A NaHCO3-Tolerant Endophyte Bacillus amyloliquefaciens ZmBA DSM7 Enhances Growth and Mitigates NaHCO3-Induced Alkaline Stress in Maize Through Multiple Mechanism
Abstract
1. Introduction
2. Results
2.1. Isolation and Identification of a NaHCO3-Tolerant Bacterium
2.1.1. Isolation of a NaHCO3-Tolerant Bacterium
2.1.2. Characterization and Sequencing Analysis of a NaHCO3-Tolerant Bacterium
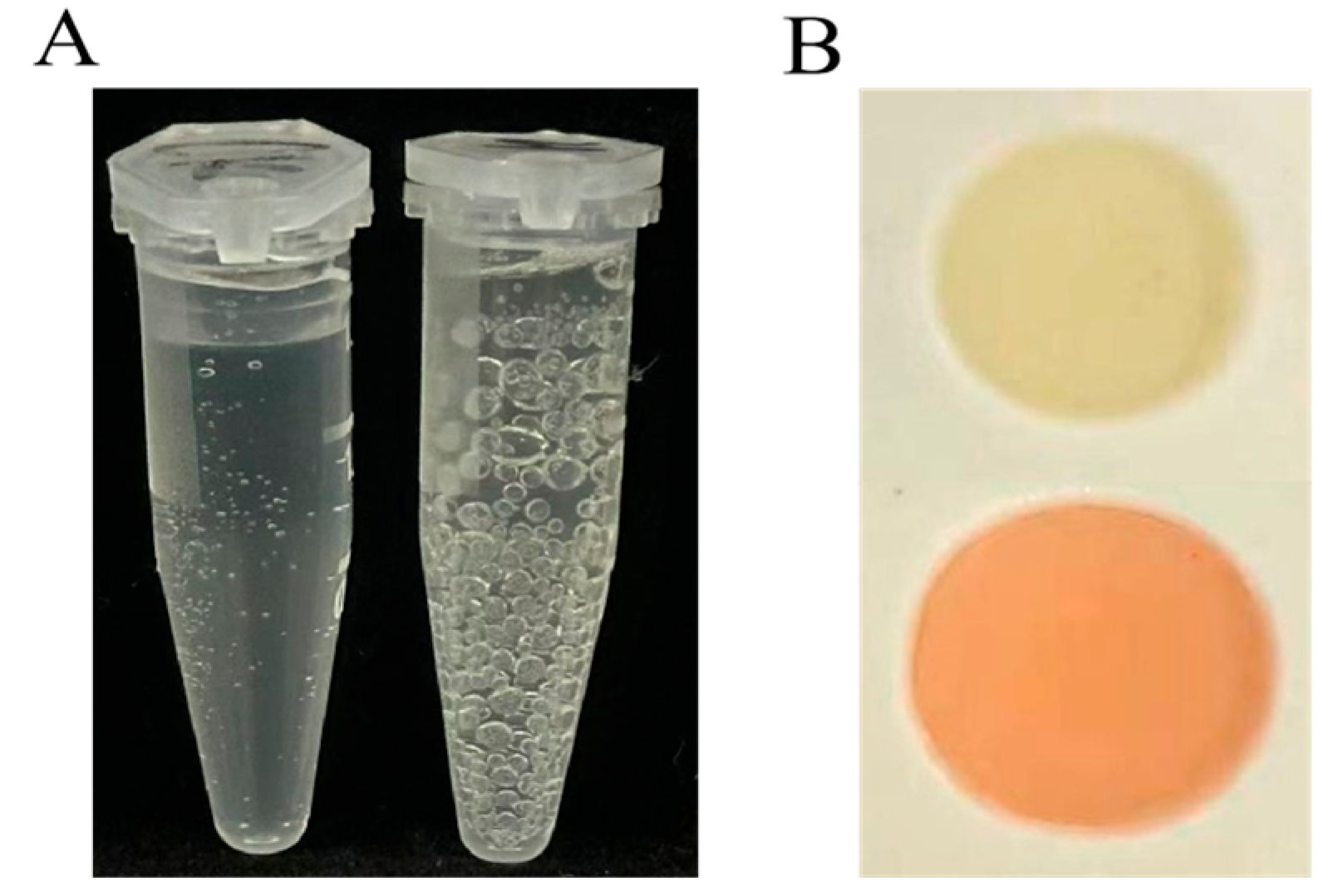
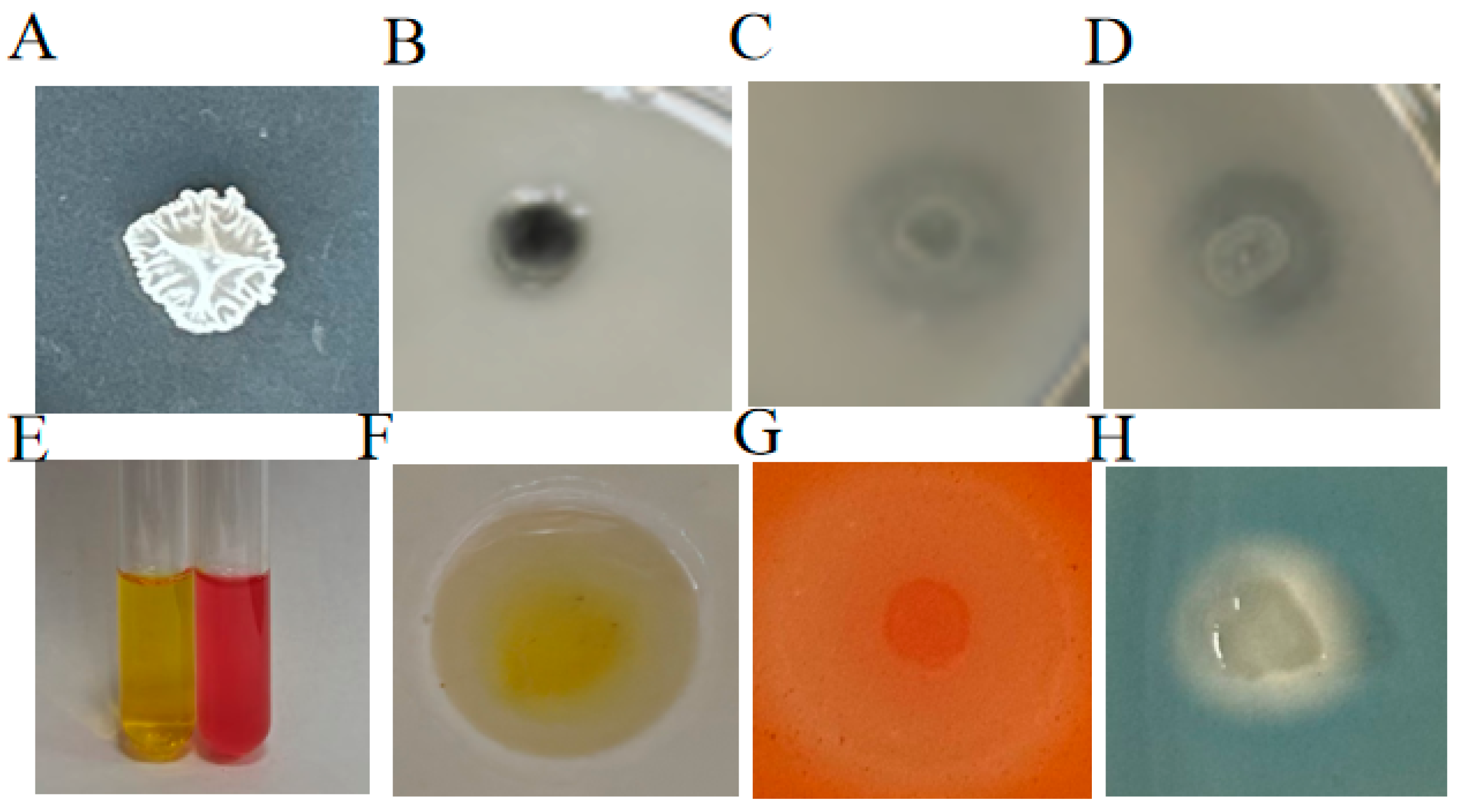
2.1.3. Assay Physiological and Biochemical Identification of ZmBA DSM7
2.1.4. Analysis of the Growth-Promoting Characteristics of ZmBA DSM7
2.1.5. The ZmBA DSM7 Growth-Promoting Traits
Growth of ZmBA DSM7 Under NaHCO3 Stress
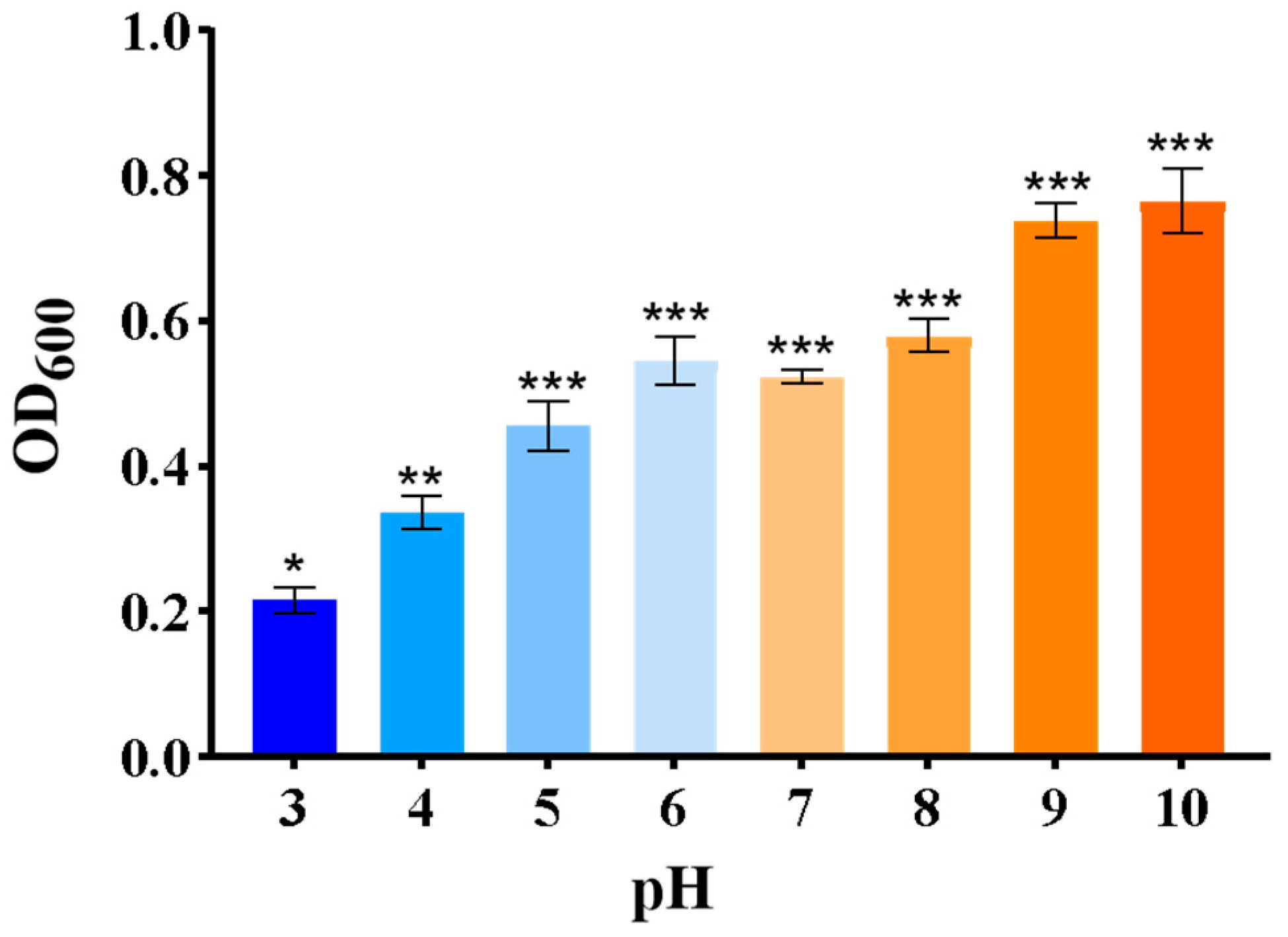
Growth of ZmBA DSM7 Under Different pH Value
2.2. Effect of ZmBA DSM7 on Growth of Maize
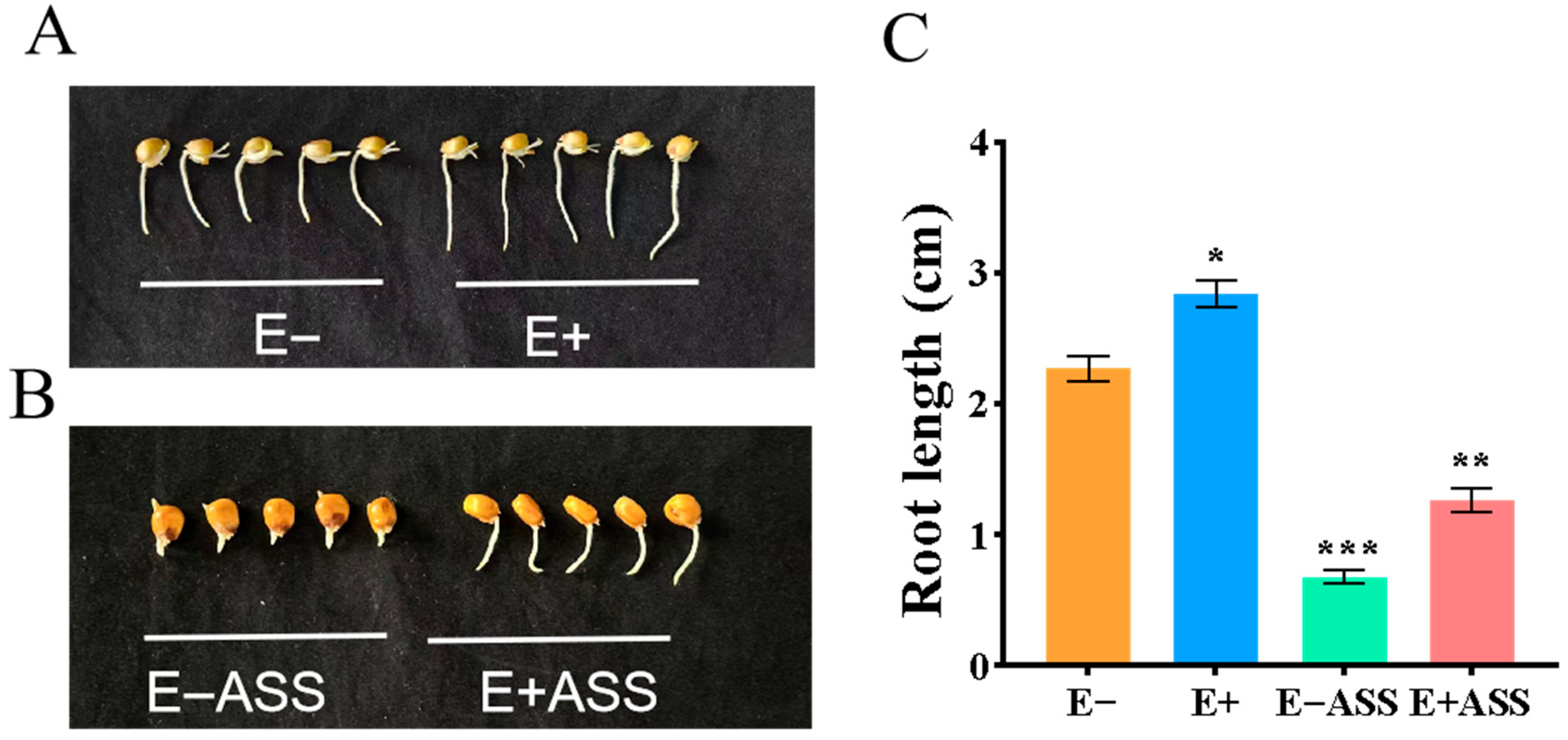
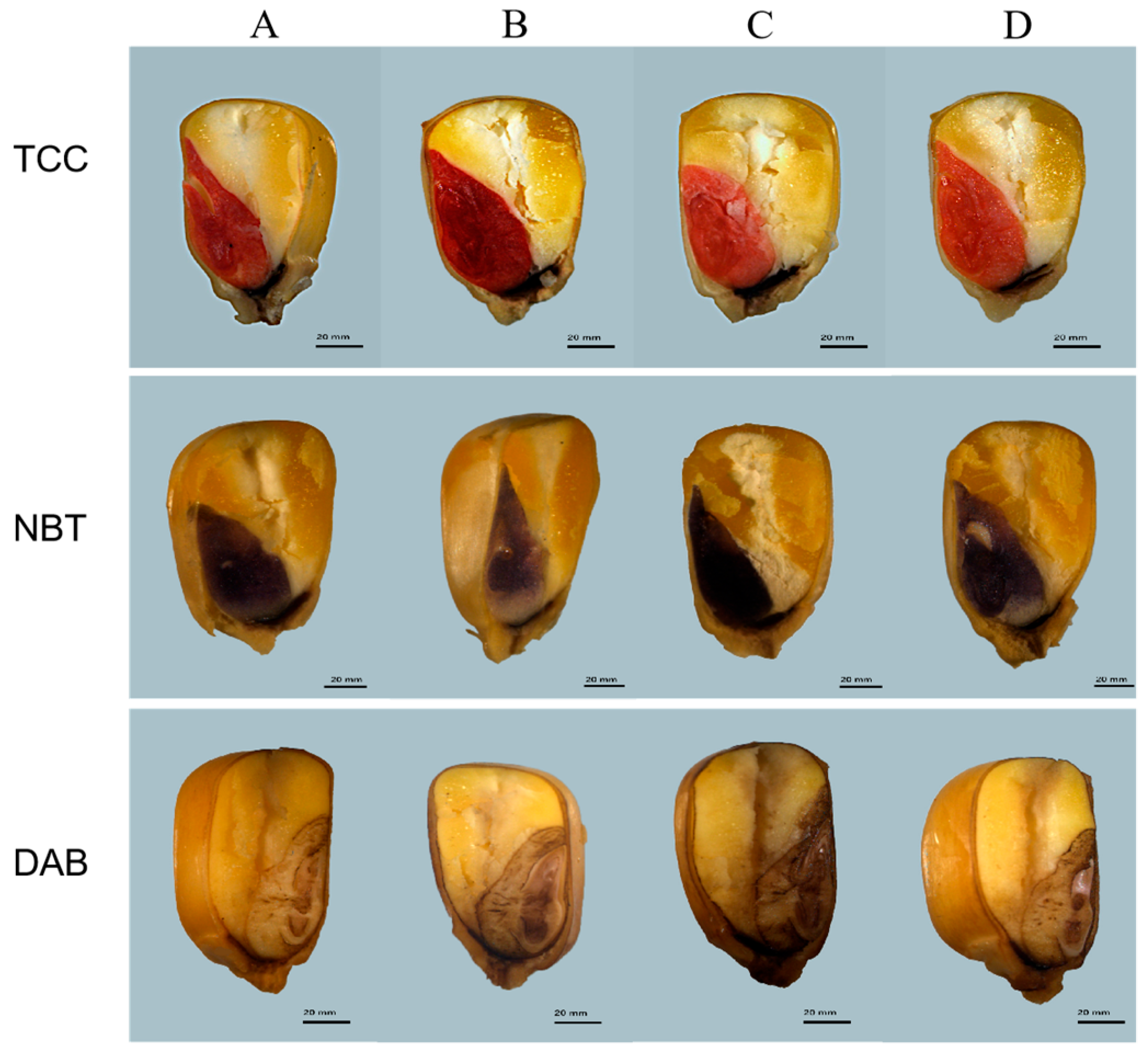
2.2.1. ZmBA DSM7 Effect on Maize Seeds Germination
2.2.2. The Effect of ZmBA DSM7 on the Maize Seedlings in the Pots
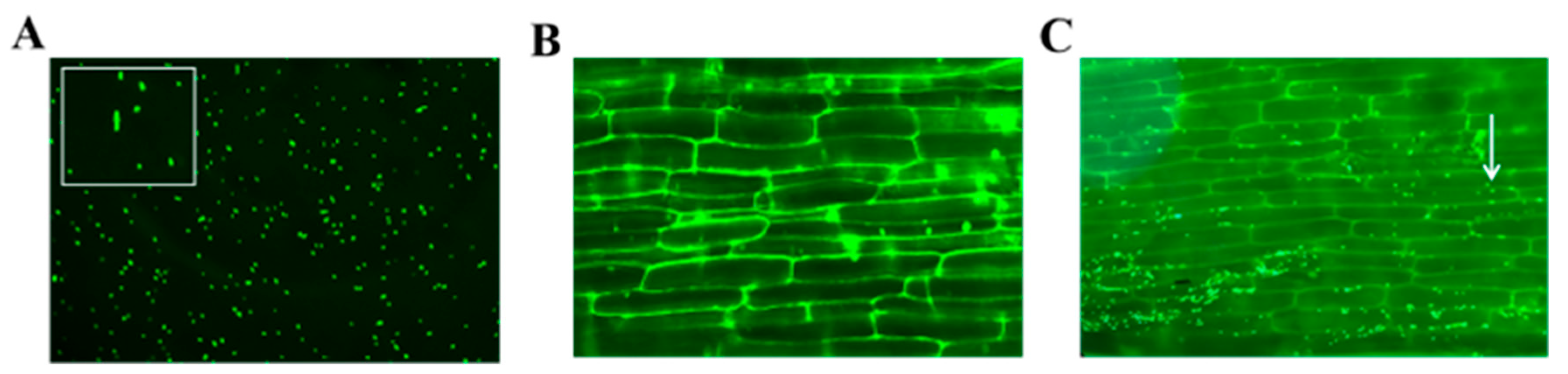
B. amyloliquefaciens Bacterial Strains to Colonize Maize Seedling
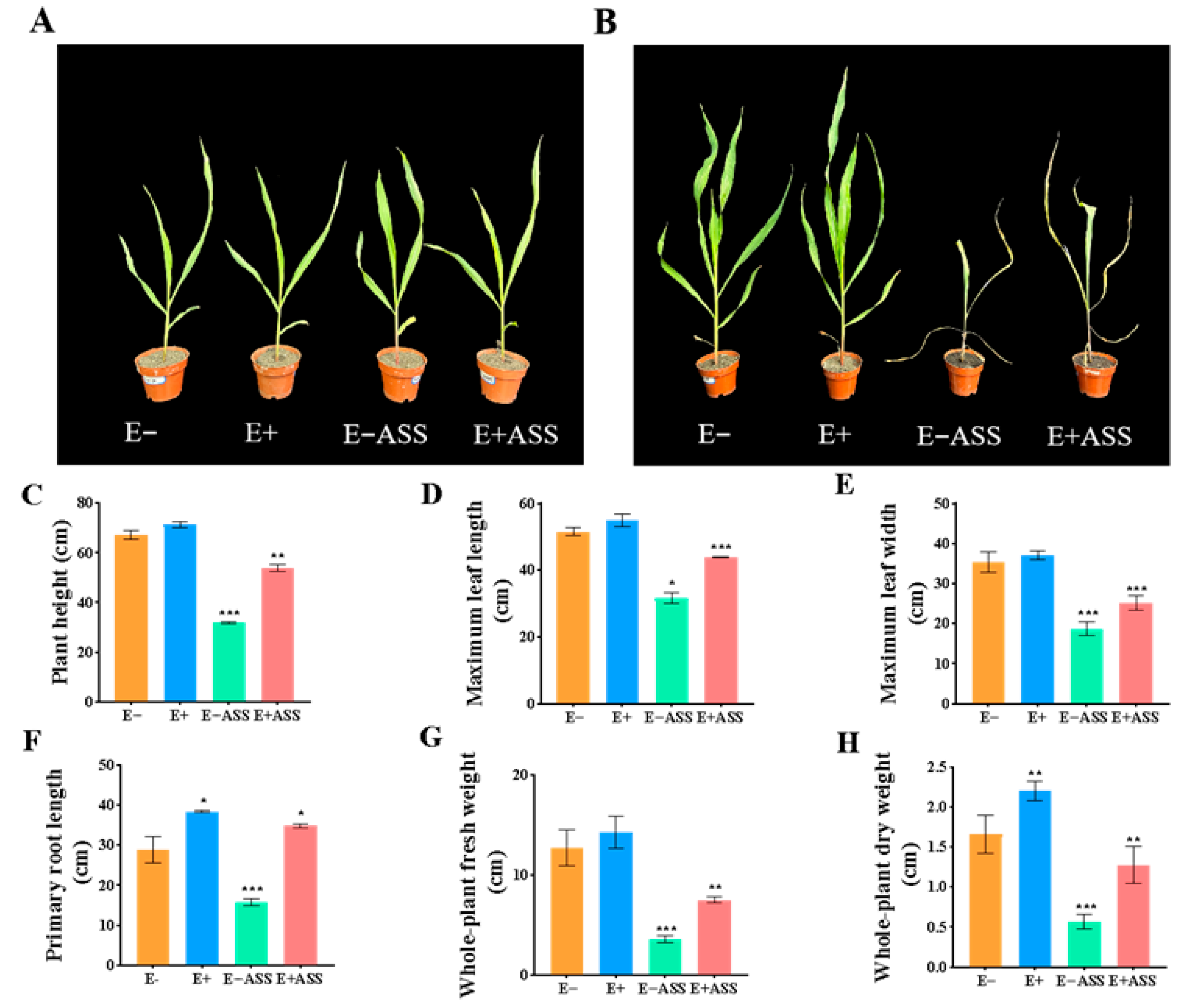
