The SlHsfC1–SlGAI3 Module Controls Tomato Growth and Development via the Gibberellin Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials, Growth Conditions, and Treatments
2.2. Generation of Transgenic Lines
2.3. Subcellular Localization Analysis
2.4. Tissue Sectioning and Cell Morphology Quantification
2.5. Measurement of Gibberellin Content
2.6. RNA-Seq and RT-PCR
2.7. Yeast One-Hybrid Assay
2.8. Transactivation Assay
2.9. Electrophoretic Mobility Shift Assay
2.10. Chromatin Immunoprecipitation (ChIP) Qpcr Assay
2.11. Data Statistics and Analysis
3. Results
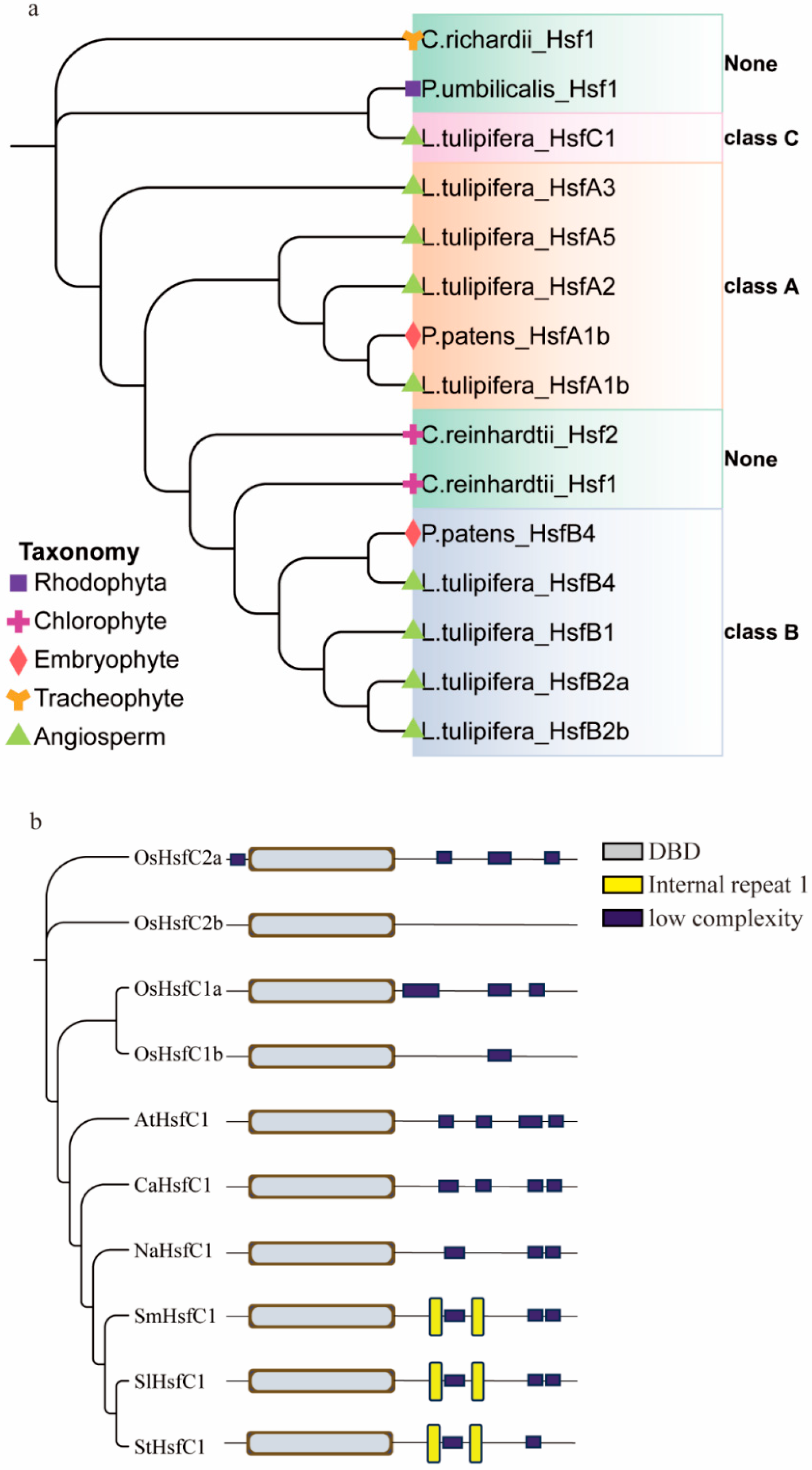
3.1. Origin and Protein Structure Analysis of HsfC Gene
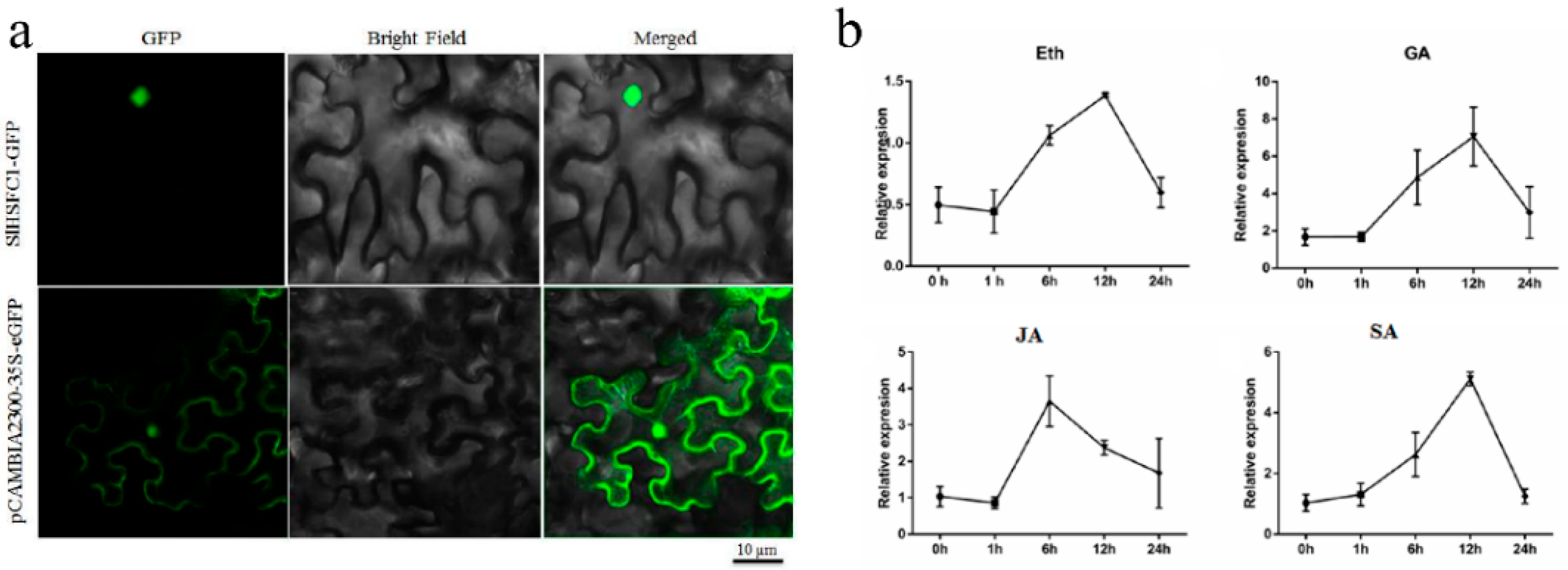
3.2. Subcellular Localization and Hormone Expression Patterns of SlHsfC1
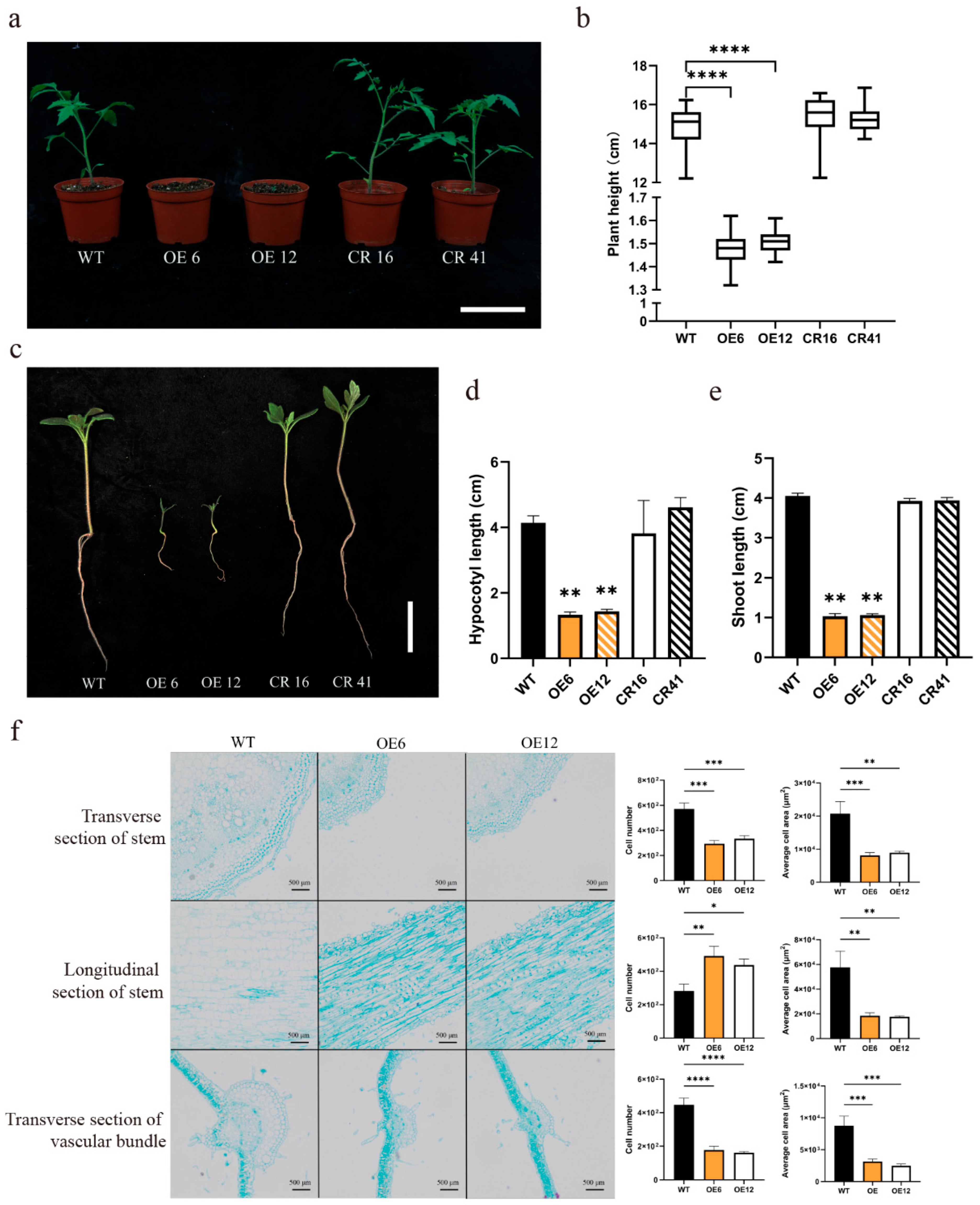
3.3. SlHsfC1 Influences Tomato Growth and Developmental Processes
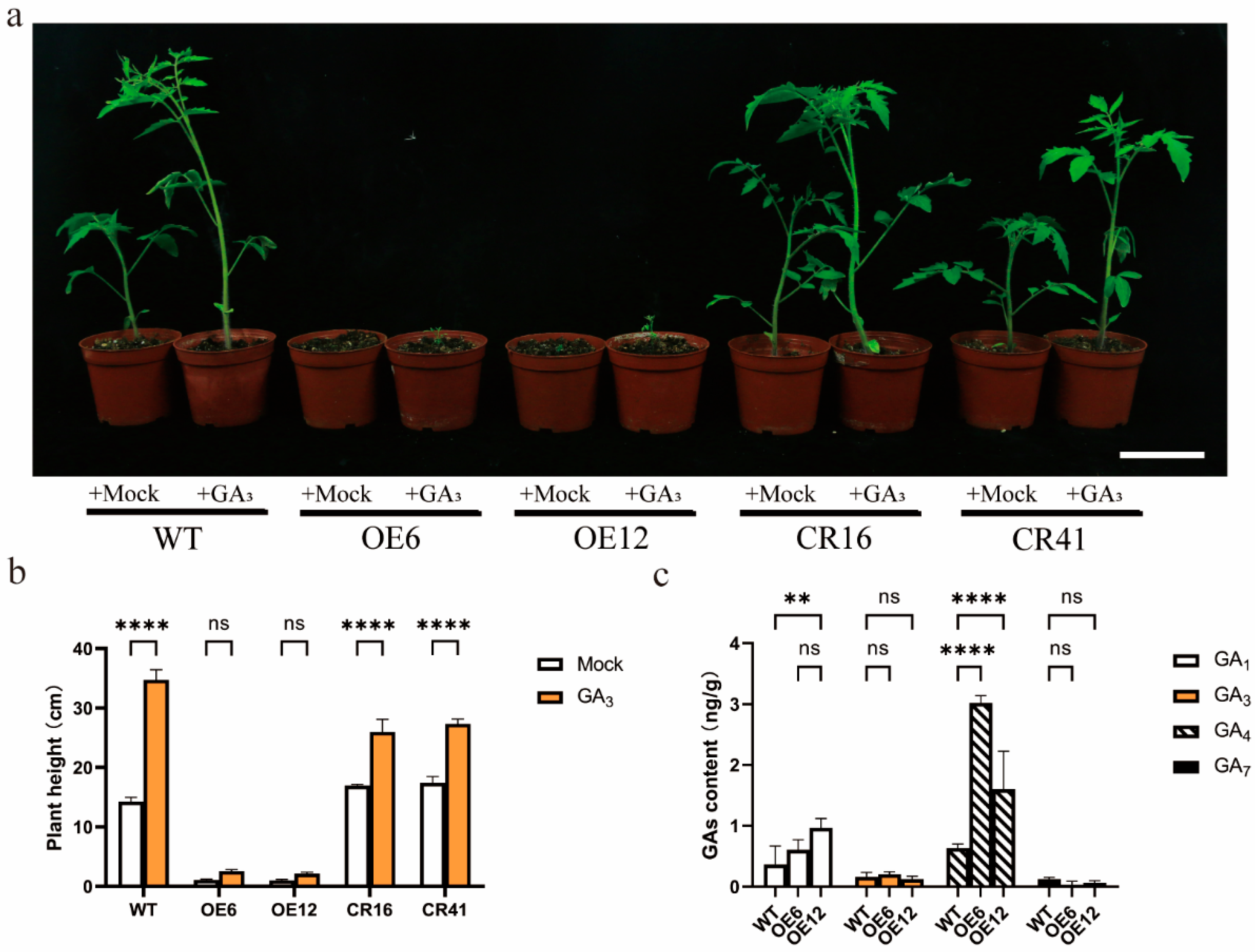
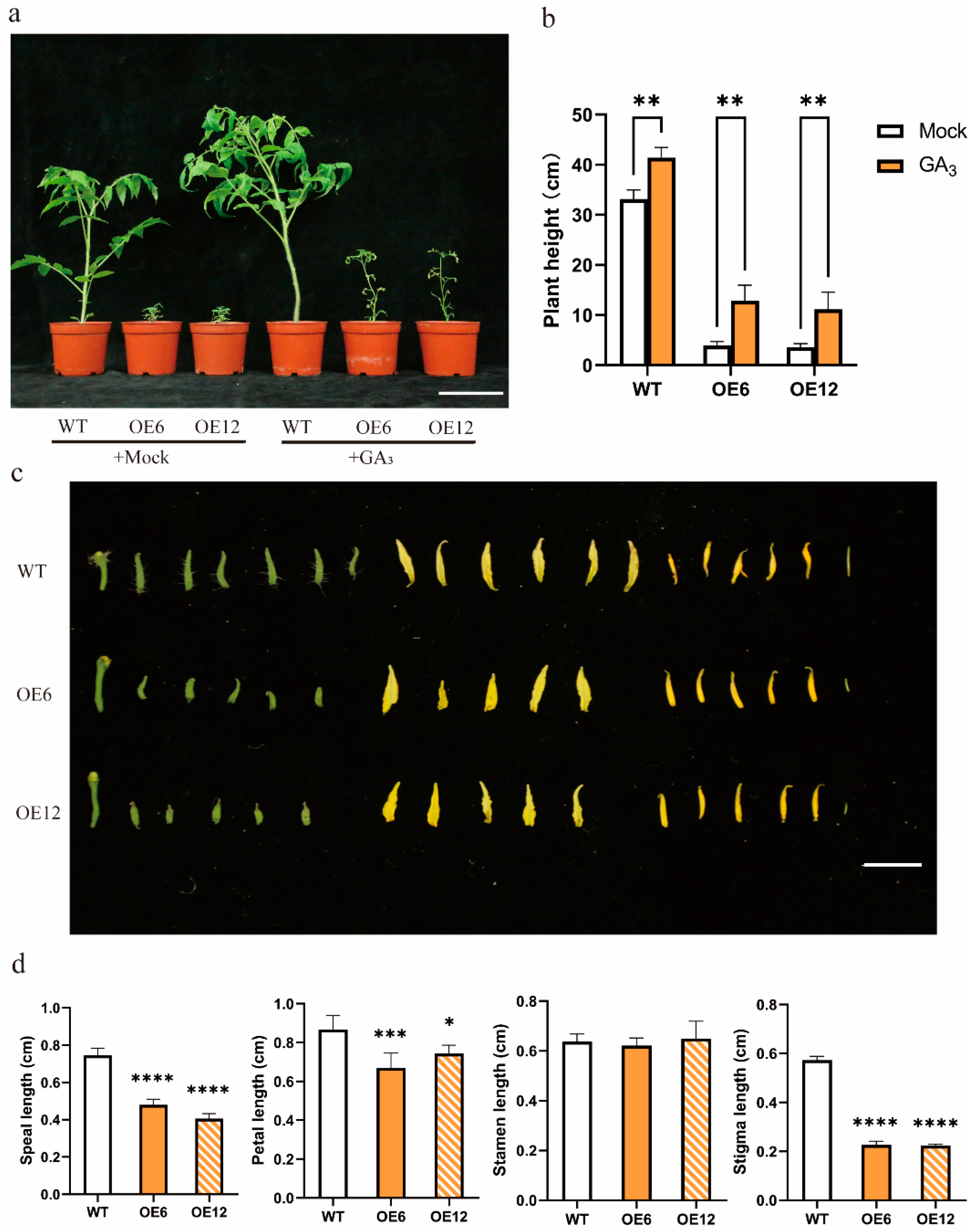
3.4. Exogenous GA3 Treatment Did Not Recover Growth, and SlHsfC1 Overexpressors Showed Increased GA Content Relative to Wild Type
3.5. Prolonged Application of GA3 Might Not Restore Growth and Development
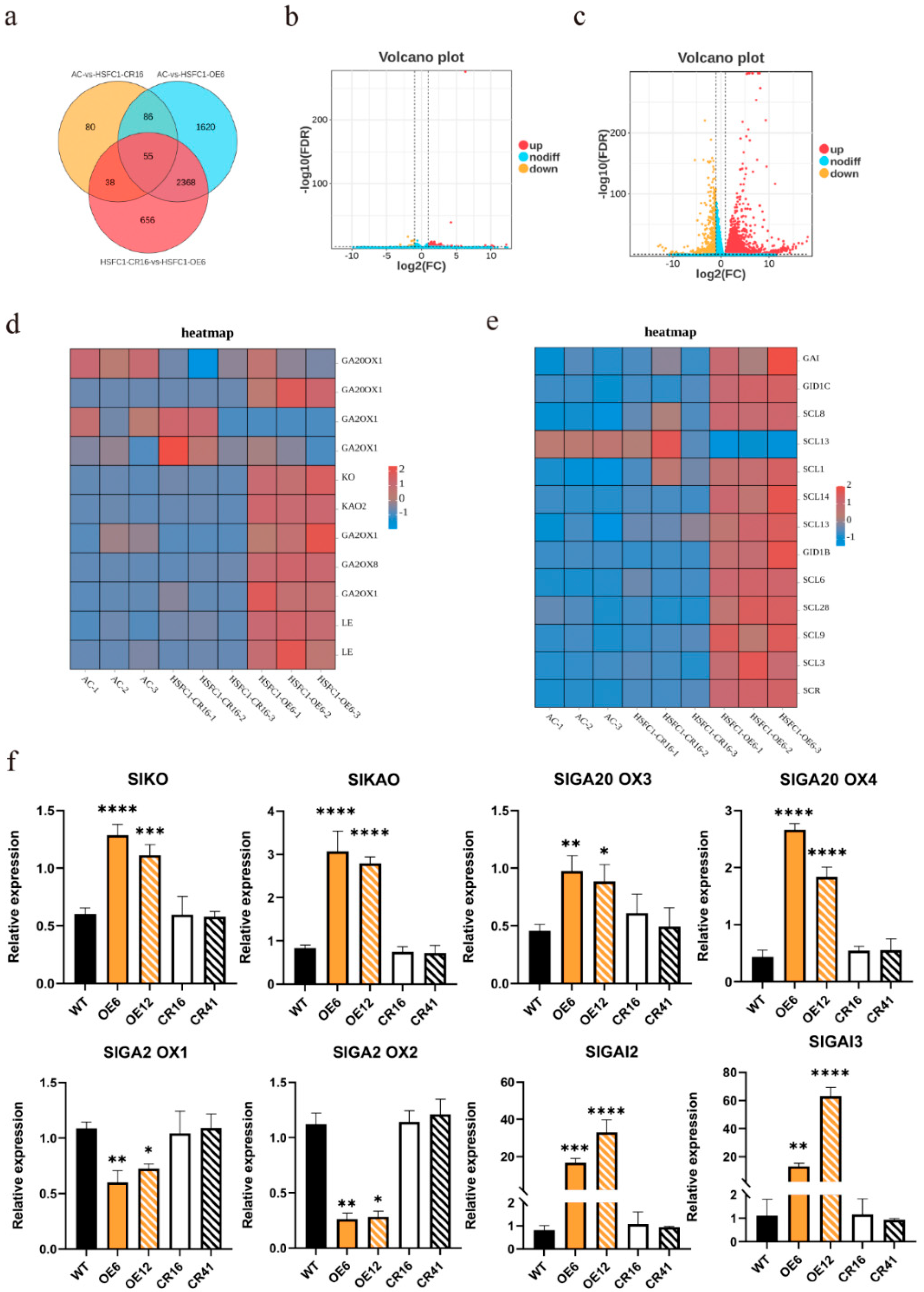
3.6. SlHsfC1 Affects GA Synthesis Pathway and Signal Transduction Pathway
3.7. SlGAI3 Is a Direct Target Gene of SlHsfC1
4. Discussion
4.1. Overexpression of SlHsfC1 Results Dwarf Phenotype but Not Due to GA Deficiency
4.2. SlHsfC1 Influences the Stature of Tomato Plants by Interacting with the Promoter Region of the Gibberellin SlGAI3 Signaling Gene
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, F.; Chen, G.; Xie, Q.; Zhou, S.; Hu, Z. Down-regulation of SlGT-26 gene confers dwarf plants and enhances drought and salt stress resistance in tomato. Plant Physiol. Biochem. 2023, 203, 108053. [Google Scholar] [CrossRef]
- Kim, P.; Xue, C.Y.; Song, H.D.; Gao, Y.; Feng, L.; Li, Y.; Xuan, Y.H. Tissue-specific activation of DOF11 promotes rice resistance to sheath blight disease and increases grain weight via activation of SWEET14. Plant Biotechnol. J. 2021, 19, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Hedden, P. The Current Status of Research on Gibberellin Biosynthesis. Plant Cell Physiol. 2020, 61, 1832–1849. [Google Scholar] [CrossRef]
- Yamaguchi, S. Gibberellin Metabolism and Its Regulation. Annu. Rev. Plant Biol. 2008, 59, 225–251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; He, L.; Zhao, B.; Zhou, S.; Li, Y.; He, H.; Bai, Q.; Zhao, W.; Guo, S.; Liu, Y.; et al. Dwarf and Increased Branching 1 controls plant height and axillary bud outgrowth in Medicago truncatula. J. Exp. Bot. 2020, 71, 6355–6365. [Google Scholar] [CrossRef]
