Doubled Haploid Production in Cucurbita pepo L. Through Ovary Culture
Abstract
1. Introduction
2. Results
2.1. Developmental Stages of Ovary-Derived Explants
2.2. Initial Screening of Culture Media
2.2.1. General Overview of Species and Floral Stage Response
2.2.2. Comparison of Morphological Responses Across Culture Medium
2.2.3. Embryo Production Across Medium
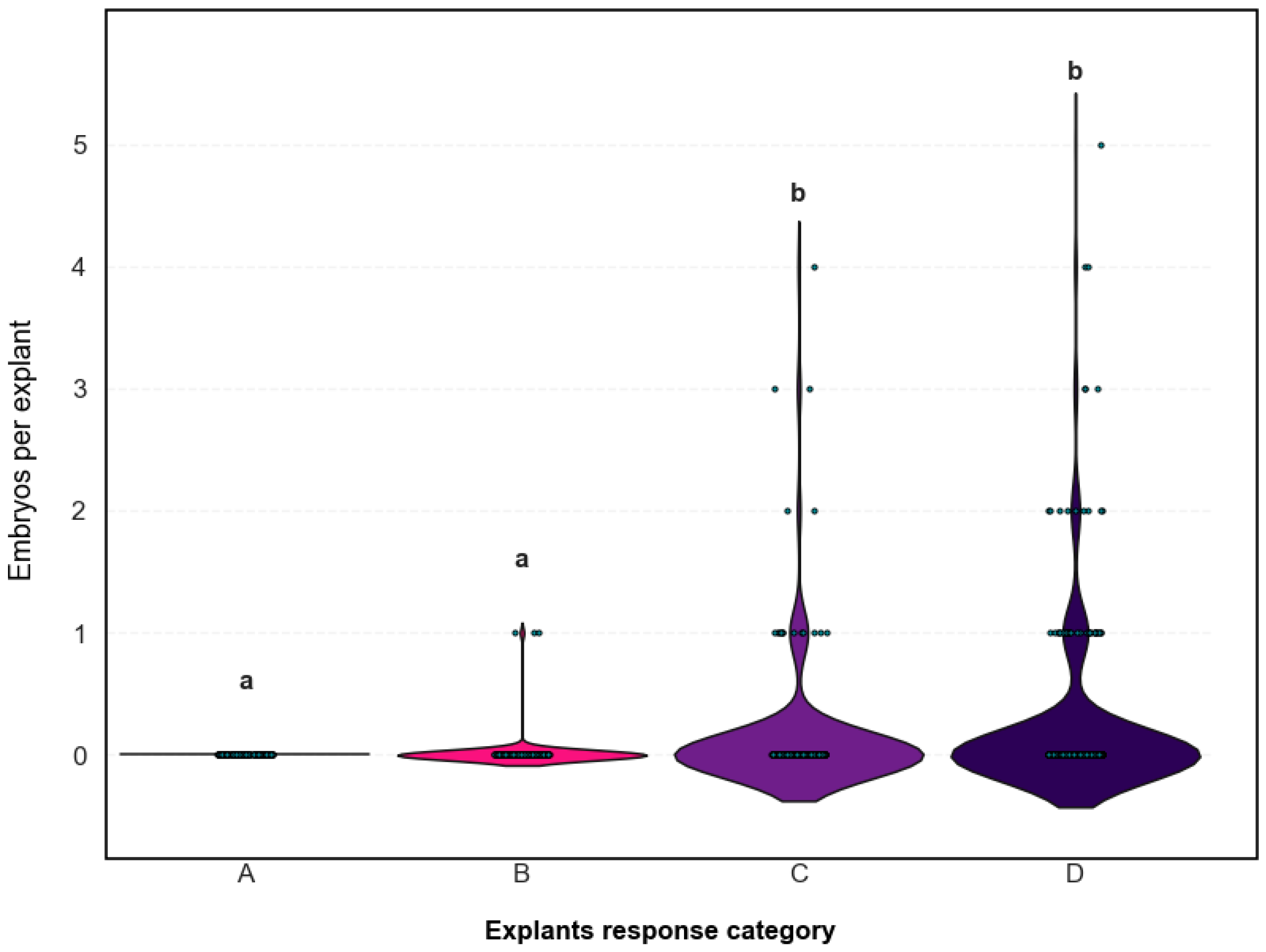
2.2.4. Association Between Morphological Response and Embryo Yield
2.3. Comparative Analysis of Zeatin Riboside-Based Media for Ovule Induction and Embryogenic Response
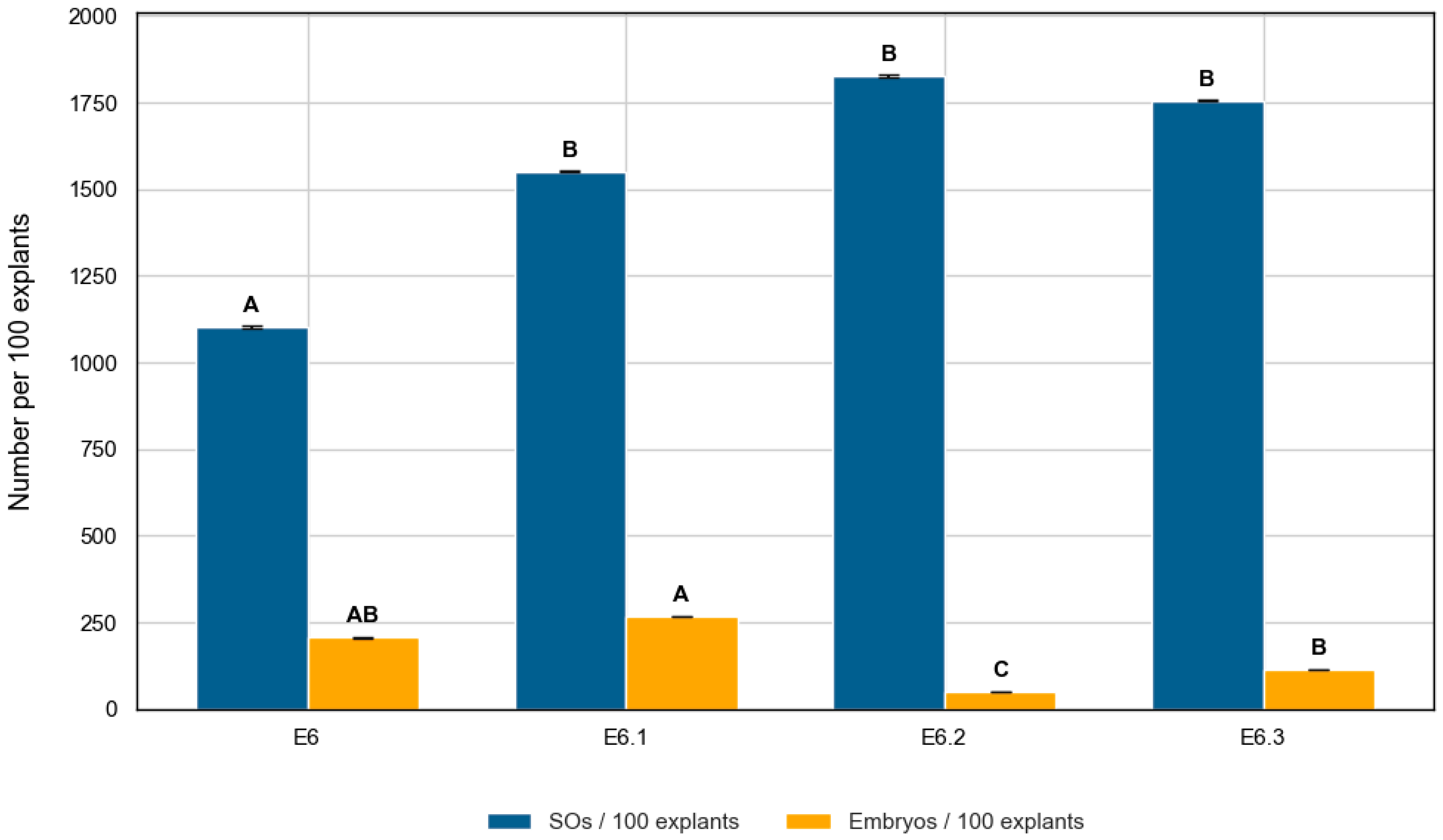
2.3.1. Induction of Swollen Ovules (SOs) in Zeatin Riboside-Based Media
2.3.2. Embryo Production in ZR-Based Media
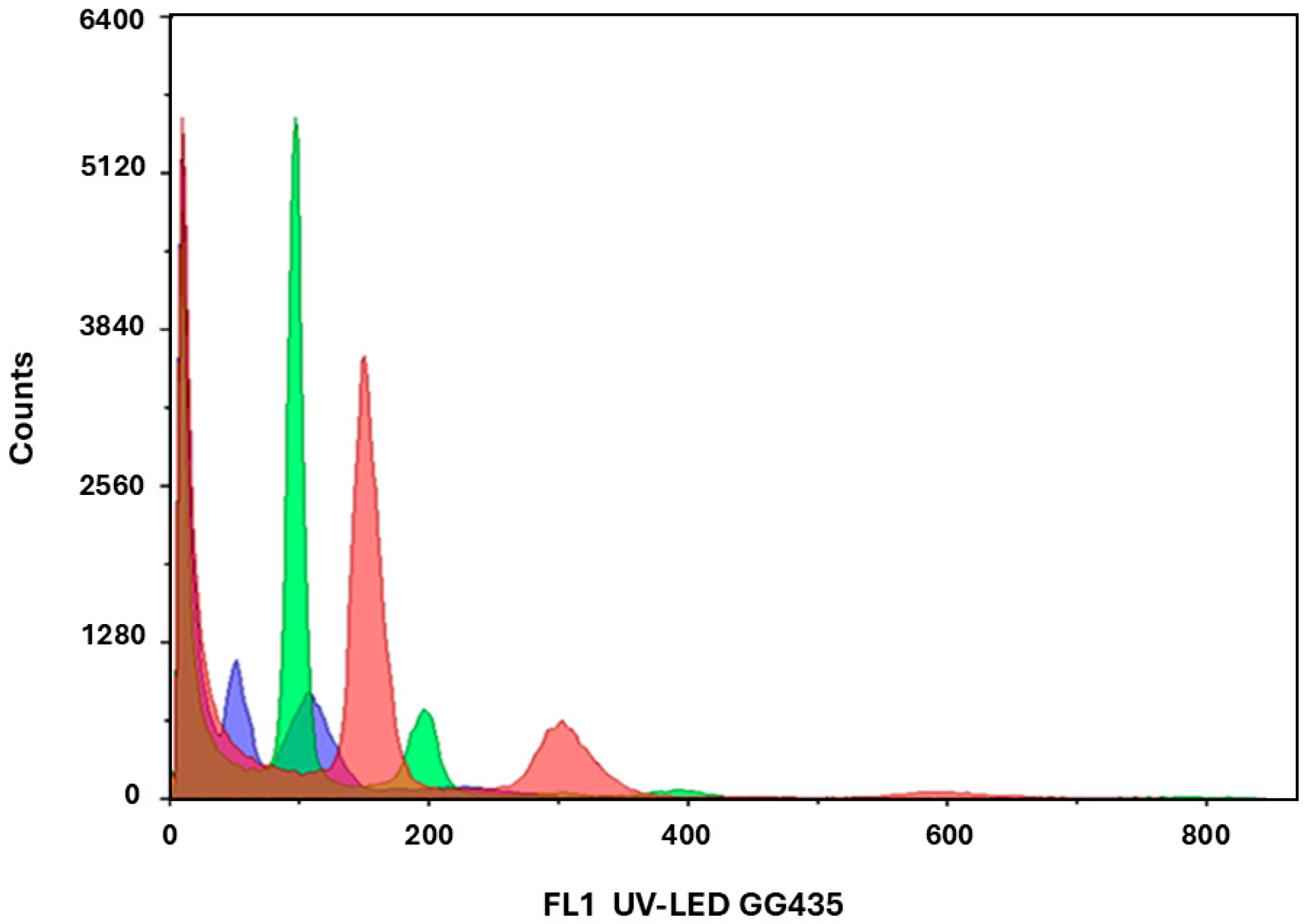
2.4. Determination of Ploidy Level and Acclimatization
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Ovary Collection and In Vitro Culture Conditions
4.3. Culture Medium and Hormonal Treatments
4.4. Experimental Design and Data Collection
4.5. Statistical Analysis
4.6. Acclimatization and Greenhouse Transfer
4.7. Flow Cytometry for Ploidy Verification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 1 DBA | One day before anthesis |
| 2,4-D | 2,4-Dichlorophenoxyacetic acid |
| BAP | 6-Benzylaminopurine |
| DH | Doubled haploid |
| ELS | Embryos-Like Structure |
| IAA | Indole-3-acetic acid |
| KT | Kinetin |
| NAA | Naphthaleneacetic acid |
| SOs | Swollen ovules |
| PCM | Picloram |
| TDZ | Thidiazuron |
| Z | Zeatin |
| ZR | Zeatin riboside |
Appendix A
| Medium | Response|Embryos | Total Explants | Total Embryos | |||
|---|---|---|---|---|---|---|
| A | B | C | D | |||
| E6 | 12|0 | 20|0 | 33|30 | 27|39 | 92 | 69 |
| E6.1 | 0|0 | 16|0 | 48|25 | 46|77 | 110 | 102 |
| E6.2 | 8|0 | 7|0 | 23|1 | 82|14 | 120 | 15 |
| E6.3 | 3|0 | 17|0 | 42|15 | 41|22 | 103 | 37 |
| Total | 23|0 | 60|0 | 146|71 | 196|152 | 425 | 223 |
References
- Food and Agriculture Organization of the United Nations. Crops and Livestock Products: Pumpkins, Squashes and Gourds. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 24 October 2025).
- Ministerio de Agricultura, Pesca y Alimentación (MAPA). Informe de Campaña 2024/2025 de Hortalizas. Available online: https://www.mapa.gob.es/dam/mapa/contenido/agricultura/temas/producciones-agricolas/frutas-y-hortalizas/frutas-y-hortalizas/informacion-subsectorial/hortalizas/informe-campana-2024-25-hortalizas--sep24-may25-.pdf (accessed on 24 October 2025).
