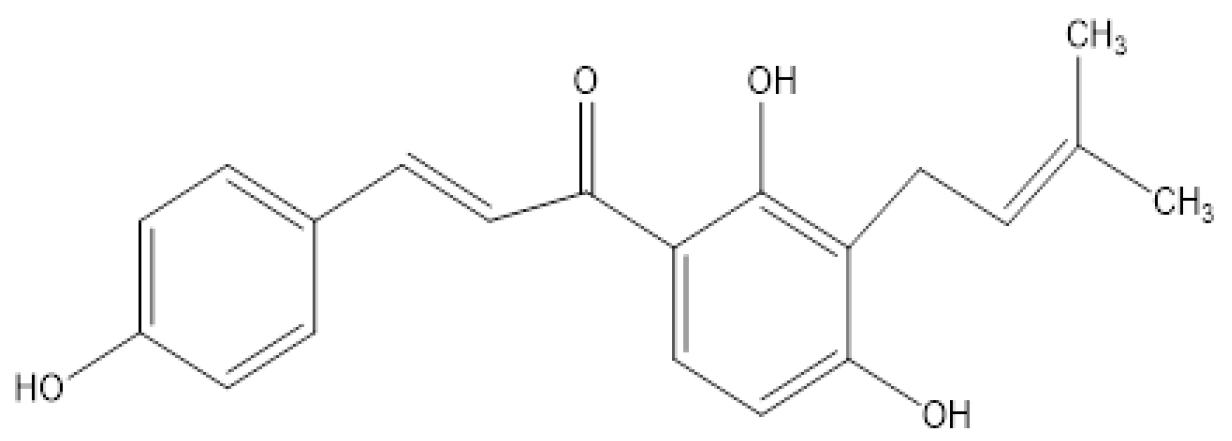
Isobavachalcone Alleviates Plant Photosynthesis Inhibition Caused by Tobacco Mosaic Virus (TMV) Infection in Tobacco
Abstract
1. Introduction
2. Results
2.1. Effects of IBC on Physiological and Biochemical Changes in Tobacco Leaves Under TMV Stress
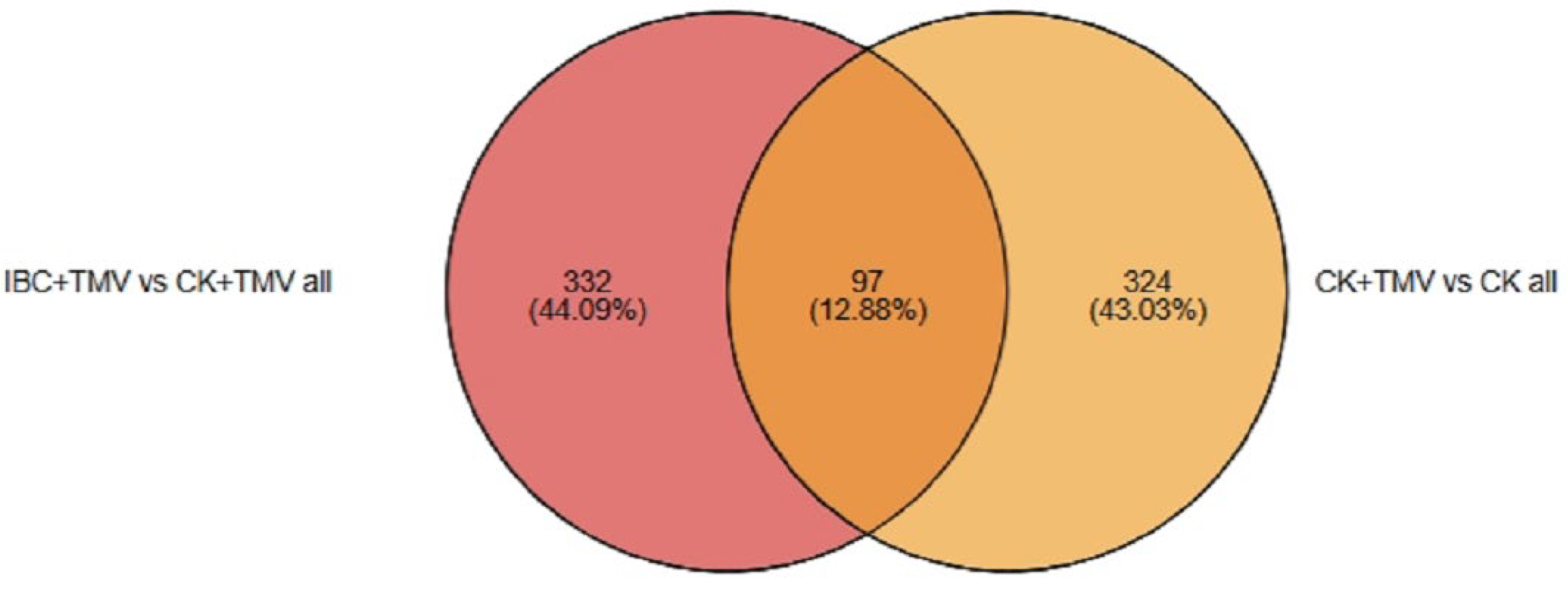
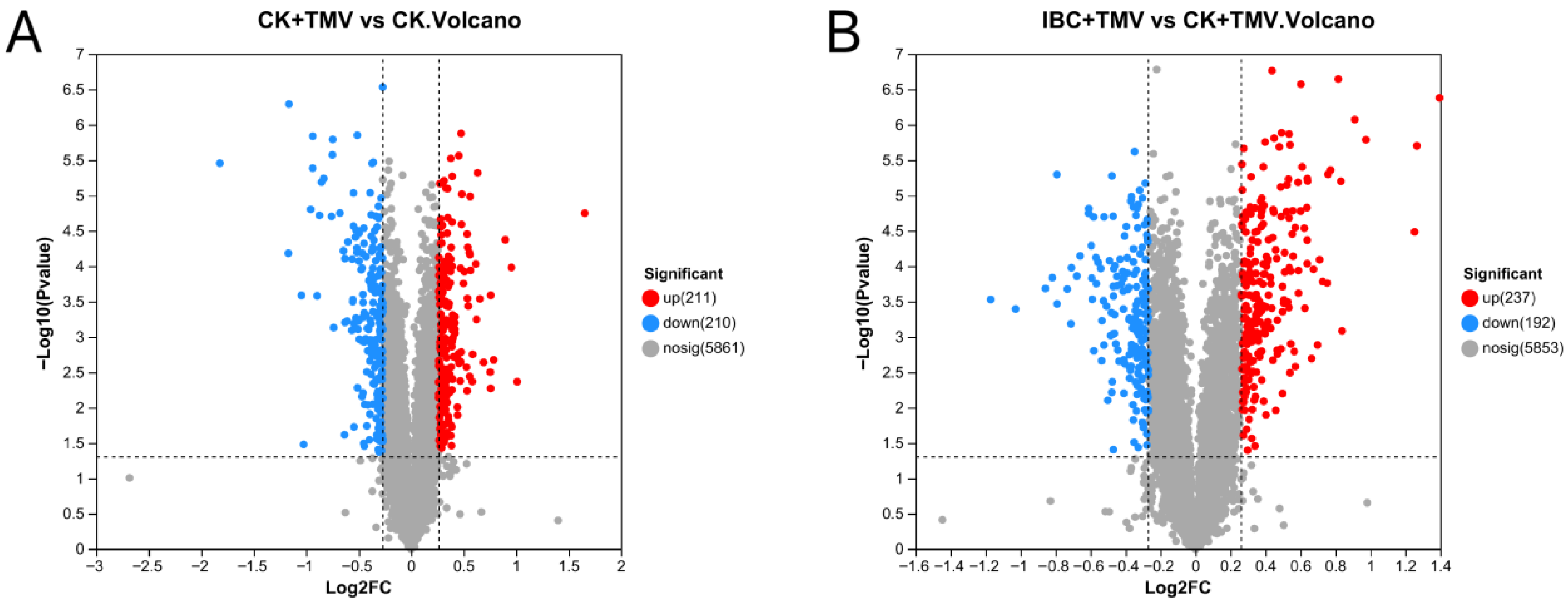
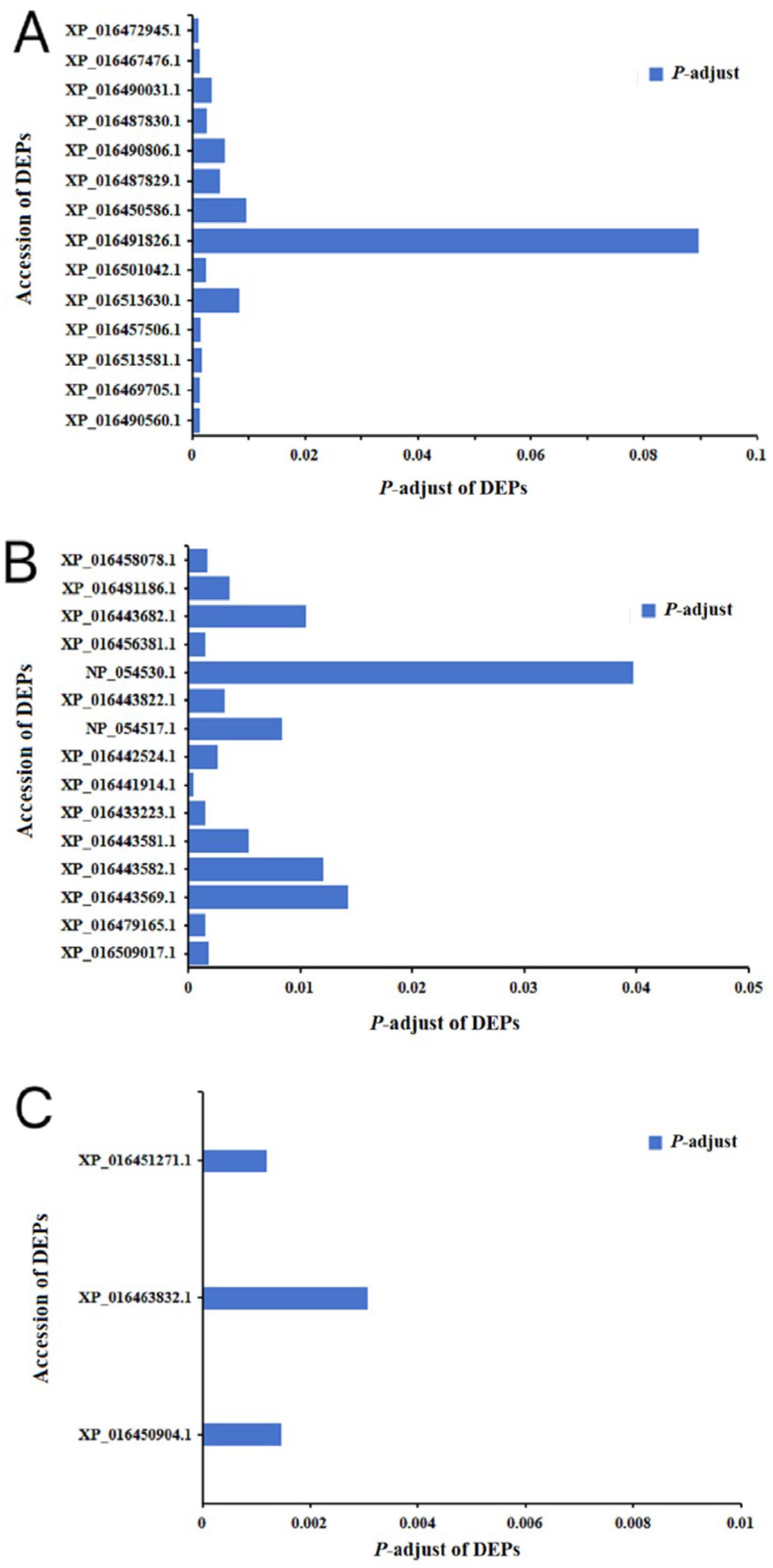
2.2. Differential Expression Protein Analysis
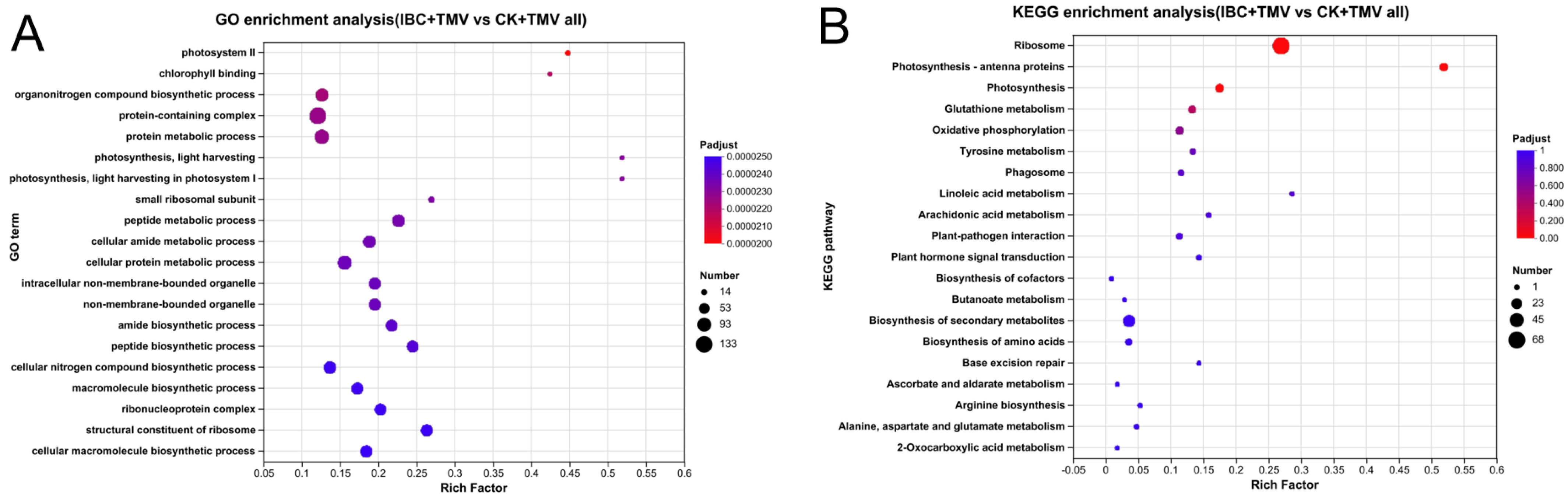
2.3. GO and KEGG Enrichment Analysis
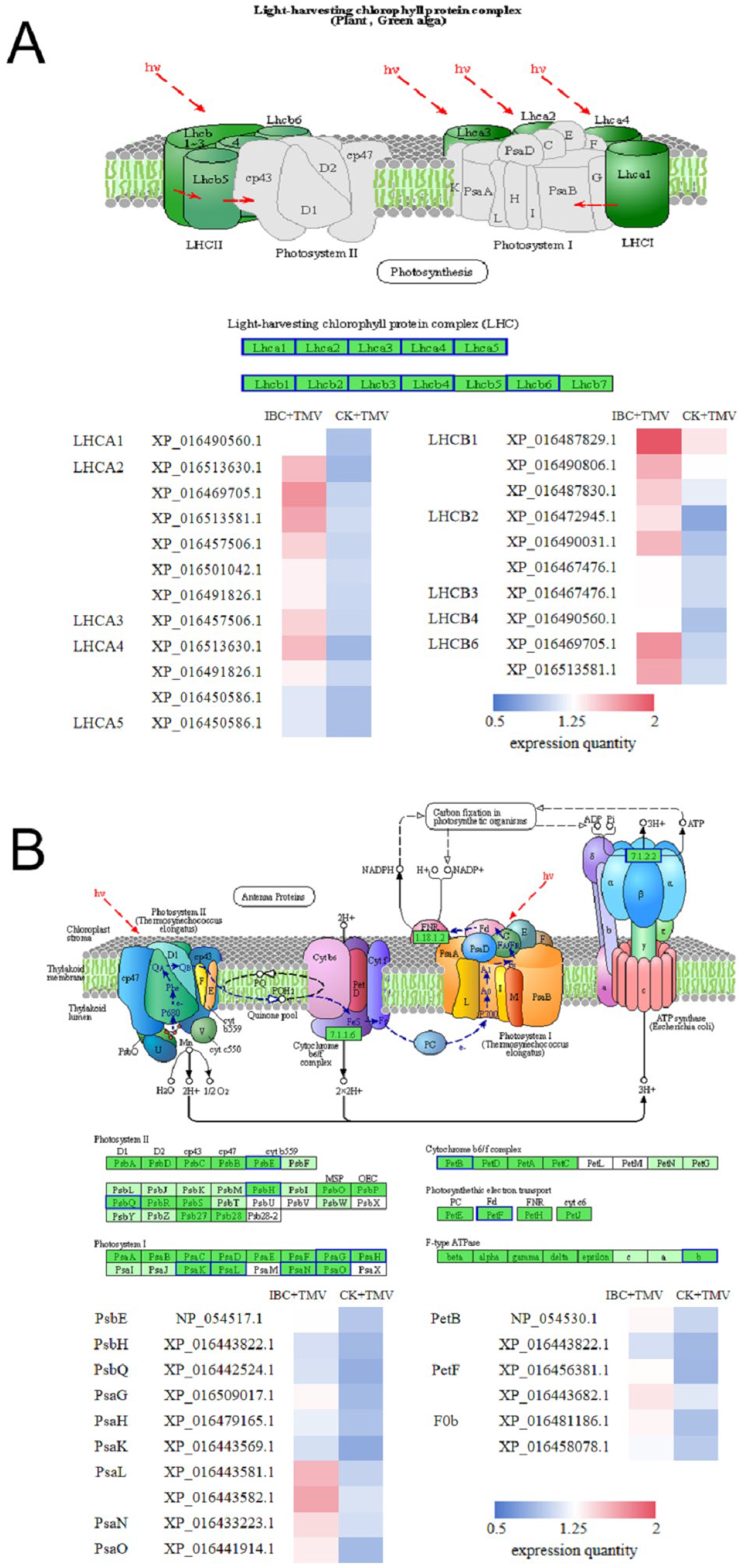
2.4. Analysis of DEPs in the Photosynthetic Antenna Proteins Pathway
2.5. Analysis of DEPs in the Photosynthesis Pathway
2.6. Analysis of DEPs in the Photosynthetic Pathway Carbon Fixation
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Virus
4.2. Sample Treatment
4.3. Determination of Chlorophyll Content
4.4. Determination of Rubisco Activity
4.5. Determination of Photosynthetic Parameters
4.6. Protein Preparation
4.7. Reduction, Alkylation and Enzymatic Digestion
4.8. TMT Labeling and Peptide Fractionation
4.9. LC-Tandem Mass Spectrometry (MS/MS) Quantitative Proteomics Analysis
4.10. Database Search
4.11. Bioinformatic Analysis
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IBC | isobavachalcone |
| TMV | tobacco mosaic virus |
| Pn | net photosynthetic rate |
| Gs | stomatal conductance |
| Ci | intercellular CO2 concentration |
| Tr | transpiration rate |
| dpi | days post inoculation |
| MYMIV | mungbean yellow mosaic India virus |
| AMV | alfalfa mosaic virus |
| LHC | light-harvesting complex |
| TYLCV | tomato yellow leaf curl virus |
| PSbMV | pea seed-borne mosaic virus |
| BCMV | bean common mosaic virus |
| DEGs | differentially expressed genes |
| PSII | Photosystem II |
| PSI | Photosystem I |
| ATPase | ATP synthase |
| MCMV | maize chlorotic mottle virus |
| SCMV | sugarcane mosaic virus |
| DEIs | differentially expressed isoforms |
| CYVCV | citrus yellow vein clearing virus |
| PVY | potato virus Y |
| MLT | melatonin |
| SA | salicylic acid |
| SOD | superoxide dismutase |
| POD | peroxidase |
| PPO | polyphenol oxidase |
| PAL | phenylalanine ammonia-lyase |
| CAT | catalase |
| BP | biological processes |
| CC | cellular components |
| MF | molecular functions |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| DEPs | differentially expressed proteins |
| PC | plastocyanin |
| Fd | ferredoxin |
