A Low Red/Far-Red Light Ratio Promotes a Reduction in Time from Sowing to Flowering in Wheat Under Speed Breeding Conditions
Abstract
1. Introduction
2. Results
2.1. The Effect of Far-Red Light on the Vegetative Period of Durum Wheat
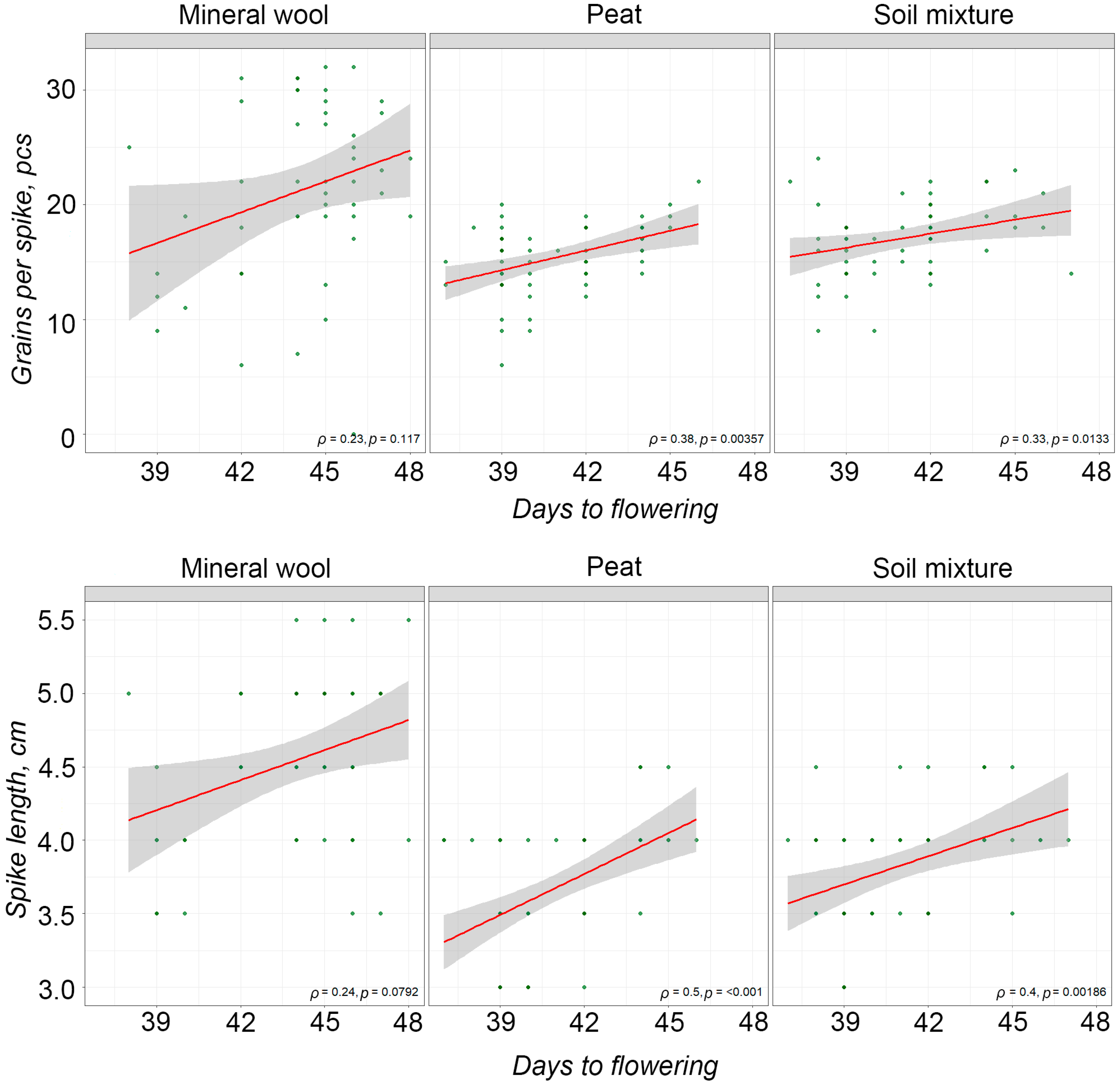
2.2. The Effect of Far-Red Light on Yield Components of Durum Wheat
2.3. The Effect of Far-Red Light on Seed Viability and Germination
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. The Influence of Far-Red Light on Plant Growth Period Duration and Productivity
- 1.
