Effects of Forest Fire on Non-Structural Carbohydrates and Carbon, Nitrogen, and Phosphorus of Pinus yunnanensis
Abstract
1. Introduction
2. Results
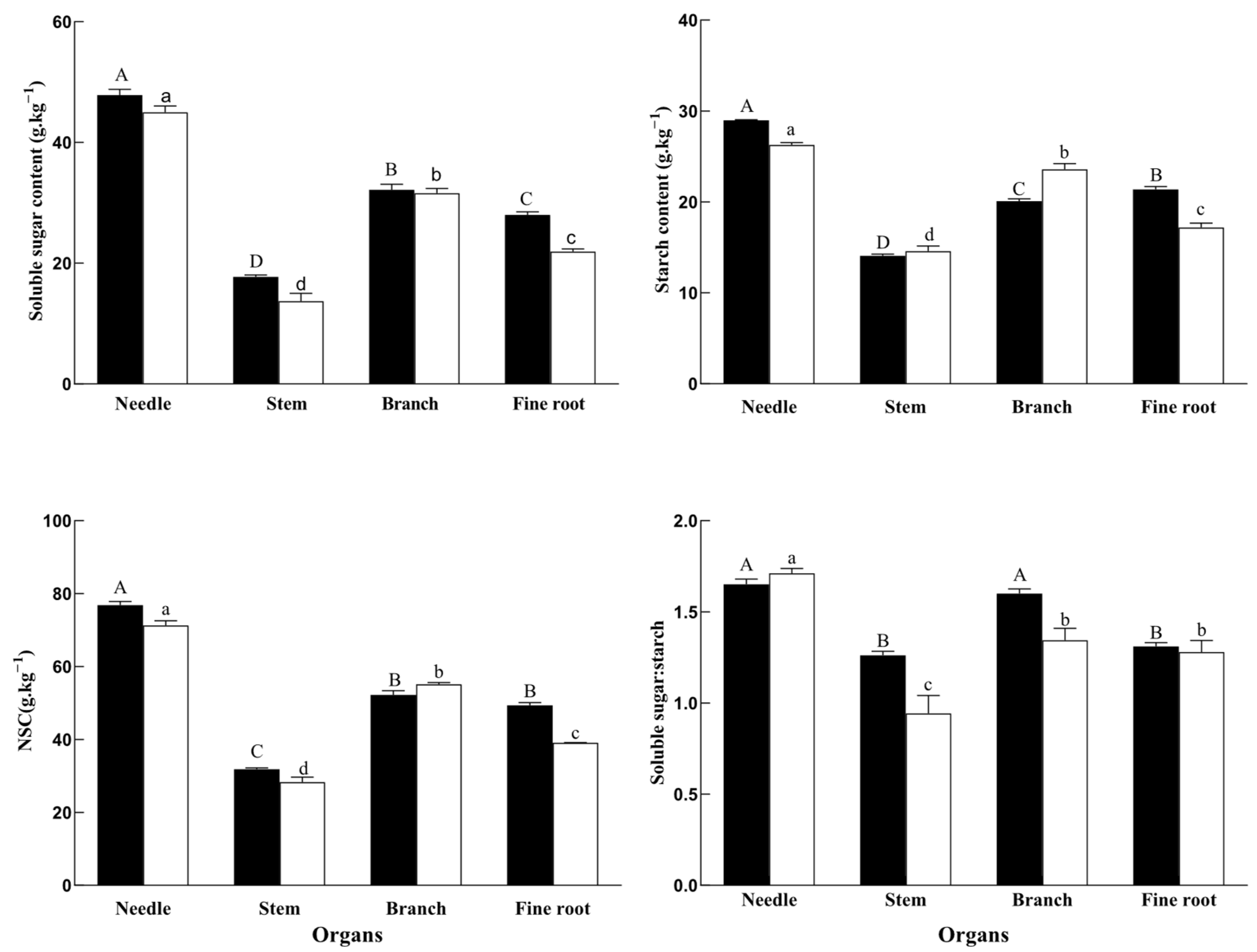
2.1. NSC
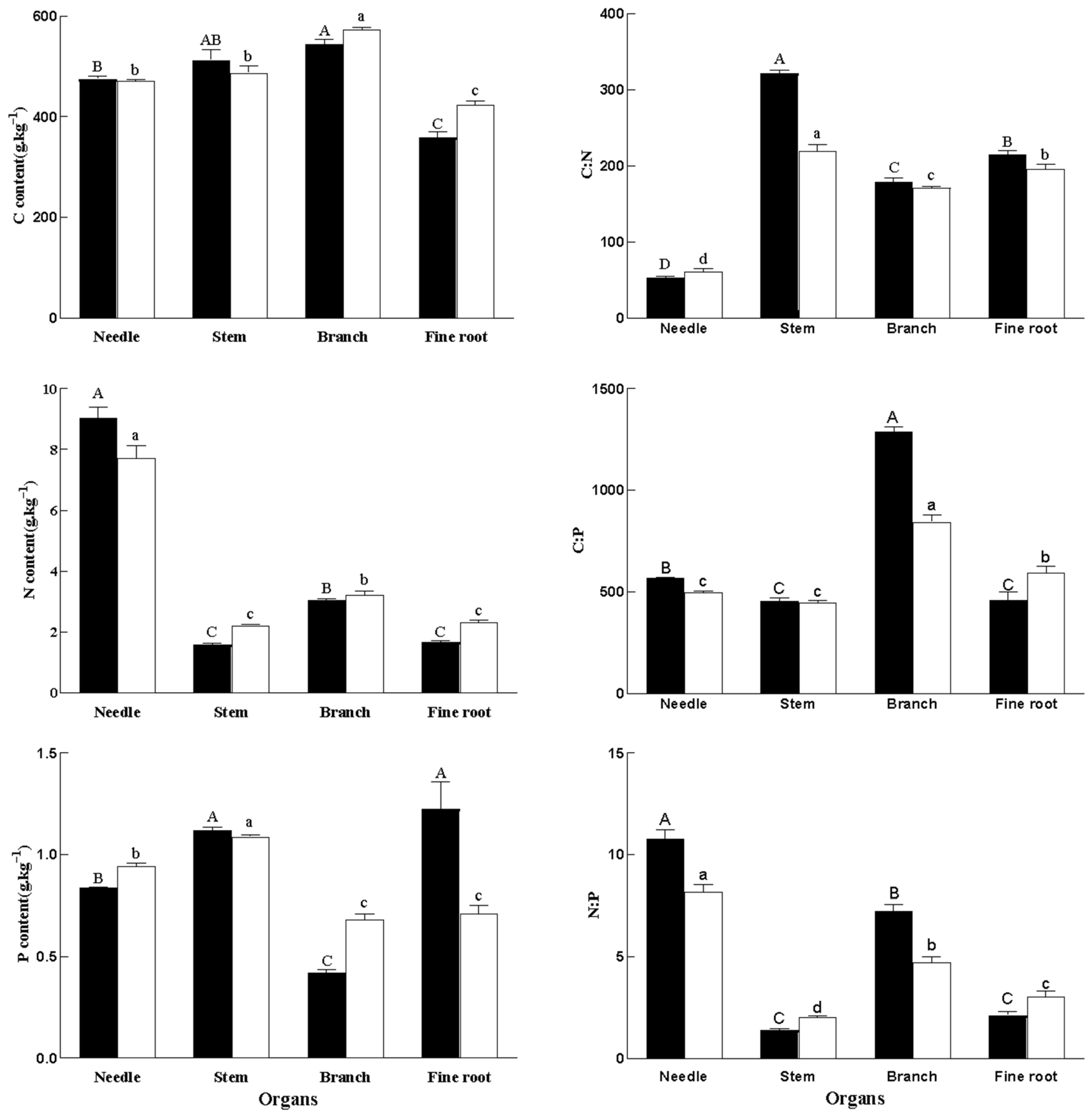
2.2. Ecological Stoichiometry Characteristics
3. Discussion
3.1. Effects of Forest Fire Disturbance on NSC Content and Distribution in P. yunnanensis Organs
3.2. Effects of Forest Fire Disturbance on Chemometric Characteristics in P. yunnanensis
3.3. Implications for Vegetation Restoration
4. Materials and Methods
4.1. Study Site and Species
4.2. Plot Setting
4.3. Sample Collection and Measurement
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, Y.H.; Shen, Y.W.; Wang, J.L.; Wei, Y.C.; Liu, Z.Y. Simulation and mapping of drought and soil erosion in central Yunnan Province, China. Adv. Space Res. 2021, 68, 4556–4572. [Google Scholar] [CrossRef]
- Tian, X.R. Forest Fire Danger Changes for Southwest China under Future Scenarios. Sci. Silvae Sin. 2012, 48, 121–125. [Google Scholar] [CrossRef]
- Matsumoto, M.; Takemi, S.; Sakai, T.; Sakata, I. Identification of motilin in Japanese fire bellied newt. Gen. Comp. Endocrinol. 2022, 323, 114031. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.J.; Yang, J.; Bai, E. Long-term effects of wildfire on available soil nutrient composition and stoichiometry in a Chinese boreal forest. Sci. Total Environ. 2018, 642, 1353–1361. [Google Scholar] [CrossRef]
- Pellegrini, A.F.A.; Ahlström, A.; Hobbie, S.E.; Reich, P.B.; Nieradzik, L.P.; Staver, A.C.; Scharenbroch, B.C.; Jumpponen, A.; Anderegg, W.R.L.; Randerson, J.T. Fire frequency drives decadal changes in soil carbon and nitrogen and ecosystem productivity. Nature 2018, 7687, 194–198. [Google Scholar] [CrossRef]
- Beals, K.K.; Scearce, A.E.; Swystun, A.T.; Schweitzer, J.A. Belowground mechanisms for oak regeneration: Interactions among fire, soil microbes, and plant community alter oak seedling growth. For. Ecol. Manag. 2022, 503, 503–509. [Google Scholar] [CrossRef]
- Wang, R.; Hu, H.Q. Physiological response of Betula platyphylla leaves to fire and the restoration after fire. J. Beijing For. Univ. 2013, 35, 1–6. [Google Scholar] [CrossRef]