| FNR | ferredoxin-NADP+ reductase |
| cytc6 | cytochrome C6 |
| CMV | Cucumber mosaic cucumovirus |
| CHLG | chlorophyll synthase |
| CLH1 | Chlorophyllase 1 |
| CLH2 | Chlorophyllase 2 |
| PPH | pheophytin pheophorbide hydrolase |
| PMMoV | pepper mild mottle virus |
| RPI3 | ribose-5-phosphate isomerase 3 |
| RPI2 | ribose-5-phosphate isomerase 2 |
References
- Cheaib, A.; Killiny, N. Photosynthesis Responses to the Infection with Plant Pathogens. Mol. Plant Microbe Interact. 2025, 38, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Prigigallo, M.I.; Picciotti, U.; Bubici, G. Resistance-breaking strains of tomato spotted wilt virus hamper photosynthesis and protein synthesis pathways in a virus accumulation-dependent manner in Sw5-carrying tomatoes. Sci. Rep. 2025, 15, 3630. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Zhang, S.; Zhang, H.; Deng, X.; Jiang, T.; Chen, X.; Dong, L.; Yan, Q.; Zang, L.; Xing, Y.; et al. The transcriptional analysis of pepper shed light on a proviral role of light-harvesting chlorophyll a/b binding protein 13 during infection of pepper mild mottle virus. Front. Plant Sci. 2025, 16, 1533151. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, J.; Bi, H.; Zhang, P. Why mosaic? Gene expression profiling of African cassava mosaic virus-infected cassava reveals the effect of chlorophyll degradation on symptom development. J. Integr. Plant Biol. 2014, 56, 122–132. [Google Scholar] [CrossRef]
- Maravi, D.K.; Kumar, S.; Sahoo, L. NMR-Based Metabolomic Profiling of Mungbean Infected with Mungbean Yellow Mosaic India Virus. Appl. Biochem. Biotechnol. 2022, 194, 5808–5826. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Bashir, S.; El-Gendi, H.; Elbeaino, T.; El-Rahim, W.M.A.; Moawad, H. Protective Activity of Rhizobium leguminosarum bv. viciae Strain 33504-Mat209 against Alfalfa Mosaic Virus Infection in Faba Bean Plants. Plants 2023, 12, 2658. [Google Scholar] [CrossRef]
- Zheng, H.; Wen, F.; Zhang, C.; Luo, R.; Wu, Z. Novel 1,3,4-Thiadiazole Derivatives: Synthesis, Antiviral Bioassay and Regulation the Photosynthetic Pathway of Tobacco against TMV Infection. Int. J. Mol. Sci. 2023, 24, 8881. [Google Scholar] [CrossRef]
- Meng, F.; Ma, M.; Li, S.; Liang, P.; Liang, Y.; Shi, H.; Huang, S.; Su, H.; Deng, Y.; Akram, M.A.; et al. Genome-wide identification of light-harvesting chlorophyll a/b-binding (LHC) gene family in tomato and functional analysis of SlLhcb1.11 and SlELIP1 under cold stress. Genomics 2025, 117, 111022. [Google Scholar] [CrossRef]
