Plant Diversity and Sustainable Landscape Management: The Case of Misiliscemi, a New Municipality in Sicily
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
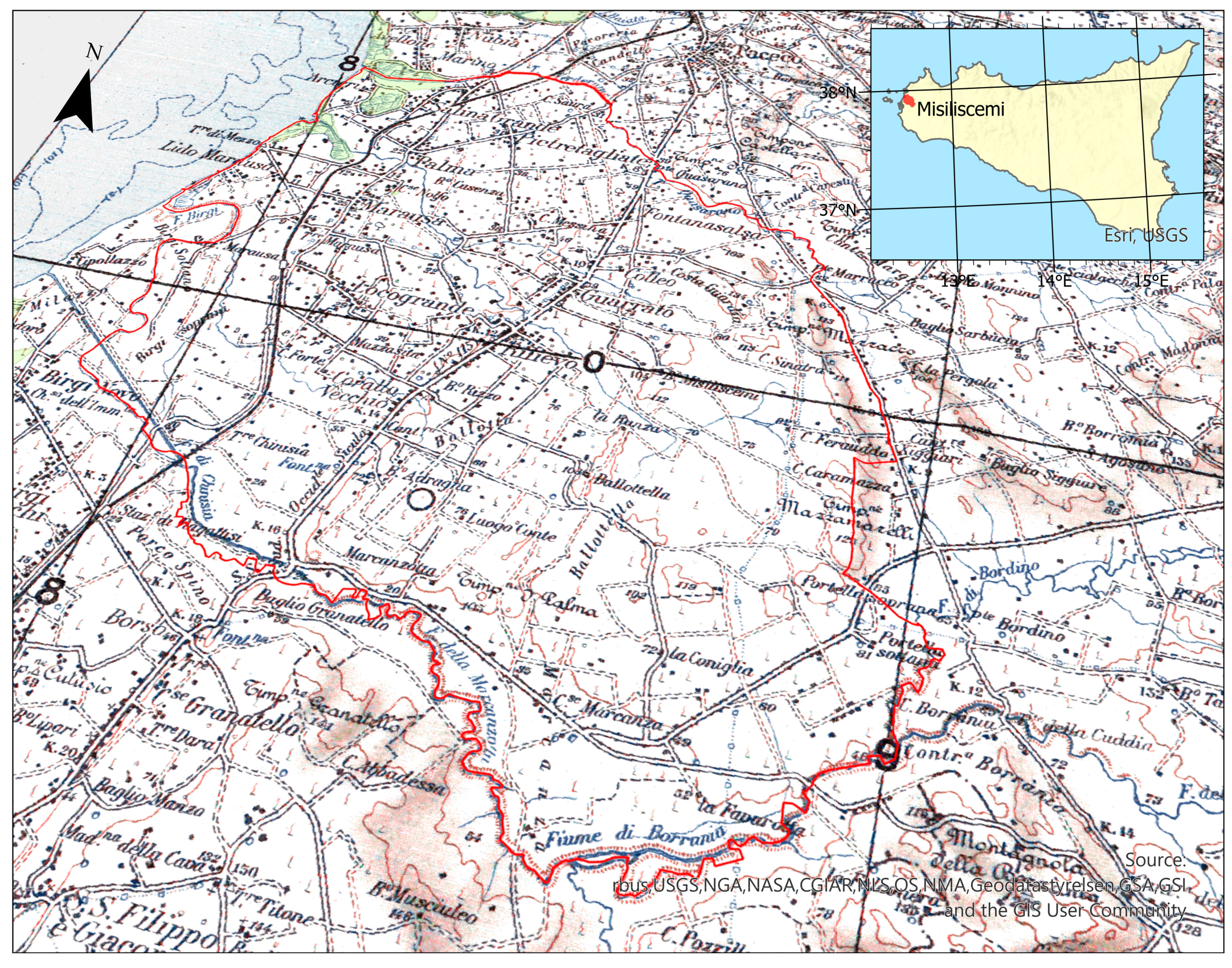
2.2. Field Surveys and Data Analysis
3. Results
3.1. Floristic Surveys and Data Analysis
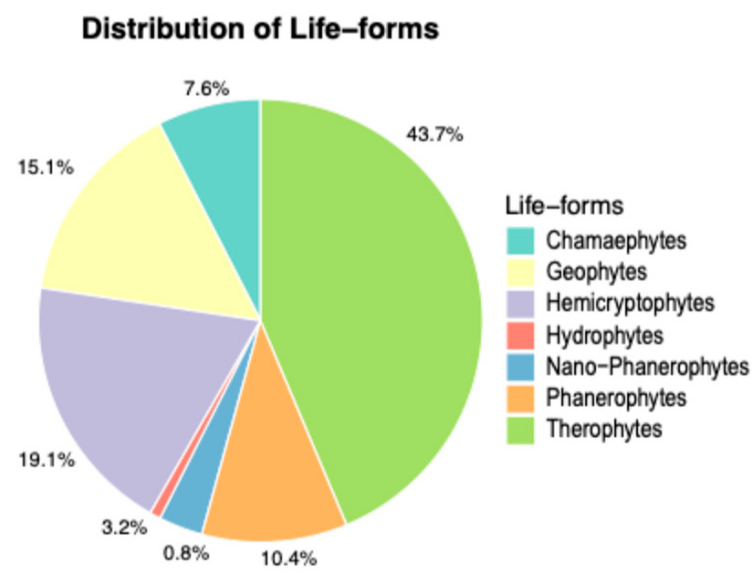
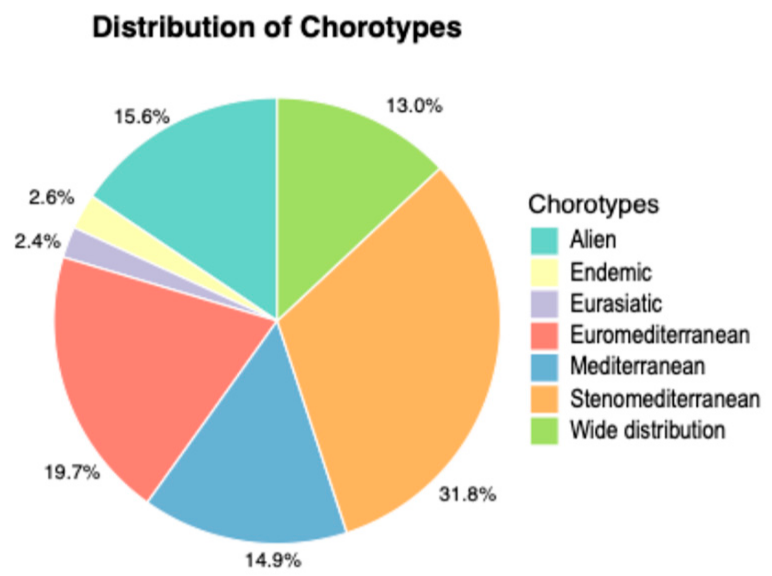
3.2. Life-Forms and Chorological Spectrum
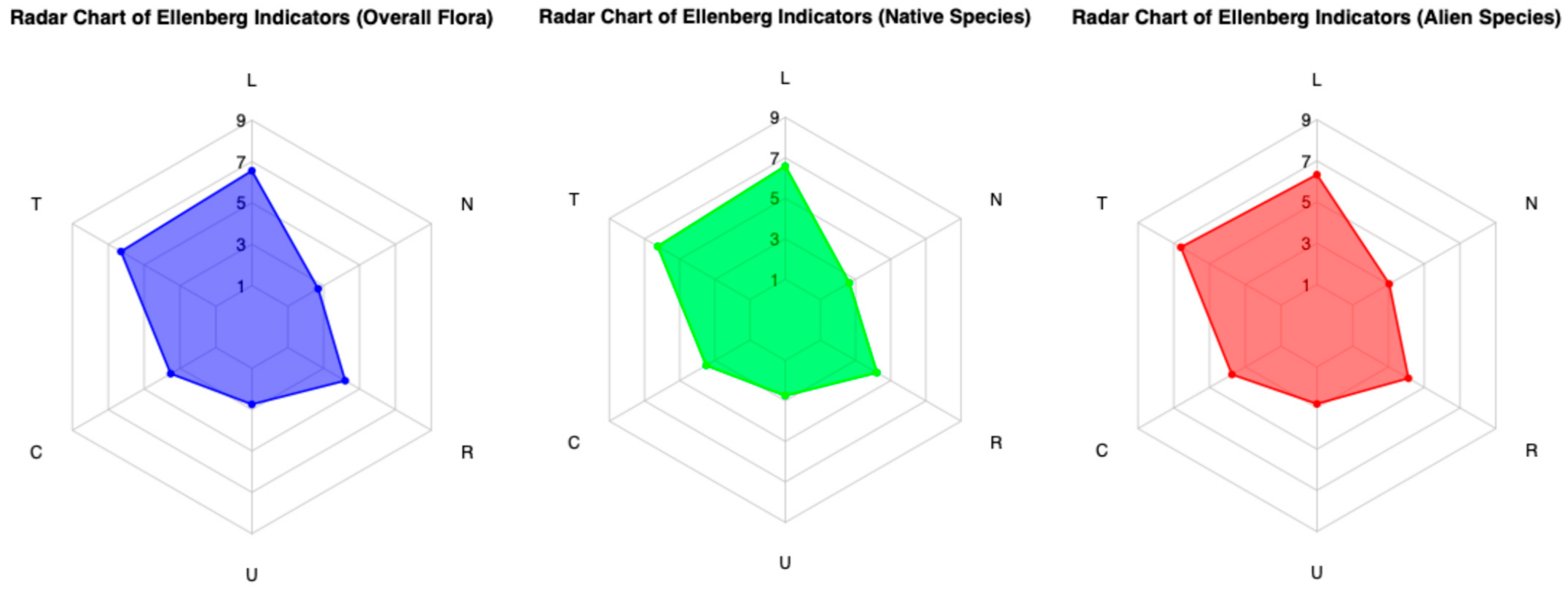
3.3. Ecological Indicators
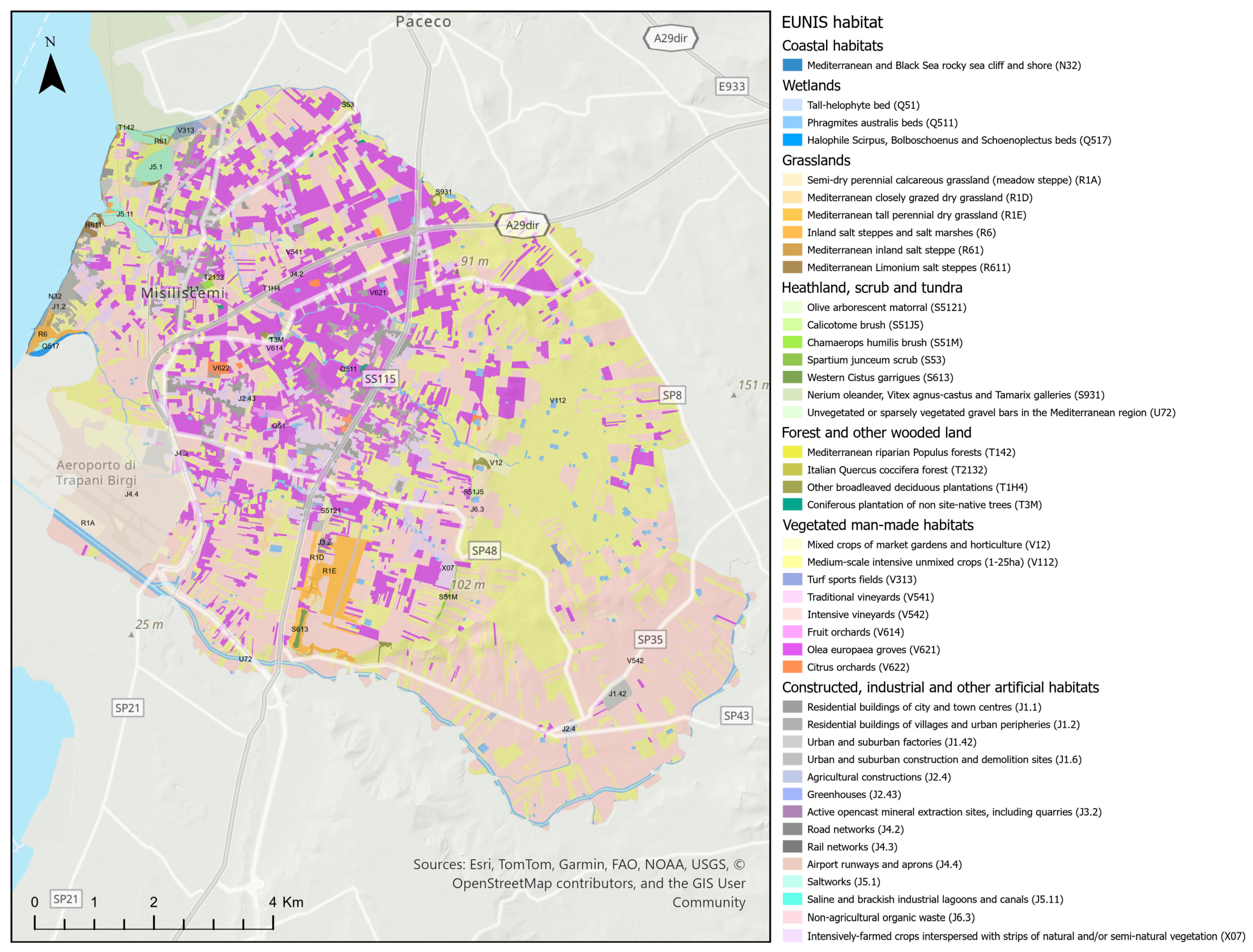
3.4. Habitat
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kier, G.; Mutke, J.; Dinerstein, E.; Ricketts, T.H.; Küper, W.; Kreft, H.; Barthlott, W. Global Patterns of Plant Diversity and Floristic Knowledge. J. Biogeogr. 2005, 32, 1107–1116. [Google Scholar] [CrossRef]