- Bensen, R.J.; Johal, G.S.; Crane, V.C.; Tossberg, J.T.; Schnable, P.S.; Meeley, R.B.; Briggs, S.P. Cloning and characterization of the maize An1 gene. Plant Cell 1995, 7, 75–84. [Google Scholar] [CrossRef]
- Chen, Y.; Hou, M.; Liu, L.; Wu, S.; Shen, Y.; Ishiyama, K.; Kobayashi, M.; McCarty, D.R.; Tan, B.C. The maize DWARF1 encodes a gibberellin 3-oxidase and is dual localized to the nucleus and cytosol. Plant Physiol. 2014, 166, 2028–2039. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Ueguchi-Tanaka, M.; Sentoku, N.; Kitano, H.; Matsuoka, M.; Kobayashi, M. Cloning and functional analysis of two gibberellin 3 beta -hydroxylase genes that are differently expressed during the growth of rice. Proc. Natl. Acad. Sci. USA 2001, 98, 8909–8914. [Google Scholar] [CrossRef]
- Monna, L.; Kitazawa, N.; Yoshino, R.; Suzuki, J.; Masuda, H.; Maehara, Y.; Tanji, M.; Sato, M.; Nasu, S.; Minobe, Y. Positional cloning of rice semidwarfing gene, sd-1: Rice “green revolution gene” encodes a mutant enzyme involved in gibberellin synthesis. DNA Res. 2002, 9, 11–17. [Google Scholar] [CrossRef]
- Qin, X.; Liu, J.H.; Zhao, W.S.; Chen, X.J.; Guo, Z.J.; Peng, Y.L. Gibberellin 20-oxidase gene OsGA20ox3 regulates plant stature and disease development in rice. Mol. Plant Microbe Interact. 2013, 26, 227–239. [Google Scholar] [CrossRef]
- Spielmeyer, W.; Ellis, M.H.; Chandler, P.M. Semidwarf (sd-1), “green revolution” rice, contains a defective gibberellin 20-oxidase gene. Proc. Natl. Acad. Sci. USA 2002, 99, 9043–9048. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; Zhai, L.; Liu, R.; Bai, W.; Wang, L.; Huo, D.; Tao, Y.; Zheng, Y.; Zhang, Z. ZmGA3ox2, a candidate gene for a major QTL, qPH3.1, for plant height in maize. Plant J. Cell Mol. Biol. 2013, 73, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.L.; Li, L.; Wu, K.; Peeters, A.J.; Gage, D.A.; Zeevaart, J.A. The GA5 locus of Arabidopsis thaliana encodes a multifunctional gibberellin 20-oxidase: Molecular cloning and functional expression. Proc. Natl. Acad. Sci. USA 1995, 92, 6640–6644. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Takeda-Kamiya, N.; Hanada, A.; Ogawa, M.; Kuwahara, A.; Seo, M.; Kamiya, Y.; Yamaguchi, S. Contribution of gibberellin deactivation by AtGA2ox2 to the suppression of germination of dark-imbibed Arabidopsis thaliana seeds. Plant Cell Physiol. 2007, 48, 555–561. [Google Scholar] [CrossRef]
- Thomas, S.G.; Phillips, A.L.; Hedden, P. Molecular cloning and functional expression of gibberellin 2- oxidases, multifunctional enzymes involved in gibberellin deactivation. Proc. Natl. Acad. Sci. USA 1999, 96, 4698–4703. [Google Scholar] [CrossRef]