- Evans, D.E.; Coleman, J.O.D.; Kearns, A. Plant Cell Culture; Illustrated; Garland Science: New York, NY, USA, 2003; ISBN 9781859963203. [Google Scholar]
- Forster, B.P.; Heberle-Bors, E.; Kasha, K.J.; Touraev, A. The Resurgence of Haploids in Higher Plants. Trends Plant Sci. 2007, 12, 368–375. [Google Scholar] [CrossRef]
- Germanà, M.A. Anther Culture for Haploid and Doubled Haploid Production. Plant Cell Tissue Organ Cult. 2011, 104, 283–300. [Google Scholar] [CrossRef]
- Gałązka, J.; Niemirowicz-Szczytt, K. Review of Research on Haploid Production in Cucumber and Other Cucurbits. Folia Hortic. 2013, 25, 67–78. [Google Scholar] [CrossRef]
- Dong, Y.Q.; Zhao, W.X.; Li, X.H.; Liu, X.C.; Gao, N.N.; Huang, J.H.; Wang, W.Y.; Xu, X.L.; Tang, Z.H. Androgenesis, Gynogenesis, and Parthenogenesis Haploids in Cucurbit Species. Plant Cell Rep. 2016, 35, 1991–2019. [Google Scholar] [CrossRef]
- Ficcadenti, N.; Sestili, S.; Annibali, S.; Di Marco, M.; Schiavi, M. In Vitro Gynogenesis to Induce Haploid Plants in Melon Cucumis melo L. J. Genet. Breed. 1999, 53, 255–257. [Google Scholar]
- Suprunova, T.; Shmykova, N. In Vitro Induction of Haploid Plants in Unpollinated Ovules, Anther and Microspore Culture of Cucumis Sativus. In Proceedings of the IXth EUCARPIA Meeting on Genetics and Breeding of Cucurbitaceae, Avignon, France, 21–24 May 2008; Pitrat, M., Ed.; INRA: Paris, France, 2008; pp. 371–374. [Google Scholar]
- Diao, W.P.; Jia, Y.Y.; Song, H.; Zhang, X.Q.; Lou, Q.F.; Chen, J.F. Efficient Embryo Induction in Cucumber Ovary Culture and Homozygous Identification of the Regenetants Using SSR Markers. Sci. Hortic. 2009, 119, 246–251. [Google Scholar] [CrossRef]
- Kurtar, E.S.; SarI, N.; Abak, K. Obtention of Haploid Embryos and Plants through Irradiated Pollen Technique in Squash (Cucurbita pepo L.). Euphytica 2002, 127, 335–344. [Google Scholar] [CrossRef]
- Metwally, E.I.; Moustafa, S.A.; El-Sawry, B.I.; Shalaby, T.A. Haploid Plantlets Derived by Anther Culture of Cucurbita pepo. Plant Cell Tissue Organ Cult. 1998, 52, 171–176. [Google Scholar] [CrossRef]
- Shalaby, T.A. Embryogenesis and Plantlets Regeneration from Anther Culture of Squash Plants (Cucurbita pepo L.) as Affected by Different Genotypes. J. Agric. Res. Tanta Univ. 2006, 32, 173–183. [Google Scholar]
