Oxidative Stress and Negative Consequences on Photosystem II Occasioned by Lead Stress Are Mitigated by 24-Epibrassinolide and Dopamine in Tomato Plants
Abstract
1. Introduction
2. Results
2.1. Pb Concentrations Were Minimized After Treatment with DOP and EBR
2.2. DOP and EBR Stimulated the Nutritional Status in Pb-Stressed Plants
2.3. Plant Growth Regulators Provided Maintenance of Photosynthetic Pigments
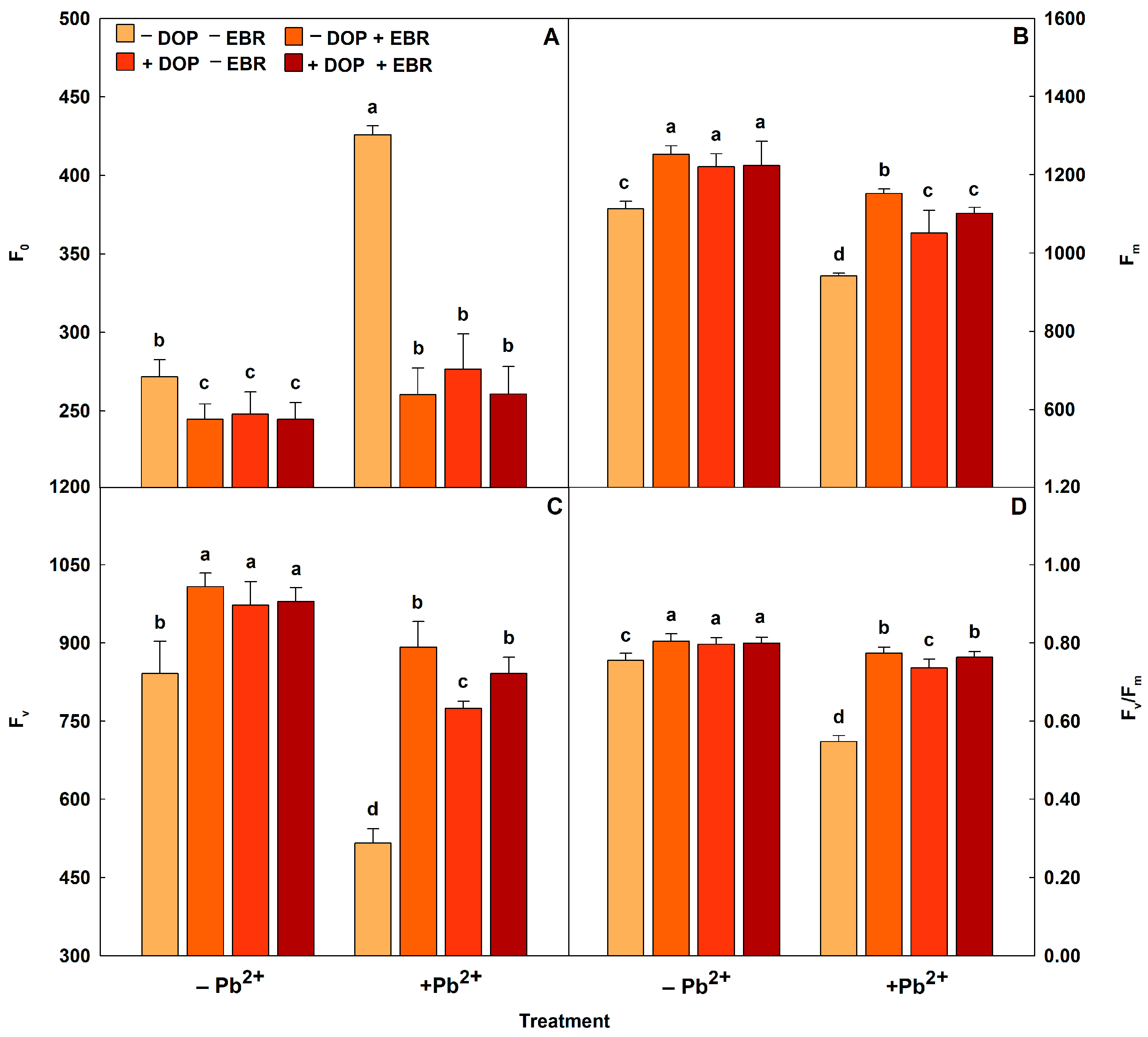
2.4. The Performance of Photosystem II Was Boosted Up After DOP and EBR Spray
2.5. Exogenous Spraying of DOP and EBR Maximized Gas Exchange
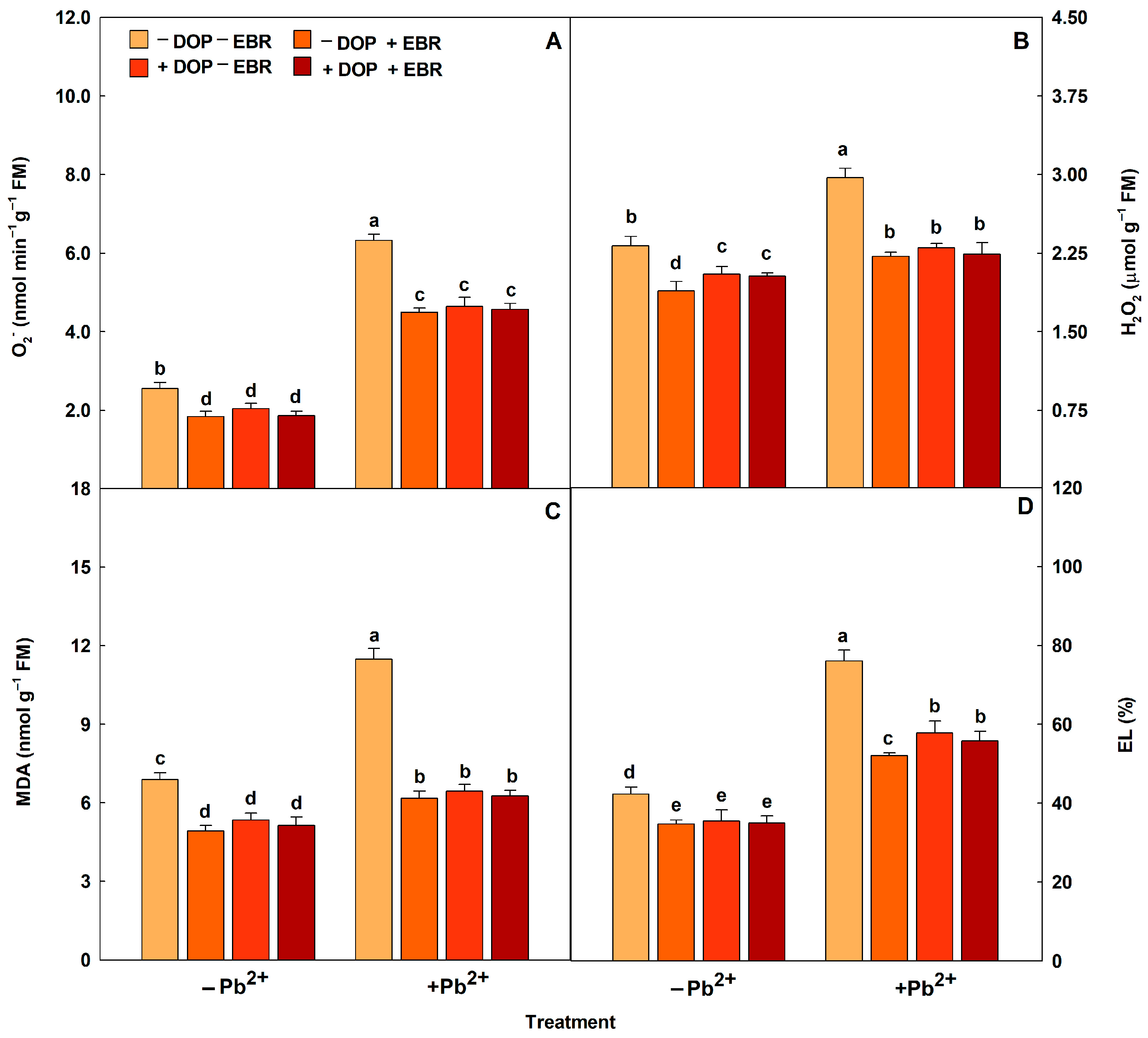
2.6. Neurotransmitter and Brassinosteroids Enhance Antioxidant Defense
2.7. DOP and EBR Promoted Higher Biomass Accumulation in Plants Exposed to Pb Excess
3. Discussion
4. Materials and Methods
4.1. Location and Growth Conditions
4.2. Plants, Containers, and Acclimation
4.3. Experimental Design
4.4. Dopamine (DOP) and 24-Epibrassinolide (EBR) Preparations and Applications
4.5. Plant Nutrition and Pb Treatment
4.6. Pb Determination
4.7. Measurement of Chlorophyll Fluorescence
4.8. Evaluation of Gas Exchange
4.9. Extraction of Antioxidant Enzymes, Superoxide and Soluble Proteins
4.10. Superoxide Dismutase Assay
4.11. Catalase Assay
4.12. Ascorbate Peroxidase Assay
4.13. Peroxidase Assay
4.14. Determination of Superoxide Concentration
4.15. Extraction of Nonenzymatic Compounds
4.16. Determination of Hydrogen Peroxide Concentration
4.17. Quantification of Malondialdehyde Concentration
4.18. Determination of Electrolyte Leakage
4.19. Determination of Photosynthetic Pigments
4.20. Measurements of Biomass
4.21. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mustafa, M.; Mwangi, R.W.; Szalai, Z.; Kappel, N.; Csambalik, L. Sustainable Responses to Open Field Tomato (Solanum lycopersicum L.) Stress Impacts. J. Agric. Food Res. 2025, 21, 101825. [Google Scholar] [CrossRef]
- Li, J.; Xian, J.; Zhang, S.; An, Y.; Li, N.; Zhou, D.; Sun, S.; Wang, J. Influence of Pigment Composition on Antioxidant Capacity of Different Tomato (Solanum lycopersicum L.). Lwt 2025, 224, 117871. [Google Scholar] [CrossRef]
- Cui, X.; Gu, J.; Liu, P.; Lu, R.; Ren, Z.; Zhang, Y.; Wang, F.; Qi, M.; Liu, Y.; Li, T. Genome-Wide Identification and Characterization of the Thioredoxin (TRX) Gene Family in Tomato (Solanum lycopersicum) and a Functional Analysis of SlTRX2 under Salt Stress. Plant Physiol. Biochem. 2025, 220, 109478. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Tabata, S.; Hirakawa, H.; Asamizu, E.; Shirasawa, K.; Isobe, S.; Kaneko, T.; Nakamura, Y.; Shibata, D.; Aoki, K.; et al. The Tomato Genome Sequence Provides Insights into Fleshy Fruit Evolution. Nature 2012, 485, 635–641. [Google Scholar] [CrossRef]
- Fao FAO—Food Agriculture Organization of the United Nations. Agricultural Production Statistics 2000–2022; FAO: Rome, Italy, 2023. [Google Scholar] [CrossRef]
- Bello, A.S.; Ahmed, T. Moringa Leaf Extract Alleviates Salt Stress in Tomato (Solanum lycopersicum L.) by Activating Antioxidant Defenses, Reducing Osmolyte Accumulation, Improving Water Status, and Enhancing Yield. Plant Stress 2024, 14, 100640. [Google Scholar] [CrossRef]
- Pizarro-Oteíza, S.; Salazar, F. Effect of UV-LED Irradiation Processing on Pectolytic Activity and Quality in Tomato (Solanum lycopersicum) Juice. Innov. Food Sci. Emerg. Technol. 2022, 80, 103097. [Google Scholar] [CrossRef]
- Piccolo, V.; Maisto, M.; Schiano, E.; Iannuzzo, F.; Keivani, N.; Manuela Rigano, M.; Santini, A.; Novellino, E.; Carlo Tenore, G.; Summa, V. Phytochemical Investigation and Antioxidant Properties of Unripe Tomato Cultivars (Solanum lycopersicum L.). Food Chem. 2024, 438, 137863. [Google Scholar] [CrossRef]
- Kabir, M.S.; Roy, U.; Suvo, S.P.; Sobhan, A.; Kamal, M.M.; Akhter, M.J.; Akter, M.S.; Ahmed, M. Comparative Assessment of Fresh and Processed Tomato (Solanum lycopersicum) Pulps: Impact of Processing on Physicochemical, Antioxidant, and Enzymatic Behavior. Appl. Food Res. 2024, 4, 100550. [Google Scholar] [CrossRef]
- Jin, L.; Jin, N.; Wang, S.; Huang, S.; Yang, X.; Xu, Z.; Jiang, S.; Lyu, J.; Yu, J. Moderate Salt Stress Aids in the Enhancement of Nutritional and Flavor Quality in Tomato (Solanum lycopersicum L.) Fruits. Food Chem. X 2025, 26, 102330. [Google Scholar] [CrossRef] [PubMed]
- El-Sorogy, A.S.; Al-kahtany, K.; Alharbi, T.; Alarifi, S.S. Distribution Patterns, Health Hazards, and Multivariate Assessment of Contamination Sources of As, Pb, Ni, Zn, and Fe in Agricultural Soils. J. King Saud. Univ.—Sci. 2024, 36, 103489. [Google Scholar] [CrossRef]