- D’Antraccoli, M.; Bedini, G.; Peruzzi, L. Next Generation Floristics: A Workflow to Integrate Novel Methods in Traditional Floristic Research. Plant Biosyst.—Int. J. Deal. All Asp. Plant Biol. 2022, 156, 594–597. [Google Scholar] [CrossRef]
- Wagensommer, R.P. Floristic Studies in the Light of Biodiversity Knowledge and Conservation. Plants 2023, 12, 2973. [Google Scholar] [CrossRef] [PubMed]
- Baiamonte, G.; Domina, G.; Raimondo, F.M.; Bazan, G. Agricultural Landscapes and Biodiversity Conservation: A Case Study in Sicily (Italy). Biodivers Conserv 2015, 24, 3201–3216. [Google Scholar] [CrossRef]
- MacGregor-Fors, I.; Escobar, F.; Rueda-Hernández, R.; Avendaño-Reyes, S.; Baena, M.; Bandala, V.; Chacón-Zapata, S.; Guillén-Servent, A.; González-García, F.; Lorea-Hernández, F.; et al. City “Green” Contributions: The Role of Urban Greenspaces as Reservoirs for Biodiversity. Forests 2016, 7, 146. [Google Scholar] [CrossRef]
- Domina, G.; Emilio, D.G.; Filippo, S.; Roberta, C.; Giuseppe, V.; Letizia, G.M. The Urban Vascular Flora of Palermo (Sicily, Italy). Plant Biosyst.—Int. J. Deal. All Asp. Plant Biol. 2020, 154, 627–634. [Google Scholar] [CrossRef]
- European Environment Agency CORINE Land Cover 2018 (Vector), Europe, 6-Yearly—Version 2020_20u1. May 2020. [CrossRef]
- Eurostat Urban-Rural Europe. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Urban-rural_Europe_-_introduction#Information_on_data (accessed on 23 October 2024).