- Chambonnet, D.; Dumas de Vaulx, R. Obtention of Embryos and Plants from In Vitro Culture of Unfertilized Ovules of Cucurbita pepo. In Proceedings of the Genetic Manipulation in Plant Breeding: Proceedings International Symposium Organized by EUCARPIA, Berlin, Germany, 8–13 September 1985; Horn, W., Jensen, C.J., Odenbach, W., Schieder, O., Eds.; De Gruyter: Berlin, Germany, 1985; pp. 8–66. [Google Scholar]
- Metwally, E.I.; Moustafa, S.A.; El-Sawy, B.I.; Haroun, S.A.; Shalaby, T.A. Production of Haploid Plants from In Vitro Culture of Unpollinated Ovules of Cucurbita pepo. Plant Cell Tissue Organ Cult. 1998, 52, 117–121. [Google Scholar] [CrossRef]
- Shalaby, T.A. Factors Affecting Haploid Induction through In Vitro Gynogenesis in Summer Squash (Cucurbita pepo L.). Sci. Hortic. 2007, 115, 1–6. [Google Scholar] [CrossRef]
- Claveria, E.; Garcia-Mas, J.; Dolcet-Sanjuan, R. Optimization of Cucumber Doubled Haploid Line Production Using In Vitro Rescue of In Vivo Induced Parthenogenic Embryos. J. Am. Soc. Hortic. Sci. 2005, 130, 555–560. [Google Scholar] [CrossRef]
- Dolcet-Sanjuan, R.; Claveria, E.; Garcia-Mas, J. Cucumber (Cucumis sativus L.) Dihaploid Line Production Using In Vitro Rescue of In Vivo Induced Parthenogenic Embryos. Acta Hortic. 2006, 725, 837–843. [Google Scholar] [CrossRef]
- Gonzalo, M.J.; Claveria, E.; Monforte, A.J.; Dolcet-Sanjuan, R. Parthenogenic Haploids in Melon: Generation and Molecular Characterization of a Doubled Haploid Line Population. J. Am. Soc. Hortic. Sci. 2011, 136, 145–154. [Google Scholar] [CrossRef]
- Gémesné-Juhász, A.; Venczel, G.; Balogh, P. Haploid Plant Induction in Zucchini (Cucurbita pepo L. Convar. Giromontiina DUCH) and in Cucumber (Cucumis sativus L.) Lines through In Vitro Gynogenesis. In Proceedings of the III International Symposium on In Vitro Culture and Horticultural Breeding, Acta Horticulturae 447; International Society for Horticultural Science: Korbeek-Lo, Belgium, 1997; Volume 447, pp. 623–624. [Google Scholar]
- Domblides, E.A.; Ermolaev, A.S.; Belov, S.N. Obtaining Doubled Haploids of Cucurbita pepo L. Veg. Crops Russ. 2021, 4, 11–26. [Google Scholar] [CrossRef]
- Min, Z.; Li, H.; Zou, T.; Tong, L.; Cheng, J.; Sun, X. Studies of in vitro Culture and Plant Regeneration of Unfertilized Ovary of Pumpkin. Chin. Bull. Bot. 2016, 51, 74–80. [Google Scholar] [CrossRef]
