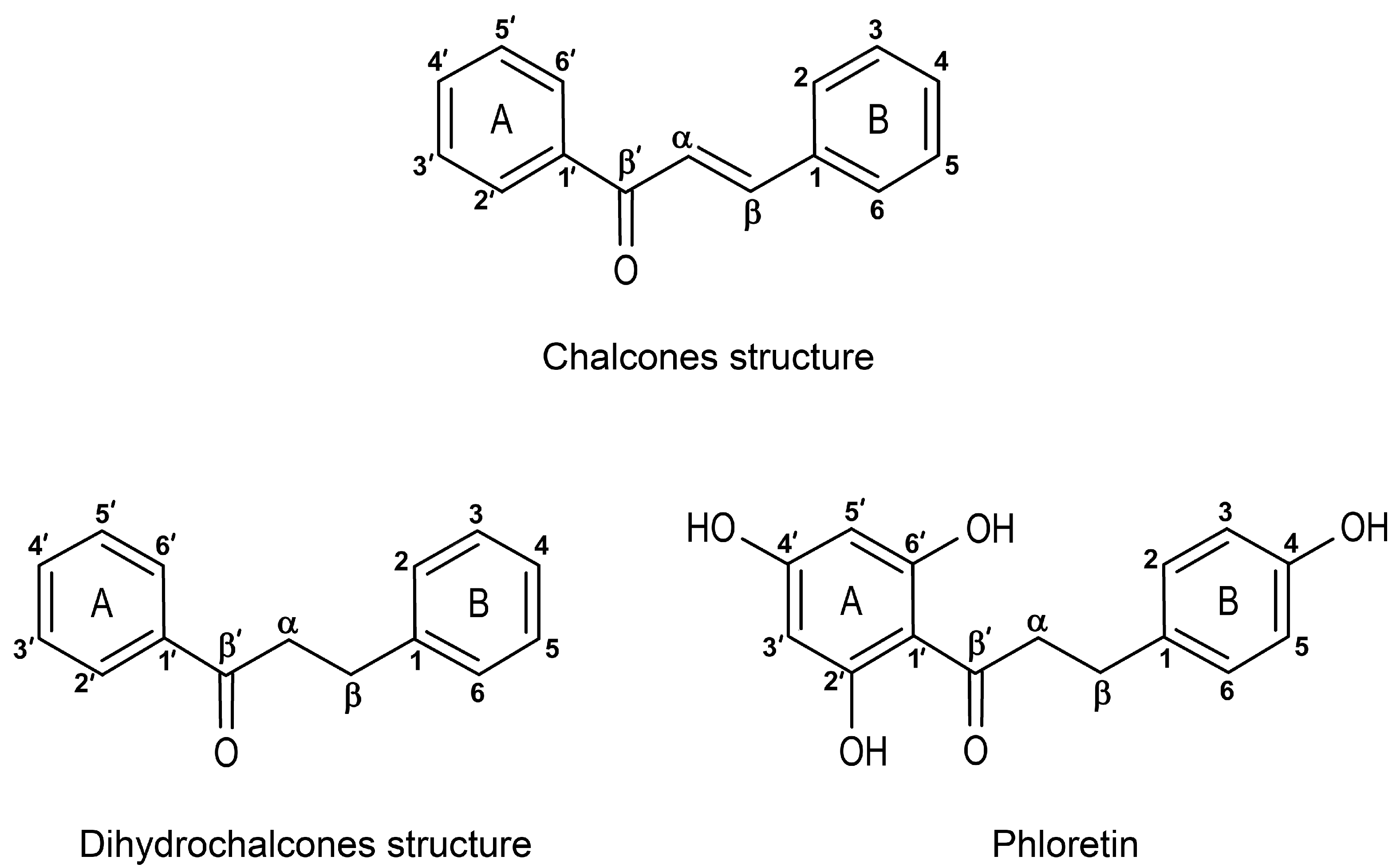
| Nepeta Species | Dominant Compounds of the EO | Species Examined for Sensitivity to EO | Experimental Design | Reported Phytotoxic Activity | Ref. |
|---|---|---|---|---|---|
| N. binaludensis Jamzad N. elymaitica Bornm N. menthoides Boiss & Buhse | 1,8-Cineole | Avena ludoviciana Dur. Sinapis arvensis L. | In vitro assay in Petri dishes on filter paper. Dose tested: 1, 2, 4, and 8 μg EO mL−1 water with Tween 20 (0.1 g 100 mL−1 of water). In a greenhouse assay, foliar application: 1.25, 2.5, 5, and 10% EO in water (v/v) on 3-week-old weeds. | Inhibition of seed germination and root and shoot length by increasing the concentration of EOs after 2 weeks of treatment. Inhibition of S. arvensis seed germination (100%) at N. binaludensis EO 4 μg mL−1 and 8 μg mL−1, and at N. menthoides EO at 8 μg mL−1. Reduction of Chl content and electrolyte leakage and increase in proline levels after 7 days of treatmnets. S. arvensis was more sensitive than A. ludoviciana. | [97] |
| N. mahanensis Jamzad & Simmonds | Nepetalactone | ||||
| N. cataria L. | trans,cis-Nepetalactone (55.0%) cis,trans-Nepetalactone (31.2%) | Avena fatua L. Hordeum spontaneum Koch Lepidium sativum L. Nepeta cataria L. Ocimum basilicum L. Taraxacum officinale (L.) Weber ex F.H.Wigg. | In vitro assay in Petri dishes on filter paper. Dose tested: 150, 300, 600, and 1200 µL EO L-1 of water. | Inhibition of seed germination (100%) at 1200 µL L-1 in all species tested except H. spontaneum, where it was 26%. Inhibition of seed germination (100%) at 600 µL L-1 in A. fatua and T. officinale. Inhibition of seedling length and fresh and dry weight with increasing EO concentration. | [94] |
| N. cataria L. | cis,trans-Nepetalactone (77%) Spathulenol (4.26%) Caryophyllene oxide (4.80%) | Ambrosia artemisiifolia L. | In vitro enclosed volatile bioassays—shoot growth and activities and abundance of antioxidant enzymes. Dose tested: 2 and 4% (v/v) EO in 99.8% methanol on A. artemisiifolia axillary buds (about 1 cm high) grown in vitro. 50 µL applied on filter paper placed on a metal holder stuck into the culture medium in a 350 mL glass jar. | Inhibition of shoot and root growth of A. artemisiifolia: 64 and 65% inhibition of shoot fresh weight with 4% N. rtanjensis EO and N. cataria EO, respectively; 100 and 80% inhibition of root fresh weight with 4% N. rtanjensis EO and N. cataria EO, respectively. Discoloration of the shoot. Suppression of CAT and SOD activity and stimulation of POX activity with both EOs tested. N. cataria EO had a stronger effect on CAT and POX activity, N. rtanjensis EO had a stronger effect on SOD activity. | [37] |
| N. rtanjensis Diklić et Milojević | trans,cis-Nepetalactone (72%) cis,trans-Nepetalactone (16%) α-pinene (3%) | ||||
