Changes in the Suitable Habitat of the Smoke Tree (Cotinus coggygria Scop.), a Species with an East Asian–Tethyan Disjunction
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Processing of Distribution Records
2.2. Environmental Variable Selection
2.3. MaxEnt Simulations and Model Accuracy Evaluation
2.4. Classification of Suitable Habitat
2.5. Simulation of the Historical Suitable Habitat
2.6. Simulation and Migration of the Future Suitable Habitat
3. Results
3.1. Environmental Variable Selection
3.2. Suitable Habitat Under Current Conditions
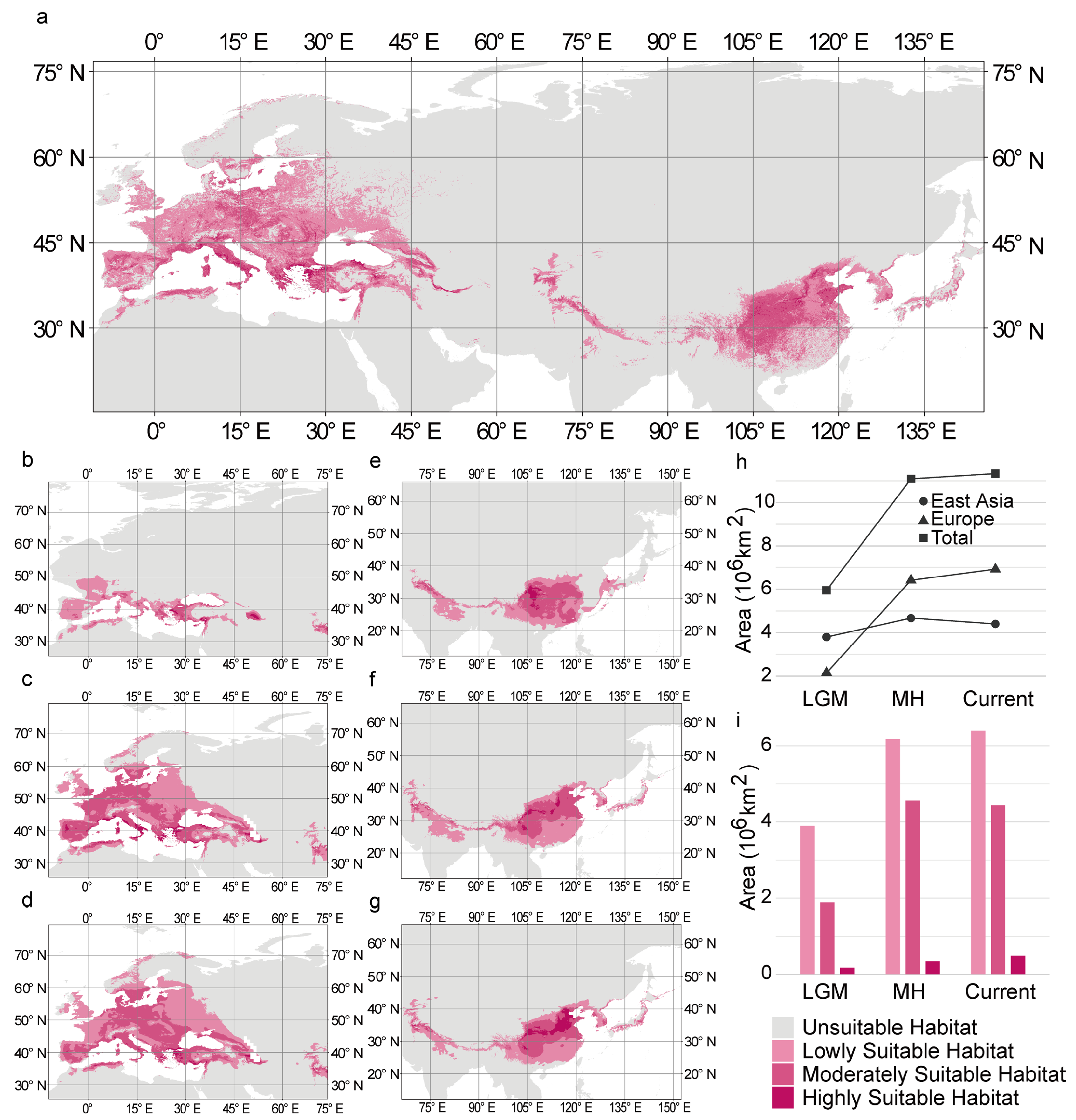
3.3. Changes in the Distribution of Historical Suitable Habitat
3.4. Changes in the Area of Historical Suitable Habitat
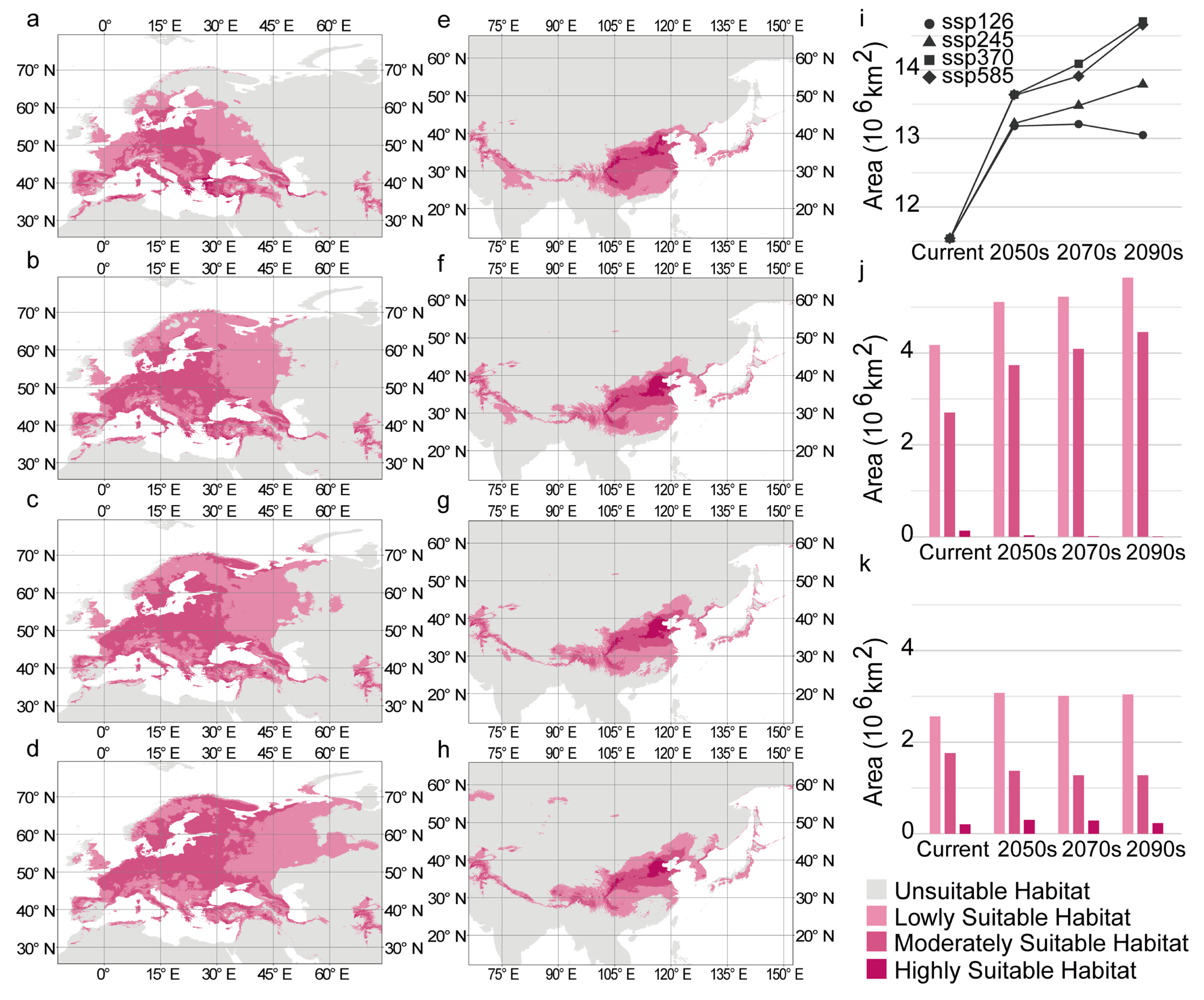
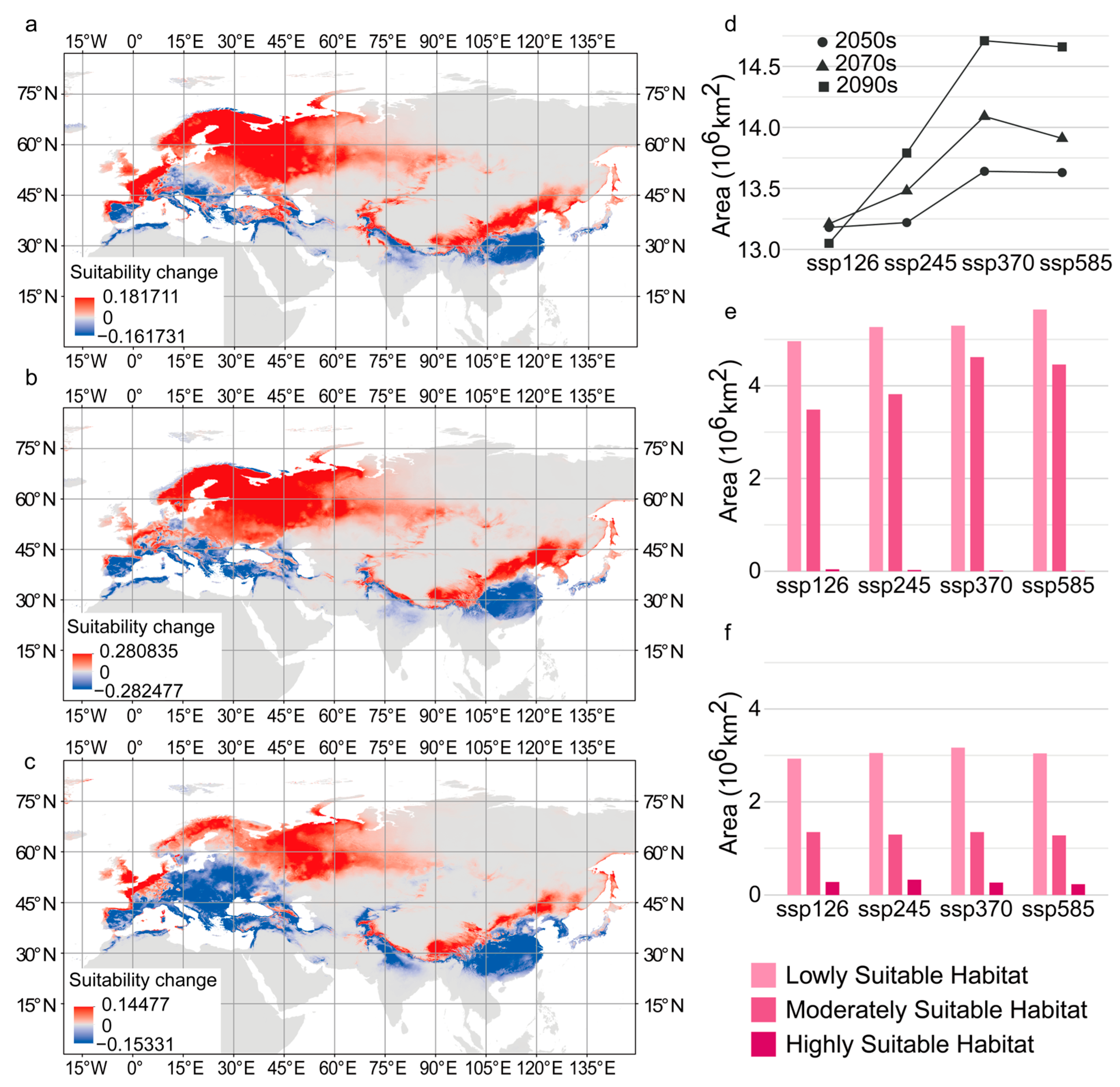
3.5. Suitable Habitat of C. coggygria Under Future Climate Change
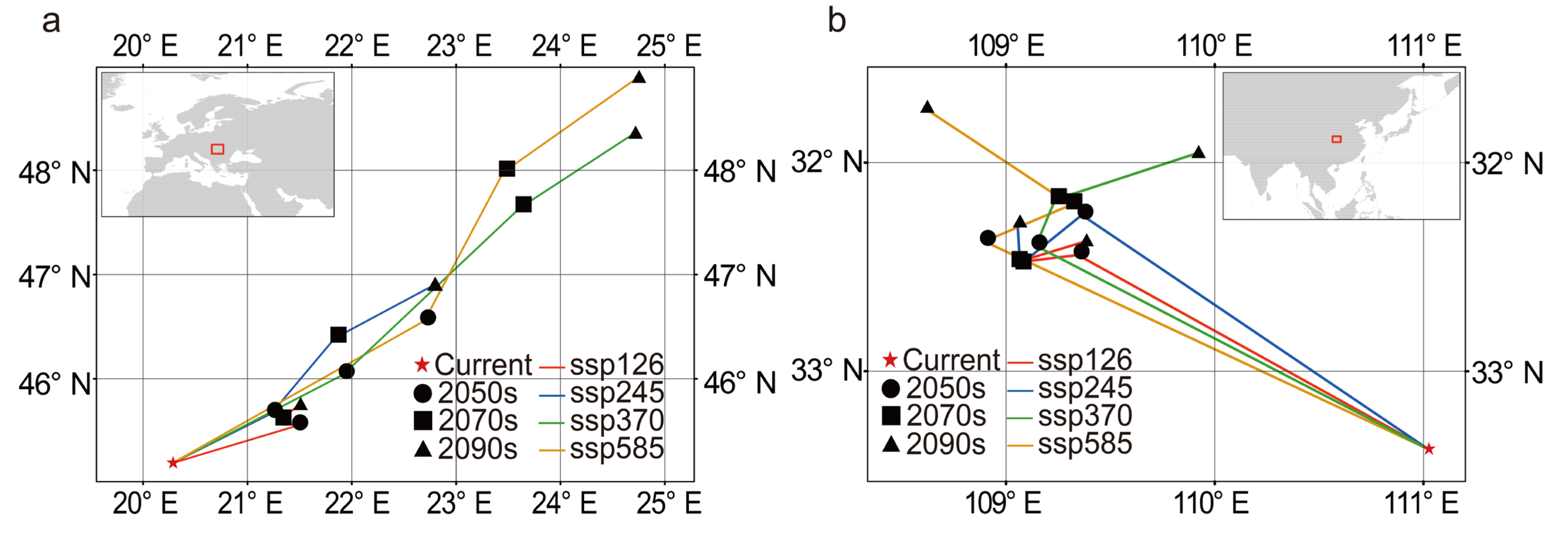
3.6. Variation in Suitable Habitat Under Different Future Climate Scenarios
4. Discussion
4.1. Differences in the Effects of Environmental Variables on C. coggygria
4.2. Changes in the Distribution of C. coggygria