- Zou, T.; Song, H.; Chu, X.; Tong, L.; Liang, S.; Gong, S.; Yang, H.; Sun, X. Efficient Induction of Gynogenesis through Unfertilized Ovary Culture with Winter Squash (Cucurbita maxima Duch.) and Pumpkin (Cucurbita moschata Duch.). Sci. Hortic. 2020, 264, 109152. [Google Scholar] [CrossRef]
- Min, Z.; Wu, Q.; Li, J.; Chen, S.; Li, Y.; Han, X.; Zou, T.; Hu, X. Optimization and Application of Induction Technology of Double Haploid in Pumpkin (Cucurbita moschata D.). Vitr. Cell. Dev. Biol. Plant 2025, 61, 67–74. [Google Scholar] [CrossRef]
- Musial, K.; Bohanec, B.; Jakše, M.; Przywara, L. The Development of Onion (Allium cepa L.) Embryo Sacs in vitro and Gynogenesis Induction in Relation to Flower Size. Vitr. Cell. Dev. Biol. Plant 2005, 41, 446–452. [Google Scholar] [CrossRef]
- Bohanec, B. Doubled Haploids via Gynogenesis. In Advances in Haploid Production in Higher Plants; Alisher Touraev, B.P., Forster, S., Jain, M., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 35–46. ISBN 978-1-4020-8853-7. [Google Scholar]
- Malik, A.A.; Cui, L.; Zhang, S.; Chen, J.-F. Efficiency of SSR Markers for Determining the Origin of Melon Plantlets Derived through Unfertilized Ovary Culture. Hort. Sci. 2011, 38, 27–34. [Google Scholar] [CrossRef]
- Grewal, R.K.; Lulsdorf, M.; Croser, J.; Ochatt, S.; Vandenberg, A.; Warkentin, T.D. Doubled-Haploid Production in Chickpea (Cicer arietinum L.): Role of Stress Treatments. Plant Cell Rep. 2009, 28, 1289–1299. [Google Scholar] [CrossRef]
- Niazian, M.; Shariatpanahi, M.E. In vitro-Based Doubled Haploid Production: Recent Improvements. Euphytica 2020, 216, 69. [Google Scholar] [CrossRef]
- Benega Garcia, R.; Cisneros, A.; Schneider, B.; Tel-Zur, N. Gynogenesis in the Vine Cacti Hylocereus and Selenicereus (Cactaceae). Plant Cell Rep. 2009, 28, 719–726. [Google Scholar] [CrossRef]
- Touraev, A.; Forster, B.P.; Jain, S.M. Advances in Haploid Production in Higher Plants, 1st ed.; Springer: Dordrecht, The Netherlands; Berlin/Heidelberg, Germany, 2009; ISBN 9781402088537. [Google Scholar]
- García-Fortea, E.; García-Pérez, A.; Gimeno-Páez, E.; Sánchez-Gimeno, A.; Vilanova, S.; Prohens, J.; Pastor-Calle, D. A Deep Learning-Based System (Microscan) for the Identification of Pollen Development Stages and Its Application to Obtaining Doubled Haploid Lines in Eggplant. Biology 2020, 9, 272. [Google Scholar] [CrossRef]