- Bär, A.; Michaletz, S.T.; Mayr, S. Fire effects on tree physiology. New Phytol. 2019, 223, 1728–1741. [Google Scholar] [CrossRef]
- Halpern, C.B.; Antos, J.A. Burn severity and pre-fire seral state interact to shape vegetation responses to fire in a young, western Cascade Range forest. For. Ecol. Manag. 2022, 507, 120028. [Google Scholar] [CrossRef]
- Xu, W.; He, H.; Huang, C.; Duan, S.; Hawbaker, T.J.; Henne, P.D.; Yu, L.; Zhu, Z. Large fires or small fires, will they differ in affecting shifts in species composition and distributions under climate change? For. Ecol. Manag. 2022, 510, 120–131. [Google Scholar] [CrossRef]
- Piper, F.I.; Paula, S. The Role of Nonstructural Carbohydrates Storage in Forest Resilience under Climate Change. Curr. For. Rep. 2020, 6, 1–13. [Google Scholar] [CrossRef]
- Gričar, J.; Hafner, P.; Lavrič, M.; Ferlan, M.; Ogrinc, N.; Krajnc, B.; Eler, K.; Vodnik, D. Post-fire effects on development of leaves and secondary vascular tissues in Quercus pubescens. Tree Physiol. 2020, 40, 796–809. [Google Scholar] [CrossRef] [PubMed]
- Michaletz, S.T. Xylem dysfunction in fires: Towards a hydraulic theory of plant responses to multiple disturbance stressors. New Phytol. 2018, 217, 1391–1393. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.J.; Leuzinger, S.; Philipson, C.D.; Tay, J.; Hector, A. Drought survival of tropical tree seedlings enhanced by non-structural carbohydrate levels. Nat. Clim. Change 2014, 4, 710–714. [Google Scholar] [CrossRef]
- Yan, W.; Zhong, Y.; Shangguan, Z. Responses of different physiological parameter thresholds to soil water availability in four plant species during prolonged drought. Agric. For. Meteorol. 2017, 247, 311–319. [Google Scholar] [CrossRef]
- He, W.; Liu, H.; Qi, Y.; Liu, F.; Zhu, X. Patterns in nonstructural carbohydrate contents at the tree organ level in response to drought duration. Glob. Change Biol. 2020, 26, 3627–3638. [Google Scholar] [CrossRef]
- Li, X.; Piao, S.; Wang, K.; Wang, X.; Wang, T.; Ciais, P.; Chen, A.; Lian, X.; Peng, S.; Peuelas, J. Temporal trade-off between gymnosperm resistance and resilience increases forest sensitivity to extreme drought. Nat. Ecol. Evol. 2020, 4, 1075–1083. [Google Scholar] [CrossRef]