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, D.; Milne, R.I.; Yang, H.; Zhang, Y.; Wang, Y.; Jia, S.; Li, J.; Mao, K. Phylogenomics shed light on the complex evolutionary history of a gymnosperm genus showing East Asian–Tethyan disjunction. J. Syst. Evol. 2025. early view. [Google Scholar] [CrossRef]
- Xie, L.; Yang, Z.-Y.; Wen, J.; Li, D.-Z.; Yi, T.-S. Biogeographic history of Pistacia (Anacardiaceae), emphasizing the evolution of the Madrean-Tethyan and the eastern Asian-Tethyan disjunctions. Mol. Phylogenetics Evol. 2014, 77, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Velitzelos, D.; Bouchal, J.M.; Denk, T. Review of the Cenozoic floras and vegetation of Greece. Rev. Palaeobot. Palynol. 2014, 204, 56–117. [Google Scholar] [CrossRef]
- Xia, M.; Cai, M.; Comes, H.P.; Zheng, L.; Ohi-Toma, T.; Lee, J.; Qi, Z.; Konowalik, K.; Li, P.; Cameron, K.M.; et al. An overlooked dispersal route of Cardueae (Asteraceae) from the Mediterranean to East Asia revealed by phylogenomic and biogeographical analyses of Atractylodes. Ann. Bot. 2022, 130, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.L.; Hipp, A.L.; Deng, M.; Su, T.; Zhou, Z.K.; Yan, M.X. East Asian origins of European holly oaks (Quercus section Ilex Loudon) via the Tibet-Himalaya. J. Biogeogr. 2019, 46, 2203–2214. [Google Scholar] [CrossRef]