- Lo, S.F.; Yang, S.Y.; Chen, K.T.; Hsing, Y.I.; Zeevaart, J.A.; Chen, L.J.; Yu, S.M. A novel class of gibberellin 2-oxidases control semidwarfism, tillering, and root development in rice. Plant Cell 2008, 20, 2603–2618. [Google Scholar] [CrossRef]
- Sakamoto, T.; Kobayashi, M.; Itoh, H.; Tagiri, A.; Kayano, T.; Tanaka, H.; Iwahori, S.; Matsuoka, M. Expression of a gibberellin 2-oxidase gene around the shoot apex is related to phase transition in rice. Plant Physiol. 2001, 125, 1508–1516. [Google Scholar] [CrossRef]
- Schomburg, F.M.; Bizzell, C.M.; Lee, D.J.; Zeevaart, J.A.; Amasino, R.M. Overexpression of a novel class of gibberellin 2-oxidases decreases gibberellin levels and creates dwarf plants. Plant Cell 2003, 15, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Sakai, M.; Sakamoto, T.; Saito, T.; Matsuoka, M.; Tanaka, H.; Kobayashi, M. Expression of novel rice gibberellin 2-oxidase gene is under homeostatic regulation by biologically active gibberellins. J. Plant Res. 2003, 116, 161–164. [Google Scholar] [CrossRef]
- Wang, H.; Caruso, L.V.; Downie, A.B.; Perry, S.E. The embryo MADS domain protein AGAMOUS-Like 15 directly regulates expression of a gene encoding an enzyme involved in gibberellin metabolism. Plant Cell 2004, 16, 1206–1219. [Google Scholar] [CrossRef]
- Sun, T.P. The molecular mechanism and evolution of the GA-GID1-DELLA signaling module in plants. Curr. Biol. CB 2011, 21, R338–R345. [Google Scholar] [CrossRef]
- Hirano, K.; Asano, K.; Tsuji, H.; Kawamura, M.; Mori, H.; Kitano, H.; Ueguchi-Tanaka, M.; Matsuoka, M. Characterization of the Molecular Mechanism Underlying Gibberellin Perception Complex Formation in Rice. Plant Cell 2010, 22, 2680–2696. [Google Scholar] [CrossRef]
- Lawit, S.J.; Wych, H.M.; Xu, D.; Kundu, S.; Tomes, D.T. Maize DELLA proteins dwarf plant8 and dwarf plant9 as modulators of plant development. Plant Cell Physiol. 2010, 51, 1854–1868. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.K.; Chang, C. Arabidopsis RGL1 encodes a negative regulator of gibberellin responses. Plant Cell 2002, 14, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Pastor, R.; Burchfiel, E.T.; Thiele, D.J. Regulation of heat shock transcription factors and their roles in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 4–19. [Google Scholar] [CrossRef]
- Guo, J.; Wu, J.; Ji, Q.; Wang, C.; Luo, L.; Yuan, Y.; Wang, Y.; Wang, J. Genome-wide analysis of heat shock transcription factor families in rice and Arabidopsis. J. Genet. Genom. 2008, 35, 105–118. [Google Scholar] [CrossRef]