- Dehkehan, M.E.; Moieni, A.; Movahedi, Z. Effects of Zeatin Riboside, Mannitol and Heat Stress on Eggplant (Solanum melongena L.) Anther Culture. Iran. J. Genet. Plant Breed. 2017, 6, 16–26. [Google Scholar]
- Bennici, A.; Cionini, P.G. Cytokinins and in vitro Development of Phaseolus coccineus Embryos. Planta 1979, 147, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Kuroha, T.; Kato, H.; Asami, T.; Yoshida, S.; Kamada, H.; Satoh, S. A Trans-zeatin Riboside in Root Xylem Sap Negatively Regulates Adventitious Root Formation on Cucumber Hypocotyls. J. Exp. Bot. 2002, 53, 2193–2200. [Google Scholar] [CrossRef]
- Kurtar, E.S.; Balkaya, A.; Ozbakir Ozer, M. Production of Callus Mediated Gynogenic Haploids in Winter Squash (Cucurbita maxima Duch.) and Pumpkin (Cucurbita moschata Duch.). Czech J. Genet. Plant Breed. 2018, 54, 9–16. [Google Scholar] [CrossRef]
- Kurtar, E.S.; Seymen, M. Gynogenesis in Cucurbita Species. Methods Mol. Biol. 2021, 2289, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Koli, S.P.; Murthy, H.N. Haploid Plant Regeneration from Unpollinated Ovules of Cucumis melo L. var. conomon Cv. Mudicode. Br. Biotechnol. J. 2013, 3, 605–613. [Google Scholar] [CrossRef]
- Sun, S.; Zhai, Q.; Hu, J.; Chen, J.; Zhang, P. Effects of Several Physiological Factors on Embryo Formation in Unpollinated Ovary Culture of Pumpkin. Plant Physiol. Commun. 2009, 45, 977–980. [Google Scholar]
- Li, J.W.; Si, S.W.; Cheng, J.Y.; Li, J.X.; Liu, J.Q. Thidiazuron and Silver Nitrate Enhanced Gynogenesis of Unfertilized Ovule Cultures of Cucumis Sativus. Biol. Plant 2013, 57, 164–168. [Google Scholar] [CrossRef]
- Kurtar, E.S.; Seymen, M. Induction of Parthenogenesis by Irradiated Pollen in Cucurbita Species. Methods Mol. Biol. 2021, 2289, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Hesami, M.; Pepe, M.; de Ronne, M.; Yoosefzadeh-Najafabadi, M.; Adamek, K.; Torkamaneh, D.; Jones, A.M.P. Transcriptomic Profiling of Embryogenic and Non-Embryogenic Callus Provides New Insight into the Nature of Recalcitrance in Cannabis. Int. J. Mol. Sci. 2023, 24, 14625. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Gao, F.; Wang, H.; Tretyakova, I.N.; Nosov, A.M.; Shen, H.; Yang, L. Morphological and Physiological Indicators for Screening Cell Lines with High Potential for Somatic Embryo Maturation at an Early Stage of Somatic Embryogenesis in Pinus koraiensis. Plants 2022, 11, 1867. [Google Scholar] [CrossRef] [PubMed]