- Reuveni, M.; Debbi, A.; Kutsher, Y.; Gelbart, D.; Zemach, H.; Belausov, E.; Levin, I.; Lapidot, M. Tomato yellow leaf curl virus effects on chloroplast biogenesis and cellular structure. Physiol. Mol. Plant Pathol. 2015, 92, 51–58. [Google Scholar] [CrossRef]
- Cerna, H.; Černý, M.; Habánová, H.; Šafářová, D.; Abushamsiya, K.; Navrátil, M.; Brzobohatý, B. Proteomics offers insight to the mechanism behind Pisum sativum L. response to pea seed-borne mosaic virus (PSbMV). J. Proteom. 2017, 153, 78–88. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, W.; Shen, W.; Li, M.; Fu, Y.; Li, Z.; Li, J.; Liu, H.; Su, X.; Zhang, B.; et al. Integrated transcriptome and microRNA sequencing analyses reveal gene responses in poplar leaves infected by the novel pathogen bean common mosaic virus (BCMV). Front. Plant Sci. 2023, 14, 1163232. [Google Scholar] [CrossRef]
- Nagao, R.; Ogawa, H.; Suzuki, T.; Dohmae, N.; Kato, K.; Nakajima, Y.; Shen, J.R. Biochemical evidence for the diversity of LHCI proteins in PSI-LHCI from the red alga Galdieria sulphuraria NIES-3638. Photosyn. Res. 2025, 163, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, J.; Min, Z.; Wang, J.; Hong, X.; Pei, X.; Rao, Z.; Xu, X. Structure of ATP synthase from an early photosynthetic bacterium Chloroflexus aurantiacus. Proc. Natl. Acad. Sci. USA 2025, 122, e2425824122. [Google Scholar] [CrossRef] [PubMed]
- Kono, M.; Kodama, H.; Tanigawa, K.; Terashima, I.; Yamori, W. Drastic Reduction in Cytochrome b6/f Complex Confers Robust PSI Photoprotection Under Fluctuating Light at the Expense of Photosynthetic Capacity. Physiol. Plant. 2025, 177, e70483. [Google Scholar] [CrossRef]
- Kono, M.; Kodama, H.; Tanigawa, K.; Terashima, I.; Yamori, W. Cyt b6/f complex fine-tunes PSI stability and photosynthetic capacity under fluctuating light. BioRxiv 2025. [Google Scholar] [CrossRef]
- Hao, K.; Yang, M.; Cui, Y.; Jiao, Z.; Gao, X.; Du, Z.; Wang, Z.; An, M.; Xia, Z.; Wu, Y. Transcriptomic and Functional Analyses Reveal the Different Roles of Vitamins C, E, and K in Regulating Viral Infections in Maize. Int. J. Mol. Sci. 2023, 24, 8012. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Braga, C.; Ramos, H.C.C.; Santos, J.S.; de Souza, G.A.R.; Rodrigues, A.S.; Pereira, M.G.; Campostrini, E. Papaya ringspot virus type P affects photochemical efficiency (JIP test parameters) in young papaya plants. Physiol. Mol. Plant Pathol. 2025, 140, 102894. [Google Scholar] [CrossRef]