- 2.
- 3.
4.3. Plant Growth Under Field Conditions
4.4. The Effect of Far-Red Light on Seed Viability
4.5. Statistical Analysis
5. Conclusions
- (1)
- Not only the presence of far-red light but also its ratio to red light affects the shortening of the vegetative period in durum wheat. The spectral composition with the highest proportion of far-red light (R/FR~0.4) had the greatest effect on reducing the sowing-to-heading period.
- (2)
- A negative impact of far-red light on durum wheat spike productivity parameters was revealed. A statistically significant positive correlation was found between the duration of the sowing-to-heading period and both spike length and the number of grains per spike.
- (3)
- An interaction between the factors of light spectral composition and substrate on plant growth rate and productivity was shown. This relationship indicates that modifying mineral nutrition could potentially be used to either enhance the effect of far-red light on shortening the vegetative period or to mitigate its negative impact on spike productivity.
- (4)
- A trend towards an increase in the 1000-grain weight was observed when a high proportion of far-red light (R/FR~0.4) was used.
- (5)
- The absence of any effect of far-red light on the regenerative capacity of isolated embryos and seed germination was demonstrated.
- (6)
- The use of a high proportion of far-red light (R/FR~0.4) could be an effective tool for modifying durum wheat speed breeding protocols, allowing the vegetative period to be shortened by 4.1–4.2 days.
- (7)
- The obtained results reveal several promising directions for future research. Firstly, modifying the light spectrum by incorporating a high proportion of far-red light holds potential for optimizing speed breeding protocols, not only for cereals but also for other crops. Secondly, this approach could form the basis for developing such protocols for plant species where speed breeding methods are currently unavailable. Finally, the identified interaction between the substrate’s mineral composition and far-red light suggests the possibility of optimizing these protocols through nutritional management. This could enhance the positive effect of far-red light on shortening the vegetative phase while mitigating its potential negative impact on productivity.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| R | Red light |
| FR | Far-red light |
| R/FR | Red-to-Far-red ratio |
| PPFD | Photosynthetic Photon Flux Density |
References