- González, G.A.; Pacheco, M.G.; Oneto, C.D.; Etchart, V.J.; Kandus, M.V.; Salerno, J.C.; Eyherabide, G.; Presello, D.; Lewi, D.M. Somatic Embryogenesis and Plant Regeneration Capacity in Argentinean Maize (Zea mays L.) Inbred Lines. Electron. J. Biotechnol. 2012, 15, 9. [Google Scholar] [CrossRef]
- Martínez-López, M.; García-Pérez, A.; Gimeno-Páez, E.; Prohens, J.; Vilanova, S.; García-Fortea, E. Screening of Suitable Plant Regeneration Protocols for Several Capsicum spp. through Direct Organogenesis. Horticulturae 2021, 7, 261. [Google Scholar] [CrossRef]
- Xie, B.; Wang, X.; Fan, Z. Improved Conditions of In Vitro Culture of Unpollinated Ovules and Production of Embryonary Sac Plants in Summer Squash (Cucurbita pepo L.). Sci. Agric. Sin. 2006, 39, 132–138. [Google Scholar]
- Moqbeli, E.; Peyvast, G.; Hamidoghli, Y.; Olfati, J.A. In Vitro Cucumber Haploid Line Generation in Several New Cultivars. Asia-Pac. J. Mol. Biol. Biotechnol. 2013, 21, 18–25. [Google Scholar]
- Gautam, N.; Gupta, A.K. Regulation of Artificial Gynogenic Embryogenesis in Plants. Plant Cell Tissue Organ Cult. 2025, 161, 30. [Google Scholar] [CrossRef]
- Ramasamy, G.; Ramasamy, S.; Ravi, N.S.; Krishnan, R.; Subramanian, R.; Raman, R.; Duraialaguraja, S.; Muthurajan, R.; Vellaichamy, J. Haploid Embryogenesis and Molecular Detection of Somatic Embryogenesis Receptor-like Kinase (TcSERK) Genes in Sliced Ovary Cultures of Cocoa (Theobroma cacao L.). Plant Biotechnol. Rep. 2022, 16, 283–297. [Google Scholar] [CrossRef]
- Celebi-Toprak, F.; Ergun, Z.; Alan, A.R. Production of Haploid and Doubled Haploid Plants of Allium tuncelianum (Kollman) Özhatay, Matthew and Şiraneci via In Vitro Gynogenesis. Vitr. Cell. Dev. Biol. Plant 2023, 59, 700–710. [Google Scholar] [CrossRef]
- Alan, A.R. Doubled Haploid Onion (Allium cepa L.) Production via In Vitro Gynogenesis. In Doubled Haploid Technology. Methods in Molecular Biology; Segui-Simarro, J.M., Ed.; Humana Press: New York, NY, USA, 2021; Volume 2287, pp. 151–169. [Google Scholar]
- Fernández, A.M.; Nakazaki, T.; Tanisaka, T. Development of Diploid and Triploid Interspecific Hybrids between Lilium longiflorum and L. concolor by Ovary Slice Culture. Plant Breed. 1996, 115, 167–171. [Google Scholar] [CrossRef]