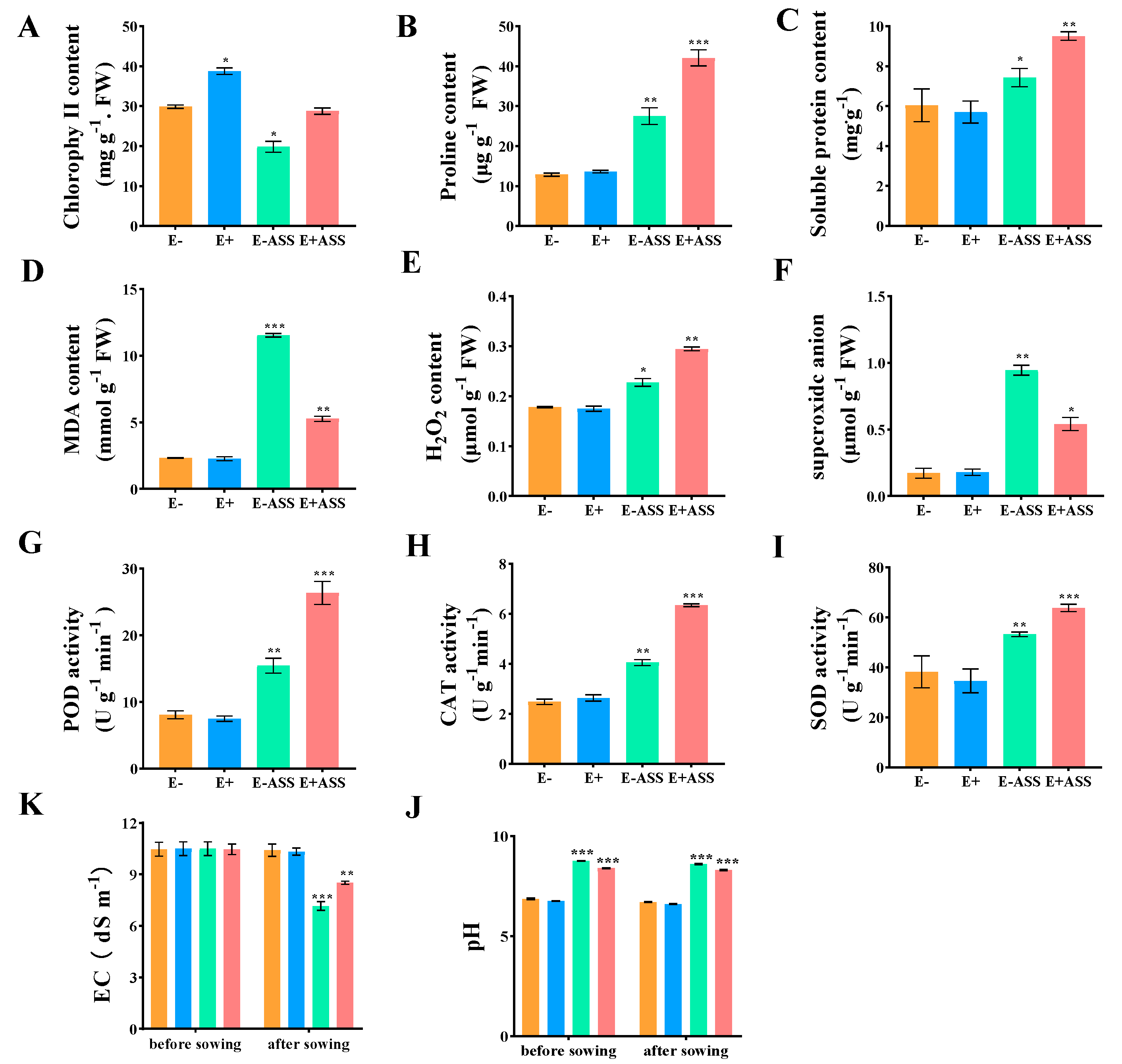
The Effect of ZmBA DSM7 on the Maize Seedling in the Pots
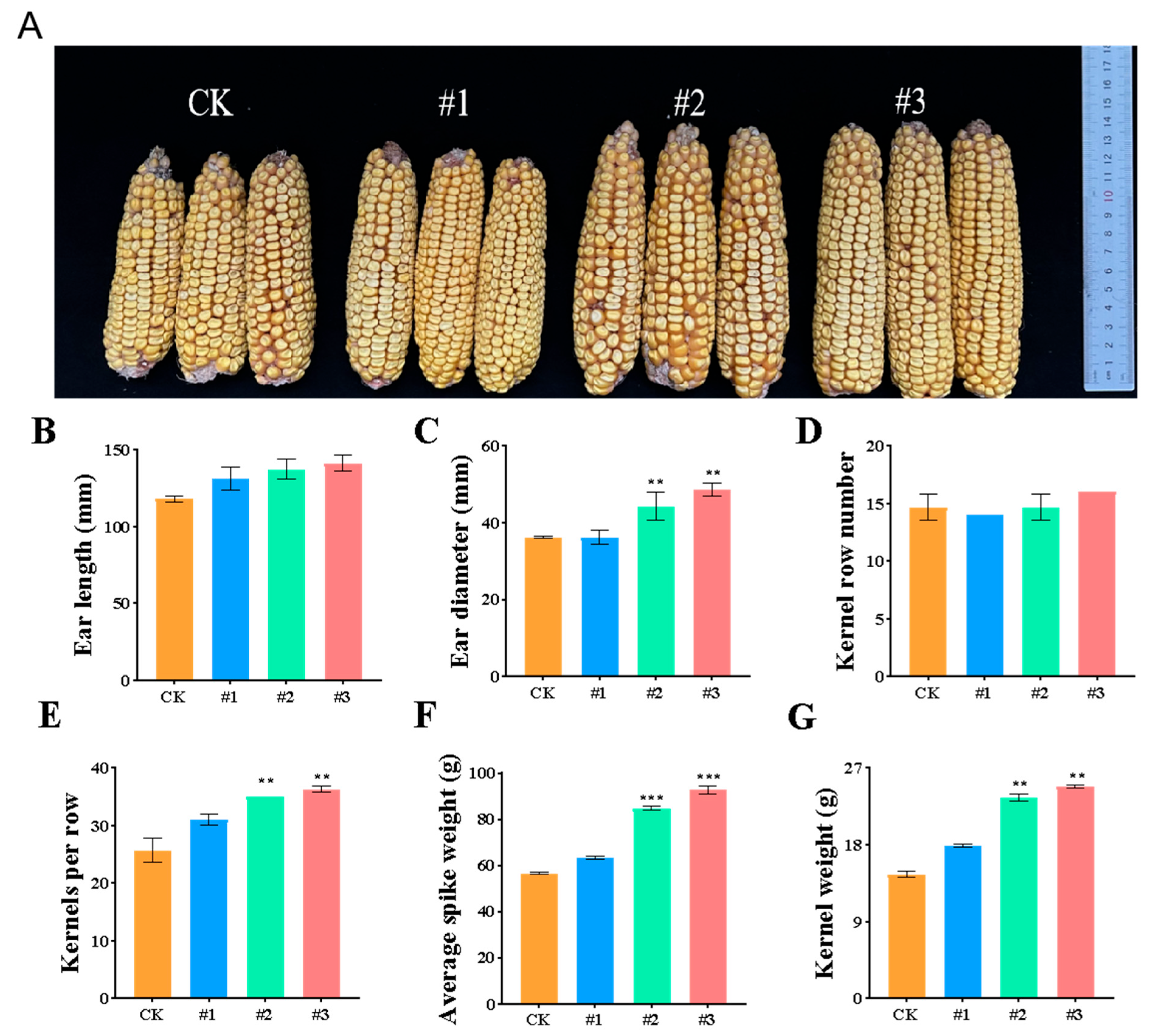
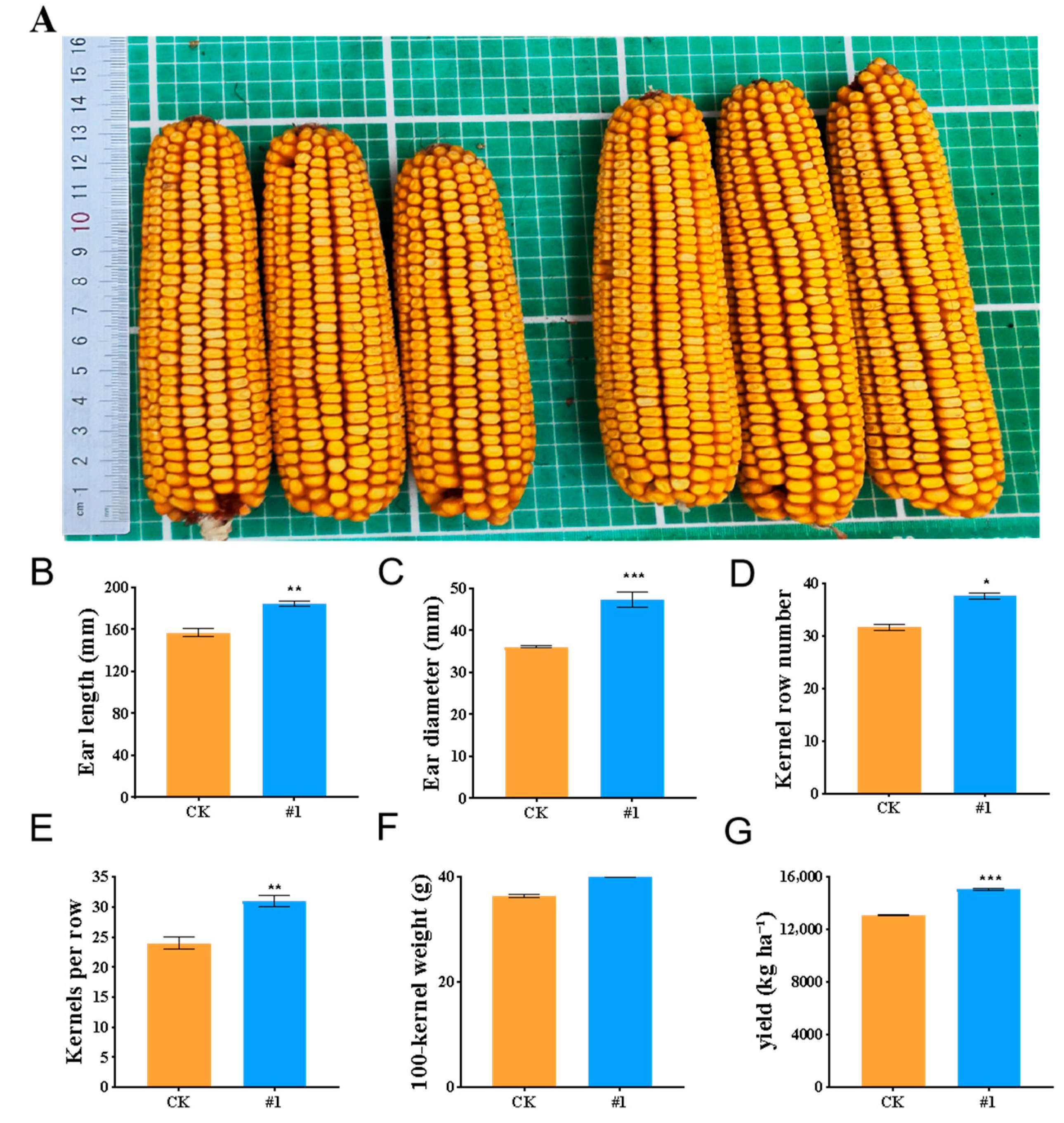
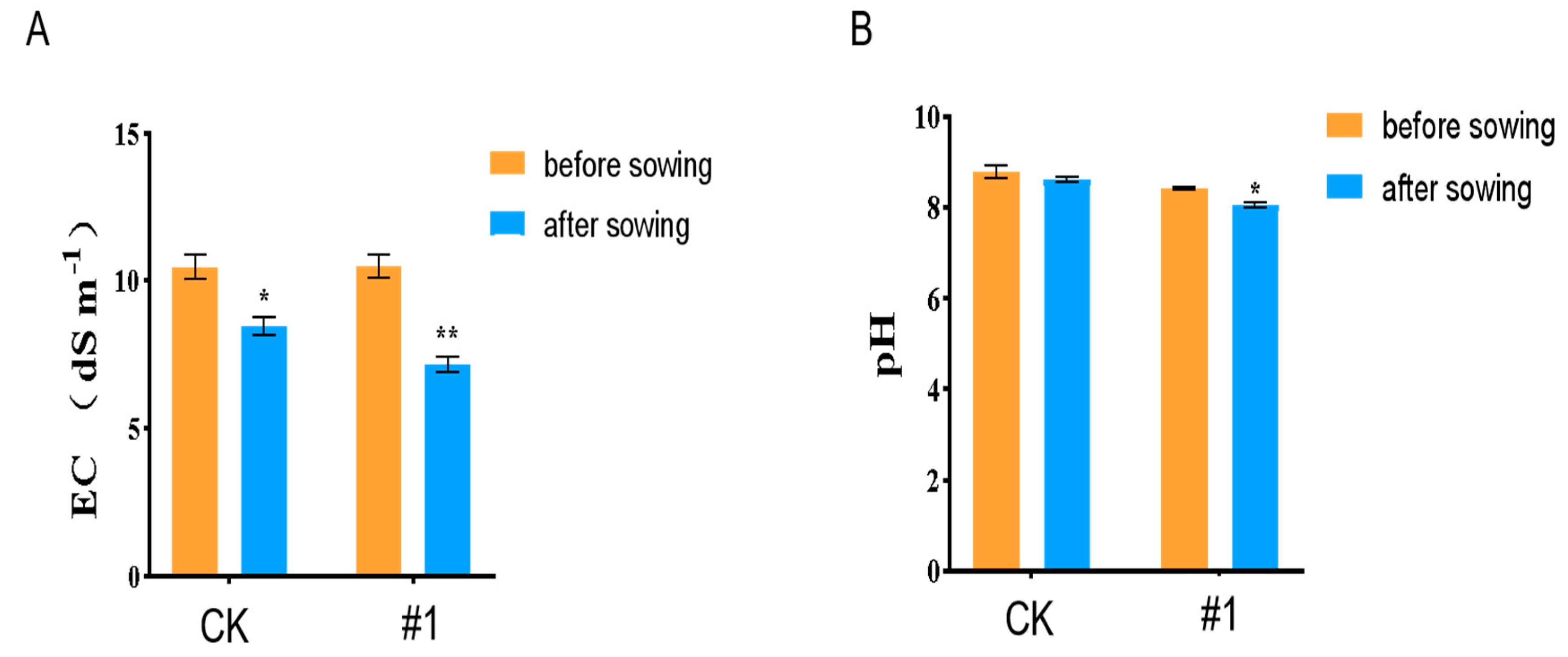
2.2.3. The Effect of ZmBA DSM7 on the Maize Seedling in the Filds
Endophytic Bacteria Improve Maize Salt Tolerance in Field Experiments
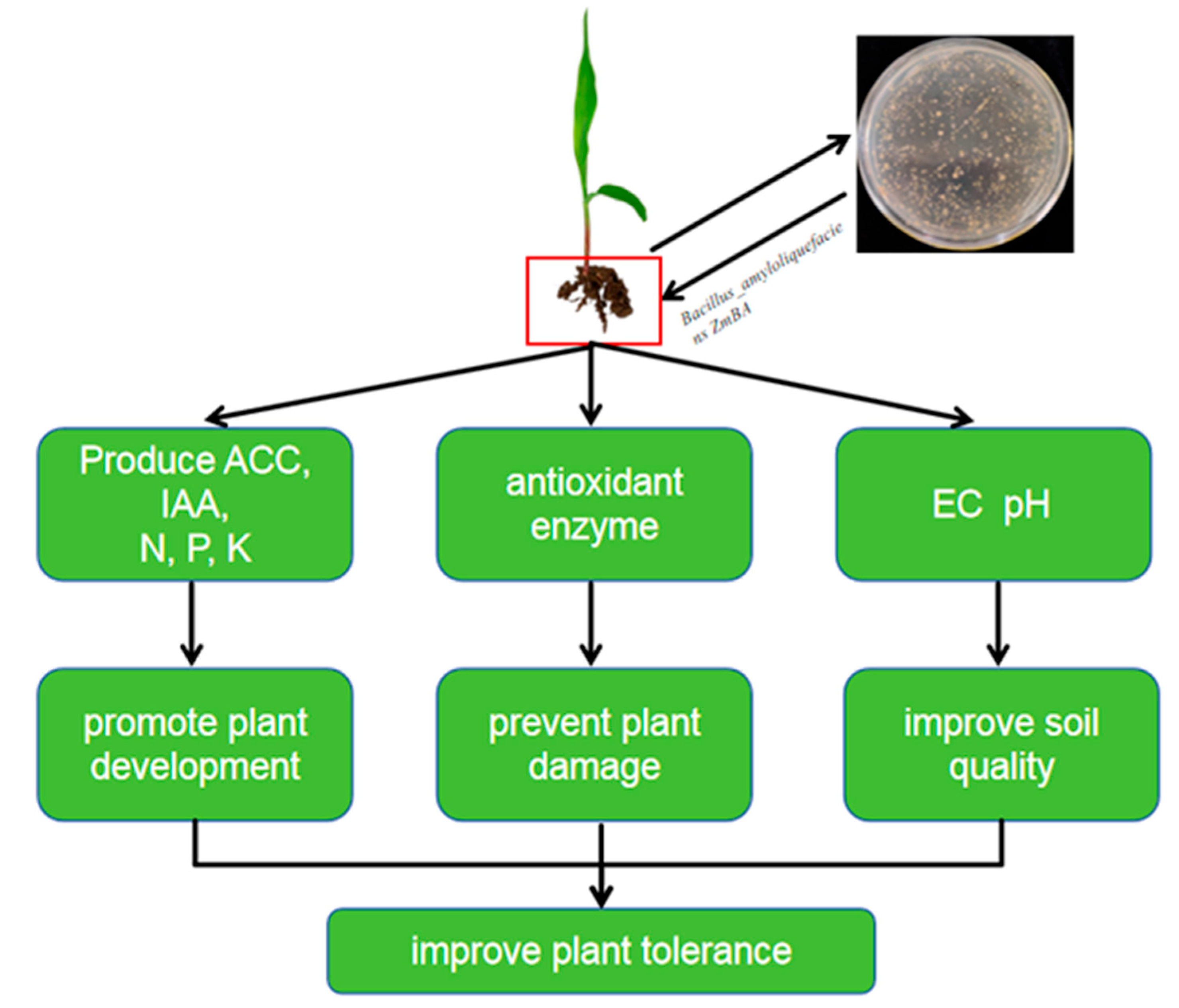
3. Discussion
4. Materials and Methods
4.1. Isolation and Identification of a NaHCO3-Tolerant Bacterium
4.1.1. Isolation of a NaHCO3-Tolerant Bacterium
4.1.2. Identification of a NaHCO3-Tolerant Bacterium
Sequencing Analysis of the 16S rRNA Gene
4.2. The Bacterial Growth-Promoting Traits Under the Normal and Alkaline Stress
4.3. In Vitro Stress-Tolerance Estimation for Bacterium Under Alkaline Stress
4.4. Plant Growth Promotion and Alkaline Stress
4.4.1. ZmBA DSM7 Effect on Maize Seed Germination
4.4.2. ZmBA DSM7 Effect on Maize Seedling
Colonization of ZmBA DSM7 in Plants
ZmBA DSM7 Effect on Maize Seedling
4.4.3. ZmBA DSM7 Effect on Maize Development in the Open Field Tests
PGP of Maize in Open Field Tests
