Nanotechnology Strategies in Plant Genetic Engineering: Intelligent Delivery and Precision Editing
Abstract
1. Introduction
2. Bottleneck Landscape of Conventional Gene-Delivery Systems
2.1. Agrobacterium Transformation
2.2. Gene Gun Delivery: Tissue Damage and Risk of Multicopy Integration
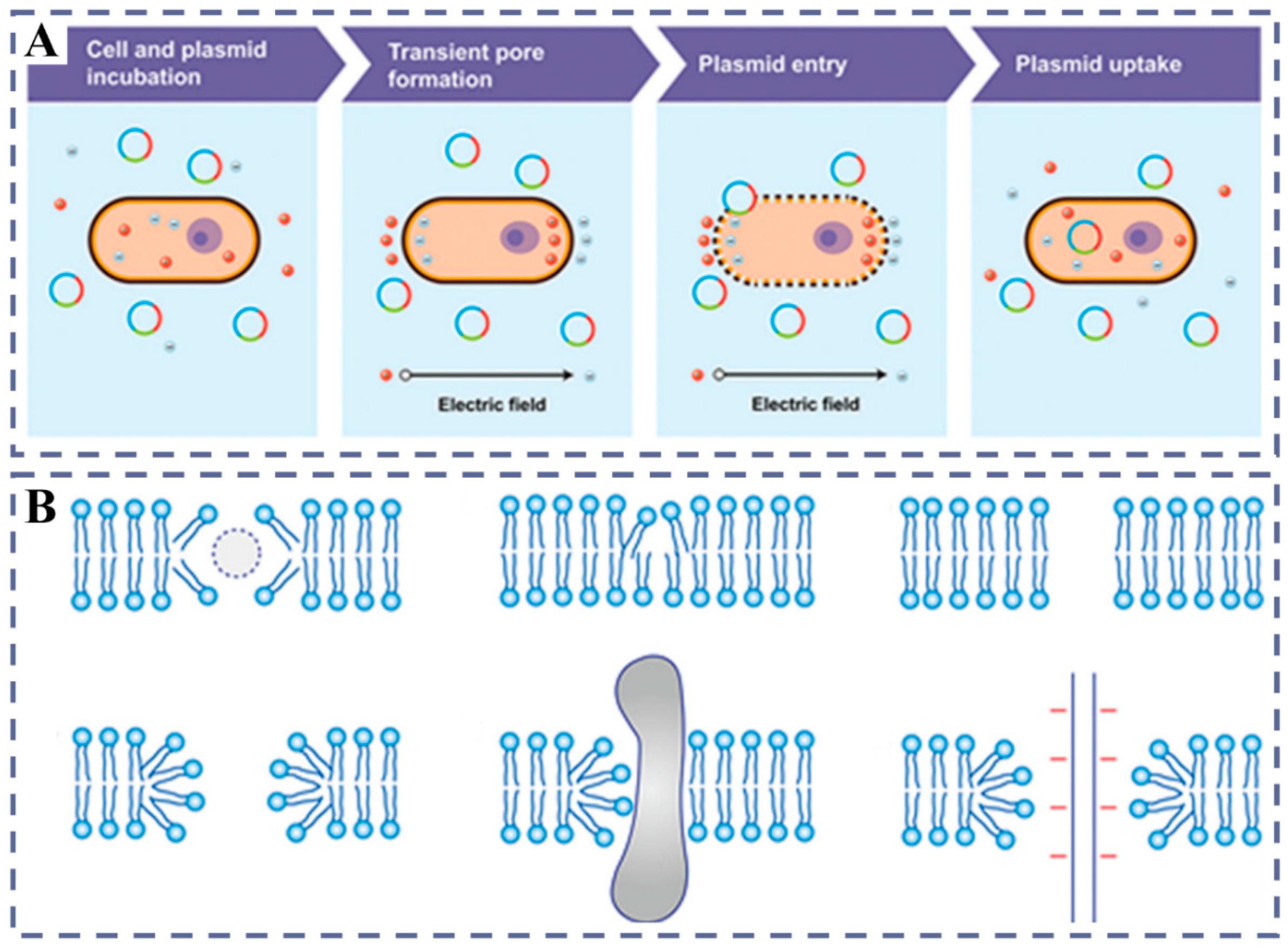
2.3. Electroporation/PEG Method: The Protoplast Regeneration Barrier Electroporation
2.4. PEG-Mediated Delivery
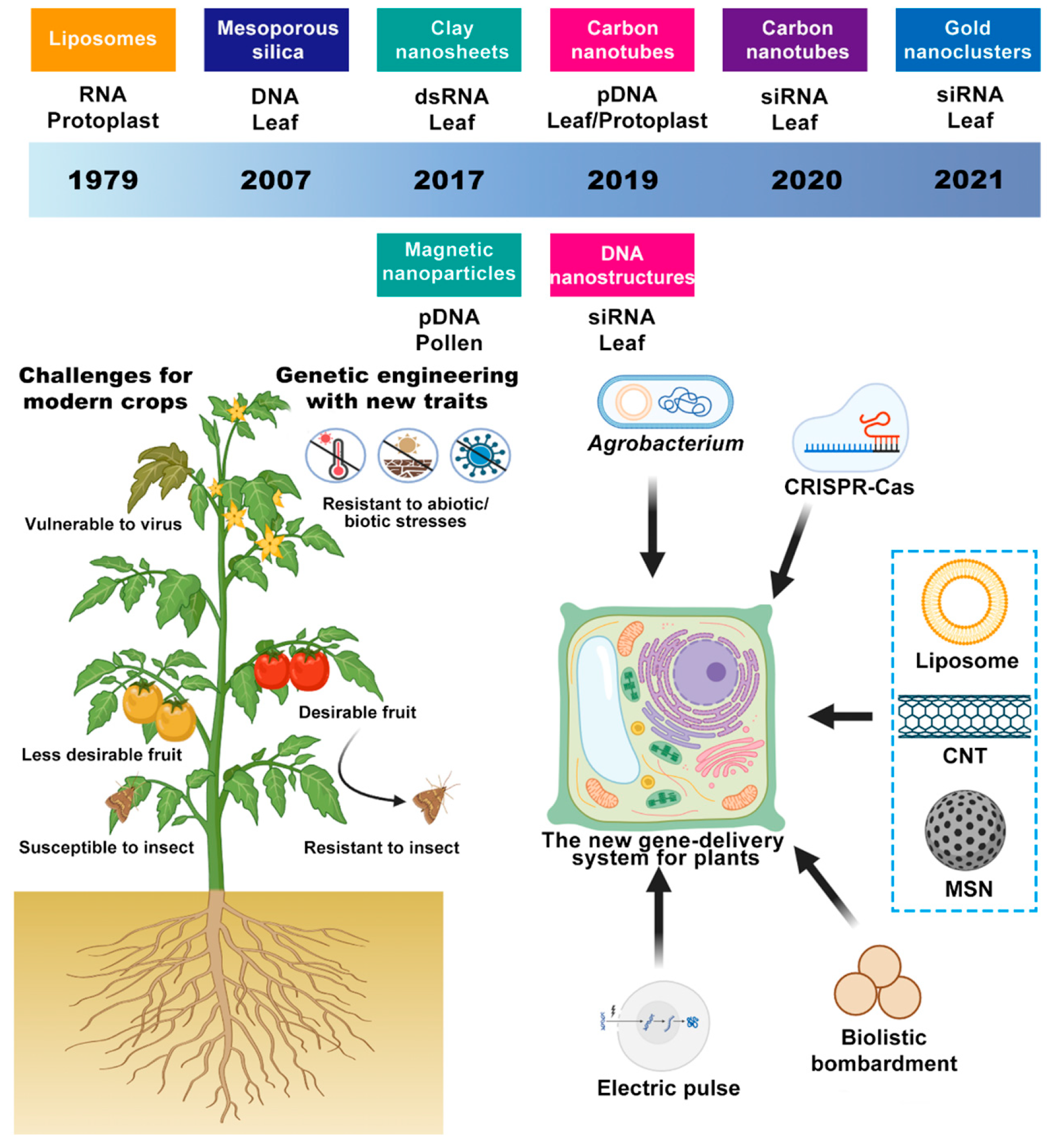
3. Nanocarrier Design: From Material Classification to Smart Responsiveness
3.1. Carbon-Based Nanoplatforms
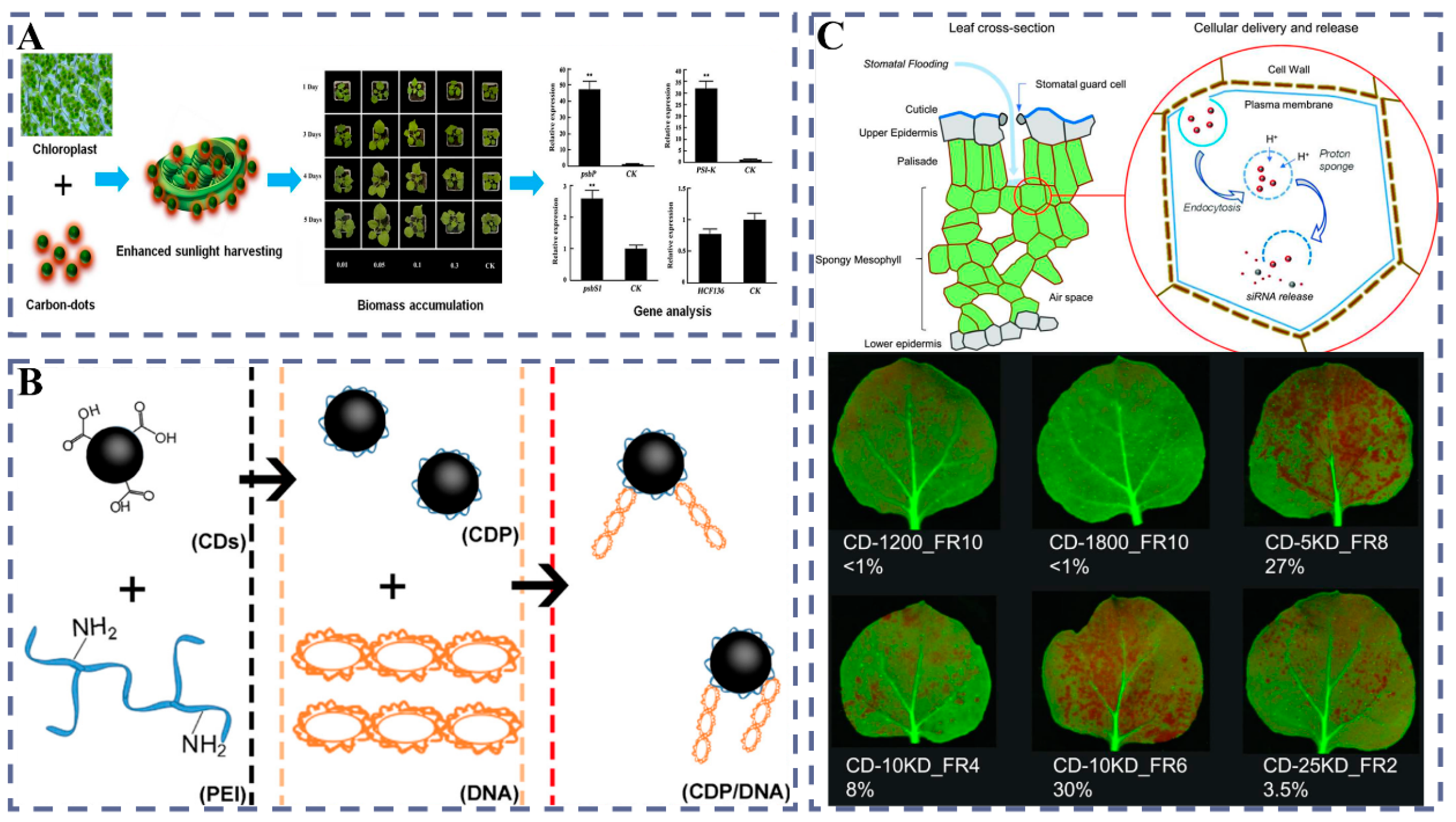
3.1.1. CDs
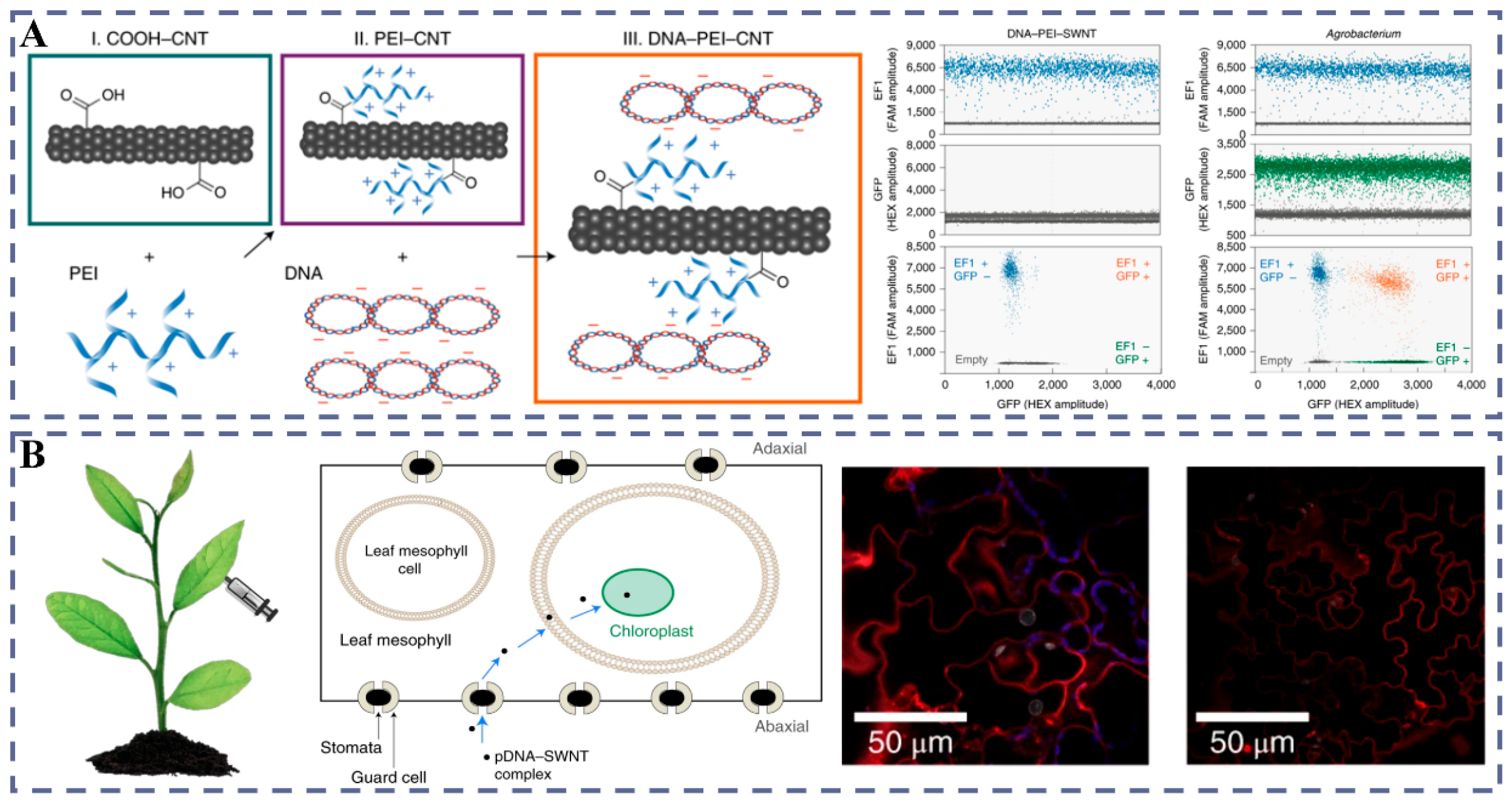
3.1.2. CNTs
3.1.3. Graphene Derivatives and Other Carbon Nanomaterials
3.2. Inorganic, Non-Carbon Carriers
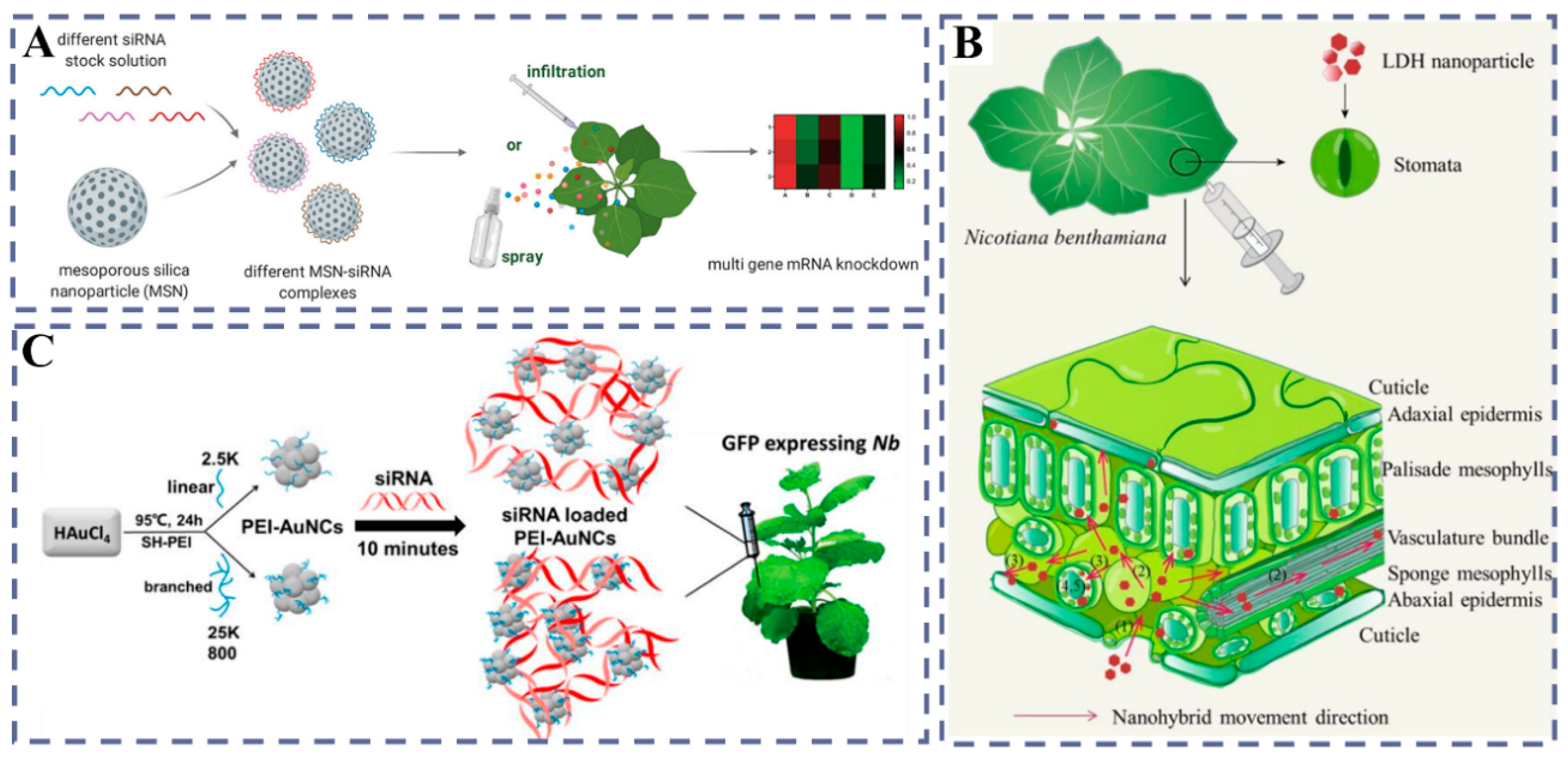
3.2.1. MSNs: Pore-Channel Encapsulation and Controlled Release
3.2.2. Innovative Applications of LDH in Plant Gene Delivery
3.2.3. AuNCs: Photothermal Responsiveness and Enhanced Gene Silencing
3.3. Organic–Biological Hybrid Systems
3.3.1. Liposomes: Membrane-Fusion Delivery
3.3.2. Chitosan-Based Nanocomposite Systems: Enhanced Cell-Wall Penetration
3.3.3. Peptide Carriers: Endosomal Escape and Nuclear Targeting

3.3.4. DNA Self-Assembled Structures: Programmable Nucleic-Acid Carriers
4. CRISPR-Cas Nano-Synergistic Editing Systems
4.1. Common Vectors: CRISPR-Cas Is Hailed as “Molecular Scissors”
4.1.1. CRISPR-Cas DNA Vectors
4.1.2. Gene Gun Bombardment Delivery (RNP)
4.1.3. Viral/Nano-Synergistic Vectors and Organelle Targeting

4.2. Editing-Efficiency Advantages and Development Potential
5. Rational Design of Nanocarriers and Multiscale Delivery
6. Summary and Prospect
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, M.; Chen, A.; Li, X.; Li, X.; Hou, X.; Liu, X. Advancements in delivery strategies and non-tissue culture regeneration systems for plant genetic transformation. Adv. Biotechnol. 2024, 2, 34. [Google Scholar] [CrossRef]
- Steinwand, M.A.; Ronald, P.C. Crop biotechnology and the future of food. Nat. Food 2020, 1, 273–283. [Google Scholar] [CrossRef]
- Duan, K.; Willig, C.J.; De Tar, J.R.; Spollen, W.G.; Zhang, Z.J. Transcriptomic Analysis of Arabidopsis Seedlings in Response to an Agrobacterium-Mediated Transformation Process. Mol. Plant Microbe Interact. 2018, 31, 445–459. [Google Scholar] [CrossRef]
- Ozyigit, I.I.; Yucebilgili Kurtoglu, K. Particle bombardment technology and its applications in plants. Mol. Biol. Rep. 2020, 47, 9831–9847. [Google Scholar] [CrossRef]
- Wang, M.; Gao, S.; Zeng, W.; Yang, Y.; Ma, J.; Wang, Y. Plant Virology Delivers Diverse Toolsets for Biotechnology. Viruses 2020, 12, 1338. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, K.; Qi, Y.; Nguyen, L.V.; Bethke, G.; Tsuda, Y.; Glazebrook, J.; Katagiri, F. An efficient Agrobacterium-mediated transient transformation of Arabidopsis. Plant J. 2012, 69, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Mayo, K.J.; Gonzales, B.J.; Mason, H.S. Genetic transformation of tobacco NT1 cells with Agrobacterium tumefaciens. Nat. Protoc. 2006, 1, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Titanji, V.P.; Ngwa, A.A.; Ngemenya, M. Applications of biotechnology techniques to the study of medicinal plants. Afr. J. Med. Med. Sci. 2007, 36, 23–29. [Google Scholar]
- Song, G.-q.; Prieto, H.; Orbovic, V. Agrobacterium-Mediated Transformation of Tree Fruit Crops: Methods, Progress, and Challenges. Front. Plant Sci. 2019, 10, 226. [Google Scholar] [CrossRef]
- Yin, H.; Kanasty, R.L.; Eltoukhy, A.A.; Vegas, A.J.; Dorkin, J.R.; Anderson, D.G. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014, 15, 541–555. [Google Scholar] [CrossRef]
- Yan, Y.; Zhu, X.; Yu, Y.; Li, C.; Zhang, Z.; Wang, F. Nanotechnology Strategies for Plant Genetic Engineering. Adv. Mater. 2022, 34, e2106945. [Google Scholar] [CrossRef] [PubMed]
- Vuong, U.T.; Iswanto, A.B.B.; Nguyen, Q.M.; Kang, H.; Lee, J.; Moon, J.; Kim, S.H. Engineering plant immune circuit: Walking to the bright future with a novel toolbox. Plant Biotechnol. J. 2023, 21, 17–45. [Google Scholar] [CrossRef] [PubMed]
- McKenna, J.F.; Tolmie, A.F.; Runions, J. Across the great divide: The plant cell surface continuum. Curr. Opin. Plant Biol. 2014, 22, 132–140. [Google Scholar] [CrossRef]
- Jonsson, K.; Hamant, O.; Bhalerao, R.P. Plant cell walls as mechanical signaling hubs for morphogenesis. Curr. Biol. 2022, 32, R334–R340. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.J. Structure and growth of plant cell walls. Nat. Rev. Mol. Cell Biol. 2024, 25, 340–358. [Google Scholar] [CrossRef]
- Schell, J.; Van Montagu, M. Transfer, maintenance, and expression of bacterial Ti-plasmid DNA in plant cells transformed with A. tumefaciens. In Brookhaven Symposium in Biology; Brookhaven National Laboratory: Upton, NY, USA, 1977; pp. 36–49. [Google Scholar]
- Chilton, M.D.; Drummond, M.H.; Merlo, D.J.; Sciaky, D.; Montoya, A.L.; Gordon, M.P.; Nester, E.W. Stable incorporation of plasmid DNA into higher plant cells: The molecular basis of crown gall tumorigenesis. Cell 1977, 11, 263–271. [Google Scholar] [CrossRef]
- Horsch, R.B.; Fry, J.E.; Hoffmann, N.L.; Wallroth, M.; Eichholtz, D.; Rogers, S.G.; Fraley, R.T. A simple and general method for transferring genes into plants. Science 1985, 227, 1229–1231. [Google Scholar] [CrossRef]
- Sunilkumar, G.; Vijayachandra, K.; Veluthambi, K. Preincubation of cut tobacco leaf explants promotes Agrobacterium-mediated transformation by increasing vir gene induction. Plant Sci. 1999, 141, 51–58. [Google Scholar] [CrossRef]
- Butler, N.M.; Carlson, A.T.; Starker, C.; Voytas, D.F. Viral-mediated delivery of morphogenic regulators enables leaf transformation in Sorghum bicolor (L.). Plant Biotechnol. J. 2025, 23, 4491–4499. [Google Scholar] [CrossRef]
- Binns, A.N. Agrobacterium-mediated gene delivery and the biology of hostrange limitations. Physiol. Plant. 1990, 79, 135–139. [Google Scholar] [CrossRef]