- Hooghvorst, I.; Nogués, S. Opportunities and Challenges in Doubled Haploids and Haploid Inducer-Mediated Genome-Editing Systems in Cucurbits. Agronomy 2020, 10, 1441. [Google Scholar] [CrossRef]
- Patel, N.B.; Jha, Z. In Vitro Culture of Haploids. In Doubled Haploids: Technological Advances and Role in Crop Improvement; Jha, Z., Verulkar, S.B., Penna, S., Eds.; Springer Nature: Singapore, 2025; pp. 57–84. ISBN 9789819623396. [Google Scholar]
- Kurtar, E.S.; Balkaya, A.; Kandemir, D. Evaluation of Haploidization Efficiency in Winter Squash (Cucurbita maxima Duch.) and Pumpkin (Cucurbita moschata Duch.) through Anther Culture. Plant Cell Tissue Organ Cult. 2016, 127, 497–511. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Rotino, G.L. Haploidy in Eggplant; Springer: Dordrecht, The Netherlands, 1996; pp. 115–141. [Google Scholar]
- García-Fortea, E.; Lluch-Ruiz, A.; Pineda-Chaza, B.J.; García-Pérez, A.; Bracho-Gil, J.P.; Plazas, M.; Gramazio, P.; Vilanova, S.; Moreno, V.; Prohens, J. A Highly Efficient Organogenesis Protocol Based on Zeatin Riboside for In Vitro Regeneration of Eggplant. BMC Plant Biol. 2020, 20, 6. [Google Scholar] [CrossRef]
- Doležel, J.; Binarová, P. The Effects of Colchicine on Ploidy Level, Morphology and Embryogenic Capacity of Alfalfa Suspension Cultures. Plant Sci. 1989, 64, 213–219. [Google Scholar] [CrossRef]
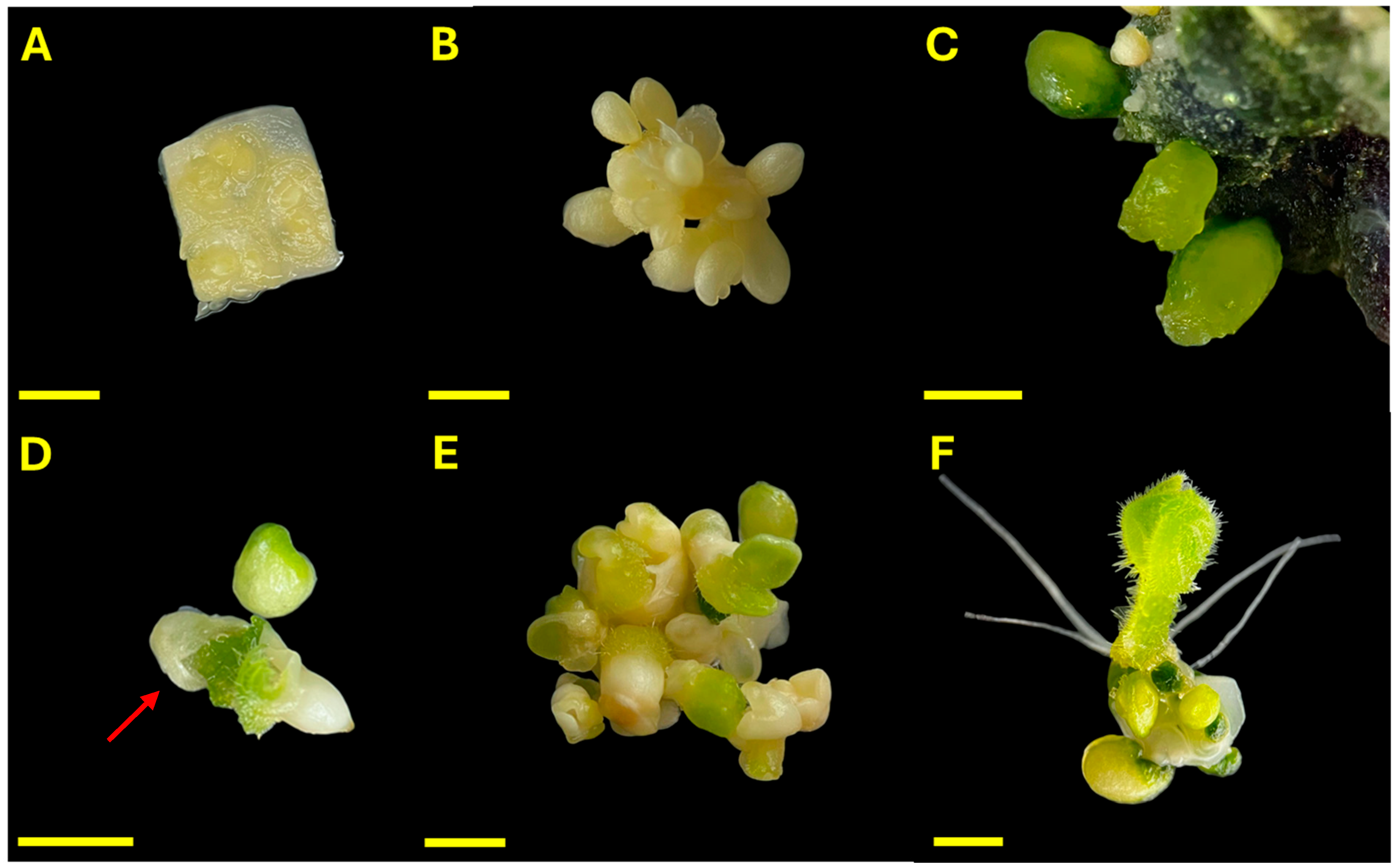

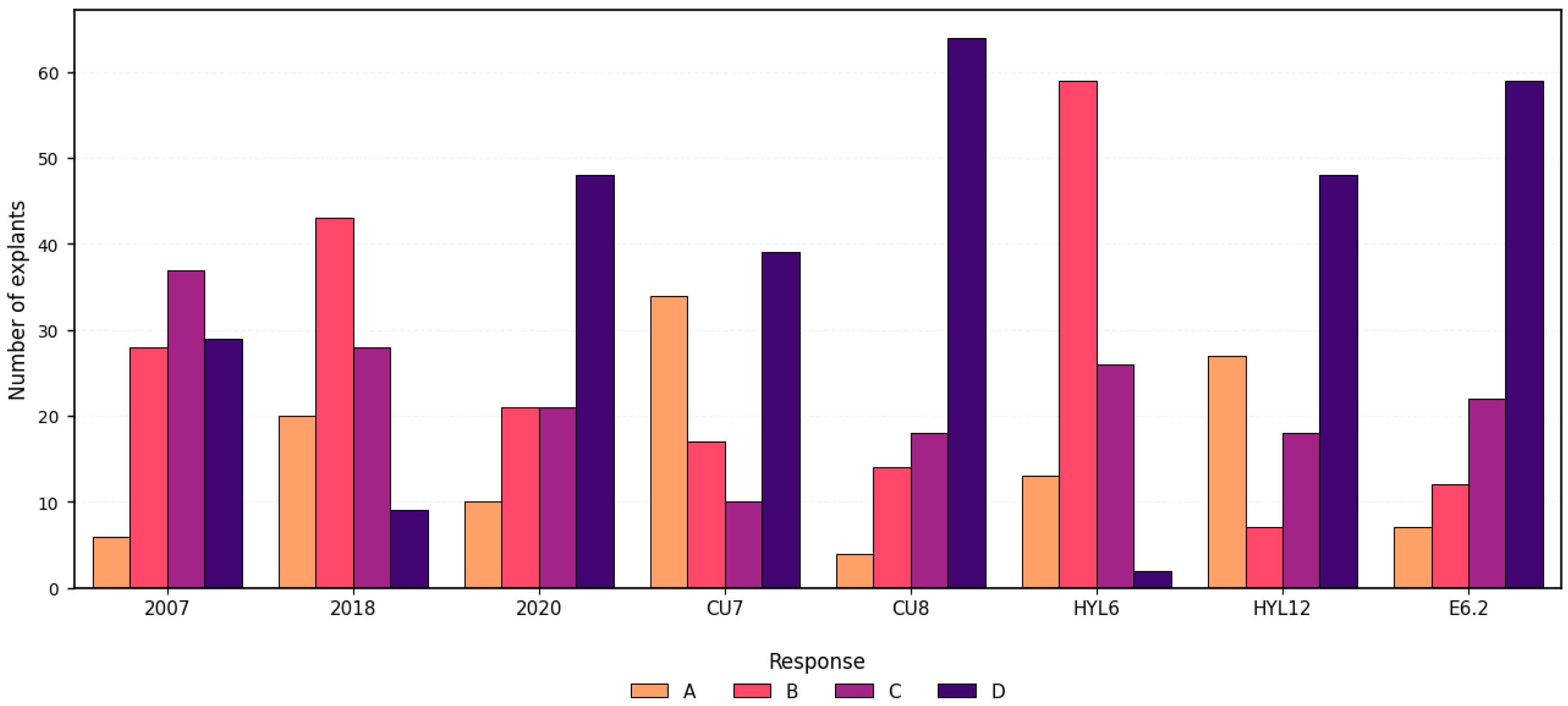

| Floral Development | Medium | Response | No. of Embryos | No. of Explants with Embryos | |||
|---|---|---|---|---|---|---|---|
| A | B | C | D | ||||
| 1 DBA | 2007 | 6 | 28 | 37 | 29 | 27 | 17 |
| 2018 | 20 | 43 | 28 | 9 | 4 | 3 | |
| 2020 | 10 | 21 | 21 | 48 | 5 | 4 | |
| CU7 | 34 | 17 | 10 | 39 | 4 | 4 | |
| CU8 | 4 | 14 | 18 | 64 | 2 | 2 | |
| HYL6 | 13 | 59 | 26 | 2 | 0 | 0 | |
| HYL12 | 27 | 7 | 18 | 48 | 3 | 2 | |
| E6.2 | 7 | 12 | 22 | 59 | 50 | 29 | |
| At anthesis | 2007 | 19 | 28 | 33 | 20 | 0 | 0 |
| 2018 | 27 | 38 | 28 | 7 | 0 | 0 | |
| 2020 | 11 | 23 | 20 | 46 | 0 | 0 | |
| CU7 | 40 | 16 | 9 | 35 | 0 | 0 | |
| CU8 | 16 | 19 | 17 | 48 | 0 | 0 | |
| HYL6 | 23 | 51 | 25 | 1 | 0 | 0 | |
| HYL12 | 33 | 8 | 16 | 43 | 0 | 0 | |
| E6.2 | 26 | 11 | 15 | 48 | 3 | 2 | |
| Medium | No. of Explants | % Responsive Explants | SOs per Explant (Mean ± SD) | SOs/100 Explants |
|---|---|---|---|---|
| E6 | 138 | 62.3% | 4.00 ± 4.18 | 400.00 |
| E6.1 | 165 | 84.9% | 5.64 ± 3.68 | 564.24 |