- Wu, Z.Q.; Li, H.Y.; Lü, L.Y.; Liang, G.J.; Wu, T.T.; Zhu, J.X. Distributions and Risk Assessment of Heavy Metals in Solid Waste in Lead-Zinc Mining Areas and Across the Soil, Water Body, Sediment and Agricultural Product Ecosystem in Their Surrounding Areas. China Geol. 2025, 8, 92–106. [Google Scholar] [CrossRef]
- Sanad, H.; Moussadek, R.; Mouhir, L.; Lhaj, M.O.; Zahidi, K.; Dakak, H.; Manhou, K.; Zouahri, A. Ecological and Human Health Hazards Evaluation of Toxic Metal Contamination in Agricultural Lands Using Multi-Index and Geostatistical Techniques across the Mnasra Area of Morocco’s Gharb Plain Region. J. Hazard. Mater. Adv. 2025, 18, 100724. [Google Scholar] [CrossRef]
- Liu, P.; Wu, Q.; Hu, W.; Tian, K.; Huang, B.; Zhao, Y. Effects of Atmospheric Deposition on Heavy Metals Accumulation in Agricultural Soils: Evidence from Field Monitoring and Pb Isotope Analysis. Environ. Pollut. 2023, 330, 121740. [Google Scholar] [CrossRef]
- Lima, F.A.; Bhattacharjee, S.; Jahangir Sarker, M.; Salam, M.A. Ecological Risk Assessment of Potentially Toxic Elements (PTEs) in Agricultural Soil, Vegetables and Fruits with Respect to Distance Gradient in Proximity to Lead-Acid Battery Industry. Environ. Nanotechnol. Monit. Manag. 2024, 21, 100932. [Google Scholar] [CrossRef]
- Anjana, K.R.; Suresh, A.; Soman, V.; Rahman, K.H. Metal Contamination in the Ashtamudi Wetland Ecosystem: Source Identification, Toxicological Risk Assessment of Ni, Cd, Cr, and Pb and Remediation Strategies. Mar. Pollut. Bull. 2025, 212, 117534. [Google Scholar] [CrossRef]
- Yu, A.; Yang, Q.; Xu, B.; Yang, Y.; Ren, Z.; Li, K. Analysis of Lead Contamination Sources in Roadside Soil via the Isotope Tracing Method. J. Environ. Chem. Eng. 2024, 12, 114205. [Google Scholar] [CrossRef]
- Dejoie, E.; Lemay, M.A.; Fenton, N.J.; DesRochers, A.; Marion, J.; Savard, M.M.; Porter, T.J.; Proulx, D.; Gennaretti, F. Spatiotemporal Assessment of Lead (Pb) and Cadmium (Cd) Contamination in Urban Tree Rings near an Industrial Smelter: High Intraspecific Variability but Limited Spatial Differentiation. Atmos. Pollut. Res. 2025, 16, 102582. [Google Scholar] [CrossRef]
- Salmen, S.H.; Alharbi, S.A. Mitigating Pb-Induced Oxidative Stress in Rice Plants by Cerium Oxide and Iron Oxide Nanoparticles. S. Afr. J. Bot. 2024, 172, 544–555. [Google Scholar] [CrossRef]
- Feng, G.; Li, S.; Yang, X.; Hu, Y.; Zhang, X.; Chen, D.; Liu, W.; Yu, G.; Nie, G.; Huang, L.; et al. Integrative Multi-Omic Analyses Reveal the Molecular Mechanisms of Silicon Nanoparticles in Enhancing Hyperaccumulator under Pb Stress. Environ. Pollut. 2025, 368, 125677. [Google Scholar] [CrossRef]
- Noreen, S.; Malik, Z.; Luqman, M.; Fatima, I.; Tahir, U.A.; Dar, M.; Rizwan, M. Effect of Bacillus Strain and Fe-Modified Biochar on Lead (Pb) Bioaccumulation and Oxidative Stress in Wheat (Triticum aestivum L.) Grown in Pb Contaminated Soil. S. Afr. J. Bot. 2024, 172, 720–735. [Google Scholar] [CrossRef]
- Timori, Z.; Amirinejad, A.A.; Ghobadi, M. Biochar Improves Pb and Cd-Induced Stress in Mung Bean (Vigna radiata L. Wilczek). Environ. Chall. 2024, 16, 100992. [Google Scholar] [CrossRef]
- Maryam, H.; Rizwan, M.; Masood, N.; Waseem, M.; Ahmed, T.; Farooq, M.; Zia-ur-rehman, M.; Aziz, H. Mitigating Lead (Pb) Toxicity in Zea mays (L.) Plants Using Green Synthesized Iron Oxide Nanoparticles. S. Afr. J. Bot. 2024, 175, 657–668. [Google Scholar] [CrossRef]
- Torabi, S.; Rahmani, F. 24-Epibrassinolide Promotes Resilience against Arsenic Stress via Modulating Amino Acid Profiles and MRNA Abundance of CYP450 and MRP Genes in Zea mays L. Plant Physiol. Biochem. 2025, 221, 109631. [Google Scholar] [CrossRef]
- Pandey, A.; Singh, G.; Pandey, S.; Singh, V.K.; Prasad, S.M. 24-Epibrassinolide Effectively Alleviates UV-B Stress-Induced Damage in the Cyanobacterium Anabaena Sp. PCC 7120 by Employing Nitric Oxide: Improved PS II Photochemistry, Antioxidant System, and Growth. Plant Physiol. Biochem. 2025, 221, 109667. [Google Scholar] [CrossRef]
- Shabab, Z.; Sarada, D.V.L. 24-Epibrassinolide Mitigates Arsenate Stress in Seedlings of Oryza sativa (IR-20) via the Induction of Phenylpropanoid Pathway. Plant Physiol. Biochem. 2024, 215, 109023. [Google Scholar] [CrossRef]