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, D.; Hu, J.; Li, Q.; Huang, Y. Research progress of salt-affected land in Songnen Plain. Chin. J. Ecol. 2025, 44, 1671. [Google Scholar] [CrossRef]
- Liu, L.; Wang, B. Protection of halophytes and their uses for cultivation of saline-alkali soil in China. Biol. Fertil. Soils 2021, 10, 353. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Arias, C.C.; Ligarreto-Moreno, G.A.; Ramírez-Godoy, A.; Restrepo-Díaz, H. Maize responses challenged by drought, elevated daytime temperature and arthropod herbivory stresses: A physiological, biochemical and molecular view. Front. Plant Sci. 2021, 12, 702841. [Google Scholar] [CrossRef]
- Patel, M.; Vurukonda, S.; Patel, A. Multi-trait halotolerant plant growth-promoting bacteria mitigate induced salt stress and enhance growth of Amaranthus viridis. J. Soil Sci. Plant Nutr. 2023, 23, 1860–1883. [Google Scholar] [CrossRef]
- Fajardo-Cavazos, P.; Maughan, H.; Nicholson, W.L. The Bacterial Spore: From Molecules to Systems; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Etesami, H.; Maheshwari, D.K. Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: Action mechanisms and future prospects. Ecotoxicol. Environ. Saf. 2018, 156, 225–246. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, R.; Wu, X.; Xu, T.; Ahmad, S.; Zhang, X.; Zhao, J.; Liu, Y. An endophytic strain of the genus Bacillus isolated from the seeds of maize (Zea mays L.) has antagonistic activity against maize pathogenic strains. Microb. Pathog. 2020, 142, 104074. [Google Scholar] [CrossRef]
- Revilla, P.; Alves, M.L.; Andelković, V.; Balconi, C.; Dinis, I.; Mendes-Moreira, P.; Redaelli, R.; Ruiz de Galarreta, J.I.; Vaz Patto, M.C.; Žilić, S.; et al. Traditional foods from maize (Zea mays L.) in Europe. Front. Nutr. 2022, 8, 683399. [Google Scholar] [CrossRef]
- Notununu, I.; Moleleki, L.; Roopnarain, A.; Adeleke, R. Effects of plant growth-promoting rhizobacteria on the molecular responses of maize under drought and heat stresses: A review. Pedosphere 2022, 32, 90–106. [Google Scholar] [CrossRef]
- Moturu, U.S.; Nunna, T.; Avula, V.G.; Jagarlamudi, V.R.; Gutha, R.R.; Tamminana, S. Investigating the diversity of bacterial endophytes in maize and their plant growth-promoting attributes. Folia Microbiol. 