| E6.2 | 180 | 85.0% | 6.09 ± 3.54 | 608.89 |
| E6.3 | 155 | 81.9% | 6.01 ± 4.16 | 600.65 |
| Medium | Total Embryos | Embryos/Explant (Mean ± SD) | Embryos/100 Explants | % Explants ≥ 1 Embryo | Conversion Rate (%) |
|---|---|---|---|---|---|
| E6 | 103 | 0.75 ± 1.42 | 74.64 | 32.6% | 18.7% |
| E6.1 | 160 | 0.97 ± 1.54 | 96.97 | 44.9% | 17.2% |
| E6.2 | 29 | 0.16 ± 0.37 | 16.11 | 16.1% | 2.7% |
| E6.3 | 60 | 0.39 ± 0.73 | 38.71 | 27.1% | 6.4% |
| Medium | Sucrose | BAP | IAA | KT | NAA | TDZ | ZR | 2,4-D | PCM |
|---|---|---|---|---|---|---|---|---|---|
| Induction | |||||||||
| 2007 [16] | 30 | - | - | 1 | - | - | - | 1 | - |
| 2018 [36] | 30 | 4 | - | - | 0.05 | 0.1 | - | - | - |
| 2020 [23] | 30 | 1 | - | - | - | - | - | - | - |
| CU7 * [57] | 120 | - | - | 5 | - | - | - | 5 | - |
| CU8 | 120 | - | - | 5 | - | - | - | - | 2 |
| HYL6 [30] | 60 | - | - | - | - | 0.5 | - | 0.2 | - |
| HYL12 * [30] | 120 | - | - | - | - | 0.5 | - | 0.2 | - |
| E6.2 * [33] | 120 | - | - | - | 3 | - | 1 | - | - |
| A | 30 | - | - | - | - | - | - | - | - |
| Others | |||||||||
| Regeneration | 30 | 1 | 0.01 | - | - | - | - | - | - |
| Medium | MS | Sucrose | ZR | NAA |
|---|---|---|---|---|
| E6 [58] | 2.2 | 15 | 2 | - |
| E6.1 [33] | 4.4 | 30 | 1 | 3 |
| E6.3 | 4.4 | 30 | 2 | - |
| E6.2 | 4.4 | 120 | 1 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Pérez, A.; Escánez, M.; Gil, S.; Miralles-Rodríguez, A.; Vilanova, S.; Bermúdez, F.; García-Fortea, E. Doubled Haploid Production in Cucurbita pepo L. Through Ovary Culture. Plants 2025, 14, 3733. https://doi.org/10.3390/plants14243733
García-Pérez A, Escánez M, Gil S, Miralles-Rodríguez A, Vilanova S, Bermúdez F, García-Fortea E. Doubled Haploid Production in Cucurbita pepo L. Through Ovary Culture. Plants. 2025; 14(24):3733. https://doi.org/10.3390/plants14243733
Chicago/Turabian StyleGarcía-Pérez, Ana, Malen Escánez, Sandra Gil, Alejandro Miralles-Rodríguez, Santiago Vilanova, Francisco Bermúdez, and Edgar García-Fortea. 2025. "Doubled Haploid Production in Cucurbita pepo L. Through Ovary Culture" Plants 14, no. 24: 3733. https://doi.org/10.3390/plants14243733
APA StyleGarcía-Pérez, A., Escánez, M., Gil, S., Miralles-Rodríguez, A., Vilanova, S., Bermúdez, F., & García-Fortea, E. (2025). Doubled Haploid Production in Cucurbita pepo L. Through Ovary Culture. Plants, 14(24), 3733. https://doi.org/10.3390/plants14243733