- Sue, J.P. Origin and evolution of the Mediterranean vegetation and climate in Europe. Nature 1984, 307, 429–432. [Google Scholar]
- Morley, R.J. Palynological evidence for Tertiary plant dispersals in the SE Asian region in relation to plate tectonics and climate. Biogeogr. Geol. Evol. SE Asia 1998, 1, 211–234. [Google Scholar]
- Pacifici, M.; Foden, W.B.; Visconti, P.; Watson, J.E.; Butchart, S.H.; Kovacs, K.M.; Scheffers, B.R.; Hole, D.G.; Martin, T.G.; Akçakaya, H.R. Assessing species vulnerability to climate change. Nat. Clim. Change 2015, 5, 215–224. [Google Scholar] [CrossRef]
- Walther, G.-R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.; Fromentin, J.-M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.L. A Treatise on Limnology, Vol. 1: Geography, Physics, and Chemistry. J. Geol. 1958, 66, 710. [Google Scholar] [CrossRef]
- Chauvier, Y.; Thuiller, W.; Brun, P.; Lavergne, S.; Descombes, P.; Karger, D.N.; Renaud, J.; Zimmermann, N.E. Influence of climate, soil, and land cover on plant species distribution in the European Alps. Ecol. Monogr. 2021, 91, e01433. [Google Scholar] [CrossRef]