2023, 68, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Peñas-Corte, M.; Bouzas, P.R.; Nieto del Río, J.; Manzanera, M.; Barros-Rodríguez, A.; Fernández-Navarro, J.R. Enhancing maize stress tolerance and productivity through synergistic application of Bacillus velezensis A6 and Lamiales plant extract, biostimulants suitable for organic farming. Biol. Fertil. Soils 2024, 13, 718. [Google Scholar] [CrossRef]
- Manjunath, M.; Khokhar, A.; Chary, G.R.; Singh, M.; Yadav, S.K.; Gopinath, K.A.; Jyothilakshmi, N.; Srinivas, K.; Prabhakar, M.; Singh, V.K. Microbial consortia enhance the yield of maize under sub-humid rainfed production system of India. Saudi J. Biol. Sci. 2023, 7, 1108492. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.; Wang, P.; Zhao, L. Seed endophytic bacterium Lysinibacillus sp. (ZM1) from maize (Zea mays L.) shapes its root architecture through modulation of auxin biosynthesis and nitrogen metabolism. Plant Physiol. Biochem. 2024, 212, 108731. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, Y.; Wang, P.; Zhao, L. Structure and diversity of endophytic bacteria in maize seeds and germinating roots. Microorganisms 2024, 12, 1348. [Google Scholar] [CrossRef]