- Park, S.H.; Morris, J.L.; Park, J.E.; Hirschi, K.D.; Smith, R.H. Efficient and genotype-independent Agrobacterium-mediated tomato transformation. J. Plant Physiol. 2003, 160, 1253–1257. [Google Scholar] [CrossRef]
- Song, G.-Q.; Sink, K.C. Agrobacterium tumefaciens-mediated transformation of blueberry (Vaccinium corymbosum L.). Plant Cell Rep. 2004, 23, 475–484. [Google Scholar] [CrossRef]
- Riaz, S.; Choudry, M.W.; Riaz, R.; Farooq, A.M.; Bakhsh, A. Agrobacterium-Mediated Transformation of Soybean (Glycine max L.) Using Split-Cotyledonary Explant. In Agrobacterium: Methods in Molecular Biology; Stange Klein, C., Ed.; Humana Press: New York, NY, USA, 2025; Volume 2911, pp. 71–82. [Google Scholar] [CrossRef]
- Stachel, S.E.; Messens, E.; Van Montagu, M.; Zambryski, P. Identification of the signal molecules produced by wounded plant cells that activate T-DNA transfer in Agrobacterium tumefaciens. Nature 1985, 318, 624–629. [Google Scholar] [CrossRef]
- Hernalsteens, J.P.; Thia-Toong, L.; Schell, J.; Van Montagu, M. An Agrobacterium-transformed cell culture from the monocot Asparagus officinalis. EMBO J. 1984, 3, 3039–3041. [Google Scholar] [CrossRef]
- Grimsley, N.; Hohn, T.; Davies, J.W.; Hohn, B. Agrobacterium-mediated delivery of infectious maize streak virus into maize plants. Nature 1987, 325, 177–179. [Google Scholar] [CrossRef]
- Hiei, Y.; Ohta, S.; Komari, T.; Kumashiro, T. Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J. 1994, 6, 271–282. [Google Scholar] [CrossRef]
- Hiei, Y.; Komari, T. Agrobacterium-mediated transformation of rice using immature embryos or calli induced from mature seed. Nat. Protoc. 2008, 3, 824–834. [Google Scholar] [CrossRef]
- Harwood, W.A.; Bartlett, J.G.; Alves, S.C.; Perry, M.; Smedley, M.A.; Leyl, N.; Snape, J.W. Barley Transformation Using Agrobacterium-Mediated Techniques. In Transgenic Wheat, Barley and Oats: Production and Characterization Protocols; Jones, H.D., Shewry, P.R., Eds.; Humana Press: Totowa, NJ, USA, 2009; pp. 137–147. [Google Scholar]
- Wu, H.; Doherty, A.; Jones, H.D. Agrobacterium-mediated transformation of bread and durum wheat using freshly isolated immature embryos. In Transgenic Wheat, Barley and Oats: Production and Characterization Protocols; Jones, H.D., Shewry, P.R., Eds.; Humana Press: Totowa, NJ, USA, 2009; Volume 478, pp. 93–103. [Google Scholar] [CrossRef]
- Ishida, Y.; Hiei, Y.; Komari, T. Agrobacterium-mediated transformation of maize. Nat. Protoc. 2007, 2, 1614–1621. [Google Scholar] [CrossRef]
- Sangwan, R.S.; Bourgeois, Y.; Brown, S.; Vasseur, G.; Sangwan-Norreel, B. Characterization of competent cells and early events of Agrobacterium-mediated genetic transformation in Arabidopsis thaliana. Planta 1992, 188, 439–456. [Google Scholar] [CrossRef]
- Li, S.; Cong, Y.; Liu, Y.; Wang, T.; Shuai, Q.; Chen, N.; Gai, J.; Li, Y. Optimization of Agrobacterium-Mediated Transformation in Soybean. Front. Plant Sci. 2017, 8, 246. [Google Scholar] [CrossRef]
- Baron, C.; Zambryski, P.C. Plant transformation: A pilus in Agrobacterium T-DNA transfer. Curr. Biol. 1996, 6, 1567–1569. [Google Scholar] [CrossRef]
- Aliu, E.; Ji, Q.; Wlazlo, A.; Grosic, S.; Azanu, M.K.; Wang, K.; Lee, K. Enhancing Agrobacterium-mediated plant transformation efficiency through improved ternary vector systems and auxotrophic strains. Front. Plant Sci. 2024, 15, 1429353. [Google Scholar] [CrossRef]
- Klein, T.M.; Wolf, E.D.; Wu, R.; Sanford, J.C. High-velocity microprojectiles for delivering nucleic acids into living cells. Nature 1987, 327, 70–73. [Google Scholar] [CrossRef]
- Klein, T.M.; Fromm, M.; Weissinger, A.; Tomes, D.; Schaaf, S.; Sletten, M.; Sanford, J.C. Transfer of foreign genes into intact maize cells with high-velocity microprojectiles. Proc. Natl. Acad. Sci. USA 1988, 85, 4305–4309. [Google Scholar] [CrossRef]
- Mendel, R.R.; Müller, B.; Schulze, J.; Kolesnikov, V.; Zelenin, A. Delivery of foreign genes to intact barley cells by high-velocity microprojectiles. Theor. Appl. Genet. 1989, 78, 31–34. [Google Scholar] [CrossRef]
- Boynton, J.E.; Gillham, N.W.; Harris, E.H.; Hosler, J.P.; Johnson, A.M.; Jones, A.R.; Randolph-Anderson, B.L.; Robertson, D.; Klein, T.M.; Shark, K.B.; et al. Chloroplast transformation in Chlamydomonas with high velocity microprojectiles. Science 1988, 240, 1534–1538. [Google Scholar] [CrossRef]
- Vasil, V.; Srivastava, V.; Castillo, A.M.; Fromm, M.E.; Vasil, I.K. Rapid Production of Transgenic Wheat Plants by Direct Bombardment of Cultured Immature Embryos. Nat. Biotechnol. 1993, 11, 1553–1558. [Google Scholar] [CrossRef]
- Caimi, P.G.; McCole, L.M.; Klein, T.M.; Kerr, P.S. Fructan Accumulation and Sucrose Metabolism in Transgenic Maize Endosperm Expressing a Bacillus amyloliquefaciens SacB Gene. Plant Physiol. 1996, 110, 355–363. [Google Scholar] [CrossRef]
- Bidney, D.; Scelonge, C.; Martich, J.; Burrus, M.; Sims, L.; Huffman, G. Microprojectile bombardment of plant tissues increases transformation frequency by Agrobacterium tumefaciens. Plant Mol. Biol. 1992, 18, 301–313. [Google Scholar] [CrossRef]
- Mesa, M.C.d.; Jiménez-Bermúdez, S.; Pliego-Alfaro, F.; Quesada, M.A.; Mercado, J.A. Agrobacterium cells as microprojectile coating: A novel approach to enhance stable transformation rates in strawberry. Funct. Plant Biol. 2000, 27, 1093–1100. [Google Scholar] [CrossRef]
- Galbraith, D.W. Nanobiotechnology: Silica breaks through in plants. Nat. Nanotechnol. 2007, 2, 272–273. [Google Scholar] [CrossRef]
- Carsono, N.; Yoshida, T. Transient Expression of Green Fluorescent Protein in Rice Calluses: Optimization of Parameters for Helios Gene Gun Device. Plant Prod. Sci. 2008, 11, 88–95. [Google Scholar] [CrossRef]
- Hay, I.; Lachance, D.; Aderkas, P.V.; Charest, P.J. Transient chimeric gene expression in pollen of five conifer species following microparticle bombardment. Can. J. For. Res. 1994, 24, 2417–2423. [Google Scholar] [CrossRef]
- Pawlowski, W.P.; Somers, D.A. Transgene inheritance in plants genetically engineered by microprojectile bombardment. Mol. Biotechnol. 1996, 6, 17–30. [Google Scholar] [CrossRef]