- Berz, J.; Simm, S.; Schuster, S.; Scharf, K.D.; Schleiff, E.; Ebersberger, I. HEATSTER: A Database and Web Server for Identification and Classification of Heat Stress Transcription Factors in Plants. Bioinform. Biol. Insights 2019, 13, 1177932218821365. [Google Scholar] [CrossRef]
- Xue, G.P.; Sadat, S.; Drenth, J.; McIntyre, C.L. The heat shock factor family from Triticum aestivum in response to heat and other major abiotic stresses and their role in regulation of heat shock protein genes. J. Exp. Bot. 2014, 65, 539–557. [Google Scholar] [CrossRef] [PubMed]
- Scharf, K.D.; Berberich, T.; Ebersberger, I.; Nover, L. The plant heat stress transcription factor (Hsf) family: Structure, function and evolution. Biochim. Biophys. Acta 2012, 1819, 104–119. [Google Scholar] [CrossRef]
- Nover, L.; Bharti, K.; Döring, P.; Mishra, S.K.; Ganguli, A.; Scharf, K.D. Arabidopsis and the heat stress transcription factor world: How many heat stress transcription factors do we need? Cell Stress Chaperones 2001, 6, 177–189. [Google Scholar] [CrossRef]
- Zhuang, L.; Cao, W.; Wang, J.; Yu, J.; Yang, Z.; Huang, B. Characterization and Functional Analysis of FaHsfC1b from Festuca arundinacea Conferring Heat Tolerance in Arabidopsis. Int. J. Mol. Sci. 2018, 19, 2702. [Google Scholar] [CrossRef]
- Lu, Y.; Ouyang, B.; Zhang, J.; Wang, T.; Lu, C.; Han, Q.; Zhao, S.; Ye, Z.; Li, H. Genomic organization, phylogenetic comparison and expression profiles of annexin gene family in tomato (Solanum lycopersicum). Gene 2012, 499, 14–24. [Google Scholar] [CrossRef]
- Xing, H.L.; Dong, L.; Wang, Z.P.; Zhang, H.Y.; Han, C.Y.; Liu, B.; Wang, X.C.; Chen, Q.J. A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biol. 2014, 14, 327. [Google Scholar] [CrossRef]
- Li, Y.; Chu, Z.; Luo, J.; Zhou, Y.; Cai, Y.; Lu, Y.; Xia, J.; Kuang, H.; Ye, Z.; Ouyang, B. The C2H2 zinc-finger protein SlZF3 regulates AsA synthesis and salt tolerance by interacting with CSN5B. Plant Biotechnol. J. 2018, 16, 1201–1213. [Google Scholar] [CrossRef]
- Song, J.; Shang, L.; Li, C.; Wang, W.; Wang, X.; Zhang, C.; Ai, G.; Ye, J.; Yang, C.; Li, H.; et al. Variation in the fruit development gene POINTED TIP regulates protuberance of tomato fruit tip. Nat. Commun. 2022, 13, 5940. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Suzuki, T.; Murata, S.; Nakamura, S.; Hino, T.; Maeo, K.; Tabata, R.; Kawai, T.; Tanaka, K.; Niwa, Y.; et al. Improved Gateway binary vectors: High-performance vectors for creation of fusion constructs in transgenic analysis of plants. Biosci. Biotechnol. Biochem. 