- Kämpfer, P.; Lipski, A.; Lamothe, L.; Clermont, D.; Criscuolo, A.; McInroy, J.A.; Glaeser, S.P. Paenibacillus plantiphilus sp. nov. from the plant environment of Zea mays. Antonie Leeuwenhoek 2023, 116, 883–892. [Google Scholar] [CrossRef]
- Li, X.; Zhou, C.; Li, M.; Zhang, Q.; Su, L.; Li, X. Paracoccus broussonetiae subsp. drimophilus subsp. nov., a Novel Subspecies Salt-Tolerant Endophytic Bacterium from Maize Root in Hunan. Life 2025, 15, 354. [Google Scholar] [CrossRef]
- Mpanga, I.K.; Ludewig, U.; Dapaah, H.K.; Neumann, G. Acquisition of rock phosphate by combined application of ammonium fertilizers and Bacillus amyloliquefaciens FZB42 in maize as affected by soil pH. J. Appl. Microbiol. 2020, 129, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Birgit, K.; Birgit, H.; Sabine, K.; Alemayehu, W.; Junge, H.; Schmiedeknecht, G.; Rita, G.; Bochow Hand Mária, H. Use of Bacillus subtilis as biocontrol agent. I. Activities and characterization of Bacillus subtilis strains/Anwendung von Bacillus subtilis als Mittel für den biologischen Pflanzenschutz. I. Aktivitäten und Charakterisierung von Bacillus sibtills-Stämmen. J. Plant Dis. 1998, 105, 181–197. Available online: https://www.jstor.org/stable/43215232 (accessed on 2 December 2025).