| N. curviflora Boiss. collected in North Lebanon | β-Caryophyllene (41.6%) Caryophyllene oxide (9.5%) trans-β-Farnesene (6.2%) cis-β-Farnesene (4.8%) | Lepidium sativum L. Raphanus sativus L. | In vitro assay in Petri dishes on filter paper. Dose tested: 2.5, 1.25, 0.625, and 0.25 mg EO mL−1 water–acetone mixture (99.5:0.5). | No effect on seed germination. Inhibition (49 and 42%) of R. sativus radicle length with N. nuda subsp. albiflora—1800 masl EO at concentrations of 1.25 and 2.5 mg mL−1, respectively. | [98] |
| N. nuda L. subsp. albiflora (Boiss.) Gams. collected in Mount Lebanon at 1400 masl | β-Bisabolene (11.8%) Pulegone (10.8%) trans,cis-Nepetalactone (8.0%) trans-β-Farnesene (7.1%) Caryophyllene oxide (6.9%) | ||||
| N. nuda L. subsp. albiflora (Boiss.) Gams. collected in North Lebanon at 1800 masl | Hexadecanoic acid (10.1%) β-Bisabolene (7.8%) Caryophyllene oxide (7.3%) Pulegone (7.2%) trans,cis-Nepetalactone (4.4%) | ||||
| N. x faassenii Bergmans ex Stearn | cis,trans-Nepetalactone (73.05%) germacrene B (10.25%) trans,cis-Nepetalactone (5.79%) | Lepidium sativum L. | In vitro enclosed volatile bioassays on filter paper. Dose tested: 5, 10, and 20 g of leaves in cheesecloth per 500 mL glass flask. | Inhibition of shoot growth (21 and 48%) at 5 g and 10 g of foliage, respectively. Inhibition of root elongation (7 and 44%) at 5 g and 10 g of foliage, respectively. Inhibition of radicle elongation and shoot growth (100%, both) at 20 g of foliage. | [99] |
| N. flavida | Linalool (37.64%) 1,8-Cineole (30.80%) | Eruca sativa Mill. Lepidium sativum L. Raphanus sativus L. | In vitro assay in Petri dishes on filter paper. Dose tested: 0.25, 0.5, 1.0, 2.0, 4.0, and 8.0 μL EO mL−1 Tween 80–water solution (0.5%, v/v). | Inhibition of seed germination (100%) at 4 μL and 8 μL EO mL−1 in all tested weeds. Inhibition of seed germination (84, 83, and 75%) at 2 μL EO mL−1 in E. sativa, R. sativus, and L. sativum, respectively. Inhibition of radicle and plumule length of R. sativus (93 and 91%), L. sativum (89 and 88%), and E. sativa (93 and 97%) at 2 μL EO mL−1. | [100] |
| N. glocephalata Rech.f | 1,8-Cineole (34.1%) β-Pinene (21.5%) α-Pinene (8.1%) Sabinene (7.8%) (Z)-β-Ocimene (7.6%) (E)-β-Ocimene (6.9%) | Amaranthus retroflexus L. Chenopodium album L. Echinochloa crus-galli (L.) Beauv Phalaris canariensis L. | In vitro assay in Petri dishes on filter paper. Dose tested: 1, 2, 4, and 8 μL EO mL−1 water solution. In a greenhouse assay, foliar application: 1.25, 2.5, 5, and 10% EO in water (v/v) on 3-week-old plants. | Inhibition of seed germination and root and shoot length by increasing the concentration of the tested EOs. A. retroflexus and C. album were most sensitive to N. isphanica EO and N. glocephalata