- Qazi, A.W.; Saqib, Z.; Zaman-ul-Haq, M. Trends in species distribution modelling in context of rare and endemic plants: A systematic review. Ecol. Process. 2022, 11, 40. [Google Scholar] [CrossRef]
- Wani, Z.A.; Negi, V.S.; Bhat, J.A.; Satish, K.; Kumar, A.; Khan, S.; Dhyani, R.; Siddiqui, S.; Al-Qthanin, R.N.; Pant, S. Elevation, aspect, and habitat heterogeneity determine plant diversity and compositional patterns in the Kashmir Himalaya. Front. For. Glob. Change 2023, 6, 1019277. [Google Scholar]
- Karami, R.; Mehrabi, H.R.; Ariapoor, A. The effect of altitude and slope in the species diversity of herbaceous plants (case study: Watershed Miandar Qarootag—Gilangharb). J. Appl. Environ. Biol. Sci. 2015, 5, 197–204. [Google Scholar]
- Franklin, J. Species distribution modelling supports the study of past, present and future biogeographies. J. Biogeogr. 2023, 50, 1533–1545. [Google Scholar] [CrossRef]
- Araújo, M.B.; Guisan, A. Five (or so) challenges for species distribution modelling. J. Biogeogr. 2006, 33, 1677–1688. [Google Scholar] [CrossRef]
- Franklin, J. Moving beyond static species distribution models in support of conservation biogeography. Divers. Distrib. 2010, 16, 321–330. [Google Scholar] [CrossRef]
- Sánchez-Mercado, A.; Ferrer-Paris, J.; Franklin, J. Mapping species distributions: Spatial inference and prediction. Oryx 2010, 44, 615. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Evans, J.S.; Murphy, M.A.; Holden, Z.A.; Cushman, S.A. Modeling species distribution and change using random forest. In Predictive Species and Habitat Modeling in Landscape Ecology: Concepts and Applications; Springer: New York, NY, USA, 2011; pp. 139–159. [Google Scholar]
- Elith, J.; Leathwick, J.R.; Hastie, T. A working guide to boosted regression trees. J. Anim. Ecol. 2008, 77, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Wang, Y.; Huang, T.; Lyu, Y.; Zhao, Y.; Liu, J. Maximum Entropy Analysis of Bird Diversity and Environmental Variables in Nanjing Megapolis, China. Sustainability 2024, 16, 2139. [Google Scholar] [CrossRef]
- Tripp, K.E. Considering Cotinus. 1994. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/19950309901 (accessed on 4 February 2025).