- Kim, Y.O.; Lee, J.K.; Kim, H.K.; Yu, J.H.; Oh, T.K. Cloning of the thermostable phytase gene (phy) from Bacillus sp. DS11 and its overexpression in Escherichia coli. FEMS Microbiol. Lett. 1998, 162, 185–191. [Google Scholar] [CrossRef]
- Borriss, R. Use of plant-associated Bacillus strains as biofertilizers and biocontrol agents in agriculture. In Bacteria in Agrobiology: Plant Growth Responses; Springer: New York, NY, USA, 2011; pp. 41–76. [Google Scholar]
- Dunlap, C.A.; Kim, S.J.; Kwon, S.W.; Rooney, A.P. Bacillus velezensis is not a later heterotypic synonym of Bacillus amyloliquefaciens. Int. J. Syst. Evol. Microbiol. 2016, 66, 1212–1217. [Google Scholar] [CrossRef]
- Welker, N.E.; Campbell, L.L. Unrelatedness of Bacillus amyloliquefaciens and Bacillus subtilis. J. Bacteriol. 1967, 94, 1124–1130. [Google Scholar] [CrossRef]
- William, B.; Whitman, J.W.S. Bergey’s Manual of Determinative Bacteriology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1994; Volume 3. [Google Scholar]
- Marag, P.S.; Suman, A. Growth stage and tissue specific colonization of endophytic bacteria having plant growth promoting traits in hybrid and composite maize (Zea mays L.). Microbiol. Res. 2018, 214, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Jiao, R.; Munir, S.; He, P.; Yang, H.; Wu, Y.; Wang, J.; He, P.; Cai, Y.; Wang, G.; He, Y. Biocontrol potential of the endophytic Bacillus amyloliquefaciens YN201732 against tobacco powdery mildew and its growth promotion. Biol. Control 2020, 143, 104160. [Google Scholar] [CrossRef]
- Kierul, K.; Voigt, B.; Albrecht, D.; Chen, X.H.; Carvalhais, L.C.; Borriss, R. Influence of root exudates on the extracellular proteome of the plant growth-promoting bacterium Bacillus amyloliquefaciens FZB42. Microbiology 2015, 161, 131–147. [Google Scholar] [CrossRef]
- Liu, S.C.; Yang, J.; Ma, L.; Zhang, C.; He, Y.; Pu, L. Effect of Bacillus amyloliquefaciens B9601–Y2 on growth and yield Promotion of maize. J. Maize Sci. 2010, 18, 85. [Google Scholar] [CrossRef]
- Idriss, E.E.; Makarewicz, O.; Farouk, A.; Rosner, K.; Greiner, R.; Bochow, H.; Richter, T.; Borriss, R. Extracellular phytase activity of Bacillus amyloliquefaciens FZB45 contributes to its plant-growth-promoting effect. Microbiology 2002, 148, 2097–2109. [Google Scholar] [CrossRef]
- Chen, X.H.; Koumoutsi, A.; Scholz, R.; Eisenreich, A.; Schneider, K.; Heinemeyer, I.; Morgenstern, B.; Voss, B.; Hess, W.R.; Reva, O.; et al. Comparative analysis of the complete genome sequence of the plant growth–promoting bacterium Bacillus amyloliquefaciens FZB42. Nat. Biotechnol. 2007, 25, 1007–1014. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y.; Wu, G.; Veronican Njeri, K.; Shen, Q.; Zhang, N.; Zhang, R. Induced maize salt tolerance by rhizosphere inoculation of Bacillus amyloliquefaciens SQR9. Physiol. Plant. 2016, 158, 34–44. [Google Scholar] [CrossRef]
- Mpanga, I.K.; Nkebiwe, P.M.; Kuhlmann, M.; Cozzolino, V.; Piccolo, A.; Geistlinger, J.; Berger, N.; Ludewig, U.; Neumann, G. The form of N supply determines plant growth promotion by P-solubilizing microorganisms in maize. Microorganisms 2019, 7, 38. [Google Scholar] [CrossRef]
- Nkebiwe, P.M.; Neumann, G.; Müller, T. Densely rooted rhizosphere hotspots induced around subsurface NH4+-fertilizer depots: A home for soil PGPMs? Chem. Biol. Technol. Agric. 2017, 4, 29. [Google Scholar] [CrossRef]
- Borriss, R. Towards a new generation of commercial microbial disease control and plant growth promotion products. In Principles of Plant-Microbe Interactions: Microbes for Sustainable Agriculture; Springer: New York, NY, USA, 2014; pp. 329–337. [Google Scholar] [CrossRef]
- Abrar, M.; Zhu, Y.; Li, W.S.; Aqeel, M.; Ashraf, U.; Rehman, M.M.U.; Li, J.M.; Gong, X.F.; Khan, W.; Wang, W.; et al. Stage-dependent synergistic impacts of AM fungi and rhizobacteria on phytohormone mediation and field productivity in dryland maize. Field Crops Res. 2025, 330, 109967. [Google Scholar] [CrossRef]
- Lü, Z.Y.; Shao, D.K.; Zhang, C.Y.; Lai, Z.C.; Li, W.H.; Zhou, R.P.; Liu, Z.Y.; Wang, R.B.; Lin, Z.X.; Lu, G.D.; et al. Isolation and identification of a saline-alkali resistant bacterium and its growth-promoting function on plant. Nat. Sci. Ed. 2023, 52, 41–47. [Google Scholar]
- Bi, Y.; Zhou, B.; Ren, P.; Chen, X.; Zhou, D.; Yao, S.; Fan, D.; Chen, X. Effects of Bacillus subtilis on cotton physiology and growth under water and salt stress. Agric. Water Manag. 2024, 303, 109038. [Google Scholar] [CrossRef]
- Onchwari, R.G. Isolation and characterization of rhizosphere bacteria with potential to improve the plant growth of banana plants in Juja, Kenya. Biocatal. Agric. Biotechnol. 2016, 27, 101685. [Google Scholar] [CrossRef]
- Eswaran, S.U.D.; Sundaram, L.; Perveen, K.; Bukhari, N.A.; Sayyed, R.Z. Osmolyte-producing microbial biostimulants regulate the growth of Arachis hypogaea L. under drought stress. BMC Microbiol. 2024, 24, 165. [Google Scholar] [CrossRef]
- Ming, L.W.; Ma, T.Y.; Xiao, S.S.; Yang, K.J.; Wang, Y.F. Growth-promoting effect of Bacillus on maize seedlings under salt-alkali stress. Chin. J. Ecol. 2022, 41, 2352. [Google Scholar] [CrossRef]
- Zafar-Ul-Hye, M.; Farooq, U.; Danish, S.; Hussain, S.; Shaaban, M.; Qayyum, M.F.; Rehim, A. Bacillus amyloliquefaciens and alcaligenes faecalis with biogas slurry improved maize growth and yield in saline-sodic field. Pak. J. Bot. 2020, 52, 1839–1847. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, Z.; Ling, N.; Yuan, Y.; Zheng, X.; Shen, B.; Shen, Q. Bacillus subtilis SQR 9 can control Fusarium wilt in cucumber by colonizing plant roots. Biol. Fertil. Soils 2011, 47, 495–506. [Google Scholar] [CrossRef]
- Qiu, M.; Zhang, R.; Xue, C.; Zhang, S.; Li, S.; Zhang, N.; Shen, Q. Application of bio-organic fertilizer can control Fusarium wilt of cucumber plants by regulating microbial community of rhizosphere soil. Biol. Fertil. Soils 2012, 48, 807–816. [Google Scholar] [CrossRef]
- White, J.F., Jr.; Torres, M.S.; Sullivan, R.F.; Jabbour, R.E.; Chen, Q.; Tadych, M.; Irizarry, I.; Bergen, M.S.; Havkin-Frenkel, D.; Belanger, F.C. Occurrence of Bacillus amyloliquefaciens as a systemic endophyte of vanilla orchids. Microsc. Res. Tech. 2014, 77, 874–885. [Google Scholar] [CrossRef]
- Fan, B.; Carvalhais, L.C.; Becker, A.; Fedoseyenko, D.; von Wirén, N.; Borriss, R. Transcriptomic profiling of Bacillus amyloliquefaciens FZB42 in response to maize root exudates. BMC Microbiol. 2012, 12, 116. [Google Scholar] [CrossRef]
- Fernando Devasahayam, B.R.; Uthe, H.; Poeschl, Y.; Deising, H.B. Confrontations of the Pathogenic Fungus Colletotrichum graminicola with a Biocontrol Bacterium or a Ubiquitous Fungus Trigger Synthesis of Secondary Metabolites with Lead Structures of Synthetic Fungicides. Environ. Microbiol. 2025, 27, e70145. [Google Scholar] [CrossRef]