- He, Y.; Wang, X.; Pan, Y.; Wang, Y.; Xu, W. Treatment with 24-Epibrassinolide Protects ‘Youhou’ Sweet Persimmon Fruit against Chilling Injury During Cold Storage. Sci. Hortic. 2025, 343, 114087. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, Z.; Xu, Z.; Li, Z.; Gao, X.; Yang, X.; Xie, Y.; Meng, X.; Jin, N.; Jin, L.; et al. Enhancing Industrial Value of Coriander (Coriandrum sativum L.): Effects of 2,4-Epibrassinolide on Aroma and Bioactive Compounds. Ind. Crops Prod. 2025, 230, 121139. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Sun, S.; Qu, K.; Chen, J.; Dong, Y.; Liu, A.; Chen, S. Hydrogen Peroxide Signaling Mediates Dopamine-Induced Chromium Stress Tolerance in Tomato. Environ. Pollut. 2025, 371, 125949. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; Li, X. Dopamine-Induced Abiotic Stress Tolerance in Horticultural Plants. Sci. Hortic. 2023, 307, 111506. [Google Scholar] [CrossRef]
- Cao, Y.; Du, P.; Shang, Y.; Ji, J.; Tan, L.; Zhang, X.; Xu, J.; Liang, B. Melatonin and Dopamine Alleviate Waterlogging Stress in Apples by Recruiting Beneficial Endophytes to Enhance Physiological Resilience. J. Integr. Agric. 2024, 23, 2270–2291. [Google Scholar] [CrossRef]
- Li, B.B.; Fu, Y.S.; Li, X.X.; Yin, H.N.; Xi, Z.M. Brassinosteroids Alleviate Cadmium Phytotoxicity by Minimizing Oxidative Stress in Grape Seedlings: Toward Regulating the Ascorbate-Glutathione Cycle. Sci. Hortic. 2022, 299, 111002. [Google Scholar] [CrossRef]
- Wen, J.; Tang, Y.; Li, J.; He, T.; Xiao, J.; Nangia, V.; Liu, Y. Effects of Exogenous Brassinosteroids on the Starch Structure, Physicochemical Properties and Digestibility of Wheat Under High-Temperature Stress at the Early Grain-Filling Stage. Int. J. Biol. Macromol. 2024, 283, 137690. [Google Scholar] [CrossRef]
- Barooti, S.; Edalat, M.; Oveisi, M.; Kazemeini, S.A.; Naderi, R. Does the Exogenous Application of Brassinosteroids Affect the Photosynthetic, Morphological Characteristics, and THC Concentrations of Cannabis sativa L. under Drought Stress? J. Appl. Res. Med. Aromat. Plants 2025, 46, 100635. [Google Scholar] [CrossRef]
- Cao, Y.; Du, P.; Zhang, J.; Ji, J.; Xu, J.; Liang, B. Dopamine Alleviates Cadmium Stress in Apple Trees by Recruiting Beneficial Microorganisms to Enhance the Physiological Resilience Revealed by High-Throughput Sequencing and Soil Metabolomics. Hortic. Res. 2023, 10, uhad112. [Google Scholar] [CrossRef]
- Zhang, Z.; Tang, Z.; Jing, G.; Gao, S.; Liu, C.; Ai, S.; Liu, Y.; Liu, Q.; Li, C.; Ma, F. Dopamine Confers Cadmium Tolerance in Apples by Improving Growth, Reducing Reactive Oxygen Species, and Changing Secondary Metabolite Levels. Environ. Exp. Bot. 2023, 208, 105264. [Google Scholar] [CrossRef]
- Niu, K.; Xiao, H.; Wang, Y.; Cui, T.; Zhao, C. A Meta-Analysis on Plant Growth and Heavy Metals Uptake with the Application of 2,4-Epibrassinolide in Contaminated Soils. Ecotoxicol. Environ. Saf. 2025, 289, 117439. [Google Scholar] [CrossRef]
- Ji, Z.Y.; Liu, Z.Y.; Shi, L.M.; Lu, X.Y.; Han, Y.Y.; Sun, Y. Mitigation Effect of Exogenous Dopamine Treatment on Downy Mildew-Infected Cucumber. J. Plant Growth Regul. 2024, 43, 2615–2631. [Google Scholar] [CrossRef]
- Du, J.; Xu, H.; Zhang, D.X.; Feng, S. Chelation and Nanoparticle Delivery of Monomeric Dopamine to Increase Plant Salt Stress Resistance. Nat. Commun. 2025, 16, 4157. [Google Scholar] [CrossRef]
- Lv, M.; Li, J. Molecular Mechanisms of Brassinosteroid-Mediated Responses to Changing Environments in Arabidopsis. Int. J. Mol. Sci. 2020, 21, 2737. [Google Scholar] [CrossRef] [PubMed]
- Niu, K.; Zhu, R.; Wang, Y.; Zhao, C.; Ma, H. 24-Epibrassinolide Improves Cadmium Tolerance and Lateral Root Growth Associated with Regulating Endogenous Auxin and Ethylene in Kentucky Bluegrass. Ecotoxicol. Environ. Saf. 2023, 249, 114460. [Google Scholar] [CrossRef] [PubMed]
- Jiao, C.; Lan, G.; Sun, Y.; Wang, G.; Sun, Y. Dopamine Alleviates Chilling Stress in Watermelon Seedlings via Modulation of Proline Content, Antioxidant Enzyme Activity, and Polyamine Metabolism. J. Plant Growth Regul. 2021, 40, 277–292. [Google Scholar] [CrossRef]
- Liu, Z.; Ji, Z.; Han, Y.; Sun, Y. The Mitigation Effects of Exogenous Dopamine Treatment on Continuous Cropping Obstacles in Watermelon. J. Soil. Sci. Plant Nutr. 2023, 23, 4233–4249. [Google Scholar] [CrossRef]
- Sun, H.F.; Wang, X.N.; Li, Y.N.; Wang, L.L.; Li, Y.Y.; Ma, L.J.; Li, X.M. Long Non-Coding RNAs Modulate Glutathione Metabolism Gene Expression and Tolerance to Pb Stress in Root Tissue of Endophyte-Infected Rice Seedling. Ecotoxicol. Environ. Saf. 2025, 291, 117872. [Google Scholar] [CrossRef]
- Phuong, H.T.; Ba, V.N.; Thien, B.N.; Truong Thi Hong, L. Accumulation and Distribution of Nutrients, Radionuclides and Metals by Roots, Stems and Leaves of Plants. Nucl. Eng. Technol. 2023, 55, 2650–2655. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Govindasamy, C.; Sharma, A. Heavy Metal Accumulation in Root and Shoot Tapioca Plant Biomass Grown in Agriculture Land Situated around the Magnesite Mine Tailings. Environ. Res. 2024, 257, 119287. [Google Scholar] [CrossRef]