- Xynias, I.N.; Mylonas, I.; Korpetis, E.G.; Ninou, E.; Tsaballa, A.; Avdikos, I.D.; Mavromatis, A.G. Durum wheat breeding in the Mediterranean region: Current status and future prospects. Agronomy 2020, 10, 432. [Google Scholar] [CrossRef]
- Peleg, Z.; Cakmak, I.; Ozturk, L.; Yazici, A.; Jun, Y.; Budak, H.; Korol, A.B.; Fahima, T.; Saranga, Y. Quantitative trait loci conferring grain mineral nutrient concentrations in durum wheat× wild emmer wheat RIL population. Theor. Appl. Genet. 2009, 119, 353–369. [Google Scholar] [CrossRef]
- Yan, J.; Xue, W.-T.; Yang, R.-Z.; Qin, H.-B.; Zhao, G.; Tzion, F.; Cheng, J.-P. Quantitative trait loci conferring grain selenium nutrient in durum wheat× wild emmer wheat RIL population. Czech J. Genet. Plant Breed. 2018, 54, 52–58. [Google Scholar] [CrossRef]
- Watanabe, N. Genetic collection and development of near-isogenic lines in durum wheat. Инфoрмациoнный Вестник ВОГиС 2008, 12, 636–643. [Google Scholar]
- Amagai, Y.; Watanabe, N.; Kuboyama, T. Genetic mapping and development of near-isogenic lines with genes conferring mutant phenotypes in Aegilops tauschii and synthetic hexaploid wheat. Euphytica 2015, 205, 859–868. [Google Scholar] [CrossRef]
- Weyen, J. Applications of doubled haploids in plant breeding and applied research. In Doubled Haploid Technology: Volume 1: General Topics, Alliaceae, Cereals; Springer: New York, NY, USA, 2021; pp. 23–39. [Google Scholar] [CrossRef]
- Jauhar, P.P. Haploid and doubled haploid production in durum wheat by anther culture. In Doubled Haploid Production in Crop Plants: A Manual; Springer: Berlin/Heidelberg, Germany, 2003; pp. 167–172. [Google Scholar]
- Slama Ayed, O.; De Buyser, J.; Picard, E.; Trifa, Y.; Amara, H.S. Effect of pre-treatment on isolated microspores culture ability in durum wheat (Triticum turgidum subsp. durum Desf.). J. Plant Breed. Crop Sci. 2010, 2, 030–038. [Google Scholar]
- Slama-Ayed, O.; Bouhaouel, I.; Ayed, S.; De Buyser, J.; Picard, E.; Amara, H.S. Efficiency of three haplomethods in durum wheat (Triticum turgidum subsp. durum Desf.): Isolated microspore culture, gynogenesis and wheat × maize crosses. Czech J. Genet. Plant Breed. 2019, 55, 101. [Google Scholar] [CrossRef]
- Royo, C.; Elias, E.M.; Manthey, F.A. Durum wheat breeding. In Cereals; Springer: Berlin/Heidelberg, Germany, 2009; pp. 199–226. [Google Scholar]