2007, 71, 2095–2100. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Yuan, Z.; Zhang, J.; Zhong, Y.; Wang, L. MdWRKY71 as a positive regulator involved in 5-aminolevulinic acid-induced salt tolerance in apple. Hortic. Plant J. 2025, 11, 1397–1413. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, J.; Sun, H.; Liu, J.; Wang, J.; Wang, Q.; Qin, Q.; Mei, S.; Zhao, C.; Yang, X.; et al. CR Cistrome: A ChIP-Seq database for chromatin regulators and histone modification linkages in human and mouse. Nucleic Acids Res. 2014, 42, D450–D458. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, H.; Lu, X.; Wang, C.; Yang, W.; Li, F. An asymmetric bulge enhances artificial microRNA-mediated virus resistance. Plant Biotechnol. J. 2020, 18, 608–610. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J.; Lu, W.; Deng, D. Gibberellin in plant height control: Old player, new story. Plant Cell Rep. 2017, 36, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, W.; Wang, J.; Yang, M.; Wei, K.; Liu, X.; Qiu, Z.; van Giang, T.; Wang, X.; Guo, Y.; et al. SlGID1a Is a Putative Candidate Gene for qtph1.1, a Major-Effect Quantitative Trait Locus Controlling Tomato Plant Height. Front. Genet. 2020, 11, 881. [Google Scholar] [CrossRef]
- Chu, Y.; Xu, N.; Wu, Q.; Yu, B.; Li, X.; Chen, R.; Huang, J. Rice transcription factor OsMADS57 regulates plant height by modulating gibberellin catabolism. Rice 2019, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Kang, K.; Lee, S.H.; Lee, I.J.; Paek, N.C. OsWOX3A is involved in negative feedback regulation of the gibberellic acid biosynthetic pathway in rice (Oryza sativa). J. Exp. Bot. 2016, 67, 1677–1687. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, N.; Sun, T.P.; Gubler, F. Gibberellin signaling: Biosynthesis, catabolism, and response pathways. Plant Cell 2002, 14 (Suppl. 1), S61–S80. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, X.; Bai, Q.; Zhao, W.; Fang, Y.; Zhou, S.; Zhao, B.; He, L.; Chen, J. Cloning and Functional Analysis of Dwarf Gene Mini Plant 1 (MNP1) in Medicago truncatula. Int. J. Mol. Sci. 2020, 21, 4968. [Google Scholar] [CrossRef] [PubMed]
- Regnault, T.; Davière, J.M.; Heintz, D.; Lange, T.; Achard, P. The gibberellin biosynthetic genes AtKAO1 and AtKAO2 have overlapping roles throughout Arabidopsis development. Plant J. Cell Mol. Biol. 2014, 80, 462–474. [Google Scholar] [CrossRef]
- Chen, H.I.; Li, P.F.; Yang, C.H. NAC-Like Gene GIBBERELLIN SUPPRESSING FACTOR Regulates the Gibberellin Metabolic Pathway in Response to Cold and Drought Stresses in Arabidopsis. Sci. Rep. 2019, 9, 19226. [Google Scholar] [CrossRef]