- da Silva, J.A.T.; Pacholczak, A.; Ilczuk, A. Smoke tree (Cotinus coggygria Scop.) propagation and biotechnology: A mini-review. South Afr. J. Bot. 2018, 114, 232–240. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, N.; Dong, W.; Zhao, L. Molecular evolution and phylogenomic analysis of complete chloroplast genomes of Cotinus (Anacardiaceae). Ecol. Evol. 2023, 13, e10134. [Google Scholar] [CrossRef]
- Emelyanova, O.; Firsov, A. Ecological and biological features and prospects of using Cotinus coggygria in breeding. BIO Web Conf. 2021, 36, 01016. [Google Scholar] [CrossRef]
- Wang, W.; Tian, C.Y.; Li, Y.H.; Li, Y.; Peeters, T. Molecular data and ecological niche modelling reveal the phylogeographic pattern of Cotinus coggygria(Anacardiaceae) in China’s warm-temperate zone. Plant Biol. 2014, 16, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.-K.; Wang, W.; Liu, Y.-P.; He, D.; Li, Y. Adaptive genetic variation in the smoke tree (Cotinus coggygria Scop.) is driven by precipitation. Biochem. Syst. Ecol. 2015, 59, 63–69. [Google Scholar] [CrossRef]
- GBIF.org. GBIF Occurrence Download. Available online: https://doi.org/10.15468/dl.zmgrye (accessed on 24 October 2023).
- Brown, J.L.; Anderson, B. SDMtoolbox: A python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. Methods Ecol. Evol. 2014, 5, 694–700. [Google Scholar] [CrossRef]
- Gavinet, J.; Santonja, M.; Baldy, V.; Hashoum, H.; Peano, S.; Tchong, T.; Gros, R.; Greff, S.; Fernandez, C.; Bousquet-Mélou, A. Phenolics of the understory shrub Cotinus coggygria influence Mediterranean oak forests diversity and dynamics. For. Ecol. Manag. 2019, 441, 262–270. [Google Scholar] [CrossRef]
- Zhao, Q.; Mi, Z.Y.; Lu, C.; Zhang, X.F.; Chen, L.J.; Wang, S.Q.; Niu, J.F.; Wang, Z.Z. Predicting potential distribution of Ziziphus spinosa (Bunge) HH Hu ex FH Chen in China under climate change scenarios. Ecol. Evol. 2022, 12, e8629. [Google Scholar] [CrossRef] [PubMed]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Zhang, K.; Yao, L.; Meng, J.; Tao, J. Maxent modeling for predicting the potential geographical distribution of two peony species under climate change. Sci. Total Environ. 2018, 634, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef]
- Canran, L.; Graeme, N.; Matt, W. On the selection of thresholds for predicting species occurrence with presence-only data. Ecol. Evol. 2016, 6, 337–348. [Google Scholar]
- Eyring, V.; Bony, S.; Meehl, G.A.; Senior, C.A.; Stevens, B.; Stouffer, R.J.; Taylor, K.E. Overview of the Coupled Model Intercomparison Project Phase 6 (CMIP6) experimental design and organization. Geosci. Model Dev. 2016, 9, 1937–1958. [Google Scholar] [CrossRef]
- Van Vuuren, D.P.; Stehfest, E.; Gernaat, D.E.; Doelman, J.C.; Van den Berg, M.; Harmsen, M.; de Boer, H.S.; Bouwman, L.F.; Daioglou, V.; Edelenbosch, O.Y. Energy, land-use and greenhouse gas emissions trajectories under a green growth paradigm. Glob. Environ. Change 2017, 42, 237–250. [Google Scholar] [CrossRef]