- Ghosh, S.; Watson, A.; Gonzalez-Navarro, O.E.; Ramirez-Gonzalez, R.H.; Yanes, L.; Mendoza-Suárez, M.; Simmonds, J.; Wells, R.; Rayner, T.; Green, P.; et al. Speed breeding in growth chambers and glasshouses for crop breeding and model plant research. Nat. Protoc. 2018, 13, 2944–2963. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.; Ghosh, S.; Williams, M.J.; Cuddy, W.S.; Simmonds, J.; Rey, M.-D.; Asyraf Md Hatta, M.; Hinchliffe, A.; Steed, A.; Reynolds, D. Speed breeding is a powerful tool to accelerate crop research and breeding. Nat. Plants 2018, 4, 23–29. [Google Scholar] [CrossRef]
- Blinkov, A.; Kroupin, P.; Dmitrieva, A.; Kocheshkova, A.A.; Karlov, G.; Divashuk, M. Speed Breeding: Protocols, application and achievements. Front. Plant Sci. 2025, 16, 1680955. [Google Scholar] [CrossRef]
- Alahmad, S.; Dinglasan, E.; Leung, K.M.; Riaz, A.; Derbal, N.; Voss-Fels, K.P.; Able, J.A.; Bassi, F.M.; Christopher, J.; Hickey, L.T. Speed breeding for multiple quantitative traits in durum wheat. Plant Methods 2018, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Özkan, R.; Bayhan, M.; Yorulmaz, L.; Öner, M.; Albayrak, Ö.; Yıldırım, M.; Akıncı, C. Genotypic Responses of Some Cereal Species to Speed Breeding Conditions. Pak. J. Agric. Sci. 2025, 62, 9–18. [Google Scholar]
- Bayhan, M.; Özkan, R.; Albayrak, O.; Akıncı, C.; Yıldırım, M. Cultivation factors affecting durum wheat performance under speed breeding conditions. Front. Plant Sci. 2025, 16, 1630915. [Google Scholar] [CrossRef] [PubMed]
- Kigoni, M.; Choi, M.; Arbelaez, J.D. ‘Single-Seed-SpeedBulks: ’a protocol that combines ‘speed breeding’ with a cost-efficient modified single-seed descent method for rapid-generation-advancement in oat (Avena sativa L.). Plant Methods 2023, 19, 92. [Google Scholar] [CrossRef]
- Marenkova, A.G.; Blinkov, A.O.; Radzeniece, S.; Kocheshkova, A.A.; Karlov, G.I.; Lavygina, V.A.; Patrushev, M.V.; Divashuk, M.G. Testing and Modification of the Protocol for Accelerated Growth of Malting Barley under Speed Breeding Conditions. Nanobiotechnol. Rep. 2024, 19, 808–814. [Google Scholar] [CrossRef]
- Taku, M.; Saini, M.; Kumar, R.; Debbarma, P.; Rathod, N.K.K.; Onteddu, R.; Sharma, D.; Pandey, R.; Gaikwad, K.; Lal, S.K.; et al. Modified speed breeding approach reduced breeding cycle to less than half in vegetable soybean [Glycine max (L.) Merr.]. Physiol. Mol. Biol. Plants 2024, 30, 1463–1473. [Google Scholar] [CrossRef]
- Bursakov, S.A.; Karlov, G.I.; Kroupin, P.Y.; Divashuk, M.G. Microorganisms as Potential Accelerators of Speed Breeding: Mechanisms and Knowledge Gaps. Plants 2025, 14, 2628. [Google Scholar] [CrossRef]