- He, Y.; Hou, X.; Wu, X.; Duan, C.; Liu, C.; Yin, L.; Zhang, M.; Fu, D. Fertilization and Intercropping Reduce Pb Accumulation in Plants by Influencing Rhizosphere Soil Phosphorus Forms in Soil-Plant Systems. Ecotoxicol. Environ. Saf. 2025, 293, 118011. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.Y.; Guo, R.; Jiang, Q.; Chen, C.Z.; Gao, Y.Q.; Jiang, M.M.; Shen, R.F.; Zhu, X.F.; Huang, J. Brassinosteroid Decreases Cadmium Accumulation via Regulating Gibberellic Acid Accumulation and Cd Fixation Capacity of Root Cell Wall in Rice (Oryza sativa). J. Hazard. Mater. 2024, 469, 133862. [Google Scholar] [CrossRef]
- Haider, F.U.; Zulfiqar, U.; Mehmood, T.; Shahzad, B.; Liqun, C.; Yong, J.W.H.; Binobead, M.A. Effects of Titanium Oxide Nanoparticles and 24-Epibrassinosteroid to Mitigate the Toxicity of Cadmium (Cd) and Improve Physio-Morphological Traits of Soybean (Glycine max L.) Cultivated Under Cd-Contaminated Soil. Environ. Technol. Innov. 2024, 36, 103811. [Google Scholar] [CrossRef]
- Yildirim, E.; Ekinci, M.; Turan, M.; Yuce, M.; Ors, S.; Araz, O.; Torun, U.; Argin, S. Exogenous Dopamine Mitigates the Effects of Salinity Stress in Tomato Seedlings by Alleviating the Oxidative Stress and Regulating Phytohormones. Acta Physiol. Plant. 2024, 46, 59. [Google Scholar] [CrossRef]
- Niu, G.; Wang, R.; Zhou, H.; Yang, J.; Lu, X.; Han, X.; Huang, J. Nitrogen Addition and Mowing Had Only Weak Interactive Effects on Macronutrients in Plant-Soil Systems of a Typical Steppe in Inner Mongolia. J. Environ. Manag. 2023, 347, 119121. [Google Scholar] [CrossRef]
- Umar, A.W.; Naeem, M.; Hussain, H.; Ahmad, N.; Xu, M. Starvation from Within: How Heavy Metals Compete with Essential Nutrients, Disrupt Metabolism, and Impair Plant Growth. Plant Sci. 2025, 353, 112412. [Google Scholar] [CrossRef]
- Muneer, M.A.; Afridi, M.S.; Saddique, M.A.B.; Chen, X.; Zaib-Un-Nisa; Yan, X.; Farooq, I.; Munir, M.Z.; Yang, W.; Ji, B.; et al. Nutrient Stress Signals: Elucidating Morphological, Physiological, and Molecular Responses of Fruit Trees to Macronutrients Deficiency and Their Management Strategies. Sci. Hortic. 2024, 329, 112985. [Google Scholar] [CrossRef]
- Ma, L.; Zhao, X.; Lin, G.; Shi, H.; Li, Z.; Shen, L. Bioaccessibility Assessment of Mn, Cu, Fe, and Cd in Henan Province Wheat Using Physiologically Based Extraction. Environ. Monit. Assess. 2025, 197, 600. [Google Scholar] [CrossRef]
- Yusuf, M.; Khan, T.A.; AlBlooshi, F.S.; Alharmoudi, A.; AlAlawi, M.S.; Alshkeili, A.M.; Albedwawi, A.; Masih, J.; Buty Alghfeli, M.M. Unveiling the Protective Role of Silicon Dioxide Nanoparticles against Copper-Induced Oxidative Damage in Soybean Plants through Altered Proline Metabolism and Antioxidants. Plant Nano Biol. 2025, 12, 100149. [Google Scholar] [CrossRef]
- Xian, Z.; Guo, F.; Chen, M.; Wang, Y.; Zhang, Z.; Wu, H.; Dai, J.; Zhang, X.; Chen, Y. Plant-Microbe Involvement: How Manganese Achieves Harmonious Nitrogen-Removal and Carbon-Reduction in Constructed Wetlands. Bioresour. Technol. 2024, 402, 130794. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.R.; Dubey, K.; Goswami, S.; Hasija, S.; Pandey, R.; Singh, P.K.; Singh, B.; Sareen, S.; Rai, G.K.; Singh, G.P.; et al. Heterologous Expression and Characterization of Novel Manganese Superoxide Dismutase (Mn-SOD)—A Potential Biochemical Marker for Heat Stress-Tolerance in Wheat (Triticum aestivum). Int. J. Biol. Macromol. 2020, 161, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zhang, Y.; Liu, S.; Wang, H.; Gao, F.; Shao, G. 24-Epibrassinolide Mitigates Cadmium Toxicity in Rice Plants by Activating the Plant Detoxification System and Regulating Genes Expression Involved in Cd/Fe Uptake and Translocation. Plant Stress 2024, 12, 100485. [Google Scholar] [CrossRef]
- Ferreyroa, G.V.; Lagorio, M.G.; Trinelli, M.A.; Lavado, R.S.; Molina, F.V. Lead Effects on Brassica Napus Photosynthetic Organs. Ecotoxicol. Environ. Saf. 2017, 140, 123–130. [Google Scholar] [CrossRef]
- Dhir, B.; Sharmila, P.; Saradhi, P.P.; Nasim, S.A. Physiological and Antioxidant Responses of Salvinia Natans Exposed to Chromium-Rich Wastewater. Ecotoxicol. Environ. Saf. 2009, 72, 1790–1797. [Google Scholar] [CrossRef]
- Gillet, S.; Decottignies, P.; Chardonnet, S.; Le Maréchal, P. Cadmium Response and Redoxin Targets in Chlamydomonas Reinhardtii: A Proteomic Approach. Photosynth. Res. 2006, 89, 201–211. [Google Scholar] [CrossRef]