- Dong, O.X.; Ronald, P.C. Targeted DNA insertion in plants. Proc. Natl. Acad. Sci. USA 2021, 118, e2004834117. [Google Scholar] [CrossRef]
- Thorpe, C.; Luo, W.; Ji, Q.; Eggenberger, A.L.; Chicowski, A.S.; Xu, W.; Sandhu, R.; Lee, K.; Whitham, S.A.; Qi, Y.; et al. Enhancing biolistic plant transformation and genome editing with a flow guiding barrel. Nat. Commun. 2025, 16, 5624. [Google Scholar] [CrossRef]
- Fromm, M.; Taylor, L.P.; Walbot, V. Expression of genes transferred into monocot and dicot plant cells by electroporation. Proc. Natl. Acad. Sci. USA 1985, 82, 5824–5828. [Google Scholar] [CrossRef]
- Gehl, J. Electroporation: Theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol. Scand. 2003, 177, 437–447. [Google Scholar] [CrossRef]
- Furuhata, Y.; Sakai, A.; Murakami, T.; Morikawa, M.; Nakamura, C.; Yoshizumi, T.; Fujikura, U.; Nishida, K.; Kato, Y. A method using electroporation for the protein delivery of Cre recombinase into cultured Arabidopsis cells with an intact cell wall. Sci. Rep. 2019, 9, 2163. [Google Scholar] [CrossRef]
- Barampuram, S.; Zhang, Z.J. Recent advances in plant transformation. In Plant Chromosome Engineering: Methods and Protocols; Birchler, J.A., Ed.; Humana Press: Totowa, NJ, USA, 2011; Volume 701, pp. 1–35. [Google Scholar] [CrossRef]
- Veronese, F.M.; Pasut, G. PEGylation, successful approach to drug delivery. Drug Discov. Today 2005, 10, 1451–1458. [Google Scholar] [CrossRef]
- Kao, K.N.; Constabel, F.; Michayluk, M.R.; Gamborg, O.L. Plant protoplast fusion and growth of intergeneric hybrid cells. Planta 1974, 120, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Krens, F.A.; Molendijk, L.; Wullems, G.J.; Schilperoort, R.A. In vitro transformation of plant protoplasts with Ti-plasmid DNA. Nature 1982, 296, 72–74. [Google Scholar] [CrossRef]
- Lentz, B.R.; Lee, J.K. Poly(ethylene glycol) (PEG)-mediated fusion between pure lipid bilayers: A mechanism in common with viral fusion and secretory vesicle release? Mol. Membr. Biol. 1999, 16, 279–296. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, C.; Liu, Y.; Zeng, J.; Yu, H.; Tong, Z.; Yuan, X.; Sui, X.; Fang, D.; Xiao, B.; et al. CRISPR/Cas9-mediated seamless gene replacement in protoplasts expands the resistance spectrum to TMV-U1 strain in regenerated Nicotiana tabacum. Plant Biotechnol. J. 2023, 21, 2641–2653. [Google Scholar] [CrossRef] [PubMed]
- Santana, I.; Wu, H.; Hu, P.; Giraldo, J.P. Targeted delivery of nanomaterials with chemical cargoes in plants enabled by a biorecognition motif. Nat. Commun. 2020, 11, 2045. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, Y.; Xu, D.; Goh, N.S.; Demirer, G.S.; Cestellos-Blanco, S.; Chen, Y.; Landry, M.P.; Yang, P. Gold-Nanocluster-Mediated Delivery of siRNA to Intact Plant Cells for Efficient Gene Knockdown. Nano Lett. 2021, 21, 5859–5866. [Google Scholar] [CrossRef]
- Ali, Z.; Serag, M.F.; Demirer, G.S.; Torre, B.; di Fabrizio, E.; Landry, M.P.; Habuchi, S.; Mahfouz, M. DNA–Carbon Nanotube Binding Mode Determines the Efficiency of Carbon Nanotube-Mediated DNA Delivery to Intact Plants. ACS Appl. Nano Mater. 2022, 5, 4663–4676. [Google Scholar] [CrossRef]
- He, T.; Wang, J.; Hu, D.; Yang, Y.; Chae, E.; Lee, C. Epidermal electronic-tattoo for plant immune response monitoring. Nat. Commun. 2025, 16, 3244. [Google Scholar] [CrossRef]
- Baker, S.N.; Baker, G.A. Luminescent carbon nanodots: Emergent nanolights. Angew. Chem. Int. Ed. Engl. 2010, 49, 6726–6744. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Yang, B. Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef] [PubMed]
- Akbar, K.; Moretti, E.; Vomiero, A. Carbon Dots for Photocatalytic Degradation of Aqueous Pollutants: Recent Advancements. Adv. Opt. Mater. 2021, 9, 2100532. [Google Scholar] [CrossRef]
- Gan, Z.; Wu, X.; Zhou, G.; Shen, J.; Chu, P.K. Is There Real Upconversion Photoluminescence from Graphene Quantum Dots? Adv. Opt. Mater. 2013, 1, 554–558. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Z.; Wang, X.; Peng, X.; Zheng, J. Fluorescent carbon-dots enhance light harvesting and photosynthesis by overexpressing PsbP and PsiK genes. J. Nanobiotechnol. 2021, 19, 260. [Google Scholar] [CrossRef]
- Wang, B.; Huang, J.; Zhang, M.; Wang, Y.; Wang, H.; Ma, Y.; Zhao, X.; Wang, X.; Liu, C.; Huang, H.; et al. Carbon Dots Enable Efficient Delivery of Functional DNA in Plants. ACS Appl. Bio Mater. 2020, 3, 8857–8864. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.H.; Hendrix, B.; Hoffer, P.; Sanders, R.A.; Zheng, W. Carbon dots for efficient small interfering RNA delivery and gene silencing in plants. Plant Physiol. 2019, 184, 647–657. [Google Scholar] [CrossRef]
- Liang, C.; Diao, S.; Wang, C.; Gong, H.; Liu, T.; Hong, G.; Shi, X.; Dai, H.; Liu, Z. Tumor metastasis inhibition by imaging-guided photothermal therapy with single-walled carbon nanotubes. Adv. Mater. 2014, 26, 5646–5652. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Jia, G.; Wang, H.; Sun, H.; Wang, X.; Yang, S.; Wang, T.; Liu, Y. Translocation and fate of multi-walled carbon nanotubes in vivo. Carbon 2007, 45, 1419–1424. [Google Scholar] [CrossRef]
- Zheng, M.; Jagota, A.; Semke, E.D.; Diner, B.A.; McLean, R.S.; Lustig, S.R.; Richardson, R.E.; Tassi, N.G. DNA-assisted dispersion and separation of carbon nanotubes. Nat. Mater. 2003, 2, 338–342. [Google Scholar] [CrossRef]
- Ferrier, D.C.; Honeychurch, K.C. Carbon Nanotube (CNT)-Based Biosensors. Biosensors 2021, 11, 486. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-B.; Premkumar, T.; Lee, K.; Geckeler, K.E. A Facile Approach to Single-Wall Carbon Nanotube/Poly(allylamine) Nanocomposites. Macromol. Rapid Commun. 2007, 28, 276–280. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, B.; Wang, Q.; Shi, X.; Xiao, Z.; Lin, J.; Fang, X. Carbon nanotubes as molecular transporters for walled plant cells. Nano Lett. 2009, 9, 1007–1010. [Google Scholar] [CrossRef]