- Blinkov, A.O.; Nagamova, V.M.; Minkova, Y.V.; Svistunova, N.Y.; Radzeniece, S.; Kocheshkova, A.A.; Sleptsov, N.N.; Freymans, A.V.; Panchenko, V.V.; Chernook, A.G.; et al. A higher far-red intensity promotes the transition to flowering in triticale grown under speed breeding conditions. Vavilov J. Genet. Breed. 2025, 29, 896–904. [Google Scholar] [CrossRef]
- Smith, H. Phytochromes and light signal perception by plants—An emerging synthesis. Nature 2000, 407, 585–591. [Google Scholar] [CrossRef]
- Demotes-Mainard, S.; Péron, T.; Corot, A.; Bertheloot, J.; Le Gourrierec, J.; Pelleschi-Travier, S.; Crespel, L.; Morel, P.; Huché-Thélier, L.; Boumaza, R.; et al. Plant responses to red and far-red lights, applications in horticulture. Environ. Exp. Bot. 2016, 121, 4–21. [Google Scholar] [CrossRef]
- Sheerin, D.J.; Hiltbrunner, A. Molecular mechanisms and ecological function of far-red light signalling. Plant Cell Environ. 2017, 40, 2509–2529. [Google Scholar] [CrossRef]
- Ugarte, C.C.; Trupkin, S.A.; Ghiglione, H.; Slafer, G.; Casal, J.J. Low red/far-red ratios delay spike and stem growth in wheat. J. Exp. Bot. 2010, 61, 3151–3162. [Google Scholar] [CrossRef]
- Toyota, M.; Tatewaki, N.; Morokuma, M.; Kusutani, A. Tillering responses to high red/far-red ratio of four Japanese wheat cultivars. Plant Prod. Sci. 2014, 17, 124–130. [Google Scholar] [CrossRef]
- Lei, K.; Tan, Q.; Zhu, L.; Xu, L.; Yang, S.; Hu, J.; Gao, L.; Hou, P.; Shao, Y.; Jiang, D. Low red/far-red ratio can induce cytokinin degradation resulting in the inhibition of tillering in wheat (Triticum aestivum L.). Front. Plant Sci. 2022, 13, 971003. [Google Scholar] [CrossRef]
- Dreccer, M.F.; Zwart, A.B.; Schmidt, R.-C.; Condon, A.G.; Awasi, M.A.; Grant, T.J.; Galle, A.; Bourot, S.; Frohberg, C. Wheat yield potential can be maximized by increasing red to far-red light conditions at critical developmental stages. Plant Cell Environ. 2022, 45, 2652–2670. [Google Scholar] [CrossRef] [PubMed]
- Jähne, F.; Hahn, V.; Würschum, T.; Leiser, W.L. Speed breeding short-day crops by LED-controlled light schemes. Theor. Appl. Genet. 2020, 133, 2335–2342. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Back, S.; Kim, G.W.; Lee, K.; Venkatesh, J.; Lee, H.B.; Kwon, J.-K.; Kang, B.-C. Development of a speed breeding protocol with flowering gene investigation in pepper (Capsicum annuum). Front. Plant Sci. 2023, 14, 1151765. [Google Scholar] [CrossRef]
- Kegge, W.; Ninkovic, V.; Glinwood, R.; Welschen, R.A.M.; Voesenek, L.A.C.J.; Pierik, R. Red:far-red light conditions affect the emission of volatile organic compounds from barley (Hordeum vulgare), leading to altered biomass allocation in neighbouring plants. Ann. Bot. 2015, 115, 961–970. [Google Scholar] [CrossRef]