- Zeng, S.; Liu, F.; Zhao, M.; Wang, G.; Chen, X. Effects of fire disturbance intensities on soil physiochemical properties of pour subtropical forest types. Acta Ecol. Sin. 2020, 40, 233–246. [Google Scholar] [CrossRef]
- Wildy, D.T.; Pate, J.S. Quantifying Above—And Below—Ground Growth Responses of the Western Australian Oil Mallee, Eucalyptus kochii subsp. plenissima, to Contrasting Decapitation Regimes. Ann. Bot. 2002, 90, 185–197. [Google Scholar] [CrossRef]
- Cruz, A.; Pérez, B.; Moreno, J.M. Plant stored reserves do not drive resprouting of the lignotuberous shrub Erica australis. New Phytol. 2003, 157, 251–261. [Google Scholar] [CrossRef]
- Schutz, A.E.N.; Bond, W.J.; Cramer, M.D. Juggling carbon: Allocation patterns of a dominant tree in a fire-prone savanna. Oecologia 2009, 160, 235–246. [Google Scholar] [CrossRef]
- Pratt, R.B.; Jacobsen, A.L.; Ramirez, A.R.; Helms, A.M.; Traugh, C.A.; Tobin, M.F.; Heffner, M.S.; Davis, S.D. Mortality of resprouting chaparral shrubs after a fire and during a record drought: Physiological mechanisms and demographic consequences. Glob. Change Biol. 2016, 20, 893–907. [Google Scholar] [CrossRef]
- Zhao, Y.; Cao, Y.; Chen, Y.; Peng, S. Ecological stoichiometry in a forest ecosystem in the hilly-gully area of the Loess Plateau. Acta Ecol. Sin. 2017, 37, 5451–5460. [Google Scholar]
- Butler, O.M.; Elser, J.J.; Lewis, T.; Maunsell, S.C.; Rashti, M.R.; Chen, C. The multi-element stoichiometry of wet eucalypt forest is transformed by recent, frequent fire. Plant Soil 2020, 447, 447–461. [Google Scholar] [CrossRef]
- Schafer, J.L.; Mack, M.C. Short-term effects of fire on soil and plant nutrients in palmetto flatwoods. Plant Soil 2010, 334, 433–447. [Google Scholar] [CrossRef]
- Hume, A.; Chen, H.Y.H.; Taylor, A.R.; Kayahara, G.J.; Man, R. Soil C:N:P dynamics during secondary succession following fire in the boreal forest of central Canada. For. Ecol. Manag. 2016, 369, 1–9. [Google Scholar] [CrossRef]