- Serag, M.F.; Kaji, N.; Gaillard, C.; Okamoto, Y.; Terasaka, K.; Jabasini, M.; Tokeshi, M.; Mizukami, H.; Bianco, A.; Baba, Y. Trafficking and subcellular localization of multiwalled carbon nanotubes in plant cells. ACS Nano 2011, 5, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Demirer, G.S.; Zhang, H.; Matos, J.L.; Goh, N.S.; Cunningham, F.J.; Sung, Y.; Chang, R.; Aditham, A.J.; Chio, L.; Cho, M.-J.; et al. High aspect ratio nanomaterials enable delivery of functional genetic material without DNA integration in mature plants. Nat. Nanotechnol. 2019, 14, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.-Y.; Lew, T.T.S.; Sweeney, C.J.; Koman, V.B.; Wong, M.H.; Bohmert-Tatarev, K.; Snell, K.D.; Seo, J.S.; Chua, N.-H.; Strano, M.S. Chloroplast-selective gene delivery and expression in planta using chitosan-complexed single-walled carbon nanotube carriers. Nat. Nanotechnol. 2019, 14, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Demirer, G.S.; Zhang, H.; Goh, N.S.; Pinals, R.L.; Chang, R.; Landry, M.P. Carbon nanocarriers deliver siRNA to intact plant cells for efficient gene knockdown. Sci. Adv. 2020, 6, eaaz0495. [Google Scholar] [CrossRef]
- Sutter, E.; Huang, Y.; Komsa, H.P.; Ghorbani-Asl, M.; Krasheninnikov, A.V.; Sutter, P. Electron-Beam Induced Transformations of Layered Tin Dichalcogenides. Nano Lett. 2016, 16, 4410–4416. [Google Scholar] [CrossRef]
- Demirer, G.S.; Zhang, H.; Goh, N.S.; González-Grandío, E.; Landry, M.P. Carbon nanotube-mediated DNA delivery without transgene integration in intact plants. Nat. Protoc. 2019, 14, 2954–2971. [Google Scholar] [CrossRef]
- Wu, K.; Xu, C.; Li, T.; Ma, H.; Gong, J.; Li, X.; Sun, X.; Hu, X. Application of Nanotechnology in Plant Genetic Engineering. Int. J. Mol. Sci. 2023, 24, 14836. [Google Scholar] [CrossRef]
- Martin-Ortigosa, S.; Peterson, D.J.; Valenstein, J.S.; Lin, V.S.; Trewyn, B.G.; Lyznik, L.A.; Wang, K. Mesoporous silica nanoparticle-mediated intracellular cre protein delivery for maize genome editing via loxP site excision. Plant Physiol. 2014, 164, 537–547. [Google Scholar] [CrossRef]
- Kocsisova, Z.; Coneva, V. Strategies for delivery of CRISPR/Cas-mediated genome editing to obtain edited plants directly without transgene integration. Front. Genome Ed. 2023, 5, 1209586. [Google Scholar] [CrossRef]
- Tang, L.; Wang, Y.; Li, J. The graphene/nucleic acid nanobiointerface. Chem. Soc. Rev. 2015, 44, 6954–6980. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Hu, D.; Lin, C.T.; Li, J.; Lin, Y. Aptamer/graphene oxide nanocomplex for in situ molecular probing in living cells. J. Am. Chem. Soc. 2010, 132, 9274–9276. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Lam, R.; Xu, X.; Chow, E.K.; Kim, H.J.; Ho, D. Multimodal nanodiamond drug delivery carriers for selective targeting, imaging, and enhanced chemotherapeutic efficacy. Adv. Mater. 2011, 23, 4770–4775. [Google Scholar] [CrossRef]
- Grichko, V.; Grishko, V.; Shenderova, O. Nanodiamond bullets and their biological targets. NanoBiotechnology 2006, 2, 37–42. [Google Scholar] [CrossRef]
- Burkhardt, J.; Basi, S.; Pariyar, S.; Hunsche, M. Stomatal penetration by aqueous solutions—An update involving leaf surface particles. New Phytol. 2012, 196, 774–787. [Google Scholar] [CrossRef]
- Rastogi, A.; Tripathi, D.K.; Yadav, S.; Chauhan, D.K.; Živčák, M.; Ghorbanpour, M.; El-Sheery, N.I.; Brestic, M. Application of silicon nanoparticles in agriculture. 3Biotech 2019, 9, 90. [Google Scholar] [CrossRef]
- Jambhrunkar, S.; Qu, Z.; Popat, A.; Yang, J.; Noonan, O.; Acauan, L.; Ahmad Nor, Y.; Yu, C.; Karmakar, S. Effect of surface functionality of silica nanoparticles on cellular uptake and cytotoxicity. Mol. Pharm. 2014, 11, 3642–3655. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.I.; Yi, Z.; Rookes, J.E.; Kong, L.X.; Cahill, D.M. Mesoporous silica nanoparticles as a biomolecule delivery vehicle in plants. J. Nanoparticle Res. 2013, 15, 1676. [Google Scholar] [CrossRef]
- Torney, F.; Trewyn, B.G.; Lin, V.S.-Y.; Wang, K. Mesoporous silica nanoparticles deliver DNA and chemicals into plants. Nat. Nanotechnol. 2007, 2, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Martin-Ortigosa, S.; Valenstein, J.S.; Sun, W.; Moeller, L.; Fang, N.; Trewyn, B.G.; Lin, V.S.; Wang, K. Parameters affecting the efficient delivery of mesoporous silica nanoparticle materials and gold nanorods into plant tissues by the biolistic method. Small 2012, 8, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Liu, Z.; Wang, H.; Meng, H.; Cao, Y. Mesoporous Silica Nanoparticles Mediate SiRNA Delivery for Long-Term Multi-Gene Silencing in Intact Plants. Adv. Sci. 2024, 11, e2301358. [Google Scholar] [CrossRef]
- Yong, J.; Wu, M.; Zhang, R.; Bi, S.; Mann, C.W.G.; Mitter, N.; Carroll, B.J.; Xu, Z.P. Clay nanoparticles efficiently deliver small interfering RNA to intact plant leaf cells. Plant Physiol. 2022, 190, 2187–2202. [Google Scholar] [CrossRef]
- Choy, J.-H.; Kwak, S.-Y.; Jeong, Y.-J.; Park, J.-S. Inorganic Layered Double Hydroxides as Nonviral Vectors. Angew. Chem. Int. Ed. 2000, 39, 4041–4045. [Google Scholar] [CrossRef]
- Li, P.; Duan, X.; Kuang, Y.; Li, Y.; Zhang, G.; Liu, W.; Sun, X. Tuning Electronic Structure of NiFe Layered Double Hydroxides with Vanadium Doping toward High Efficient Electrocatalytic Water Oxidation. Adv. Energy Mater. 2018, 8, 1703341. [Google Scholar] [CrossRef]
- Fan, G.; Li, F.; Evans, D.G.; Duan, X. Catalytic applications of layered double hydroxides: Recent advances and perspectives. Chem. Soc. Rev. 2014, 43, 7040–7066. [Google Scholar] [CrossRef]
- Li, X.; Du, D.; Zhang, Y.; Xing, W.; Xue, Q.; Yan, Z. Layered double hydroxides toward high-performance supercapacitors. J. Mater. Chem. A 2017, 5, 15460–15485. [Google Scholar] [CrossRef]
- Sajid, M.; Basheer, C. Layered double hydroxides: Emerging sorbent materials for analytical extractions. TrAC Trends Anal. Chem. 2016, 75, 174–182. [Google Scholar] [CrossRef]
- Bao, W.; Wang, J.; Wang, Q.; O’Hare, D.; Wan, Y. Layered Double Hydroxide Nanotransporter for Molecule Delivery to Intact Plant Cells. Sci. Rep. 2016, 6, 26738. [Google Scholar] [CrossRef] [PubMed]