- Kundu, S.; Chakraborty, D.; Pal, A. Proteomic analysis of salicylic acid induced resistance to Mungbean Yellow Mosaic India Virus in Vigna mungo. J. Proteom. 2011, 74, 337–349. [Google Scholar] [CrossRef]
- Souza, P.F.; Silva, F.D.; Carvalho, F.E.; Silveira, J.A.; Vasconcelos, I.M.; Oliveira, J.T. Photosynthetic and biochemical mechanisms of an EMS-mutagenized cowpea associated with its resistance to cowpea severe mosaic virus. Plant Cell Rep. 2017, 36, 219–234. [Google Scholar] [CrossRef]
- Reinero, A.; Beachy, R.N. Reduced Photosystem II Activity and Accumulation of Viral Coat Protein in Chloroplasts of Leaves Infected with Tobacco Mosaic Virus. Plant Physiol. 1989, 89, 111–116. [Google Scholar] [CrossRef]
- Bin, Y.; Zhang, Q.; Su, Y.; Wang, C.; Jiang, Q.; Song, Z.; Zhou, C. Transcriptome analysis of Citrus limon infected with Citrus yellow vein clearing virus. BMC Genom. 2023, 24, 65. [Google Scholar] [CrossRef] [PubMed]
- Ribalet, B.; John, S.; Milner, M.G.; Salongo, L.; Bertholet, A.M. Imaging of mitochondrial matrix pH dynamics reveals a functional interaction between the ADP/ATP carrier and ATP synthase to regulate H+ distribution. Pharmacol. Res. 2025, 221, 107973. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Jin, Y.; Ma, D.; Li, H.; Zhang, Z.; Dong, J.; Wang, T. Interaction between PVY HC-Pro and the NtCF1β-subunit reduces the amount of chloroplast ATP synthase in virus-infected tobacco. Sci. Rep. 2015, 5, 15605. [Google Scholar] [CrossRef] [PubMed]
- Perdomo, J.A.; Scales, J.C.; Lee, W.S.; Kanyuka, K.; Carmo-Silva, E. Down-regulation of wheat Rubisco activase isoforms expression by virus-induced gene silencing. Plant Direct 2024, 8, e583. [Google Scholar] [CrossRef]
- Xu, M.; Chen, J.; Huang, Y.; Shen, D.; Sun, P.; Xu, Y.; Tao, X. Dynamic Transcriptional Profiles of Arabidopsis thaliana Infected by tomato spotted wilt virus. Phytopathology 2020, 110, 153–163. [Google Scholar] [CrossRef]
- Real, N.; Garcia-Molina, A.; Stolze, S.C.; Harzen, A.; Nakagami, H.; Martín-Hernández, A.M. Comprehensive proteomic profiling of cucumber mosaic virus infection: Identifying key proteins and pathways involved in resistance and susceptibility in melon. BMC Plant Biol. 2025, 25, 434. [Google Scholar] [CrossRef]