- Capotorti, G.; Zavattero, L.; Anzellotti, I.; Burrascano, S.; Frondoni, R.; Marchetti, M.; Marignani, M.; Smiraglia, D.; Blasi, C. Do National Parks Play an Active Role in Conserving the Natural Capital of Italy? Plant Biosyst.—Int. J. Deal. All Asp. Plant Biol. 2012, 146, 258–265. [Google Scholar] [CrossRef]
- Löfvenhaft, K.; Björn, C.; Ihse, M. Biotope Patterns in Urban Areas: A Conceptual Model Integrating Biodiversity Issues in Spatial Planning. Landsc. Urban Plan. 2002, 58, 223–240. [Google Scholar] [CrossRef]
- Gussone, G. Florae Siculae Prodromus, Sive, Plantarum in Sicilia Ulteriori Nascentium Enumeratio Secundum Systema Linnaeanum Disposita; Ex Regia Typographia: Neapoli, Greek, 1827; Volume 1. [Google Scholar]
- Gussone, G. Florae Siculae Synopsis, Exhibens Plantas Vasculares in Sicilia Insulisque Adjacentibus Huc Usque Detectas Secundum Systema Linneanum Dispositas; Ex Typis Tramater: Neapoli, Greek, 1842; Volume 1. [Google Scholar]
- Lojacono Pojero, M. Flora Sicula o Descrizione Delle Piante Vascolari Spontanee o Indigenate in Sicilia; Stabilimento Tipografico Virzì: Palermo, Italy, 1888; Volume 1, p. 1. [Google Scholar]
- Lojacono Pojero, M. Flora Sicula o Descrizione Delle Piante Vascolari Spontanee o Indigenate in Sicilia; Tipografia dello Statuto: Palermo, Italy, 1891; Volume 1, p. 2. [Google Scholar]
- Lojacono Pojero, M. Flora Sicula o Descrizione Delle Piante Vascolari Spontanee o Indigenate in Sicilia. Tipo-litografia, S. Bizzarrilli: Palermo, Italy, 1902; Volume 2, p. 1. [Google Scholar]
- Lojacono Pojero, M. Flora Sicula o Descrizione Delle Piante Vascolari Spontanee o Indigenate in Sicilia; Tipo-litografia Salvatore Bizzarrilli: Palermo, Italy, 1904; Volume 2, p. 2. [Google Scholar]
- Lojacono Pojero, M. Flora Sicula o Descrizione Delle Piante Vascolari Spontanee o Indigenate in Sicilia; Scuola Tip. Boccone del Povero: Palermo, Italy, 1908; Volume 3. [Google Scholar]
- Ponzo, A. Contributo Alla Conoscenza Dei Caratteri Biologici Della Flora Trapanese; Tipografia Sciarrino: Palermo, Italy, 1900. [Google Scholar]
- Ponzo, A. La Flora Trapanese; Tipografia Puccio: Palermo, Italy, 1900. [Google Scholar]
- Ponzo, A. Le Plantule Della Flora Trapanese: Primo Contributo. G. Bot. Ital. 1926, 33, 341–389. [Google Scholar]
- Ponzo, A. Le Plantule Della Flora Trapanese: Secondo Contributo. G. Bot. Ital. 1927, 34, 546–592. [Google Scholar]
- Ponzo, A. Le Plantule Della Flora Trapanese: Terzo Contributo. G. Bot. Ital. 1928, 35, 169–213. [Google Scholar]
- Ponzo, A. Le Plantule Della Flora Trapanese: Quarto Contributo. G. Bot. Ital. 1940, 47, 579–590. [Google Scholar] [CrossRef]
- Ottonello, D.; Catanzaro, F. Contributo Alla Flora Del Trapanese. Il Nat. Sicil. 1986, 9, 89–99. [Google Scholar]
- Ottonello, D.; Aleo, M.; Romano, S. La Macchia Mediterranea a Quercus Calliprinos Webb Di Marausa (TP): Un’area Da Conservare. G. Bot. Ital. 1991, 145, 435. [Google Scholar]
- Aleo, M.; Bazan, G.; Cordì, R. Le Piante Vascolari Del Litorale Trapanese: Da Capo Lilibeo a Ronciglio. Quad. Di Bot. Ambient. E Appl. 2005, 15, 83–98. [Google Scholar]
- Aleo, M.; Bazan, G.; Quattrocchi, U. Le Piante Vascolari Del Litorale Trapanese: Da Ronciglio a Capo San Vito. Quad. Di Bot. Ambient. Appl. 2011, 22, 101–116. [Google Scholar]
- Istituto Nazionale di Statistica Confini Delle Unità Amministrative a Fini Statistici 2024. Available online: https://www.istat.it/notizia/confini-delle-unita-amministrative-a-fini-statistici-al-1-gennaio-2018-2/ (accessed on 23 October 2024).
- Regione Siciliana Carta Dell’Uso Del Suolo Secondo Corine Land Cover Dell’intero Territorio Siciliano Sulla Base Delle CTR Regionali a Scala 1:10.000. Available online: https://map.sitr.regione.sicilia.it/orbs/rest/services/carta_habitat_10000/cartausosuolo_corinelandcover_CLC/MapServer (accessed on 19 December 2024).
- Ministero dell’Ambiente Geoportale Nazionale. Available online: https://gn.mase.gov.it/portale/servizio-di-consultazione-wms (accessed on 2 February 2022).
- Duro, A.; Piccione, V.; Scalia, C.; Zampino, S. Precipitazioni e Temperature Medie Mensili in Sicilia Relative al Sessantennio 1926-1985. Atti 5° Workshop Progr. Strat. C.N.R. Clima Amb. Terr. Mezzogiorno; C.N.R. 1996, 1, 17–109. [Google Scholar]
- Rivas-Martínez, S.; Rivas-Saenz, S.; Penas, A. Worldwide Bioclimatic Classification System. Glob. Geobot. 2002, 1, 1–634. [Google Scholar]
- Bazan, G.; Marino, P.; Guarino, R.; Domina, G.; Schicchi, R. Bioclimatology and Vegetation Series in Sicily: A Geostatistical Approach. Ann. Bot. Fenn. 2015, 52, 1–18. [Google Scholar] [CrossRef]
- Basilone, L. Lithostratigraphy of Sicily; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 3-319-73941-7. [Google Scholar]
- Fierotti, G.; Dazzi, C.; Raimondi, S. Carta Dei Suoli Della Sicilia (Scala 1: 250.000). Regione Siciliana, Assessorato Territorio e Ambiente; Università degli studi di Palermo, Facoltà di Agraria. Istituto di Agronomia Generale, Cattedra di Pedologia: Palermo, Italy, 1988. [Google Scholar]
- Sestini, A. Il Paesaggio; Conosci l’Italia; Touring Club Italiano: Milano, Italy, 1963; Volume 7. [Google Scholar]
- Biondi, E.; Blasi, C. Manuale Italiano d’interpretazione. Available online: http://vnr.unipg.it/habitat/ (accessed on 18 December 2024).