- Mitter, N.; Worrall, E.A.; Robinson, K.E.; Li, P.; Jain, R.G.; Taochy, C.; Fletcher, S.J.; Carroll, B.J.; Lu, G.Q.; Xu, Z.P. Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nat. Plants 2017, 3, 16207. [Google Scholar] [CrossRef] [PubMed]
- Christou, P. Morphological Description of Transgenic Soybean Chimeras Created by the Delivery, Integration and Expression of Foreign DNA Using Electric Discharge Particle Acceleration. Ann. Bot. 1990, 66, 379–386. [Google Scholar] [CrossRef]
- Zhang, H.; Goh, N.S.; Wang, J.W.; Pinals, R.L.; González-Grandío, E.; Demirer, G.S.; Butrus, S.; Fakra, S.C.; Del Rio Flores, A.; Zhai, R.; et al. Nanoparticle cellular internalization is not required for RNA delivery to mature plant leaves. Nat. Nanotechnol. 2022, 17, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Fraley, R.T.; Dellaporta, S.L.; Papahadjopoulos, D. Liposome-mediated delivery of tobacco mosaic virus RNA into tobacco protoplasts: A sensitive assay for monitoring liposome-protoplast interactions. Proc. Natl. Acad. Sci. USA 1982, 79, 1859–1863. [Google Scholar] [CrossRef] [PubMed]
- Karny, A.; Zinger, A.; Kajal, A.; Shainsky-Roitman, J.; Schroeder, A. Therapeutic nanoparticles penetrate leaves and deliver nutrients to agricultural crops. Sci. Rep. 2018, 8, 7589. [Google Scholar] [CrossRef]
- Fukunaga, Y.; Nagata, T.; Takebe, I. Liposome-mediated infection of plant protoplasts with tobacco mosaic virus RNA. Virology 1981, 113, 752–760. [Google Scholar] [CrossRef]
- Nagata, T. [4] Interaction of plant protoplast and liposome. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1987; Volume 148, pp. 34–39. [Google Scholar]
- Lurquin, P.F.; Rollo, F. [31] Liposome-mediated delivery of nucleic acids into plant protoplasts. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1993; Volume 221, pp. 409–415. [Google Scholar]
- Deshayes, A.; Herrera-Estrella, L.; Caboche, M. Liposome-mediated transformation of tobacco mesophyll protoplasts by an Escherichia coli plasmid. EMBO J. 1985, 4, 2731–2737. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, Y.; Nagata, T.; Takebe, I.; Kakehi, T.; Matsui, C. An ultrastructural study of the interaction of liposomes with plant protoplasts. Exp. Cell Res. 1983, 144, 181–189. [Google Scholar] [CrossRef]
- Rosenberg, N.; Dekel-Reichenbach, M.; Navot, N.; Gad, A.E.; Altman, A.; Czosnek, H. Liposome-Mediated Introduction of DNA Into Plant Protoplasts and Calli. In I International Symposium on In Vitro Culture and Horticultural Breeding; International Society for Horticultural Science (ISHS): Leuven, Belgium, 1990; pp. 509–516. [Google Scholar]
- Gad, A.E.; Rosenberg, N.; Altman, A. Liposome-mediated gene delivery into plant cells. Physiol. Plant. 1990, 79, 177–183. [Google Scholar] [CrossRef]
- Iqbal, Y.; Ahmed, I.; Irfan, M.F.; Chatha, S.A.S.; Zubair, M.; Ullah, A. Recent advances in chitosan-based materials; The synthesis, modifications and biomedical applications. Carbohydr. Polym. 2023, 321, 121318. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, S.B.; Wu, F.L.; Mao, Z.B.; Yu, C.L. Transfection of primary chondrocytes using chitosan-pEGFP nanoparticles. J. Control. Release 2006, 112, 223–228. [Google Scholar] [CrossRef]
- Thanou, M.; Florea, B.I.; Geldof, M.; Junginger, H.E.; Borchard, G. Quaternized chitosan oligomers as novel gene delivery vectors in epithelial cell lines. Biomaterials 2002, 23, 153–159. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, C.; Xushi; Zhang, C.; Tang, T.; Dai, K. Direct chitosan-mediated gene delivery to the rabbit knee joints in vitro and in vivo. Biochem. Biophys. Res. Commun. 2006, 341, 202–208. [Google Scholar] [CrossRef]
- Khalili, N.; Oraei, M.; Gohari, G.; Panahirad, S.; Nourafcan, H.; Hano, C. Chitosan-Enriched Salicylic Acid Nanoparticles Enhanced Anthocyanin Content in Grape (Vitis vinifera L. cv. Red Sultana) Berries. Polymers 2022, 14, 3349. [Google Scholar] [CrossRef]
- Jayanudin; Lestari, R.S.D.; Kustiningsih, I.; Irawanto, D.; Bahaudin, R.; Wardana, R.L.A.; Muhammad, F.; Suyuti, M.; Luthfi, M. Preparation of chitosan microspheres as carrier material to controlled release of urea fertilizer. S. Afr. J. Chem. Eng. 2021, 38, 70–77. [Google Scholar] [CrossRef]
- Karayianni, M.; Sentoukas, T.; Skandalis, A.; Pippa, N.; Pispas, S. Chitosan-Based Nanoparticles for Nucleic Acid Delivery: Technological Aspects, Applications, and Future Perspectives. Pharmaceutics 2023, 15, 1849. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, M.; Kodama, Y.; Yoshizumi, T.; Sudesh, K.; Numata, K. Rapid and efficient gene delivery into plant cells using designed peptide carriers. Biomacromolecules 2013, 14, 10–16. [Google Scholar] [CrossRef]
- Fonseca, S.B.; Pereira, M.P.; Kelley, S.O. Recent advances in the use of cell-penetrating peptides for medical and biological applications. Adv. Drug Deliv. Rev. 2009, 61, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Chou, J.C.; Lee, H.J. Cellular internalization of fluorescent proteins via arginine-rich intracellular delivery peptide in plant cells. Plant Cell Physiol. 2005, 46, 482–488. [Google Scholar] [CrossRef]
- Chen, C.P.; Chou, J.C.; Liu, B.R.; Chang, M.; Lee, H.J. Transfection and expression of plasmid DNA in plant cells by an arginine-rich intracellular delivery peptide without protoplast preparation. FEBS Lett. 2007, 581, 1891–1897. [Google Scholar] [CrossRef]
- Chuah, J.-A.; Numata, K. Stimulus-Responsive Peptide for Effective Delivery and Release of DNA in Plants. Biomacromolecules 2018, 19, 1154–1163. [Google Scholar] [CrossRef]
- Yoshizumi, T.; Oikawa, K.; Chuah, J.-A.; Kodama, Y.; Numata, K. Selective Gene Delivery for Integrating Exogenous DNA into Plastid and Mitochondrial Genomes Using Peptide-DNA Complexes. Biomacromolecules 2018, 19, 1582–1591. [Google Scholar] [CrossRef]
- Thagun, C.; Chuah, J.-A.; Numata, K. Targeted Gene Delivery into Various Plastids Mediated by Clustered Cell-Penetrating and Chloroplast-Targeting Peptides. Adv. Sci. 2019, 6, 1902064. [Google Scholar] [CrossRef] [PubMed]