EO: 100% inhibition of seed germination at 4 μg N. isphanica EO/mL and 100% inhibition at 8 μg N. glocephalata EO mL−1. N. ispahanica EO inhibited the root length of all tested weeds more strongly than N. glocephalata EO. Shoot length was less affected in N. isphanica EO and N. glocephalata EO than the root length of all tested weeds. Chlorosis to necrosis of the weeds 7 days after spraying. Reduction in the dry mass of the seedlings and the Chl a and Chl b content 7 days after spraying. About 10% higher inhibition of dry mass and about 5% higher inhibition of Chl a and Chl b content with N. isphanica EO (10%) compared to N. glocephalata EO (10%). Monocotyledonous weeds were more resistant than the dicotyledonous weeds tested. | [96] |
| N. ispahanica Boiss | 1,8-Cineole (66.4%) β-Pinene (10.7%) α-Pinene (3.1%) | ||||
| N. meyeri Benth. | trans,cis-Nepetalactone (80.3%) cis,trans-Nepetalactone (10.3%) trans-Pulegol (3.1%) 1,8-Cineole (3.0%) β-Bourbonene (2.0%) | Amaranthus retroflexus L. Chenopodium album L. Cirsium arvense (L.) Scop. Sinapis arvensis L. | In vitro assay in Petri dishes on filter paper. Dose tested: 0.5, 1.0, and 2 mg µL−1 DMSO–water solution (10%, v/v). In a greenhouse assay, spraying weeds at the 3–4 leaves stage with 20 mg EO mL−1 of DMSO–water solution (10%, v/v). | Inhibition of seed germination of A. retroflexus, C. album, C. arvense, and S. arvensis (100%) at all tested concentrations. Mortality of plants from 53.33 ± 6.36% (S. arvensis) to 64.00 ± 5.29% (A. retroflexus) 48h after treatments with 20 mg EO mL−1. | [93] |
| N. meyeri Benth. | trans,cis-Nepetalactone (83.4%) cis,trans-Nepetalactone (8.83%) | Amaranthus retroflexus L. Bromus danthoniae Trin. Bromus intermedius Guss. Chenopodium album L. Cynodon dactylon L. Lactuca serriola L. Portulaca oleracea L. | In vitro assay in Petri dishes on filter paper. Dose tested: 0.01 and 0.02% EO in Tween 20–water solution (0.01%, v/v). | Inhibition of seed germination (100%) at 0.02% EO in A. retroflexus, B. danthoniae, B. intermedius, and L. serriola. Inhibition of seed germination (more than 70%) at 0.02% EO in C. album and C. dactylon. CAT activity increased and SOD activity decreased in all weed species except A. retroflexus. H2O2 concentration and level of lipid peroxidation increased in all tested weeds. | [95] |
| N. nervosa Royle & Bentham. | Nepetalactone (a very low concentration) | Lepidium sativum L. | In vitro enclosed volatile bioassays—seed germination and growth of seedlings, as well as activities of antioxidant enzymes. Cocultivation of 1, 3, 5, 7, and 9 4-week-old plants of N. rtanjensis, N. sibirica, and N. nervosa with L. sativum seeds in a 350 mL glass vessel with culture medium for 5 days. | Inhibition of seed germination and seedling growth when co-cultured with N. rtanjensis and N. sibirica. N. rtanjensis had stronger effects than N. sibirica. No effect on seed germination when co-cultured with N. nervosa. Inhibition of CAT and POX activity and changes in the profiles of Fe- and Cu/Zn- isoforms of SOD when co-cultured with N. rtanjensis and N. sibirica. | [35,36] |