- Britton, A.J.; Helliwell, R.C.; Fisher, J.M.; Gibbs, S. Interactive effects of nitrogen deposition and fire on plant and soil chemistry in an alpine heathland. Environ. Pollut. 2008, 156, 409–416. [Google Scholar] [CrossRef]
- Cui, Q.; Lü, X.T.; Wang, Q.B.; Han, X.G. Nitrogen fertilization and fire act independently on foliar stoichiometry in a temperate steppe. Plant Soil 2010, 334, 209–219. [Google Scholar] [CrossRef]
- Pellegrini, A.F.A.; Staver, A.C.; Hedin, L.O.; Govender, N.; Charles-Dominique, T. Fire alters ecosystem carbon and nutrients but not plant nutrient stoichiometry or composition in tropical savanna. Ecology 2015, 96, 1275–1285. [Google Scholar] [CrossRef]
- Guo, X.; Peng, C.; Li, T.; Huang, J.; Wang, M. The Effects of Drought and Re-Watering on Non-Structural Carbohydrates of Pinus tabulaeformis Seedlings. Biology 2021, 10, 281. [Google Scholar] [CrossRef]
- Pavlovic, N.B.; Leicht-Young, S.A.; Grundel, R. Oriental bittersweet (Celastrus orbiculatus): Spreading by fire. For. Ecol. Manag. 2016, 364, 183–194. [Google Scholar] [CrossRef]
- Butler, O.M.; Lewis, T.; Chen, C. Prescribed fire alters foliar stoichiometry and nutrient resorption in the understorey of a subtropical eucalypt forest. Plant Soil 2017, 410, 181–191. [Google Scholar] [CrossRef]
- Sala, A.; Piper, F.; Hoch, G. Physiological mechanisms of drought-induced tree mortality are far from being resolved. New Phytol. 2010, 186, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhang, W.; Li, R.; Xu, M.; Wang, S. Different responses of non-structural carbohydrates in above-ground tissues/organs and root to extreme drought and re-watering in Chinese fir (Cunninghamia lanceolata) saplings. Trees 2016, 30, 1863–1871. [Google Scholar] [CrossRef]
- Zhang, P.; Zhou, X.; Fu, Y.; Shao, J.; McDowell, N.G. Differential effects of drought on nonstructural carbohydrate storage in seedlings and mature trees of four species in a subtropical forest. For. Ecol. Manag. 2020, 469, 118–159. [Google Scholar] [CrossRef]
- Chu, X.; Di, X.Y.; Yang, G. Impacts of forest fire on root biomass, carbon and nitrogen concentration of Larix gmelinii. J. Beijing For. Univ. 2013, 35, 10–16. [Google Scholar] [CrossRef]