- Numata, K.; Horii, Y.; Oikawa, K.; Miyagi, Y.; Demura, T.; Ohtani, M. Library screening of cell-penetrating peptide for BY-2 cells, leaves of Arabidopsis, tobacco, tomato, poplar, and rice callus. Sci. Rep. 2018, 8, 10966. [Google Scholar] [CrossRef]
- Miyamoto, T.; Tsuchiya, K.; Numata, K. Dual Peptide-Based Gene Delivery System for the Efficient Transfection of Plant Callus Cells. Biomacromolecules 2020, 21, 2735–2744. [Google Scholar] [CrossRef]
- Nummelin, S.; Kommeri, J.; Kostiainen, M.A.; Linko, V. Evolution of Structural DNA Nanotechnology. Adv. Mater. 2018, 30, e1703721. [Google Scholar] [CrossRef]
- Wang, Y.; Benson, E.; Fördős, F.; Lolaico, M.; Baars, I.; Fang, T.; Teixeira, A.I.; Högberg, B. DNA Origami Penetration in Cell Spheroid Tissue Models is Enhanced by Wireframe Design. Adv. Mater. 2021, 33, e2008457. [Google Scholar] [CrossRef]
- Zhan, P.; Peil, A.; Jiang, Q.; Wang, D.; Mousavi, S.; Xiong, Q.; Shen, Q.; Shang, Y.; Ding, B.; Lin, C.; et al. Recent Advances in DNA Origami-Engineered Nanomaterials and Applications. Chem. Rev. 2023, 123, 3976–4050. [Google Scholar] [CrossRef]
- Zhang, H.; Demirer, G.S.; Zhang, H.; Ye, T.; Goh, N.S.; Aditham, A.J.; Cunningham, F.J.; Fan, C.; Landry, M.P. DNA nanostructures coordinate gene silencing in mature plants. Proc. Natl. Acad. Sci. USA 2019, 116, 7543–7548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, H.; Demirer, G.S.; González-Grandío, E.; Fan, C.; Landry, M.P. Engineering DNA nanostructures for siRNA delivery in plants. Nat. Protoc. 2020, 15, 3064–3087. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-T.; Gray, M.A.; Xuan, S.; Lin, Y.; Byrnes, J.; Nguyen, A.I.; Todorova, N.; Stevens, M.M.; Bertozzi, C.R.; Zuckermann, R.N.; et al. DNA origami protection and molecular interfacing through engineered sequence-defined peptoids. Proc. Natl. Acad. Sci. USA 2020, 117, 6339–6348. [Google Scholar] [CrossRef]
- Ishino, Y.; Shinagawa, H.; Makino, K.; Amemura, M.; Nakata, A. Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. J. Bacteriol. 1987, 169, 5429–5433. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Ming, M.; Ren, Q.; Pan, C.; He, Y.; Zhang, Y.; Liu, S.; Zhong, Z.; Wang, J.; Malzahn, A.A.; Wu, J.; et al. CRISPR-Cas12b enables efficient plant genome engineering. Nat. Plants 2020, 6, 202–208. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, Y.; Ye, J.; Cao, X.; Xu, C.; Chen, B.; An, H.; Jiao, Y.; Zhang, F.; Yang, X.; et al. Establishing CRISPR/Cas13a immune system conferring RNA virus resistance in both dicot and monocot plants. Plant Biotechnol. J. 2019, 17, 1185–1187. [Google Scholar] [CrossRef]
- Yin, X.; Biswal, A.K.; Dionora, J.; Perdigon, K.M.; Balahadia, C.P.; Mazumdar, S.; Chater, C.; Lin, H.C.; Coe, R.A.; Kretzschmar, T.; et al. CRISPR-Cas9 and CRISPR-Cpf1 mediated targeting of a stomatal developmental gene EPFL9 in rice. Plant Cell Rep. 2017, 36, 745–757. [Google Scholar] [CrossRef]
- Ito, Y.; Nishizawa-Yokoi, A.; Endo, M.; Mikami, M.; Toki, S. CRISPR/Cas9-mediated mutagenesis of the RIN locus that regulates tomato fruit ripening. Biochem. Biophys. Res. Commun. 2015, 467, 76–82. [Google Scholar] [CrossRef]
- Tang, X.; Eid, A.; Zhang, R.; Cheng, Y.; Liu, A.; Chen, Y.; Chen, P.; Zhang, Y.; Qi, Y. Genome editing in rice and tomato with a small Un1Cas12f1 nuclease. Plant Genome 2024, 17, e20465. [Google Scholar] [CrossRef]
- Gong, Z.; Previtera, D.A.; Wang, Y.; Botella, J.R. Geminiviral-induced genome editing using miniature CRISPR/Cas12j (CasΦ) and Cas12f variants in plants. Plant Cell Rep. 2024, 43, 71. [Google Scholar] [CrossRef]
- Nekrasov, V.; Staskawicz, B.; Weigel, D.; Jones, J.D.; Kamoun, S. Targeted mutagenesis in the model plant Nicotiana benthamiana using Cas9 RNA-guided endonuclease. Nat. Biotechnol. 2013, 31, 691–693. [Google Scholar] [CrossRef]
- Shan, Q.; Wang, Y.; Li, J.; Zhang, Y.; Chen, K.; Liang, Z.; Zhang, K.; Liu, J.; Xi, J.J.; Qiu, J.L.; et al. Targeted genome modification of crop plants using a CRISPR-Cas system. Nat. Biotechnol. 2013, 31, 686–688. [Google Scholar] [CrossRef]
- Feng, Z.; Mao, Y.; Xu, N.; Zhang, B.; Wei, P.; Yang, D.L.; Wang, Z.; Zhang, Z.; Zheng, R.; Yang, L.; et al. Multigeneration analysis reveals the inheritance, specificity, and patterns of CRISPR/Cas-induced gene modifications in Arabidopsis. Proc. Natl. Acad. Sci. USA 2014, 111, 4632–4637. [Google Scholar] [CrossRef] [PubMed]
- Nishitani, C.; Hirai, N.; Komori, S.; Wada, M.; Okada, K.; Osakabe, K.; Yamamoto, T.; Osakabe, Y. Efficient Genome Editing in Apple Using a CRISPR/Cas9 system. Sci. Rep. 2016, 6, 31481. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Liu, X.; Zhang, Z.; Wang, Y.; Duan, W.; Li, S.; Liang, Z. CRISPR/Cas9-mediated efficient targeted mutagenesis in Chardonnay (Vitis vinifera L.). Sci. Rep. 2016, 6, 32289. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.W.; Kim, J.; Kwon, S.I.; Corvalán, C.; Cho, S.W.; Kim, H.; Kim, S.G.; Kim, S.T.; Choe, S.; Kim, J.S. DNA-free genome editing in plants with preassembled CRISPR-Cas9 ribonucleoproteins. Nat. Biotechnol. 2015, 33, 1162–1164. [Google Scholar] [CrossRef]
- Liang, Z.; Chen, K.; Li, T.; Zhang, Y.; Wang, Y.; Zhao, Q.; Liu, J.; Zhang, H.; Liu, C.; Ran, Y.; et al. Efficient DNA-free genome editing of bread wheat using CRISPR/Cas9 ribonucleoprotein complexes. Nat. Commun. 2017, 8, 14261. [Google Scholar] [CrossRef]
- Yamada, H.; Kato, N.; Ichikawa, M.; Mannen, K.; Kiba, T.; Osakabe, Y.; Sakakibara, H.; Matsui, M.; Okamoto, T. DNA- and Selectable-Marker-Free Genome-Editing System Using Zygotes from Recalcitrant Maize Inbred B73. Plant Cell Physiol. 2024, 65, 729–736. [Google Scholar] [CrossRef]
- Liu, D.; Ellison, E.E.; Myers, E.A.; Donahue, L.I.; Xuan, S.; Swanson, R.; Qi, S.; Prichard, L.E.; Starker, C.G.; Voytas, D.F. Heritable gene editing in tomato through viral delivery of isopentenyl transferase and single-guide RNAs to latent axillary meristematic cells. Proc. Natl. Acad. Sci. USA 2024, 121, e2406486121. [Google Scholar] [CrossRef]