- Cui, L.; Yang, C.; Wang, Y.; Ma, T.; Cai, F.; Wei, L.; Jin, M.; Osei, R.; Zhang, J.; Tang, M. Potential of an endophytic bacteria Bacillus amyloliquefaciens 3–5 as biocontrol agent against potato scab. Microb. Pathog. 2022, 163, 105382. [Google Scholar] [CrossRef]
- Lugtenberg, B.J. Signal Molecules in Plants and Plant-Microbe Interactions; Springer: New York, NY, USA, 2014; Volume 36. [Google Scholar]
- Dubois, M.; Van den Broeck, L.; Inzé, D. The pivotal role of ethylene in plant growth. Trends Plant Sci. 2018, 23, 311–323. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Trivellini, A.; Fatma, M.; Masood, A.; Francini, A.; Iqbal, N.; Ferrante, A.; Khan, N.A. Role of ethylene in responses of plants to nitrogen availability. Front. Plant Sci. 2015, 6, 927. [Google Scholar] [CrossRef]
- Chen, X.H.; Koumoutsi, A.; Scholz, R.; Borriss, R. More than anticipated–production of antibiotics and other secondary metabolites by Bacillus amyloliquefaciens FZB42. J. Mol. Microbiol. Biotechnol. 2009, 16, 14–24. [Google Scholar] [CrossRef]
- IIdris, E.E.; Iglesias, D.J.; Talon, M.; Borriss, R. Tryptophan-dependent production of indole-3-acetic acid (IAA) affects level of plant growth promotion by Bacillus amyloliquefaciens FZB42. Mol. Plant-Microbe Interact. 2007, 20, 619–626. [Google Scholar] [CrossRef]
- Fan, B.; Chen, X.H.; Budiharjo, A.; Bleiss, W.; Vater, J.; Borriss, R. Efficient colonization of plant roots by the plant growth promoting bacterium Bacillus amyloliquefaciens FZB42, engineered to express green fluorescent protein. J. Biotechnol. 2011, 151, 303–311. [Google Scholar] [CrossRef]
- Shao, J.; Xu, Z.; Zhang, N.; Shen, Q.; Zhang, R. Contribution of indole-3-acetic acid in the plant growth promotion by the rhizospheric strain Bacillus amyloliquefaciens SQR9. Biol. Fertil. Soils 2015, 51, 321–330. [Google Scholar] [CrossRef]
- Mpanga, I.K.; Dapaah, H.K.; Geistlinger, J.; Ludewig, U.; Neumann, G. Soil type-dependent interactions of P-solubilizing microorganisms with organic and inorganic fertilizers mediate plant growth promotion in tomato. Agronomy 2018, 8, 213. [Google Scholar] [CrossRef]
- Batista, B.D.; Bonatelli, M.L.; Quecine, M.C. Fast screening of bacteria for plant growth promoting traits. In The Plant Microbiome: Methods and Protocols; Springer: New York, NY, USA, 2020; pp. 61–75. [Google Scholar] [CrossRef]
- Jain, R.; Bhardwaj, P.; Pandey, S.S.; Kumar, S. Arnebia euchroma, a plant species of cold desert in the Himalayas, harbors beneficial cultivable endophytes in roots and leaves. Front. Microbiol. 2021, 12, 696667. [Google Scholar] [CrossRef] [PubMed]
- Mohammad Mu’az Hashim, M.M.A.H.; Mohd Khanif Yusop, M.K.Y.; Radziah Othman, R.O.; Samsuri Abd Wahid, S.A.W. Field evaluation of newly-developed controlled release fertilizer on rice production and nitrogen uptake. Sains Malays. 2017, 46, 925–932. [Google Scholar] [CrossRef]
- Vinci, G.; Cozzolino, V.; Mazzei, P.; Monda, H.; Savy, D.; Drosos, M.; Piccolo, A. Effects of Bacillus amyloliquefaciens and different phosphorus sources on Maize plants as revealed by NMR and GC-MS based metabolomics. Plant Soil 2018, 429, 437–450. [Google Scholar] [CrossRef]
- Fan, B.; Borriss, R.; Bleiss, W.; Wu, X. Gram-positive rhizobacterium Bacillus amyloliquefaciens FZB42 colonizes three types of plants in different patterns. J. Microbiol. 2012, 50, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Reva, O.N.; Dixelius, C.; Meijer, J.; Priest, F.G. Taxonomic characterization and plant colonizing abilities of some bacteria related to Bacillus amyloliquefaciens and Bacillus subtilis. FEMS Microbiol. Ecol. 2004, 48, 249–259. [Google Scholar] [CrossRef]
- Peng, J.; Ma, J.; Wei, X.; Zhang, C.; Jia, N.; Wang, X.; Wang, E.T.; Hu, D.; Wang, Z. Accumulation of beneficial bacteria in the rhizosphere of maize (Zea mays L.) grown in a saline soil in responding to a consortium of plant growth promoting rhizobacteria. Ann. Microbiol. 2021, 71, 40. [Google Scholar] [CrossRef]
- Liu, X.W.H. Effect of Bacillus amyloliquefaciens YM6 on Growth Promotion of Maize Under Salt Stress. aBIOTECH 2019, 35, 45–49. [Google Scholar]
- Wang, Q.L.; Zhang, Z.D.; Wang, J.; Deng, B.; Zhou, L.; Wei, L. Effects of plant growth-promoting bacterial on the growth of maize and the IAA secrete ability detection. J. Yunnan Agric. Univ. 2015, 30, 494–498. [Google Scholar]
- Li, G.; Shi, M.; Wan, W.; Wang, Z.; Ji, S.; Yang, F.; Jin, S.; Zhang, J. Maize endophytic plant growth-promoting bacteria Peribacillus simplex can alleviate plant saline and alkaline stress. Int. J. Mol. Sci. 2024, 25, 10870. [Google Scholar] [CrossRef]
- AlAli, H.A.; Khalifa, A.; Almalki, M. Plant growth-promoting rhizobacteria from Ocimum basilicum improve growth of Phaseolus vulgaris and Abelmoschus esculentus. S. Afr. J. Bot. 2021, 139, 200–209. [Google Scholar] [CrossRef]
- Mehta, S.; Nautiyal, C.S. An efficient method for qualitative screening of phosphate-solubilizing bacteria. Curr. Microbiol. 2001, 43, 51–56. [Google Scholar] [CrossRef]
- Bric, J.M.; Bostock, R.M.; Silverstone, S.E. Rapid in situ assay for indoleacetic acid production by bacteria immobilized on a nitrocellulose membrane. Appl. Environ. Microbiol. 1991, 57, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Yeole, R.D.; Dave, B.P.; Dube, H.C. Siderophore production by fluorescent pseudomonads colonizing roots of certain crop plants. Indian J. Exp. Biol. 2001, 39, 464–468. [Google Scholar] [PubMed]
- Guo, L.; Zhang, X.; Zhao, J.; Zhang, A.; Pang, Q. Enhancement of sulfur metabolism and antioxidant machinery confers Bacillus sp. Jrh14-10–induced alkaline stress tolerance in plant. Plant Physiol. Biochem. 2023, 203, 108063. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Wan, W.; Shi, M.; Cui, H.; Yang, F.; Yang, W.; Jin, S. A NaHCO3-Tolerant Endophyte Bacillus amyloliquefaciens ZmBA DSM7 Enhances Growth and Mitigates NaHCO3-Induced Alkaline Stress in Maize Through Multiple Mechanism. Plants 2025, 14, 3742. https://doi.org/10.3390/plants14243742
Li G, Wan W, Shi M, Cui H, Yang F, Yang W, Jin S. A NaHCO3-Tolerant Endophyte Bacillus amyloliquefaciens ZmBA DSM7 Enhances Growth and Mitigates NaHCO3-Induced Alkaline Stress in Maize Through Multiple Mechanism. Plants. 2025; 14(24):3742. https://doi.org/10.3390/plants14243742
Chicago/Turabian StyleLi, Guoliang, Wenhao Wan, Miaoxin Shi, Huitao Cui, Fengshan Yang, Wei Yang, and Shumei Jin. 2025. "A NaHCO3-Tolerant Endophyte Bacillus amyloliquefaciens ZmBA DSM7 Enhances Growth and Mitigates NaHCO3-Induced Alkaline Stress in Maize Through Multiple Mechanism" Plants 14, no. 24: 3742. https://doi.org/10.3390/plants14243742
APA StyleLi, G., Wan, W., Shi, M., Cui, H., Yang, F., Yang, W., & Jin, S. (2025). A NaHCO3-Tolerant Endophyte Bacillus amyloliquefaciens ZmBA DSM7 Enhances Growth and Mitigates NaHCO3-Induced Alkaline Stress in Maize Through Multiple Mechanism. Plants, 14(24), 3742. https://doi.org/10.3390/plants14243742