- Pignatti, S. Flora d’Italia; Edagricole: Bologna, Italy, 1982; Volume 1–3. [Google Scholar]
- Pignatti, S. Flora d’Italia; Seconda edizione in 4 volumi; Edagricole: Milano, Italy, 2017; Volume 1–4. [Google Scholar]
- Bartolucci, F.; Peruzzi, L.; Galasso, G.; Alessandrini, A.; Ardenghi, N.M.G.; Bacchetta, G.; Banfi, E.; Barberis, G.; Bernardo, L.; Bouvet, D.; et al. A Second Update to the Checklist of the Vascular Flora Native to Italy. Plant Biosyst. —Int. J. Deal. All Asp. Plant Biol. 2024, 158, 219–296. [Google Scholar] [CrossRef]
- Galasso, G.; Conti, F.; Peruzzi, L.; Alessandrini, A.; Ardenghi, N.M.G.; Bacchetta, G.; Banfi, E.; Barberis, G.; Bernardo, L.; Bouvet, D.; et al. A Second Update to the Checklist of the Vascular Flora Alien to Italy. Plant Biosyst.—Int. J. Deal. All Asp. Plant Biol. 2024, 158, 297–340. [Google Scholar] [CrossRef]
- Martellos, S.; Nimis, P.L. Portale Della Flora d’Italia/ Portal to the Flora of Italy. Available online: http://dryades.units.it/floritaly (accessed on 20 December 2024).
- Chamberlain, S.A.; Szöcs, E. Taxize: Taxonomic Search and Retrieval in R. F1000Res 2013, 2, 191. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Qian, H.V. PhyloMaker2: An Updated and Enlarged R Package That Can Generate Very Large Phylogenies for Vascular Plants. Plant Divers. 2022, 44, 335–339. [Google Scholar] [CrossRef]
- Yu, G.; Smith, D.K.; Zhu, H.; Guan, Y.; Lam, T.T. ggtree: An R Package for Visualization and Annotation of Phylogenetic Trees with Their Covariates and Other Associated Data. Methods Ecol Evol 2017, 8, 28–36. [Google Scholar] [CrossRef]
- Wirth, V. Ökologische Zeigerwerte von Flechten—Erweiterte und Aktualisierte Fassung. Herzogia 2010, 23, 229–248. [Google Scholar] [CrossRef]
- Pignatti, S.; Menegoni, P.; Pietrosanti, S. Biondicazione Attraverso Le Piante Vascolari. Valori di indicazione secondo Ellenberg (Zeigerwerte) per le specie della Flora d’Italia. Braun-Blanquetia 2005, 39, 1–97. [Google Scholar]
- Domina, G.; Galasso, G.; Bartolucci, F.; Guarino, R. Ellenberg Indicator Values for the Vascular Flora Alien to Italy. Electronic Supplementary File 1. Fl. Medit. 2018, 28, 53–61. [Google Scholar] [CrossRef]
- Regione Siciliana. S.I.T.R—Sistema Informativo Territoriale Regionale. Available online: https://www.sitr.regione.sicilia.it/ (accessed on 2 February 2024).
- Davies, C.E.; Moss, D.; Hill, M.O. EUNIS Habitat Classification; European Environment Agency: Copenhagen, Denmark, 2004. [Google Scholar]
- ESRI ArcGIS Pro 2024.
- Bazan, G.; Brullo, S.; Raimondo, F.M.; Schicchi, R. Le Serie Di Vegetazione Della Regione Sicilia. In La vegetazione d’Italia; Palombi Editori: Roma, Italy, 2010; pp. 429–470. ISBN 88-6060-290-4. [Google Scholar]