- Kaur, M.; Sandhu, K.S.; Singh, N. Comparative Study of the Functional, Thermal and Pasting Properties of Flours from Different Field Pea (Pisum sativum L.) and Pigeon Pea (Cajanus cajan L.) Cultivars. Food Chem. 2007, 104, 259–267. [Google Scholar] [CrossRef]
- Jan, S.; Noman, A.; Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. 24-Epibrassinolide Alleviates the Injurious Effects of Cr(VI) Toxicity in Tomato Plants: Insights into Growth, Physio-Biochemical Attributes, Antioxidant Activity and Regulation of Ascorbate–Glutathione and Glyoxalase Cycles. J. Plant Growth Regul. 2020, 39, 1587–1604. [Google Scholar] [CrossRef]
- Liang, B.; Li, C.; Ma, C.; Wei, Z.; Wang, Q.; Huang, D.; Chen, Q.; Li, C.; Ma, F. Dopamine Alleviates Nutrient Deficiency-Induced Stress in Malus Hupehensis. Plant Physiol. Biochem. 2017, 119, 346–359. [Google Scholar] [CrossRef]
- Silva, S.; Pinto, G.; Santos, C. Low Doses of Pb Affected Lactuca Sativa Photosynthetic Performance. Photosynthetica 2017, 55, 50–57. [Google Scholar] [CrossRef]
- Figlioli, F.; Sorrentino, M.C.; Memoli, V.; Arena, C.; Maisto, G.; Giordano, S.; Capozzi, F.; Spagnuolo, V. Overall Plant Responses to Cd and Pb Metal Stress in Maize: Growth Pattern, Ultrastructure, and Photosynthetic Activity. Environ. Sci. Pollut. Res. 2018, 26, 1781–1790. [Google Scholar] [CrossRef]
- Lan, G.; Jiao, C.; Wang, G.; Sun, Y.; Sun, Y. Effects of Dopamine on Growth, Carbon Metabolism, and Nitrogen Metabolism in Cucumber under Nitrate Stress. Sci. Hortic. 2020, 260, 108790. [Google Scholar] [CrossRef]
- Kohli, S.K.; Handa, N.; Bali, S.; Khanna, K.; Arora, S.; Sharma, A.; Bhardwaj, R. Current Scenario of Pb Toxicity in Plants: Unraveling Plethora of Physiological Responses. In Reviews of Environmental Contamination and Toxicology; Springer: Cham, Switzerland, 2019; Volume 249, pp. 153–197. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Ji, G. Responses of Biomass Allocation and Photosynthesis in Mulberry to Pb-Contaminated Soil. Acta Physiol. Plant. 2022, 44, 43. [Google Scholar] [CrossRef]
- Burzyński, M.; Kłobus, G. Changes of Photosynthetic Parameters in Cucumber Leaves Under Cu, Cd, and Pb Stress. Photosynthetica 2004, 42, 505–510. [Google Scholar] [CrossRef]
- Pereira-Matos, Y.C.; Lima, E.J.d.F.; Ribeiro, A.T.; Lange, C.N.; Batista, B.L.; El-Beltagi, H.S.; Bajguz, A.; da Silva Lobato, A.K. Exogenous 24-Epibrassinolide Reverses Disturbances in Zinc-Stressed Tomato by Synergistically Stimulating Leaf Structures, Photosynthesis and Growth. S. Afr. J. Bot. 2023, 159, 447–460. [Google Scholar] [CrossRef]
- Shu, S.; Tang, Y.; Yuan, Y.; Sun, J.; Zhong, M.; Guo, S. The Role of 24-Epibrassinolide in the Regulation of Photosynthetic Characteristics and Nitrogen Metabolism of Tomato Seedlings Under a Combined Low Temperature and Weak Light Stress. Plant Physiol. Biochem. 2016, 107, 344–353. [Google Scholar] [CrossRef]
- Cunha, L.F.S.; Oliveira, V.P.; Nascimento, A.W.S.; Silva, B.R.S.; Batista, B.L.; Alsahli, A.A.; da Silva Lobato, A.K. Leaf Application of 24-Epibrassinolide Mitigates Cadmium Toxicity in Young Eucalyptus Urophylla Plants by Modulating Leaf Anatomy and Gas Exchange. Physiol. Plant. 2020, 173, 67–87. [Google Scholar] [CrossRef]
- Mohanta, T.K.; Bashir, T.; Hashem, A.; Abd Allah, E.F.; Khan, A.L.; Al-Harrasi, A.S. Early Events in Plant Abiotic Stress Signaling: Interplay Between Calcium, Reactive Oxygen Species and Phytohormones. J. Plant Growth Regul. 2018, 37, 1033–1049. [Google Scholar] [CrossRef]
- Mehla, N.; Sindhi, V.; Josula, D.; Bisht, P.; Wani, S. Reactive Oxygen Species and Antioxidant Systems in Plants: Role and Regulation Under Abiotic Stress; Springer: Singapore, 2017; pp. 1–329. [Google Scholar] [CrossRef]
- Lan, G.; Shi, L.; Lu, X.; Liu, Z.; Sun, Y. Effects of Dopamine on Antioxidation, Mineral Nutrients, and Fruit Quality in Cucumber Under Nitrate Stress. J. Plant Growth Regul. 2022, 41, 2918–2929. [Google Scholar] [CrossRef]
- Gupta, D.K.; Palma, J.M.; Corpas, F.J. Revisiting Carotenoids and Their Role in Plant Stress Responses: From Biosynthesis to Plant Signaling Mechanisms during Stress. In Antioxidants and Antioxidant Enzymes in Higher Plants; Springer International Publishing: Cham, Switzerlands, 2018; pp. 207–232. [Google Scholar] [CrossRef]
- Berni, R.; Luyckx, M.; Xu, X.; Legay, S.; Sergeant, K.; Hausman, J.-F.; Lutts, S.; Cai, G.; Guerriero, G. Reactive Oxygen Species and Heavy Metal Stress in Plants: Impact on the Cell Wall and Secondary Metabolism. Environ. Exp. Bot. 2019, 161, 98–106. [Google Scholar] [CrossRef]