- Sofy, A.R.; Sofy, M.R.; Hmed, A.A.; Dawoud, R.A.; Refaey, E.E.; Mohamed, H.I.; El-Dougdoug, N.K. Molecular Characterization of the alfalfa mosaic virus Infecting Solanum melongena in Egypt and the Control of Its Deleterious Effects with Melatonin and Salicylic Acid. Plants 2021, 10, 459. [Google Scholar] [CrossRef]
- Khan, S.; Alvi, A.F.; Fatma, M.; Al-Hashimi, A.; Sofo, A.; Khan, N.A. Relative effects of melatonin and hydrogen sulfide treatments in mitigating salt damage in wheat. Front. Plant Sci. 2024, 15, 1406092. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Z.; Zhou, B.; Yang, W.; Wang, Y. Salicylic acid improving salinity tolerance by enhancing photosynthetic capacity, osmotic adjustment and maintenance of Na/K homeostasis in faba bean seedlings. Chem. Biol. Technol. Agric 2025, 12, 89. [Google Scholar] [CrossRef]
- Lucas, J.A.; Ramos-Solano, B.; Gutierrez-Mañero, F.J.; Garcia-Villaraco, A. Enhancing tomato plant resistance to pathogens: The role of melatonin in boosting innate immunity and antioxidant defences. Plant Growth Regul. 2024, 104, 1435–1447. [Google Scholar] [CrossRef]
- An, L.; Guan, L. Study on antifungal activity of Psoralea corylifolia L. extract to Valsa mali Miygabe et Yamada. J. South Fruits China 2021, 50, 130–133. [Google Scholar] [CrossRef]
- Liu, X.; Guan, L. The Effect of Isobavachalcone on the Cell Wall Membrane of the Pathogenic Fungus of Rice Blast. Jiangsu Agric. Sci. 2019, 47, 117–121. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, H.; Sun, L.; Guan, L. Isobavachalcone Induces Tobacco Mosaic Virus (TMV) Resistance in Tobacco and Its Impact on the Activity of Several Defense Enzymes. Pesticide 2022, 61, 767–770. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, J.; Liu, L.; Zhou, Z.; Gao, H.; Zhang, Z.; Guan, L. Study on Tobacco Growth Promotion of Psoralea corylifolia L. Extract. Contemp. Chem. Ind. 2024, 53, 1134–1139. [Google Scholar] [CrossRef]
- Croft, H.; Chen, J.M.; Luo, X.; Bartlett, P.; Chen, B.; Staebler, R.M. Leaf chlorophyll content as a proxy for leaf photosynthetic capacity. Glob. Chang. Biol. 2017, 23, 3513–3524. [Google Scholar] [CrossRef]
- Li, Z.; Tang, Y.; Lan, G.; Yu, L.; Ding, S.; He, Z.; She, X. Cucumber Green Mottle Mosaic Virus Decreases Chlorophyll a Content in Cucurbit Crops by Upregulating the Key Gene in Chlorophyll Catabolic Pathway, Chlorophyllase 1. Plants 2025, 14, 3086. [Google Scholar] [CrossRef]