- Gianguzzi, L.; Bazan, G. The Olea Europaea L. Var. Sylvestris (Mill.) Lehr. Forests in the Mediterranean Area. Plant Sociol. 2019, 56, 3–34. [Google Scholar] [CrossRef]
- Gianguzzi, L.; Bazan, G. A Phytosociological Analysis of the Olea Europaea L. Var. Sylvestris (Mill.) Lehr. Forests in Sicily. Plant Biosyst. -Int. J. Deal. All Asp. Plant Biol. 2020, 154, 705–725. [Google Scholar]
- Rivieccio, G.; Aleffi, M.; Angiolini, C.; Bagella, S.; Bazan, G.; Bonini, F.; Caria, M.C.; Casavecchia, S.; Castello, M.; Dagnino, D.; et al. New National and Regional Annex I Habitat Records: From #26 to #36. Plant Sociol. 2021, 58, 77–98. [Google Scholar] [CrossRef]
- La Mantia, T.; Pasta, S.; Troia, A. Declino degli ambienti umidi in sicilia: Primo elenco delle zone scomparse. Naturalista Siciliano 2023, 46, 159–202. [Google Scholar] [CrossRef]
- Franzén, M.; Schweiger, O.; Betzholtz, P.-E. Species-Area Relationships Are Controlled by Species Traits. PLoS ONE 2012, 7, e37359. [Google Scholar] [CrossRef] [PubMed]
- De Natale, A.; La Valva, V. La flora di Napoli: I quartieri della città. Webbia 2000, 54, 271–373. [Google Scholar] [CrossRef]
- Peruzzi, L. The Vascular Flora of Empoli (Tuscany, Central Italy). IB 2023, 15, 21–33. [Google Scholar] [CrossRef]
- Fanelli, G.; Tescarollo, P.; Testi, A. Ecological Indicators Applied to Urban and Suburban Floras. Ecol. Indic. 2006, 6, 444–457. [Google Scholar] [CrossRef]
- Gianguzzi, L.A.; La Mantia, A.; Ottonello, D.; Romano, S. La Flora Vascolare Della Riserva Naturale Monte Cofano (Sicilia Occidentale). Nat. Sicil. 2005, 29, 107–152. [Google Scholar]
- Aleo, N.; Amato, F.; Aleo, M. Le Piante Tossiche Della Flora Trapanese (Sicilia). Quad. Di Bot. Ambient. E Appl. 2011, 11, 31–49. [Google Scholar]
- Dylewski, Ł.; Banaszak-Cibicka, W.; Maćkowiak, Ł.; Dyderski, M.K. How Do Urbanization and Alien Species Affect the Plant Taxonomic, Functional, and Phylogenetic Diversity in Different Types of Urban Green Areas? Environ. Sci. Pollut. Res. 2023, 30, 92390–92403. [Google Scholar] [CrossRef]
- Rusterholz, H.; Wirz, D.; Baur, B. Garden Waste Deposits as a Source for Non-native Plants in Mixed Deciduous Forests. Appl. Veg. Sci. 2012, 15, 329–337. [Google Scholar] [CrossRef]
- Gaggini, L.; Rusterholz, H.-P.; Baur, B. Settlements as a Source for the Spread of Non-Native Plants into Central European Suburban Forests. Acta Oecologica 2017, 79, 18–25. [Google Scholar] [CrossRef]
- Hügin, G. Chamaesyce Serpens Subsp. Fissistipula—Neubewertung von Euphorbia Serpens Var. Fissistipula (Euphorbiaceae). Feddes Repert. 1998, 109, 509–519. [Google Scholar] [CrossRef]