- Zhong, W.; Xie, C.; Hu, D.; Pu, S.; Xiong, X.; Ma, J.; Sun, L.; Huang, Z.; Jiang, M.; Li, X. Effect of 24-Epibrassinolide on Reactive Oxygen Species and Antioxidative Defense Systems in Tall Fescue Plants under Lead Stress. Ecotoxicol. Environ. Saf. 2020, 187, 109831. [Google Scholar] [CrossRef]
- Kour, J.; Kohli, S.; Khanna, K.; Bakshi, P.; Sharma, P.; Singh, A.D.; Ibrahim, M.; Devi, K.; Sharma, N.; Ohri, P.; et al. Brassinosteroid Signaling, Crosstalk and Physiological Functions in Plants Under Heavy Metal Stress. Front. Plant Sci. 2021, 12, 608061. [Google Scholar] [CrossRef]
- Santos, L.R.; da Silva, B.R.S.; Pedron, T.; Batista, B.L.; Lobato, A.K.D.S. 24-Epibrassinolide Improves Root Anatomy and Antioxidant Enzymes in Soybean Plants Subjected to Zinc Stress. J. Soil. Sci. Plant Nutr. 2020, 20, 105–124. [Google Scholar] [CrossRef]
- Kumar, A.; Prasad, M.N.V. Plant-Lead Interactions: Transport, Toxicity, Tolerance, and Detoxification Mechanisms. Ecotoxicol. Environ. Saf. 2018, 166, 401–418. [Google Scholar] [CrossRef]
- Yüce, M.; Ekinci, M.; Yildirim, E.; Turan, M.; Ors, S. Changes in Bean Plant Growth in Lead-Contaminated Soil. In Proceedings of the International Conference on Agriculture, Virtual, 11–12 August 2022; Volume 7, pp. 29–33. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants Without Soil, 2nd ed.; California Agricultural Experiment Station: Berkeley, CA, USA, 1950; 58p. [Google Scholar]
- Jiao, X.; Li, Y.; Zhang, X.; Liu, C.; Liang, W.; Li, C.; Ma, F.; Li, C. Exogenous Dopamine Application Promotes Alkali Tolerance of Apple Seedlings. Plants 2019, 8, 580. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; Choudhary, S.P.; Chen, S.; Xia, X.; Shi, K.; Zhou, Y.; Yu, J. Role of Brassinosteroids in Alleviation of Phenanthrene–Cadmium Co-Contamination-Induced Photosynthetic Inhibition and Oxidative Stress in Tomato. J. Exp. Bot. 2013, 64, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Maia, C.F.; da Silva, B.R.S.; Batista, B.L.; Bajguz, A.; Lobato, A.K.D.S. 24-Epibrassinolide Simultaneously Stimulates Photosynthetic Machinery and Biomass Accumulation in Tomato Plants Under Lead Stress: Essential Contributions Connected to the Antioxidant System and Anatomical Structures. Agronomy 2022, 12, 1985. [Google Scholar] [CrossRef]
- Paniz, F.P.; Pedron, T.; Freire, B.M.; Torres, D.P.; Silva, F.F.; Batista, B.L. Effective Procedures for the Determination of As, Cd, Cu, Fe, Hg, Mg, Mn, Ni, Pb, Se, Th, Zn, U and Rare Earth Elements in Plants and Foodstuffs. Anal. Methods 2018, 10, 4094–4103. [Google Scholar] [CrossRef]
- Ma, C.C.; Gao, Y.B.; Guo, H.Y.; Wang, J.L. Photosynthesis, Transpiration, and Water Use Efficiency of Caragana microphylla, C. intermedia, and C. korshinskii. Photosynthetica 2004, 42, 65–70. [Google Scholar] [CrossRef]
- Aragão, R.M.; Silva, E.N.; Vieira, C.F.; Silveira, J.A.G. High Supply of NO3−Mitigates Salinity Effects Through an Enhancement in the Efficiency of Photosystem II and CO2 Assimilation in Jatropha curcas Plants. Acta Physiol. Plant. 2012, 34, 2135–2143. [Google Scholar] [CrossRef]
- Badawi, G.H.; Yamauchi, Y.; Shimada, E.; Sasaki, R.; Kawano, N.; Tanaka, K.; Tanaka, K. Enhanced Tolerance to Salt Stress and Water Deficit by Overexpressing Superoxide Dismutase in Tobacco (Nicotiana tabacu) Chloroplasts. Plant Sci. 2004, 166, 919–928. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases: I. Occurrence in Higher Plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Havir, E.A.; McHale, N.A. Biochemical and Developmental Characterization of Multiple Forms of Catalase in Tobacco Leaves. Plant Physiol. 1987, 84, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen Peroxide Is Scavenged by Ascorbate-Specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Cakmak, I.; Marschner, H. Magnesium Deficiency and High Light Intensity Enhance Activities of Superoxide Dismutase, Ascorbate Peroxidase, and Glutathione Reductase in Bean Leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef]
- Elstner, E.F.; Heupel, A. Inhibition of Nitrite Formation from Hydroxylammoniumchloride: A Simple Assay for Superoxide Dismutase. Anal. Biochem. 1976, 70, 616–620. [Google Scholar] [CrossRef]