| N. rtanjensis Diklić et Milojević | trans,cis-Nepetalactone (5–23 ppbV) | ||||
| N. sibirica L. | cis,trans-Nepetalactone (3–25 ppbV) | ||||
| N. nuda L. subsp. albiflora | trans,cis-Nepetalactone (74.27%) 2(1H)-Naphthalenone, octahydro8a-methyl-trans- (10.09%) | Lactuca sativa L. Lepidium sativum L. Portulaca oleracea L. Raphanus sativus L. Triticum aestivum L. | In vitro assay in Petri dishes on filter paper. Dose tested: 0.015, 0.031, 0.062, 0.125, 0.25, 0.5, 1, and 2 μL EO mL−1 Tween 80–water solution (0.5%, v/v) | Inhibition of seed germination of T. aestivum, L. sativa, R. sativus, and L. sativum (100%) at 0.5 µL EO mL−1 and at higher concentrations. Inhibition of radicle and pulmule growth of R. sativus (88 and 74%), L. sativa (81 and 86%), T. aestivum (75 and 73%), P. oleracea (71 and 64%), and L. sativum (56 and 64%), respectively, at 0.25 μL EO mL−1. Inhibition of fresh and dry weight of T. aestivum (85 and 82%), L. sativa (77 and 84%), R. sativus (70 and 70%), L. sativum (66 and 47%), and P. oleracea (53 and 41%) at 0.25 μL EO mL−1. | [101] |
| N. pannonica L. | 1,8-Cineole (28.9%) trans,cis-Nepetalactone (14.3%) | Agrostis stolonifera L. cv. Pencross Lactuca sativa L. cv. Iceberg | In vitro assay in 24-well plates. Dose tested: 0.01, 0,03, 0,1, 0.3, and 1 mg EO mL−1 water; 2 mg trans,cis-nepetalactone mL−1 water. | Inhibition of growth of A. stolonifera (100%) at 0.3 and 1.0 mg EO mL−1. No effect on seed germination at 2 mg trans,cis-nepetalactone mL−1. | [102] |
| N. rtanjensis Diklić et Milojević | trans,cis-Nepetalactone (72%) cis,trans-Nepetalactone (16%) α-Pinene (3%) β-Pinene (0.4%) | Arabidopsis thaliana (L.) Heynh. Brassica napus L. cv. napus Lactuca sativa L. cv. May Queen Lepidium sativum L. Lotus corniculatus L. cv. Bokor Rumex crispus L. Stellaria media (L.) Vill. | In vitro enclosed volatile bioassays in Petri dishes—seed germination. Dose tested: 0.1, 0.3, 0.5, 1, 2, and 4% of N. rtanjensis EO, α-pinene, and β-pinene in 99.8% methanol on seeds germinated on culture media. 30 µL of N. rtanjensis EO, α-pinene, and β-pinene applied to filter paper in Petri dishes that are not in direct contact with the culture medium. | Among the crop plants, L. sativa was the most sensitive, while B. napus showed the highest tolerance to N. rtanjensis EO and α- and β-pinene. Among the weeds, S. media was the most sensitive, while R. crispus was the most tolerant to N. rtanjensis EO and α- and β-pinene. α- and β-pinene exhibited lower phytotoxicity than N. rtanjensis EO. α-Pinene exhibited greater phytotoxicity than β-pinene. α-Pinene particularly affected the seed germination of L. sativa, L. corniculatus, B. napus, and S. media. | [38] |