- Hedden, P.; Phillips, A.L. Gibberellin metabolism: New insights revealed by the genes. Trends Plant Sci. 2000, 5, 523–530. [Google Scholar] [CrossRef]
- Davière, J.M.; Achard, P. Gibberellin signaling in plants. Development 2013, 140, 1147–1151. [Google Scholar] [CrossRef]
- Livne, S.; Lor, V.S.; Nir, I.; Eliaz, N.; Aharoni, A.; Olszewski, N.E.; Eshed, Y.; Weiss, D. Uncovering DELLA-Independent Gibberellin Responses by Characterizing New Tomato procera Mutants. Plant Cell 2015, 27, 1579–1594. [Google Scholar] [CrossRef]
- Alabadí, D.; Sun, T.P. Green Revolution DELLA Proteins: Functional Analysis and Regulatory Mechanisms. Annu. Rev. Plant Biol. 2025, 76, 373–400. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.J.; Chen, D.; Lynne Mclntyre, C.; Fernanda Dreccer, M.; Zhang, Z.B.; Drenth, J.; Kalaipandian, S.; Chang, H.; Xue, G.P. Heat shock factor C2a serves as a proactive mechanism for heat protection in developing grains in wheat via an ABA-mediated regulatory pathway. Plant Cell Environ. 2018, 41, 79–98. [Google Scholar] [CrossRef]
- Wu, Z.; Li, T.; Ding, L.; Wang, C.; Teng, R.; Xu, S.; Cao, X.; Teng, N. Lily LlHSFC2 coordinates with HSFAs to balance heat stress response and improve thermotolerance. New Phytol. 2024, 241, 2124–2142. [Google Scholar] [CrossRef]
- Wen, D.-D.; Li, X.-M.; Hong, J.-D.; Meng, S.; Yu, J.-F.; Wu, M.; Li, N.; Cheng, L.-J. C-repeat binding factor (CBF) identification and expression pattern to abiotic stress response in Casuarina equisetifolia and overexpression of CeCBF4 increase stress tolerance in Arabidopsis. Ind. Crops Prod. 2024, 222, 119593. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, S.; Xing, B.; Peng, C.; Zhu, J.; Shao, Q.; Lv, A. An HD-Zip transcription factor ArHDZ22 regulates plant height and decreases salt tolerance in Anoectochilus roxburghii. Ind. Crops Prod. 2025, 223, 120251. [Google Scholar] [CrossRef]
- Nelson, D.R.; Schuler, M.A.; Paquette, S.M.; Werck-Reichhart, D.; Bak, S. Comparative genomics of rice and Arabidopsis. Analysis of 727 cytochrome P450 genes and pseudogenes from a monocot and a dicot. Plant Physiol. 2004, 135, 756–772. [Google Scholar] [CrossRef]
- Chiang, H.H.; Hwang, I.; Goodman, H.M. Isolation of the Arabidopsis GA4 locus. Plant Cell 1995, 7, 195–201. [Google Scholar] [CrossRef]
- Margis-Pinheiro, M.; Zhou, X.R.; Zhu, Q.H.; Dennis, E.S.; Upadhyaya, N.M. Isolation and characterization of a Ds-tagged rice (Oryza sativa L.) GA-responsive dwarf mutant defective in an early step of the gibberellin biosynthesis pathway. Plant Cell Rep. 2005, 23, 819–833. [Google Scholar] [CrossRef]