- Huber, M.; de Boer, H.J.; Romanowski, A.; van Veen, H.; Buti, S.; Kahlon, P.S.; van der Meijden, J.; Koch, J.; Pierik, R. Far-red light enrichment affects gene expression and architecture as well as growth and photosynthesis in rice. Plant Cell Environ. 2024, 47, 2936–2953. [Google Scholar] [CrossRef]
- Jang, I.T.; Lee, J.H.; Shin, E.J.; Nam, S.Y. Evaluation of growth, flowering, and chlorophyll fluorescence responses of Viola cornuta cv. Penny Red Wing according to spectral power distributions. J. People Plants Environ. 2023, 26, 335–349. [Google Scholar] [CrossRef]
- Leschevin, M.; Ksas, B.; Baltenweck, R.; Hugueney, P.; Caffarri, S.; Havaux, M. Photosystem rearrangements, photosynthetic efficiency, and plant growth in far red-enriched light. Plant J. 2024, 120, 2536–2552. [Google Scholar] [CrossRef]
- Kasperbauer, M.J. Far-red light reflection from green leaves and effects on phytochrome-mediated assimilate partitioning under field conditions. Plant Physiol. 1987, 85, 350–354. [Google Scholar] [CrossRef]
- De Simone, S.; Oka, Y.; Nishioka, N.; Tadano, S.; Inoue, Y. Evidence of phytochrome mediation in the low-pH-induced root hair formation process in lettuce (Lactuca sativa L. cv. Grand Rapids) seedlings. J. Plant Res. 2000, 113, 45–53. [Google Scholar] [CrossRef]
- De Simone, S.; Oka, Y.; Inoue, Y. Effect of light on root hair formation in Arabidopsis thaliana phytochrome-deficient mutants. J. Plant Res. 2000, 113, 63–69. [Google Scholar] [CrossRef]
- De la Rosa, T.M.; Aphalo, P.J.; Lehto, T. Effects of far-red light on the growth, mycorrhizas and mineral nutrition of Scots pine seedlings. Plant Soil 1998, 201, 17–25. [Google Scholar] [CrossRef]
- Lei, K.; Hu, H.; Chang, M.; Sun, C.; Ullah, A.; Yu, J.; Dong, C.; Gao, Q.; Jiang, D.; Cao, W. A low red/far-red ratio restricts nitrogen assimilation by inhibiting nitrate reductase associated with downregulated TaNR1. 2 and upregulated TaPIL5 in wheat (Triticum aestivum L.). Plant Physiol. Biochem. 2024, 206, 107850. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Duan, X.; Wang, P.; Li, X.; Yuan, X.; Wang, Z.; Wan, L.; Yang, G.; Hong, D. Comprehensive speed breeding: A high-throughput and rapid generation system for long-day crops. Plant Biotechnol. J. 2021, 20, 13. [Google Scholar] [CrossRef]
- Ficht, A.; Bruch, A.; Rajcan, I.; Pozniak, C.; Lyons, E.M. Evaluation of the impact of photoperiod and light intensity on decreasing days to maturity in winter wheat. Crop Sci. 2023, 63, 812–821. [Google Scholar] [CrossRef]