- Farahat, A.S.; El-Morsi, A.A.; Soweha, H.E.; Sofy, A.R.; Refaey, E.E. Metabolic changes of cucumber plants due to two CMV Egyptian isolates. Arab Uni. J. Agric. Sci. 2018, 26, 2019–2028. [Google Scholar] [CrossRef]
- Sofy, A.R.; Dawoud, R.A.; Sofy, M.R.; Mohamed, H.I.; Hmed, A.A.; El-Dougdoug, N.K. Improving Regulation of Enzymatic and Non-Enzymatic Antioxidants and Stress-Related Gene Stimulation in Cucumber mosaic cucumovirus-Infected Cucumber Plants Treated with Glycine Betaine, Chitosan and Combination. Molecules 2020, 25, 2341. [Google Scholar] [CrossRef]
- Xie, D.; Shi, J.; Zhang, A.; Lei, Z.; Zu, G.; Fu, Y.; Gan, X.; Yin, L.; Song, B.; Hu, D. Syntheses, antiviral activities and induced resistance mechanisms of novel quinazoline derivatives containing a dithioacetal moiety. Bioorg. Chem. 2018, 80, 433–443. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Qari, S.H.; Abu-Saied, M.A.A.; Khalil, A.M.; Younes, H.A.; Nehela, Y.; Behiry, S.I. Chitosan Nanoparticles Inactivate Alfalfa Mosaic Virus Replication and Boost Innate Immunity in Nicotiana glutinosa Plants. Plants 2021, 10, 2701. [Google Scholar] [CrossRef]
- Sinijadas, K.; Paul, A.; Radhika, N.S.; Johnson, J.M.; Manju, R.V.; Anuradha, T. Piriformospora indica suppresses the symptoms produced by Banana bract mosaic virus by inhibiting its replication and manipulating chlorophyll and carotenoid biosynthesis and degradation in banana. 3 Biotech. 2024, 14, 141. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, S.; Hurry, V.M.; Kelley, S.E.; Osmond, C.B.; Robinson, S.A.; Rohozinski, J.; Seaton, G.G.R.; Sims, D.A. Concepts of plant biotic stress. Some insights into the stress physiology of virus-infected plants, from the perspective of photosynthesis. Physiol. Plant 1997, 100, 203–213. [Google Scholar] [CrossRef]
- Tahjib-Ul-Arif, M.; Siddiqui, M.N.; Sohag, A.A.M.; Sakil, M.A.; Rahman, M.M.; Polash, M.A.S.; Mostofa, M.G.; Tran, L.S.P. Salicylic acid-mediated enhancement of photosynthesis attributes and antioxidant capacity contributes to yield improvement of maize plants under salt stress. J. Plant Growth Regul. 2018, 37, 1318–1330. [Google Scholar] [CrossRef]
- Pineda, M.; Gáspár, L.; Morales, F.; Szigeti, Z.; Barón, M. Multicolor fluorescence imaging of leaves--a useful tool for visualizing systemic viral infections in plants. Photochem. Photobiol. 2008, 84, 1048–1060. [Google Scholar] [CrossRef]