- Wu, Q.-S.; Xia, R.-X.; Zou, Y.-N. Reactive Oxygen Metabolism in Mycorrhizal and Non-Mycorrhizal Citrus (Poncirus trifoliata) Seedlings Subjected to Water Stress. J. Plant Physiol. 2006, 163, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative Stress and Some Antioxidant Systems in Acid Rain-Treated Bean Plants Protective Role of Exogenous Polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Cakmak, I.; Horst, W.J. Effect of Aluminium on Lipid Peroxidation, Superoxide Dismutase, Catalase, and Peroxidase Activities in Root Tips of Soybean (Glycine max). Physiol. Plant. 1991, 83, 463–468. [Google Scholar] [CrossRef]
- Gong, M.; Li, Y.-J.; Chen, S.-Z. Abscisic Acid-Induced Thermotolerance in Maize Seedlings Is Mediated by Calcium and Associated with Antioxidant Systems. J. Plant Physiol. 1998, 153, 488–496. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV—VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Steel, R.G.; Torrie, J.H.; Dickey, D.A. Principles and Procedures of Statistics: A Biometrical Approach, 3rd ed.; Academic Internet Publishers: Moorpark, CA, USA, 2006. [Google Scholar]
- Venables, W.N.; Smith, D.M.; R Core Team. An Introduction to R; R Core Team: Vienna, Austria, 2021; 105p. [Google Scholar]

| Pb2+ | DOP | EBR | Pb in Leaf (µg g DM−1) | Pb in Stem (µg g DM−1) | Pb in Root (µg g DM−1) |
|---|---|---|---|---|---|
| − | − | − | 0.000 ± 0.000 d | 0.000 ± 0.000 d | 0.000 ± 0.000 d |
| − | − | + | 0.000 ± 0.000 d | 0.000 ± 0.000 d | 0.000 ± 0.000 d |
| − | + | − | 0.000 ± 0.000 d | 0.000 ± 0.000 d | 0.000 ± 0.000 d |
| − | + | + | 0.000 ± 0.000 d | 0.000 ± 0.000 d | 0.000 ± 0.000 d |
| + | − | − | 0.106 ± 0.004 a | 0.011 ± 0.001 a | 0.132 ± 0.008 a |
| + | − | + | 0.031 ± 0.007 d | 0.004 ± 0.001 c | 0.073 ± 0.003 c |
| + | + | − | 0.057 ± 0.004 b | 0.007 ± 0.001 b | 0.093 ± 0.003 b |
| + | + | + | 0.044 ± 0.003 c | 0.006 ± 0.001 b | 0.076 ± 0.006 c |
| Pb2+ | DOP | EBR | Mg (mg g DM−1) | K (mg g DM−1) | Ca (mg g DM−1) | Cu (µg g DM−1) | Zn (µg g DM−1) | Mn (µg g DM−1) |
|---|---|---|---|---|---|---|---|---|
| Contents in root | ||||||||
| − | − | − | 6.84 ± 0.26 c | 35.22 ± 3.04 d | 3.50 ± 0.15 c | 10.17 ± 0.10 d | 33.81 ± 0.94 c | 118.82 ± 2.18 d |
| − | − | + | 8.38 ± 0.22 a | 47.70 ± 2.35 a | 5.52 ± 0.17 a | 13.45 ± 0.20 a | 41.19 ± 1.42 a | 174.52 ± 5.13 a |
| − | + | − | 7.42 ± 0.12 b | 38.27 ± 0.21 c | 4.51 ± 0.18 b | 11.49 ± 0.27 c | 36.34 ± 1.66 b | 143.84 ± 4.41 c |
| − | + | + | 7.54 ± 0.26 b | 41.05 ± 1.73 b | 5.34 ± 0.20 a | 12.55 ± 0.30 b | 37.14 ± 1.57 b | 163.77 ± 1.76 b |
| + | − | − | 3.31 ± 0.15 f | 0.83 ± 0.08 e | 1.57 ± 0.10 f | 7.06 ± 0.53 f | 9.60 ± 1.54 g | 44.94 ± 1.95 f |
| + | − | + | 4.45 ± 0.15 d | 2.46 ± 0.13 e | 2.29 ± 0.17 d | 8.73 ± 0.17 e | 18.17 ± 0.95 d | 57.95 ± 2.13 e |
| + | + | − | 3.75 ± 0.28 e | 1.13 ± 0.07 e | 1.83 ± 0.07 e | 8.40 ± 0.12 e | 12.31 ± 0.69 f | 48.53 ± 2.26 f |
| + | + | + | 4.31 ± 0.14 d | 1.69 ± 0.12 e | 2.19 ± 0.16 d | 8.71 ± 0.15 e | 15.75 ± 1.04 e | 55.88 ± 1.72 e |
| Contents in leaf | ||||||||
| − | − | − | 6.40 ± 0.25 d | 46.16 ± 2.24 b | 13.24 ± 0.90 c | 8.27 ± 0.09 d | 30.78 ± 1.60 c | 46.33 ± 1.33 c |
| − | − | + | 8.36 ± 0.08 a | 55.01 ± 2.62 a | 18.62 ± 0.97 a | 10.50 ± 0.21 a | 37.58 ± 1.37 a | 58.25 ± 1.72 a |
| − | + | − | 7.17 ± 0.14 c | 52.58 ± 0.66 a | 15.80 ± 0.84 b | 9.27 ± 0.15 c | 34.55 ± 2.05 b | 51.33 ± 1.06 b |
| − | + | + | 7.52 ± 0.26 b | 53.94 ± 1.65 a | 17.82 ± 0.83 a | 9.63 ± 0.11 b | 35.78 ± 1.36 b | 56.52 ± 0.69 a |
| + | − | − | 5.53 ± 0.28 e | 31.03 ± 1.79 d | 11.00 ± 1.00 f | 5.53 ± 0.04 f | 7.71 ± 1.54 f | 37.95 ± 1.91 d |
| + | − | + | 7.78 ± 0.37 b | 46.91 ± 1.62 b | 13.45 ± 0.72 d | 8.18 ± 0.45 d | 13.91 ± 1.33 d | 47.96 ± 2.33 c |
| + | + | − | 6.34 ± 0.14 d | 35.43 ± 1.93 c | 11.91 ± 0.86 e | 7.01 ± 0.13 e | 10.49 ± 1.13 e | 46.36 ± 1.15 c |
| + | + | + | 6.52 ± 0.39 d | 44.84 ± 1.39 b | 12.48 ± 1.05 d | 8.02 ± 0.38 d | 12.40 ± 0.96 d | 47.26 ± 1.40 c |
| Pb2+ | DOP | EBR | Chl a (mg g–1 FM) | Chl b (mg g–1 FM) | Total Chl (mg g–1 FM) | Car (mg g–1 FM) | Ratio Chl a/Chl b | Ratio Total Chl/Car |
|---|---|---|---|---|---|---|---|---|
| − | − | − | 9.04 ± 0.26 c | 5.48 ± 0.11 c | 14.52 ± 0.17 d | 0.74 ± 0.03 c | 1.65 ± 0.08 b | 19.54 ± 0.81 d |
| − | − | + | 10.20 ± 0.16 a | 7.24 ± 0.21 a | 17.44 ± 0.15 a | 0.96 ± 0.02 a | 1.41 ± 0.06 c | 18.14 ± 0.47 e |
| − | + | − | 9.72 ± 0.13 b | 6.70 ± 0.16 b | 16.42 ± 0.20 c | 0.89 ± 0.02 b | 1.45 ± 0.04 c | 18.42 ± 0.25 e |
| − | + | + | 9.92 ± 0.19 b | 6.90 ± 0.07 b | 16.82 ± 0.22 b | 0.92 ± 0.02 a | 1.44 ± 0.03 c | 18.21 ± 0.31 e |
| + | − | − | 5.70 ± 0.23 f | 3.08 ± 0.13 f | 8.78 ± 0.26 g | 0.33 ± 0.1 f | 1.85 ± 0.12 a | 26.46 ± 0.82 a |
| + | − | + | 8.08 ± 0.20 d | 4.66 ± 0.11 d | 12.74 ± 0.15 e | 0.58 ± 0.02 d | 1.74 ± 0.08 b | 22.07 ± 0.88 c |
| + | + | − | 7.63 ± 0.12 e | 4.24 ± 0.11 e | 11.87 ± 0.20 f | 0.51 ± 0.01 e | 1.80 ± 0.04 a | 23.28 ± 0.39 b |