- Oh, Y.; Nagalakshmi, U.; Dahlbeck, D.; Koehler, N.; Cho, M.J.; Dinesh-Kumar, S.P.; Staskawicz, B.J. Heritable virus-induced germline editing in tomato. Plant J. 2025, 122, e70115. [Google Scholar] [CrossRef]
- Demirer, G.S.; Silva, T.N.; Jackson, C.T.; Thomas, J.B.; Ehrhardt, D.W.; Rhee, S.Y.; Mortimer, J.C.; Landry, M.P. Nanotechnology to advance CRISPR-Cas genetic engineering of plants. Nat. Nanotechnol. 2021, 16, 243–250. [Google Scholar] [CrossRef]
- Qiu, F.; Xue, C.; Liu, J.; Li, B.; Gao, Q.; Liang, R.; Chen, K.; Gao, C. An efficient mRNA delivery system for genome editing in plants. Plant Biotechnol. J. 2025, 23, 1348–1358. [Google Scholar] [CrossRef]
- Liu, X.; Gu, D.; Zhang, Y.; Jiang, Y.; Xiao, Z.; Xu, R.; Qin, R.; Li, J.; Wei, P. Conditional knockdown of OsMLH1 to improve plant prime editing systems without disturbing fertility in rice. Genome Biol. 2024, 25, 131. [Google Scholar] [CrossRef] [PubMed]
- Ni, P.; Zhao, Y.; Zhou, X.; Liu, Z.; Huang, Z.; Ni, Z.; Sun, Q.; Zong, Y. Efficient and versatile multiplex prime editing in hexaploid wheat. Genome Biol. 2023, 24, 156. [Google Scholar] [CrossRef]
- Lowry, G.V.; Giraldo, J.P.; Steinmetz, N.F.; Avellan, A.; Demirer, G.S.; Ristroph, K.D.; Wang, G.J.; Hendren, C.O.; Alabi, C.A.; Caparco, A.; et al. Towards realizing nano-enabled precision delivery in plants. Nat. Nanotechnol. 2024, 19, 1255–1269. [Google Scholar] [CrossRef]
- Jeon, S.-J.; Hu, P.; Kim, K.; Anastasia, C.M.; Kim, H.-I.; Castillo, C.; Ahern, C.B.; Pedersen, J.A.; Fairbrother, D.H.; Giraldo, J.P. Electrostatics Control Nanoparticle Interactions with Model and Native Cell Walls of Plants and Algae. Environ. Sci. Technol. 2023, 57, 19663–19677. [Google Scholar] [CrossRef] [PubMed]
- Santana, I.; Jeon, S.-J.; Kim, H.-I.; Islam, M.R.; Castillo, C.; Garcia, G.F.H.; Newkirk, G.M.; Giraldo, J.P. Targeted Carbon Nanostructures for Chemical and Gene Delivery to Plant Chloroplasts. ACS Nano 2022, 16, 12156–12173. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, Z.; Zhanakhmetova, D.; Li, W.; Chen, S.; Werner, T.; Liesche, J. Fast and simple fluorometric measurement of phloem loading exposes auxin-dependent regulation of Arabidopsis sucrose transporter AtSUC2. Plant J. 2024, 120, 2305–2318. [Google Scholar] [CrossRef]
- Chu, C.C.; Swamy, K.; Li, H.M. Tissue-Specific Regulation of Plastid Protein Import via Transit-Peptide Motifs. Plant Cell 2020, 32, 1204–1217. [Google Scholar] [CrossRef] [PubMed]
- EFSA Scientific Committee; More, S.; Bampidis, V.; Benford, D.; Bragard, C.; Halldorsson, T.; Hernández-Jerez, A.; Hougaard Bennekou, S.; Koutsoumanis, K.; Lambré, C.; et al. Guidance on risk assessment of nanomaterials to be applied in the food and feed chain: Human and animal health. EFSA J. 2021, 19, e06768. [Google Scholar] [CrossRef]
- Committee, E.S.; More, S.; Bampidis, V.; Benford, D.; Bragard, C.; Halldorsson, T.; Hernández-Jerez, A.; Bennekou, S.H.; Koutsoumanis, K.; Lambré, C.; et al. Guidance on technical requirements for regulated food and feed product applications to establish the presence of small particles including nanoparticles. EFSA J. 2021, 19, e06769. [Google Scholar] [CrossRef]
- Kagan, V.E.; Konduru, N.V.; Feng, W.; Allen, B.L.; Conroy, J.; Volkov, Y.; Vlasova, I.I.; Belikova, N.A.; Yanamala, N.; Kapralov, A.; et al. Carbon nanotubes degraded by neutrophil myeloperoxidase induce less pulmonary inflammation. Nat. Nanotechnol. 2010, 5, 354–359. [Google Scholar] [CrossRef]
- Allen, B.L.; Kotchey, G.P.; Chen, Y.; Yanamala, N.V.; Klein-Seetharaman, J.; Kagan, V.E.; Star, A. Mechanistic investigations of horseradish peroxidase-catalyzed degradation of single-walled carbon nanotubes. J. Am. Chem. Soc. 2009, 131, 17194–17205. [Google Scholar] [CrossRef]
- Forstner, C.; Orton, T.G.; Wang, P.; Kopittke, P.M.; Dennis, P.G. Effects of carbon nanotubes and derivatives of graphene oxide on soil bacterial diversity. Sci. Total Environ. 2019, 682, 356–363. [Google Scholar] [CrossRef]
- Zuo, Y.; Wei, C.; Hu, Y.; Zeng, W.; Ao, C.; Huang, J. Effect of Multi-Walled Carbon Nanotubes on the Carbon and Nitrogen Cycling Processes in Saline Soil. Agronomy 2023, 13, 2455. [Google Scholar] [CrossRef]
- Lérida-Viso, A.; Estepa-Fernández, A.; García-Fernández, A.; Martí-Centelles, V.; Martínez-Máñez, R. Biosafety of mesoporous silica nanoparticles; towards clinical translation. Adv. Drug Deliv. Rev. 2023, 201, 115049. [Google Scholar] [CrossRef] [PubMed]
- Rascol, E.; Pisani, C.; Dorandeu, C.; Nyalosaso, J.L.; Charnay, C.; Daurat, M.; Da Silva, A.; Devoisselle, J.M.; Gaillard, J.C.; Armengaud, J.; et al. Biosafety of Mesoporous Silica Nanoparticles. Biomimetics 2018, 3, 22. [Google Scholar] [CrossRef]
- Singha Roy, A.; Kesavan Pillai, S.; Ray, S.S. Layered Double Hydroxides for Sustainable Agriculture and Environment: An Overview. ACS Omega 2022, 7, 20428–20440. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Jiménez, R.; Vera, G.; Tobar, M.; Moscote, J.; Acha, G.; Herrera-Vásquez, A.; Rojas-Rivera, D.; Vidal, E.A.; Almeida, A.M.; Ahumada, M. Mg–Al LDH nanosheets as a nanotechnological tool in agriculture: An exploratory toxicity evaluation study. Environ. Sci. Nano 2024, 11, 2249–2261. [Google Scholar] [CrossRef]
- Peshkova, A.; Zinicovscaia, I.; Cepoi, L.; Rudi, L.; Chiriac, T.; Yushin, N.; Anh, T.T.; Manh Dung, H.; Corcimaru, S. Effects of Gold Nanoparticles on Mentha spicata L., Soil Microbiota, and Human Health Risks: Impact of Exposure Routes. Nanomaterials 2024, 14, 955. [Google Scholar] [CrossRef]
- Bourdineaud, J.P.; Štambuk, A.; Šrut, M.; Radić Brkanac, S.; Ivanković, D.; Lisjak, D.; Sauerborn Klobučar, R.; Dragun, Z.; Bačić, N.; Klobučar, G.I.V. Gold and silver nanoparticles effects to the earthworm Eisenia fetida—The importance of tissue over soil concentrations. Drug Chem. Toxicol. 2021, 44, 12–29. [Google Scholar] [CrossRef] [PubMed]
- Holme, M.N.; Rashid, M.H.; Thomas, M.R.; Barriga, H.M.G.; Herpoldt, K.L.; Heenan, R.K.; Dreiss, C.A.; Bañuelos, J.L.; Xie, H.N.; Yarovsky, I.; et al. Fate of Liposomes in the Presence of Phospholipase C and D: From Atomic to Supramolecular Lipid Arrangement. ACS Cent. Sci. 2018, 4, 1023–1030. [Google Scholar] [CrossRef]