| N. rtanjensis Diklić et Milojević | Nepetalactones (cis,trans- and trans,cis) with [M+1]+ at m/z 167 α- and β-Pinene with [M+1]+ at m/z 137 α-campholenal, neral, and geranial with [M+1]+ at m/z 153 γ-cadinene, δ-cadinene, cis- and trans-caryophyllene, and α-humulene with [M+1]+ at m/z 205 | Arabidopsis thaliana (L.) Heynh | In vitro enclosed volatile bioassays—plant growth, Chl, organic acid, sugar and ammonium levels, activities and abundance of GS and antioxidant enzymes, and expression of GS-encoding genes were investigated. Dose tested: 2 and 4% (v/v) EO in 99.8% methanol on 2-week-old A. thaliana plants grown in vitro. 50 µL of EO was applied to filter paper placed on a metal holder in the culture medium in 350 mL glass vessels. Nepetalactone concentrations at the beginning of the experiment: 1700 and 3400 ppbV in the atmosphere of the glass vessels with 2 and 4% EO, respectively. Nepetalactone concentration after 10 days: 18 ppbV and 29 ppbV in the atmosphere of the glass vessels with 2 and 4% EO, respectively. The same trend was observed for other traced compounds. | No effect on the Chl content (Chl a, Chl b, and Chl a+b) or the fresh weight of the roots. Decrease in fresh weight of shoots at higher EO concentrations (4%). Increase in the content of isocitric acid, malic acid, and succinic acid with a simultaneous decrease in the fructose and sucrose content in the shoots. No effects on GS activity, GS abundance, and ammonium content in the shoots. Changes in the activities of antioxidant enzymes: CAT activity decreased in both shoots and roots, with more pronounced effects in shoots, POX activity decreased in the roots, SOD remained unchanged in the shoots but showed a dose-dependent response in the roots, decreasing at lower EO concentrations and increasing at higher EO concentrations. No changes in the expression of GLN1;1, GLN1;2, GLN1;3, GLN1;4, and GLN2 in A. thaliana shoots. | [40] |
| N. rtanjensis Diklić et Milojević | trans,cis-Nepetalactone (72%) cis,trans-Nepetalactone (16%) α-pinene (3%) | Amaranthus retroflexus L. Ambrosia artemisiifolia L. Artemisia vulgaris L. Cephalaria transsylvanica (L.) Schrad. ex Roem. & Schult Stellaria media (L.) Vill. | In vitro assay in Petri dishes on filter paper. Dose tested: 0.006, 0.013, 0.025, 0.05, and 0.1% of the EO for A. retroflexus, A. artemisiifolia, and C. transsylvanica, 0.003, 0.006, 0.013, 0.025, 0.05, and 0.1% of the EO for A. vulgaris and S. media, respectively. EO:methanol:Tween 20 in a volume