- Güsewell, S.; Verhoeven, J.T.A. Litter N:P ratios indicate whether N or P limits the decomposability of graminoid leaf litter. Plant Soil 2006, 287, 131–143. [Google Scholar] [CrossRef]
- Hu, T.; Hu, H.; Li, F.; Zhao, B.; Wu, S.; Zhu, G.; Sun, L. Long-term effects of post-fire restoration types on nitrogen mineralisation in a Dahurian larch (Larix gmelinii) forest in boreal China. Sci. Total Environ. 2019, 679, 237–247. [Google Scholar] [CrossRef]
- Dijkstra, F.A.; Adams, M.A. Fire Eases Imbalances of Nitrogen and Phosphorus in Woody Plants. Ecosystems 2015, 18, 769–779. [Google Scholar] [CrossRef]
- Aerts, R.; Chapin, F.S. The Mineral Nutrition of Wild Plants Revisited: A Re-Evaluation of Processes and Patterns. Adv. Ecol. Res. 2000, 30, 1–67. [Google Scholar] [CrossRef]
- Wang, L.N.; Wu, J.W.; Dong, Q.; Shi, Z.G.; Hu, H.C.; Wu, D.Z.; Li, L.P. Effects of tending and thinning on non-structural carbon and stoichiometric characteristics of Pinus yunnanensis. J. Beijing For. Univ. 2021, 43, 70–82. [Google Scholar]
- Li, J.; Zhang, C.; Deng, Y.; Zhang, C.; Su, W.; Li, S. Spatial distribution pattern and association of Pinus yunnanensis forest formed by 40 years of fire disturbance in Jizu Mountain, Yunnan. Guihaia 2025, 45, 8. [Google Scholar]
- Wang, J.; Hong, R.C.; Ma, C.; Zhu, X.L.; Xu, S.Y.; Tang, Y.P.; Li, X.N.; Yan, X.X.; Wang, L.G.; Wang, Q.H. Effects of Prescribed Burning on Surf ace Dead Fuel and Potential Fire Behavior in Pinus yunnanensisin Central Yunnan Province, China. Forests 2023, 14, 1915. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef]
- Yeomans, J.C.; Bremner, J.M. A rapid and precise method for routine determination of organic carbon in soil 1. Commun. Soil Sci. Plant Anal. 1988, 19, 1467–1476. [Google Scholar] [CrossRef]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen-Total. In Methods of Soil Analysis; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 295–624. [Google Scholar]

| Factors | Soluble Sugar | Starch | NSC |
|---|---|---|---|
| Forest fire | 32.119 ** | 6.833 * | 39.251 ** |
| Organ | 469.289 ** | 400.407 ** | 792.687 ** |
| Forest fire × Organ | 3.730 * | 38.455 ** | 17.481 ** |
| Index | T Value | |||
|---|---|---|---|---|
| Needle | Stem | Branch | Fine Roots | |