- Mumtaz Virk, M.; Reimhult, E. Phospholipase A2-Induced Degradation and Release from Lipid-Containing Polymersomes. Langmuir 2018, 34, 395–405. [Google Scholar] [CrossRef]
- Ingle, P.U.; Shende, S.S.; Shingote, P.R.; Mishra, S.S.; Sarda, V.; Wasule, D.L.; Rajput, V.D.; Minkina, T.; Rai, M.; Sushkova, S.; et al. Chitosan nanoparticles (ChNPs): A versatile growth promoter in modern agricultural production. Heliyon 2022, 8, e11893. [Google Scholar] [CrossRef]
- Wang, X.; He, M.; Wang, X.; Liu, S.; Luo, L.; Zeng, Q.; Wu, Y.; Zeng, Y.; Yang, Z.; Sheng, G.; et al. Emerging Nanochitosan for Sustainable Agriculture. Int. J. Mol. Sci. 2024, 25, 12261. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, A.R. Nuclease resistance of DNA nanostructures. Nat. Rev. Chem. 2021, 5, 225–239. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Yu, N.-Y.; Fang, W.-D.; Tan, Q.-G.; Ji, R.; Yang, L.-Y.; Wei, S.; Zhang, X.-W.; Miao, A.-J. Photodegradation of carbon dots cause cytotoxicity. Nat. Commun. 2021, 12, 812. [Google Scholar] [CrossRef]
- Moya, S.E.; Hernández, R.R.; Angelomé, P.C. Degradation of Mesoporous Silica Materials in Biological Milieu: The Gateway for Therapeutic Applications. Adv. NanoBiomed Res. 2024, 4, 2400005. [Google Scholar] [CrossRef]
- Luo, J.; Cui, Y.; Xu, L.; Zhang, J.; Chen, J.; Li, X.; Zeng, B.; Deng, Z.; Shao, L. Layered double hydroxides for regenerative nanomedicine and tissue engineering: Recent advances and future perspectives. J. Nanobiotechnol. 2025, 23, 370. [Google Scholar] [CrossRef]
- Mehta, M.; Bui, T.A.; Yang, X.; Aksoy, Y.; Goldys, E.M.; Deng, W. Lipid-Based Nanoparticles for Drug/Gene Delivery: An Overview of the Production Techniques and Difficulties Encountered in Their Industrial Development. ACS Mater. Au 2023, 3, 600–619. [Google Scholar] [CrossRef] [PubMed]
- Won, J.; Cho, S.; Kim, K.-R.; Kim, S.; Ahn, D.-R. Engineering in vivo behavior of DNA nanostructures toward organ-targeted drug delivery. Adv. Drug Deliv. Rev. 2025, 225, 115682. [Google Scholar] [CrossRef]
- Kariuki, R.; Penman, R.; Newbold, A.D.; Mirihana, K.A.; Vaillant, P.H.A.; Shepherd, T.P.; Meftahi, N.; Bryant, G.; Voïtchovsky, K.; Contini, C.; et al. Gold Nanoparticle Adsorption and Uptake are Directed by Particle Capping Agent. Small Sci. 2025, 5, 2500060. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xie, H.; Wang, P.; Yin, H. Nanoparticles in Plants: Uptake, Transport and Physiological Activity in Leaf and Root. Materials 2023, 16, 3097. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Liu, J.; Jiang, G. Nanoparticle-specific transformations dictate nanoparticle effects associated with plants and implications for nanotechnology use in agriculture. Nat. Commun. 2024, 15, 7389. [Google Scholar] [CrossRef]
- Hou, H.; Xu, Z.; Takeda, Y.S.; Powers, M.; Yang, Y.; Hershberger, K.; Hanscom, H.; Svenson, S.; Simhadri, R.K.; Vegas, A.J. Quantitative biodistribution of nanoparticles in plants with lanthanide complexes. Sci. Rep. 2023, 13, 21440. [Google Scholar] [CrossRef]

| Classification | Systems | Characteristics | Strengths | Limitations |
|---|---|---|---|---|
| Traditional genetic transformation | Agrobacterium | Agrobacterium enters plant cells through wound infection and transfers T-DNA for insertion into the plant genome. | High efficiency; low cost; stable transformation | Limited host range |
| Biolistic particle delivery | The gene is coated onto heavy metal particles and delivered into cells via high-velocity propulsion. | Species independence; simple operation; large-size gene delivery | Low efficiency; cell or tissue damage; high cost | |
| Electroporation | The gene is transferred into the cytoplasm through transient pores created by electric field pulses. | Fast and inexpensive; high efficiency | Limited range of plant species; difficult to pass the walled cell; toxicity | |
| PEG-mediated delivery | PEG destabilizes the plant cell membrane, thereby permitting the entry of DNA into the cytoplasm. | Highly efficient protoplast transformation | Limited to protoplast | |
| Nanomaterial-mediated gene-delivery system | CNTs | Nanomaterials enable the delivery of exogenous genes into the cytoplasm or organelles via endocytic or non-endocytic pathways. | Species independence; simple operation; biocompatibility; high cargo-loading capacity; high transformation efficiency | Limited nanocarriers; affected by carriers’ physical and chemical properties |
| MSNs | ||||
| Liposomes, etc. | ||||
| Genome editing | CRISPR-Cas | The CRISPR-Cas system is delivered into the cell, where it performs gene cleavage guided by the sgRNA. | Precise genetic cutting; nanotechnology to facilitate and accelerate plant genome editing | Easy degradation for plasmid; Easy deactivation for RNP; Restricted to the delivery vectors |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, C.-M.; Xiao, X.-S.; Liu, L.-W.; Lin, X.-D.; Dou, D.-L.; Cai, H.-Y.; Mei, Z.-F.; Yang, F.; Cheng, Y.; Qin, Y. Nanotechnology Strategies in Plant Genetic Engineering: Intelligent Delivery and Precision Editing. Plants 2025, 14, 3625. https://doi.org/10.3390/plants14233625
Lai C-M, Xiao X-S, Liu L-W, Lin X-D, Dou D-L, Cai H-Y, Mei Z-F, Yang F, Cheng Y, Qin Y. Nanotechnology Strategies in Plant Genetic Engineering: Intelligent Delivery and Precision Editing. Plants. 2025; 14(23):3625. https://doi.org/10.3390/plants14233625
Chicago/Turabian StyleLai, Chun-Mei, Xiao-Shan Xiao, Li-Wei Liu, Xin-Da Lin, Dan-Lin Dou, Han-Yang Cai, Zhi-Feng Mei, Fan Yang, Yan Cheng, and Yuan Qin. 2025. "Nanotechnology Strategies in Plant Genetic Engineering: Intelligent Delivery and Precision Editing" Plants 14, no. 23: 3625. https://doi.org/10.3390/plants14233625
APA StyleLai, C.-M., Xiao, X.-S., Liu, L.-W., Lin, X.-D., Dou, D.-L., Cai, H.-Y., Mei, Z.-F., Yang, F., Cheng, Y., & Qin, Y. (2025). Nanotechnology Strategies in Plant Genetic Engineering: Intelligent Delivery and Precision Editing. Plants, 14(23), 3625. https://doi.org/10.3390/plants14233625