- Lazzaro, L.; Bolpagni, R.; Buffa, G.; Gentili, R.; Lonati, M.; Stinca, A.; Acosta, A.T.R.; Adorni, M.; Aleffi, M.; Allegrezza, M.; et al. Impact of Invasive Alien Plants on Native Plant Communities and Natura 2000 Habitats: State of the Art, Gap Analysis and Perspectives in Italy. J. Environ. Manag. 2020, 274, 111140. [Google Scholar] [CrossRef] [PubMed]
- Hardion, L.; Verlaque, R.; Saltonstall, K.; Leriche, A.; Vila, B. Origin of the Invasive Arundo Donax (Poaceae): A Trans-Asian Expedition in Herbaria. Ann. Bot. 2014, 114, 455–462. [Google Scholar] [CrossRef]
- Aleo, M.; Cambria, S.; Bazan, G. Tradizioni Etnofarmacobotaniche in Alcune Comunità Rurali Dei Monti Di Trapani (Sicilia Occidentale). Quad. Di Bot. Ambient. E Appl. 2014, 24, 27–38. [Google Scholar]

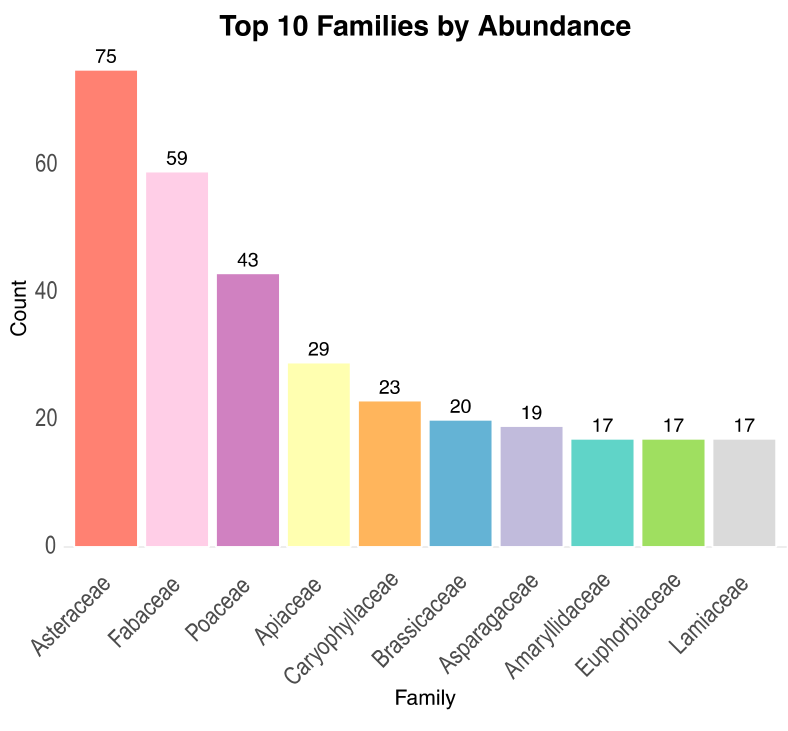
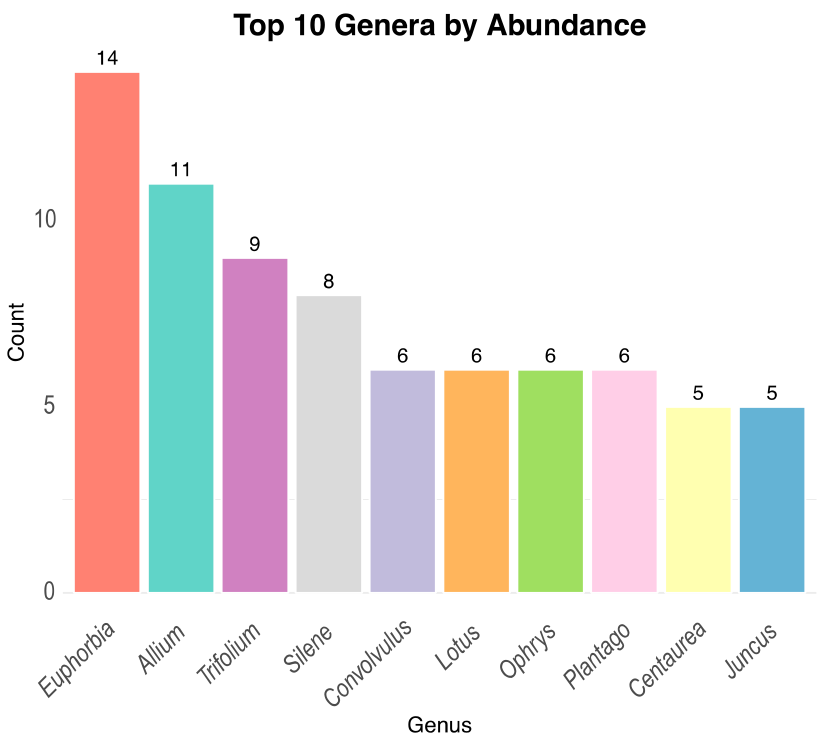
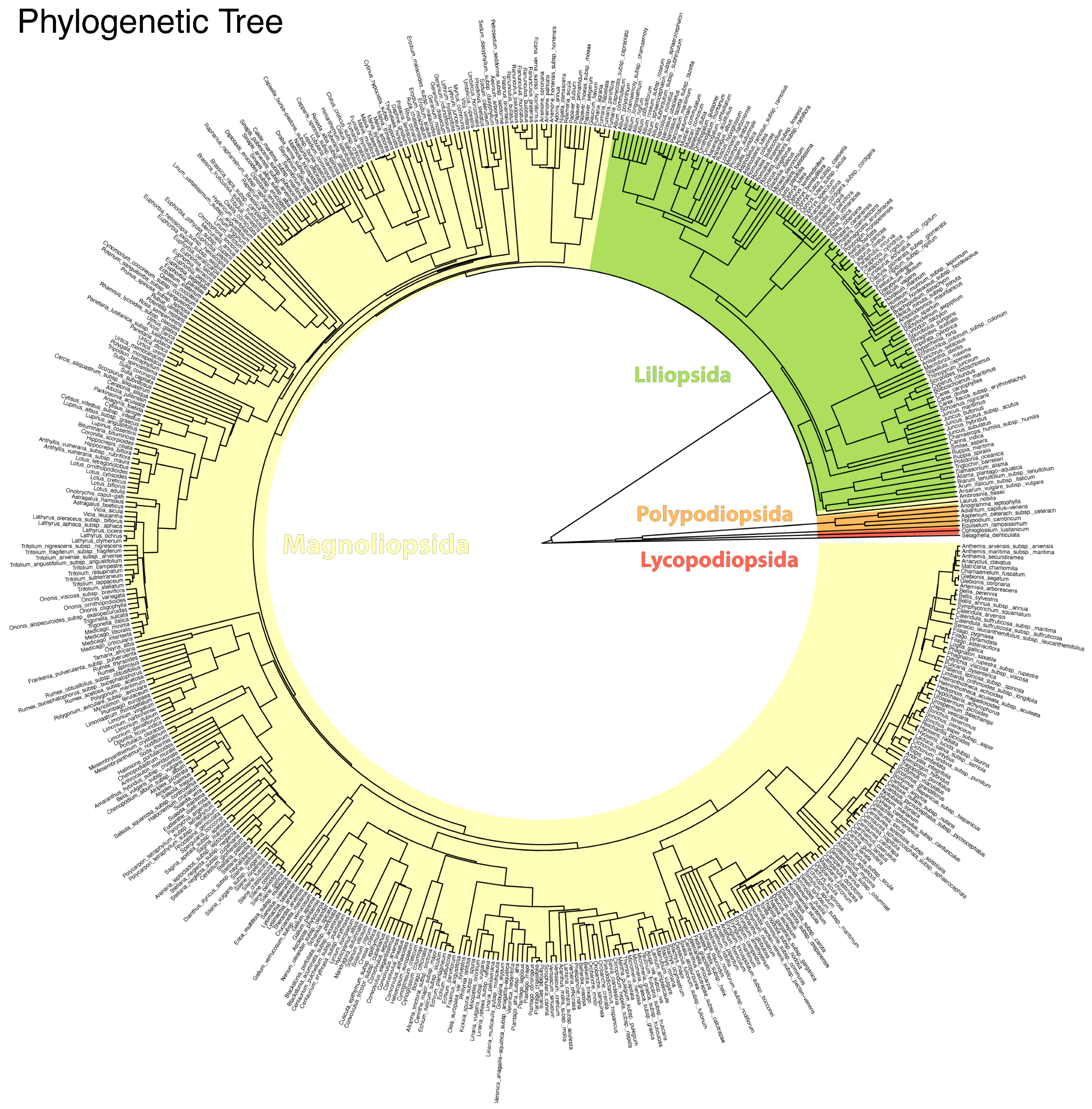

| Variable | Overall Flora | Native | Alien | Difference Native/Alien |
|---|---|---|---|---|
| Light (L) | 8.64 | 8.68 | 8.33 | 0.34 |
| Temperature (T) | 8.28 | 8.22 | 8.71 | −0.5 |
| Continentality (C) | 4.47 | 4.43 | 4.78 | −0.35 |
| Moisture (U) | 3.4 | 3.39 | 3.48 | −0.09 |
| Soil Reaction (R) | 5.4 | 5.42 | 5.29 | 0.12 |
| Nutrients (N) | 3.33 | 3.26 | 3.81 | −0.55 |
| EUNIS Habitat | Area [ha] |
|---|---|
| Coastal habitats | 5.68 |
| Mediterranean and Black Sea rocky sea cliff and shore (N32) | 5.68 |
| Wetlands | 222.38 |
| Tall-helophyte bed (Q51) | 1.44 |
| Phragmites australis beds (Q511) | 215.15 |
| Halophile Scirpus, Bolboschoenus and Schoenoplectus beds (Q517) | 5.78 |
| Grasslands | 387.13 |
| Semi-dry perennial calcareous grassland (meadow steppe) (R1A) | 231.65 |
| Mediterranean closely grazed dry grassland (R1D) | 21.56 |
| Mediterranean tall perennial dry grassland (R1E) | 100.92 |
| Inland salt steppes and salt marshes (R6) | 17.76 |
| Mediterranean inland salt steppe (R61) | 6.70 |
| Mediterranean Limonium salt steppes (R611) | 8.54 |
| Heathland, scrub and tundra | 38.61 |
| Olive arborescent matorral (S5121) | 4.45 |
| Calicotome brush (S51J5) | 1.68 |
| Chamaerops humilis brush (S51M) | 5.08 |
| Spartium junceum scrub (S53) | 1.16 |
| Western Cistus garrigues (S613) | 4.58 |
| Nerium oleander, Vitex agnus-castus and Tamarix galleries (S931) | 0.85 |
| Unvegetated or sparsely vegetated gravel bars in the Mediterranean region (U72) | 20.82 |
| Forest and other wooded land | 35.66 |
| Mediterranean riparian Populus forests (T142) | 0.58 |
| Italian Quercus coccifera forest (T2132) | 0.54 |
| Other broadleaved deciduous plantations (T1H4) | 29.45 |
| Coniferous plantation of non site-native trees (T3M) | 5.09 |
| Vegetated man-made habitats | 7404.20 |
| Mixed crops of market gardens and horticulture (V12) | 66.16 |
| Medium-scale intensive unmixed crops (1-25ha) (V112) | 2740.10 |
| Turf sports fields (V313) | 15.97 |