- O’Neill, B.C.; Kriegler, E.; Riahi, K.; Ebi, K.L.; Hallegatte, S.; Carter, T.R.; Mathur, R.; Van Vuuren, D.P. A new scenario framework for climate change research: The concept of shared socioeconomic pathways. Clim. Change 2014, 122, 387–400. [Google Scholar] [CrossRef]
- Van Vuuren, D.P.; Edmonds, J.; Kainuma, M.; Riahi, K.; Thomson, A.; Hibbard, K.; Hurtt, G.C.; Kram, T.; Krey, V.; Lamarque, J.-F. The representative concentration pathways: An overview. Clim. Change 2011, 109, 5–31. [Google Scholar] [CrossRef]
- Scott, L.M.; Janikas, M.V. Spatial statistics in ArcGIS. In Handbook of Applied Spatial Analysis: Software Tools, Methods and Applications; Springer: Berlin/Heidelberg, Germany, 2010; pp. 27–41. [Google Scholar]
- He, X.-H.; Si, J.-H.; Zhu, L.; Zhou, D.-M.; Zhao, C.-Y.; Jia, B.; Wang, C.-L.; Qin, J.; Zhu, X.-L. Modeling habitat suitability of Hippophae rhamnoides L. using MaxEnt under climate change in China: A case study of H. r. sinensis and H. r. turkestanica. Front. For. Glob. Change 2023, 5, 1095784. [Google Scholar] [CrossRef]
- Fang, J.; Shi, J.; Zhang, P.; Shao, M.; Zhou, N.; Wang, Y.; Xu, X. Potential Distribution Projections for Senegalia senegal (L.) Britton under Climate Change Scenarios. Forests 2024, 15, 379. [Google Scholar] [CrossRef]
- Bradie, J.; Leung, B. A quantitative synthesis of the importance of variables used in MaxEnt species distribution models. J. Biogeogr. 2016, 44, 1344–1361. [Google Scholar] [CrossRef]
- Miao, C.-Y.; Li, Y.; Yang, J.; Mao, R.-L. Landscape genomics reveal that ecological character determines adaptation: A case study in smoke tree (Cotinus coggygria Scop.). BMC Evol. Biol. 2017, 17, 202. [Google Scholar] [CrossRef] [PubMed]
- Beck, E.H.; Fettig, S.; Knake, C.; Hartig, K.; Bhattarai, T. Specific and unspecific responses of plants to cold and drought stress. J. Biosci. 2007, 32, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Pearce, R.S. Plant freezing and damage. Ann. Bot. 2001, 87, 417–424. [Google Scholar] [CrossRef]
- Granda, E.; Scoffoni, C.; Rubio-Casal, A.E.; Sack, L.; Valladares, F. Leaf and stem physiological responses to summer and winter extremes of woody species across temperate ecosystems. Oikos 2014, 123, 1281–1290. [Google Scholar] [CrossRef]
- Milne, R.I.; Abbott, R.J. The origin and evolution of tertiary relict floras. Adv. Bot. Res. 2002, 38, 281–314. [Google Scholar]
- Tang, C.Q.; Matsui, T.; Ohashi, H.; Dong, Y.-F.; Momohara, A.; Herrando-Moraira, S.; Qian, S.; Yang, Y.; Ohsawa, M.; Luu, H.T.; et al. Identifying long-term stable refugia for relict plant species in East Asia. Nat. Commun. 2018, 9, 4488. [Google Scholar] [CrossRef]
- Pierre Taberlet, R.C. Quaternary Refugia and Persistence of Biodiversity. Science 2002, 297, 2009–2010. [Google Scholar] [CrossRef]
- Bennett, K.D.; Tzedakis, P.C.; Willis, K.J. Quaternary refugia of north European trees. J. Biogeogr. 1991, 18, 103–115. [Google Scholar] [CrossRef]