ratio of 1:4:1 and water was added to the desired EO concentration. In the greenhouse assay, the soil was sprayed with EO (0.06, 0.13, 0.25, 0.5, and 1%) before sowing S. media seeds, and the S. media seedlings at the 2-node stage were sprayed with EO (0.06, 0.13, 0.25, 0.5, and 1%) 12 days after sowing. EO:methanol:Tween 20 at a volume ratio of 1:1:1 and water was added to the desired EO concentration. | Inhibition of seed germination of S. media (100%) at EO (from 0.013 to 0.1%). Inhibition of seed germination of A. retroflexus, A. vulgaris, and A. artemisiifolia (100, 100, and 48%, respectively) at EO (0.1%). Foliar spraying of S. media seedlings 12 days after sowing with EO (0.5 and 1%) resulted in a significant reduction in shoot height, fresh weight, and node formation. Mortality of S. media seedlings (45%) 5 days after spraying with EO (1%). | [41] |
| N. rtanjensis Diklić et Milojević | trans,cis-Nepetalactone (72%) cis,trans-Nepetalactone (16%) | Arabidopsis thaliana (L.) Heynh | In a greenhouse assay, foliar application of 10 μL of 2% EO on 10 leaves of 4-week-old A. thaliana plants | Leaf discoloration and a reduction in the fresh weight of the shoots 10 days after treatment. Chl a, Chl b, and Chl a+b content decreased slightly. Change of organic acids and reduction of glucose and fructose content in the shoots of A. thaliana 10 days after treatment. Slight increase in total GS activity without changes in ammonia levels 1 and 10 days after treatments. Upregulation of the expression of GS-encoding genes, GLN1;1 and GLN1;3, 1 day after treatment. | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dmitrović, S.; Nestorović Živković, J.; Smailagić, D.; Trajković, M.; Banjac, N.; Ninković, S.; Stanišić, M. Via Air or Rhizosphere: The Phytotoxicity of Nepeta Essential Oils and Malus Dihydrochalcones. Plants 2025, 14, 701. https://doi.org/10.3390/plants14050701
Dmitrović S, Nestorović Živković J, Smailagić D, Trajković M, Banjac N, Ninković S, Stanišić M. Via Air or Rhizosphere: The Phytotoxicity of Nepeta Essential Oils and Malus Dihydrochalcones. Plants. 2025; 14(5):701. https://doi.org/10.3390/plants14050701
Chicago/Turabian StyleDmitrović, Slavica, Jasmina Nestorović Živković, Dijana Smailagić, Milena Trajković, Nevena Banjac, Slavica Ninković, and Mariana Stanišić. 2025. "Via Air or Rhizosphere: The Phytotoxicity of Nepeta Essential Oils and Malus Dihydrochalcones" Plants 14, no. 5: 701. https://doi.org/10.3390/plants14050701
APA StyleDmitrović, S., Nestorović Živković, J., Smailagić, D., Trajković, M., Banjac, N., Ninković, S., & Stanišić, M. (2025). Via Air or Rhizosphere: The Phytotoxicity of Nepeta Essential Oils and Malus Dihydrochalcones. Plants, 14(5), 701. https://doi.org/10.3390/plants14050701