| + | + | + | 7.76 ± 0.10 e | 4.36 ± 0.13 e | 12.12 ± 0.22 f | 0.55 ± 0.04 e | 1.78 ± 0.04 a | 22.20 ± 1.45 c |
| Pb2+ | DOP | EBR | ΦPSII | qP | NPQ | ETR (µmol m−2 s−1) | EXC (µmol m−2 s−1) | ETR/PN |
|---|---|---|---|---|---|---|---|---|
| − | − | − | 0.27 ± 0.01 c | 0.71 ± 0.03 c | 0.32 ± 0.08 c | 40.54 ± 2.44 d | 0.63 ± 0.02 c | 2.83 ± 0.08 b |
| − | − | + | 0.41 ± 0.02 a | 0.89 ± 0.02 a | 0.15 ± 0.01 e | 60.58 ± 3.49 a | 0.49 ± 0.03 e | 3.52 ± 0.23 a |
| − | + | − | 0.35 ± 0.01 b | 0.79 ± 0.02 b | 0.22 ± 0.04 d | 52.56 ± 2.48 c | 0.55 ± 0.02 d | 3.35 ± 0.21 a |
| − | + | + | 0.38 ± 0.02 a | 0.87 ± 0.05 a | 0.18 ± 0.01 d | 56.56 ± 3.38 b | 0.51 ± 0.03 e | 3.40 ± 0.22 a |
| + | − | − | 0.13 ± 0.01 f | 0.48 ± 0.01 e | 0.57 ± 0.09 a | 20.00 ± 1.71 g | 0.75 ± 0.01 a | 2.17 ± 0.18 c |
| + | − | + | 0.26 ± 0.01 c | 0.68 ± 0.03 c | 0.41 ± 0.06 b | 38.22 ± 1.79 d | 0.66 ± 0.01 c | 2.85 ± 0.10 b |
| + | + | − | 0.19 ± 0.01 e | 0.62 ± 0.04 d | 0.53 ± 0.08 a | 28.52 ± 1.67 f | 0.73 ± 0.02 a | 2.61 ± 0.22 b |
| + | + | + | 0.23 ± 0.01 d | 0.63 ± 0.02 d | 0.44 ± 0.05 b | 33.82 ± 2.08 e | 0.70 ± 0.02 b | 2.69 ± 0.21 b |
| Pb2+ | DOP | EBR | PN (µmol m−2 s−1) | E (mmol m−2 s−1) | gs (mol m−2 s−1) | Ci (µmol mol−1) | WUE (µmol mmol–1) | PN/Ci (µmol m−2 s−1 Pa−1) |
|---|---|---|---|---|---|---|---|---|
| − | − | − | 14.30 ± 0.53 c | 2.87 ± 0.10 d | 0.38 ± 0.01 b | 308 ± 9 b | 4.99 ± 0.28 d | 0.046 ± 0.003 d |
| − | − | + | 17.22 ± 0.50 a | 2.72 ± 0.09 d | 0.43 ± 0.01 a | 224 ± 10 d | 6.35 ± 0.22 a | 0.077 ± 0.005 a |
| − | + | − | 15.66 ± 0.51 b | 2.83 ± 0.14 d | 0.40 ± 0.02 b | 278 ± 11 c | 5.53 ± 0.19 c | 0.056 ± 0.002 c |
| − | + | + | 16.62 ± 0.31 a | 2.79 ± 0.14 d | 0.41 ± 0.02 a | 239 ± 6 d | 5.96 ± 0.22 b | 0.070 ± 0.003 b |
| + | − | − | 9.17 ± 0.16 g | 3.77 ± 0.08 a | 0.17 ± 0.01 e | 344 ± 19 a | 2.43 ± 0.03 h | 0.027 ± 0.001 f |
| + | − | + | 13.40 ± 0.38 d | 3.06 ± 0.09 c | 0.25 ± 0.01 c | 271 ± 9 c | 4.38 ± 0.22 e | 0.050 ± 0.003 d |
| + | + | − | 10.92 ± 0.46 f | 3.33 ± 0.04 b | 0.21 ± 0.01 d | 297 ± 12 b | 3.28 ± 0.14 g | 0.037 ± 0.003 e |
| + | + | + | 12.56 ± 0.41 e | 3.11 ± 0.06 c | 0.23 ± 0.01 c | 277 ± 9 c | 4.04 ± 0.16 f | 0.045 ± 0.002 d |
| Pb2+ | DOP | EBR | LDM (g plant−1) | RDM (g plant−1) | SDM (g plant−1) | TDM (g plant−1) |
|---|---|---|---|---|---|---|
| − | − | − | 6.47 ± 0.28 d | 2.20 ± 0.04 e | 2.50 ± 0.07 d | 11.17 ± 0.36 f |
| − | − | + | 7.62 ± 0.07 a | 3.20 ± 0.14 a | 3.42 ± 0.08 a | 14.24 ± 0.16 a |
| − | + | − | 6.93 ± 0.09 c | 2.81 ± 0.08 c | 2.87 ± 0.11 c | 12.61 ± 0.09 c |
| − | + | + | 7.40 ± 0.14 b | 3.01 ± 0.11 b | 3.15 ± 0.18 b | 13.60 ± 0.30 b |
| + | − | − | 4.20 ± 0.11 e | 1.87 ± 0.07 f | 2.10 ± 0.04 e | 8.17 ± 0.20 g |
| + | − | + | 6.31 ± 0.22 d | 2.94 ± 0.17 b | 2.98 ± 0.13 c | 12.23 ± 0.27 d |
| + | + | − | 6.30 ± 0.04 d | 2.57 ± 0.15 d | 2.50 ± 0.16 d | 11.37 ± 0.24 f |
| + | + | + | 6.22 ± 0.16 d | 2.77 ± 0.12 c | 2.86 ± 0.07 c | 11.85 ± 0.35 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prestes, L.R.; Silva, S.G.A.d.; Silva, M.M.S.d.; Gonçalves, M.A.F.; Lobato, E.M.S.G.; Augusto, C.C.; Batista, B.L.; Lobato, A.K.d.S. Oxidative Stress and Negative Consequences on Photosystem II Occasioned by Lead Stress Are Mitigated by 24-Epibrassinolide and Dopamine in Tomato Plants. Plants 2025, 14, 3699. https://doi.org/10.3390/plants14233699
Prestes LR, Silva SGAd, Silva MMSd, Gonçalves MAF, Lobato EMSG, Augusto CC, Batista BL, Lobato AKdS. Oxidative Stress and Negative Consequences on Photosystem II Occasioned by Lead Stress Are Mitigated by 24-Epibrassinolide and Dopamine in Tomato Plants. Plants. 2025; 14(23):3699. https://doi.org/10.3390/plants14233699
Chicago/Turabian StylePrestes, Lohana Ribeiro, Sharon Graziela Alves da Silva, Madson Mateus Santos da Silva, Maria Andressa Fernandes Gonçalves, Elaine Maria Silva Guedes Lobato, Caroline Cristine Augusto, Bruno Lemos Batista, and Allan Klynger da Silva Lobato. 2025. "Oxidative Stress and Negative Consequences on Photosystem II Occasioned by Lead Stress Are Mitigated by 24-Epibrassinolide and Dopamine in Tomato Plants" Plants 14, no. 23: 3699. https://doi.org/10.3390/plants14233699
APA StylePrestes, L. R., Silva, S. G. A. d., Silva, M. M. S. d., Gonçalves, M. A. F., Lobato, E. M. S. G., Augusto, C. C., Batista, B. L., & Lobato, A. K. d. S. (2025). Oxidative Stress and Negative Consequences on Photosystem II Occasioned by Lead Stress Are Mitigated by 24-Epibrassinolide and Dopamine in Tomato Plants. Plants, 14(23), 3699. https://doi.org/10.3390/plants14233699