| Traditional vineyards (V541) | 5.94 |
| Intensive vineyards (V542) | 2987.24 |
| Fruit orchards (V614) | 15.46 |
| Olea europaea groves (V621) | 1557.57 |
| Citrus orchards (V622) | 15.76 |
| Constructed, industrial and other artificial habitats | 1146.94 |
| Residential buildings of city and town centres (J1.1) | 169.96 |
| Residential buildings of villages and urban peripheries (J1.2) | 128.78 |
| Urban and suburban factories (J1.42) | 42.26 |
| Agricultural constructions (J2.4) | 8.89 |
| Greenhouses (J2.43) | 4.73 |
| Active opencast mineral extraction sites, including quarries (J3.2) | 2.88 |
| Road networks (J4.2) | 28.11 |
| Rail networks (J4.3) | 14.43 |
| Airport runways and aprons (J4.4) | 303.98 |
| Saltworks (J5.1) | 65.54 |
| Saline and brackish industrial lagoons and canals (J5.11) | 1.11 |
| Non-agricultural organic waste (J6.3) | 6.25 |
| Intensively-farmed crops interspersed with strips of natural and/or semi-natural vegetation (X07) | 370.03 |
| Legenda | Area [ha]* |
|---|---|
| 1130: Estuaries | 1.85 |
| 1150 *: Coastal lagoons | 67.98 |
| 1210: Annual vegetation of drift lines | 0.30 |
| 1240: Vegetated sea cliffs of the Mediterranean coasts with endemic Limonium spp. | 4.28 |
| 1310: Salicornia and other annuals colonizing mud and sand | 3.29 |
| 1410: Mediterranean salt meadows (Juncetalia maritimi) | 11.97 |
| 1420: Mediterranean and thermo-Atlantic halophilous scrubs (Sarcocornetiea fruticosi) | 18.75 |
| 1510 *: Mediterranean salt steppes (Limonietalia) | 0.35 |
| 3280: Constantly flowing Mediterranean rivers with Paspalo-Agrostidion species and hanging curtains of Salix and Populus alba. | 0.00 |
| 3290: Intermittently flowing Mediterranean rivers of the Paspalo-Agrostidion | 20.82 |
| 5330: Thermo-Mediterranean and pre-desert scrub | 9.94 |
| 6220 *: Pseudo-steppe with grasses and annuals of the Thero-Brachypodietea | 116.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleo, M.; Bazan, G. Plant Diversity and Sustainable Landscape Management: The Case of Misiliscemi, a New Municipality in Sicily. Plants 2025, 14, 548. https://doi.org/10.3390/plants14040548
Aleo M, Bazan G. Plant Diversity and Sustainable Landscape Management: The Case of Misiliscemi, a New Municipality in Sicily. Plants. 2025; 14(4):548. https://doi.org/10.3390/plants14040548
Chicago/Turabian StyleAleo, Michele, and Giuseppe Bazan. 2025. "Plant Diversity and Sustainable Landscape Management: The Case of Misiliscemi, a New Municipality in Sicily" Plants 14, no. 4: 548. https://doi.org/10.3390/plants14040548
APA StyleAleo, M., & Bazan, G. (2025). Plant Diversity and Sustainable Landscape Management: The Case of Misiliscemi, a New Municipality in Sicily. Plants, 14(4), 548. https://doi.org/10.3390/plants14040548