- Cha, J.-K.; O’Connor, K.; Alahmad, S.; Lee, J.-H.; Dinglasan, E.; Park, H.; Lee, S.-M.; Hirsz, D.; Kwon, S.-W.; Kwon, Y. Speed vernalization to accelerate generation advance in winter cereal crops. Mol. Plant 2022, 15, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Zakieh, M.; Gaikpa, D.S.; Leiva Sandoval, F.; Alamrani, M.; Henriksson, T.; Odilbekov, F.; Chawade, A. Characterizing Winter Wheat Germplasm for Fusarium Head Blight Resistance Under Accelerated Growth Conditions. Front. Plant Sci. 2021, 12, 705006. [Google Scholar] [CrossRef]
- Baroncelli, S.; Cavallini, A.; Lercari, B.; Cionini, P.G.; D’Amato, F. Effect of light and gibberellic acid on cell division in the first foliage leaf of durum wheat (Triticum durum Desf.). Planta 1988, 173, 257–262. [Google Scholar] [CrossRef]
- Nikiforova, I.O.; Sudakov, V.L. Association between the processes of tiller formation in spring wheat and the content of far red light in the light sources. Nauchno-Tekhnicheskiĭ Byulleten’ Po Agron. Fiz. 1988, 73, 11–20. [Google Scholar]
- Colombo, M.; Montazeaud, G.; Viader, V.; Ecarnot, M.; Prosperi, J.; David, J.; Fort, F.; Violle, C.; Fréville, H. A genome-wide analysis suggests pleiotropic effects of Green Revolution genes on shade avoidance in wheat. Evol. Appl. 2022, 15, 1594–1604. [Google Scholar] [CrossRef]
- Deitzer, G.F.; Hayes, R.; Jabben, M. Kinetics and time dependence of the effect of far red light on the photoperiodic induction of flowering in Wintex barley. Plant Physiol. 1979, 64, 1015–1021. [Google Scholar] [CrossRef]
- Davis, M.H.; Simmons, S.R. Far-red light reflected from neighbouring vegetation promotes shoot elongation and accelerates flowering in spring barley plants. Plant Cell Environ. 1994, 17, 829–836. [Google Scholar] [CrossRef]
- Watson, A.; Hickey, L.T.; Christopher, J.; Rutkoski, J.; Poland, J.; Hayes, B.J. Multivariate genomic selection and potential of rapid indirect selection with speed breeding in spring wheat. Crop Sci. 2019, 59, 1945–1959. [Google Scholar] [CrossRef]
- Wanga, M.A.; Shimelis, H.; Mashilo, J.; Laing, M.D. Opportunities and challenges of speed breeding: A review. Plant Breed. 2021, 140, 185–194. [Google Scholar] [CrossRef]
- Somody, G.; Molnár, Z. Flowering Synchronization Using Artificial Light Control for Crossbreeding Hemp (Cannabis sativa L.) with Varied Flowering Times. Plants 2025, 14, 594. [Google Scholar] [CrossRef]