| Soluble sugar | 2.02 | 3.059 * | 0.458 | 8.814 ** |
| Starch | 10.287 ** | −0.881 | −5.106 ** | 7.209 ** |
| NSC | 3.384 * | 2.430 | −2.357 | 14.062 ** |
| Factors | C | N | P | C:N | C:P | N:P |
|---|---|---|---|---|---|---|
| Forest fire | 4.439 | 0.002 | 14,114.000 ** | 79.705 ** | 35.784 ** | 22.695 ** |
| Organ | 91.100 ** | 469.660 ** | 179.963 ** | 701.603 ** | 303.554 ** | 352.101 ** |
| Forest fire × Organ | 7.122 ** | 9.920 ** | 18.710 ** | 54.528 ** | 57.556 ** | 26.164 ** |
| Index | T Value | |||
|---|---|---|---|---|
| Needle | Stem | Branch | Fine Roots | |
| C | 1.056 | 1.050 | −3.320 * | −4.924 ** |
| N | 4.056 * | −11.157 ** | −1.235 | −8.361 ** |
| P | −9.059 * | 1.942 | −7.836 ** | 3.788 * |
| C:N | −3.865 * | 10.932 ** | 1.424 | 2.612 |
| C:P | 8.913 ** | 0.586 | 11.158 ** | −6.405 * |
| N:P | 5.861 ** | −13.628 ** | 6.251 ** | −5.930 ** |
| Sample Site Type | Slope Orientation | Forest Stand Depression | Average Tree Age | Average Tree Height/m | Average DBH /cm |
|---|---|---|---|---|---|
| Fire-damaged trees (F-trees) | Sunward slope | 0.8 | 28 | 15.7 | 15.4 |
| Undamaged trees (H-trees) | Sunward slope | 0.8 | 28 | 14.2 | 14.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, X.; Kou, W.; Wei, L.; Ye, J.; Wang, Q. Effects of Forest Fire on Non-Structural Carbohydrates and Carbon, Nitrogen, and Phosphorus of Pinus yunnanensis. Plants 2025, 14, 3637. https://doi.org/10.3390/plants14233637
Fu X, Kou W, Wei L, Ye J, Wang Q. Effects of Forest Fire on Non-Structural Carbohydrates and Carbon, Nitrogen, and Phosphorus of Pinus yunnanensis. Plants. 2025; 14(23):3637. https://doi.org/10.3390/plants14233637
Chicago/Turabian StyleFu, Xiaoyong, Weili Kou, Lili Wei, Jiangxia Ye, and Qiuhua Wang. 2025. "Effects of Forest Fire on Non-Structural Carbohydrates and Carbon, Nitrogen, and Phosphorus of Pinus yunnanensis" Plants 14, no. 23: 3637. https://doi.org/10.3390/plants14233637
APA StyleFu, X., Kou, W., Wei, L., Ye, J., & Wang, Q. (2025). Effects of Forest Fire on Non-Structural Carbohydrates and Carbon, Nitrogen, and Phosphorus of Pinus yunnanensis. Plants, 14(23), 3637. https://doi.org/10.3390/plants14233637