- Giacomelli, L.; Rota-Stabelli, O.; Masuero, D.; Acheampong, A.K.; Moretto, M.; Caputi, L.; Vrhovsek, U.; Moser, C. Gibberellin metabolism in Vitis vinifera L. during bloom and fruit-set: Functional characterization and evolution of grapevine gibberellin oxidases. J. Exp. Bot. 2013, 64, 4403–4419. [Google Scholar] [CrossRef]
- Hedden, P.; Phillips, A.L.; Rojas, M.C.; Carrera, E.; Tudzynski, B. Gibberellin Biosynthesis in Plants and Fungi: A Case of Convergent Evolution? J. Plant Growth Regul. 2001, 20, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Morrone, D.; Chambers, J.; Lowry, L.; Kim, G.; Anterola, A.; Bender, K.; Peters, R.J. Gibberellin biosynthesis in bacteria: Separate ent-copalyl diphosphate and ent-kaurene synthases in Bradyrhizobium japonicum. FEBS Lett. 2009, 583, 475–480. [Google Scholar] [CrossRef]
- Sun, T.P. Gibberellin metabolism, perception and signaling pathways in Arabidopsis. Arab. Book 2008, 6, e0103. [Google Scholar] [CrossRef] [PubMed]
- Phokas, A.; Meyberg, R.; Briones-Moreno, A.; Hernandez-Garcia, J.; Wadsworth, P.T.; Vesty, E.F.; Blazquez, M.A.; Rensing, S.A.; Coates, J.C. DELLA proteins regulate spore germination and reproductive development in Physcomitrium patens. New Phytol. 2023, 238, 654–672. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, M.; Wang, X.; Shao, Y.; Hu, X.; Cheng, J.; Zheng, X.; Tan, B.; Ye, X.; Wang, W.; et al. Peach DELLA Protein PpeDGYLA Is Not Degraded in the Presence of Active GA and Causes Dwarfism When Overexpressed in Poplar and Arabidopsis. Int. J. Mol. Sci. 2023, 24, 6789. [Google Scholar] [CrossRef]
- Ariizumi, T.; Murase, K.; Sun, T.P.; Steber, C.M. Proteolysis-independent downregulation of DELLA repression in Arabidopsis by the gibberellin receptor GIBBERELLIN INSENSITIVE DWARF1. Plant Cell 2008, 20, 2447–2459. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, L.; Yang, Y.; Emenecker, R.; Smoker, M.; Taylor, J.; Perkins, S.; Smith, J.; MacLean, D.; Olszewski, N.E.; Jones, J.D.G. Using CRISPR/Cas9 genome editing in tomato to create a gibberellin-responsive dominant dwarf DELLA allele. Plant Biotechnol. J. 2019, 17, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Hernández-García, J.; Sun, R.; Serrano-Mislata, A.; Inoue, K.; Vargas-Chávez, C.; Esteve-Bruna, D.; Arbona, V.; Yamaoka, S.; Nishihama, R.; Kohchi, T.; et al. Coordination between growth and stress responses by DELLA in the liverwort Marchantia polymorpha. Curr. Biol. 2021, 31, 3678–3686.e3611. [Google Scholar] [CrossRef]