- Svenning, J.C.; Normand, S.; Kageyama, M. Glacial refugia of temperate trees in Europe: Insights from species distribution modelling. J. Ecol. 2008, 96, 1117–1127. [Google Scholar] [CrossRef]
- Örücü, Ö.K.; Azadi, H.; Arslan, E.S.; Kamer Aksoy, Ö.; Choobchian, S.; Nooghabi, S.N.; Stefanie, H.I. Predicting the distribution of European Hop Hornbeam: Application of MaxEnt algorithm and climatic suitability models. Eur. J. For. Res. 2023, 142, 579–591. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, F.; Arroyo, J. Reconstructing the demise of Tethyan plants: Climate-driven range dynamics of Laurus since the Pliocene. Glob. Ecol. Biogeogr. 2008, 17, 685–695. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Li, C.; Liu, Z. Optimized maxent model predictions of climate change impacts on the suitable distribution of Cunninghamia lanceolata in China. Forests 2020, 11, 302. [Google Scholar] [CrossRef]
- Yan, H.; He, J.; Xu, X.; Yao, X.; Wang, G.; Tang, L.; Feng, L.; Zou, L.; Gu, X.; Qu, Y. Prediction of potentially suitable distributions of Codonopsis pilosula in China based on an optimized MaxEnt model. Front. Ecol. Evol. 2021, 9, 773396. [Google Scholar] [CrossRef]
- Zhao, G.; Cui, X.; Sun, J.; Li, T.; Wang, Q.I.; Ye, X.; Fan, B. Analysis of the distribution pattern of Chinese Ziziphus jujuba under climate change based on optimized biomod2 and MaxEnt models. Ecol. Indic. 2021, 132, 108256. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Z.; Zhu, B.; Cheng, X.; Yang, L.; Gao, M.; Kong, R. MaxEnt modeling based on CMIP6 models to project potential suitable zones for Cunninghamia lanceolata in China. Forests 2021, 12, 752. [Google Scholar] [CrossRef]
- Li, Y.; Shao, W.; Jiang, J. Predicting the potential global distribution of Sapindus mukorossi under climate change based on MaxEnt modelling. Environ. Sci. Pollut. Res. 2022, 29, 21751–21768. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Yan, X.; Guo, C.; Dong, W.; Zhao, L.; Liu, D. Changes in the Suitable Habitat of the Smoke Tree (Cotinus coggygria Scop.), a Species with an East Asian–Tethyan Disjunction. Plants 2025, 14, 547. https://doi.org/10.3390/plants14040547
Zhang Z, Yan X, Guo C, Dong W, Zhao L, Liu D. Changes in the Suitable Habitat of the Smoke Tree (Cotinus coggygria Scop.), a Species with an East Asian–Tethyan Disjunction. Plants. 2025; 14(4):547. https://doi.org/10.3390/plants14040547
Chicago/Turabian StyleZhang, Zichen, Xin Yan, Chang Guo, Wenpan Dong, Liangcheng Zhao, and Dan Liu. 2025. "Changes in the Suitable Habitat of the Smoke Tree (Cotinus coggygria Scop.), a Species with an East Asian–Tethyan Disjunction" Plants 14, no. 4: 547. https://doi.org/10.3390/plants14040547
APA StyleZhang, Z., Yan, X., Guo, C., Dong, W., Zhao, L., & Liu, D. (2025). Changes in the Suitable Habitat of the Smoke Tree (Cotinus coggygria Scop.), a Species with an East Asian–Tethyan Disjunction. Plants, 14(4), 547. https://doi.org/10.3390/plants14040547