- Zhou, W.; Eudes, F.; Laroche, A. Identification of differentially regulated proteins in response to a compatible interaction between the pathogen Fusarium graminearum and its host, Triticum aestivum. Proteomics 2006, 6, 4599–4609. [Google Scholar] [CrossRef]
- Ishiga, Y.; Uppalapati, S.R.; Ishiga, T.; Elavarthi, S.; Martin, B.; Bender, C.L. The phytotoxin coronatine induces light-dependent reactive oxygen species in tomato seedlings. New Phytol. 2009, 181, 147–160. [Google Scholar] [CrossRef]
- Manacorda, C.A.; Gudesblat, G.; Sutka, M.; Alemano, S.; Peluso, F.; Oricchio, P.; Baroli, I.; Asurmendi, S. TuMV triggers stomatal closure but reduces drought tolerance in Arabidopsis. Plant Cell Environ. 2021, 44, 1399–1416. [Google Scholar] [CrossRef]
- Soni, S.K.; Mishra, M.K.; Mishra, M.; Kumari, S.; Saxena, S.; Shukla, V.; Tiwari, S.; Shirke, P. Papaya Leaf Curl Virus (PaLCuV) Infection on Papaya (Carica papaya L.) Plants Alters Anatomical and Physiological Properties and Reduces Bioactive Components. Plants 2022, 11, 579. [Google Scholar] [CrossRef]
- Hasan, A.; Mutaqin, K.H.; Taufik, M.; Hidayat, S.H. The Potential of a Low-Cost Thermal Camera for Early Detection of Temperature Changes in Virus-Infected Chili Plants. J. ICT Res. Appl. 2023, 17, 17–28. [Google Scholar] [CrossRef]
- Hussain, S.; Ulhassan, Z.; Brestic, M.; Zivcak, M.; Zhou, W.; Allakhverdiev, S.I.; Yang, X.; Safdar, M.E.; Yang, W.; Liu, W. Photosynthesis research under climate change. Photosynth. Res. 2021, 150, 5–19. [Google Scholar] [CrossRef]
- Sampol, B.; Bota, J.; Riera, D.; Medrano, H.; Flexas, J. Analysis of the virus-induced inhibition of photosynthesis in malmsey grapevines. New Phyto. 2021, 160, 403–412. [Google Scholar] [CrossRef]
- Souza, P.F.N.; Garcia-Ruiz, H.; Carvalho, F.E.L. What proteomics can reveal about plant-virus interactions? Photosynthesis-related proteins on the spotlight. Thor. Exp. Plant Physiol. 2019, 31, 227–248. [Google Scholar] [CrossRef]
- Han, Y.; Luo, Y.; Qin, S.; Xi, L.; Wan, B.; Du, L. Induction of systemic resistance against tobacco mosaic virus by Ningnanmycin in tobacco. Pesticide Biochem Physiol. 2014, 111, 14–18. [Google Scholar] [CrossRef]
- Gooding, G.V., Jr.; Hebert, T.T. A Simple Technique for Purification of Tobacco Mosaic Virus in Large Quantities. Phytopathology 2025, 115, 1285. [Google Scholar] [CrossRef]
- Wintermans, J.F.; de Mots, A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim. Biophys. Acta 1965, 109, 448–453. [Google Scholar] [CrossRef]