- Wang, S.; Shen, Y.; Deng, D.; Guo, L.; Zhang, Y.; Nie, Y.; Du, Y.; Zhao, X.; Ye, X.; Huang, J.; et al. Orthogroup and phylotranscriptomic analyses identify transcription factors involved in the plant cold response: A case study of Arabidopsis BBX29. Plant Commun. 2023, 4, 100684. [Google Scholar] [CrossRef]
- Fan, S.; Li, X.; Xu, X.; Yin, Y.; Wang, G.; Shao, A.; Wang, W.; Fu, J. Chromatin Accessibility and Translational Landscapes of Bermudagrass (Cynodon dactylon) Under Chilling Stress. Physiol. Plant. 2025, 177, e70279. [Google Scholar] [CrossRef]
- Guan, Q.; Yue, X.; Zeng, H.; Zhu, J. The protein phosphatase RCF2 and its interacting partner NAC019 are critical for heat stress-responsive gene regulation and thermotolerance in Arabidopsis. Plant Cell 2014, 26, 438–453. [Google Scholar] [CrossRef]
- Lei, Y.; Chen, C.; Chen, W.; Dai, H. The MdIAA29-MdARF4 complex plays an important role in balancing plant height with salt and drought stress responses. Plant Physiol. 2024, 196, 2795–2811. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, H.; Fang, Y.; Guo, W.; Chen, H.; Zhang, X.; Dai, W.; Chen, S.; Hao, Q.; Yuan, S.; et al. Overexpression of GmMYB14 improves high-density yield and drought tolerance of soybean through regulating plant architecture mediated by the brassinosteroid pathway. Plant Biotechnol. J. 2021, 19, 702–716. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Liu, J.; Cao, B.; Liu, B.; Zhang, X.; Chen, Z.; Dong, C.; Liu, X.; Zhang, Z.; Wang, W.; et al. Reducing brassinosteroid signalling enhances grain yield in semi-dwarf wheat. Nature 2023, 617, 118–124. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Y.; Wang, M.; Tang, D.; Ni, L.; Shang, C.; Wu, L.; Pan, Y.; Li, J.; Zhang, X. The SlHsfC1–SlGAI3 Module Controls Tomato Growth and Development via the Gibberellin Signaling Pathway. Plants 2025, 14, 3617. https://doi.org/10.3390/plants14233617
Qin Y, Wang M, Tang D, Ni L, Shang C, Wu L, Pan Y, Li J, Zhang X. The SlHsfC1–SlGAI3 Module Controls Tomato Growth and Development via the Gibberellin Signaling Pathway. Plants. 2025; 14(23):3617. https://doi.org/10.3390/plants14233617
Chicago/Turabian StyleQin, Yafei, Mei Wang, Daodao Tang, Lei Ni, Chunyu Shang, Lang Wu, Yu Pan, Jinhua Li, and Xingguo Zhang. 2025. "The SlHsfC1–SlGAI3 Module Controls Tomato Growth and Development via the Gibberellin Signaling Pathway" Plants 14, no. 23: 3617. https://doi.org/10.3390/plants14233617
APA StyleQin, Y., Wang, M., Tang, D., Ni, L., Shang, C., Wu, L., Pan, Y., Li, J., & Zhang, X. (2025). The SlHsfC1–SlGAI3 Module Controls Tomato Growth and Development via the Gibberellin Signaling Pathway. Plants, 14(23), 3617. https://doi.org/10.3390/plants14233617