- Lilley, R.M.; Walker, D.A. An improved spectrophotometric assay for ribulosebisphosphate carboxylase. Biochim. Biophys. Acta 1974, 358, 226–229. [Google Scholar] [CrossRef]

| Time Point (Days) | CK + TMV | IBC + TMV |
|---|---|---|
| 3 | Vein clearing on heart leaves | No disease symptoms were observed. |
| 5 | The leaves exhibit mild mosaic symptoms, and the infected plants are stunted. | Vein clearing on heart leaves |
| 7 | Approximately 1/3 of the leaves exhibit mosaic and deformation symptoms, and the infected plants are stunted. | The heart leaves exhibited mild mosaic symptoms, and the height of the diseased plants was 1.18 times that of the CK + TMV group. |
| 9 | Approximately 1/2 to 2/3 of the leaves exhibit mosaic and deformation symptoms, and the infected plants are stunted. | Approximately 1/3 of the leaves exhibited mosaic symptoms, with a few leaves showing deformation. The height of the diseased plants was 1.27 times that of the CK + TMV group. |
| Different Groups | Total Protein | Up-Regulated Proteins | Down-Regulated Proteins |
|---|---|---|---|
| CK + TMV vs. CK | 421 | 211 | 210 |
| IBC + TMV vs. CK + TMV | 429 | 237 | 192 |
| Time (min) | B (%) |
|---|---|
| 0 | 0 |
| 1.9 | 0 |
| 2 | 5 |
| 17 | 5 |
| 18 | 10 |
| 35.5 | 30 |
| 38 | 36 |
| 39 | 42 |
| 40 | 42 |
| 44 | 100 |
| 45 | 0 |
| 48 | 0 |
| Time (min) | B (%) |
|---|---|
| 0 | 5 |
| 2 | 5 |
| 30 | 38 |
| 40 | 90 |
| 44 | Stop |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, L.; Wu, Y.; Sun, W.; Shahrajabian, M.H.; Gao, Y. Isobavachalcone Alleviates Plant Photosynthesis Inhibition Caused by Tobacco Mosaic Virus (TMV) Infection in Tobacco. Plants 2025, 14, 3638. https://doi.org/10.3390/plants14233638
Guan L, Wu Y, Sun W, Shahrajabian MH, Gao Y. Isobavachalcone Alleviates Plant Photosynthesis Inhibition Caused by Tobacco Mosaic Virus (TMV) Infection in Tobacco. Plants. 2025; 14(23):3638. https://doi.org/10.3390/plants14233638
Chicago/Turabian StyleGuan, Lijie, Yidan Wu, Wenli Sun, Mohamad Hesam Shahrajabian, and Yuan Gao. 2025. "Isobavachalcone Alleviates Plant Photosynthesis Inhibition Caused by Tobacco Mosaic Virus (TMV) Infection in Tobacco" Plants 14, no. 23: 3638. https://doi.org/10.3390/plants14233638
APA StyleGuan, L., Wu, Y., Sun, W., Shahrajabian, M. H., & Gao, Y. (2025). Isobavachalcone Alleviates Plant Photosynthesis Inhibition Caused by Tobacco Mosaic Virus (TMV) Infection in Tobacco. Plants, 14(23), 3638. https://doi.org/10.3390/plants14233638