- Suzuki, A.; Suriyagoda, L.; Shigeyama, T.; Tominaga, A.; Sasaki, M.; Hiratsuka, Y.; Yoshinaga, A.; Arima, S.; Agarie, S.; Sakai, T. Lotus japonicus nodulation is photomorphogenetically controlled by sensing the red/far red (R/FR) ratio through jasmonic acid (JA) signaling. Proc. Natl. Acad. Sci. USA 2011, 108, 16837–16842. [Google Scholar] [CrossRef]
- Kuznetsov, I.; Davletov, F.; Anokhina, N.; Akhmadullina, I.; Safin, F. Influence of weather condtion on the field peas (Pisum sativum L. ssp. sativum) vegetation period and yield. Agron. Res. 2020, 18, 472–482. [Google Scholar] [CrossRef]
- Gebeyehou, G.; Knott, D.R.; Baker, R.J. Relationships among durations of vegetative and grain filling phases, yield components, and grain yield in durum wheat cultivars. Crop Sci. 1982, 22, 287–290. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
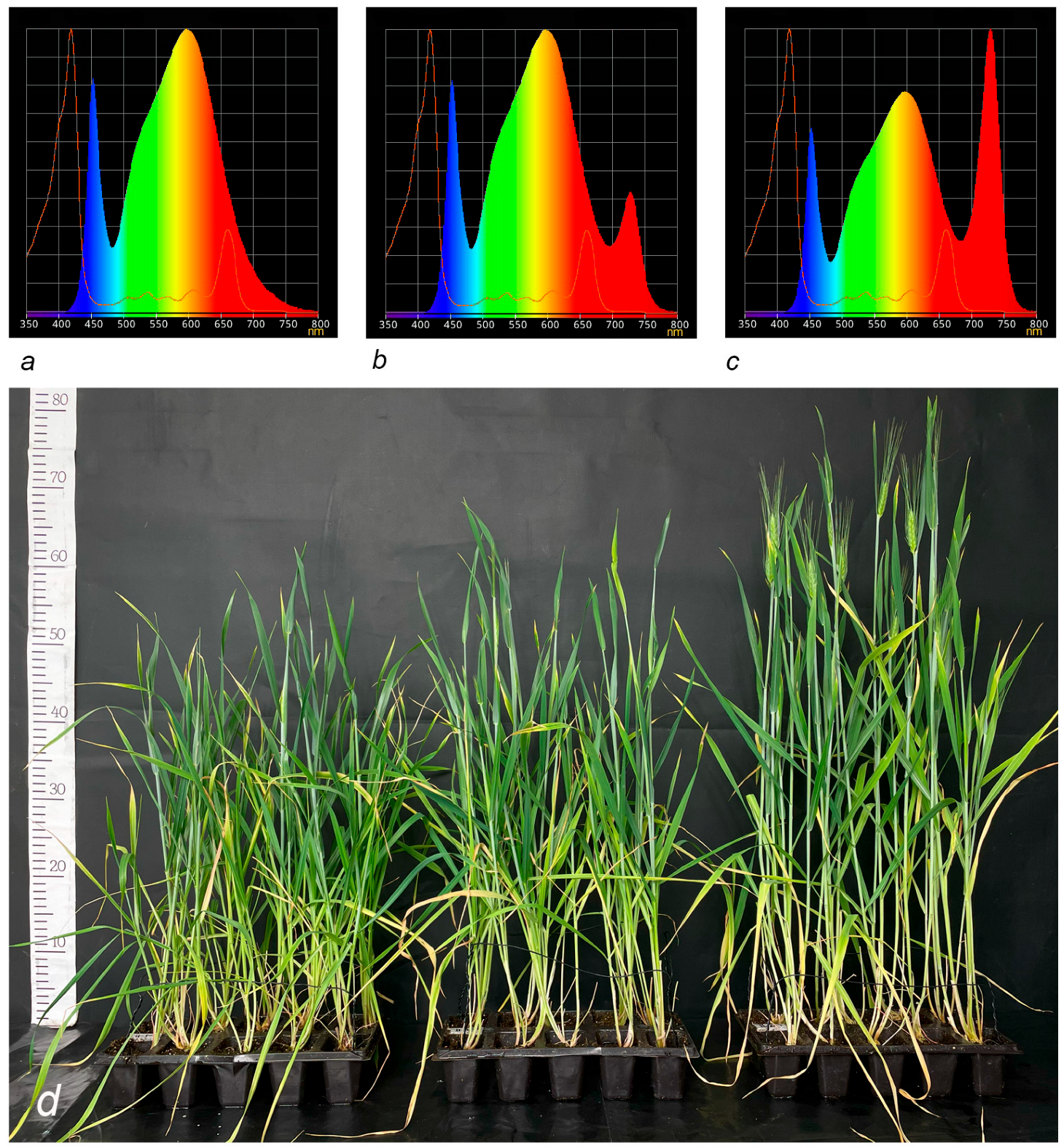
| Days from Sowing to Heading | Days from Sowing to Flowering | ||||||
|---|---|---|---|---|---|---|---|
| R/FR Ratio | Mineral Wool | Peat | Soil Mixture | R/FR Ratio | Mineral Wool | Peat | Soil Mixture |
| R/FR = 6.6 | 43.4 ± 1.0 1 d 2 | 40.5 ± 0.9 c | 41.4 ± 1.2 c | R/FR = 6.6 | 45.7 ± 1 d | 43.6 ± 0.7 c | 43.5 ± 1.0 c |
| R/FR = 1.0 | 41.4 ± 0.9 c | 37.2 ± 0.7 ab | 37.8 ± 0.7 b | R/FR = 1.0 | 44.6 ± 0.6 cd | 40.0 ± 0.8 ab | 40.9 ± 0.8 ab |
| R/FR = 0.4 | 36.3 ± 0.7 ab | 36.5 ± 0.6 ab | 35.9 ± 0.7 a | R/FR = 0.4 | 41.6 ± 1.3 b | 39.4 ± 0.6 a | 39.3 ± 0.7 a |
| R/FR Ratio | Mineral Wool | Peat | Soil Mixture |
|---|---|---|---|
| Spike length, cm | |||
| R/FR = 6.6 | 4.8 ± 0.3 ns | 4.1 ± 0.1 | 4.1 ± 0.1 |
| R/FR = 1.0 | 4.7 ± 0.2 | 3.6 ± 0.1 | 3.9 ± 0.2 |
| R/FR = 0.4 | 4.2 ± 0.2 | 3.4 ± 0.2 | 3.6 ± 0.2 |
| Vegetative weight of the dried spike, g | |||
| R/FR = 6.6 | 1.28 ± 0.12 1 d 2 | 1.00 ± 0.07 bc | 1.05 ± 0.07 bcd |
| R/FR = 1.0 | 1.12 ± 0.10 cd | 1.01 ± 0.04 bc | 1.08 ± 0.08 cd |
| R/FR = 0.4 | 0.71 ± 0.2 a | 0.84 ± 0.08 ab | 1.04 ± 0.16 bcd |
| 1000-grain weight, g | |||
| R/FR = 6.6 | 33.0 ± 2.3 a | 40.9 ± 2.5 bc | 39.6 ± 3.3 b |
| R/FR = 1.0 | 34.2 ± 1.9 a | 44.8 ± 1.7 cd | 46.6 ± 2.3 d |
| R/FR = 0.4 | 45.7 ± 1.5 cd | 43.7 ± 2.7 bcd | 45.1 ± 2.0 cd |
| Number of grains per spike, pcs. | |||
| R/FR = 6.6 | 25.8 ± 2.5 e | 17.3 ± 1.1 bc | 18.5 ± 1.6 cd |
| R/FR = 1.0 | 22.5 ± 2.8 de | 16.1 ± 0.9 abc | 16.7 ± 1.7 abc |
| R/FR = 0.4 | 13.4 ± 4.3 ab | 12.8 ± 1.5 a | 16.3 ± 1.2 abc |
| Number of spikelets per spike, pcs | |||
| R/FR = 6.6 | 11.5 ± 0.7 de | 11.6 ± 0.5 de | 11.2 ± 0.6 cde |
| R/FR = 1.0 | 11.8 ± 0.4 e | 9.4 ± 0.7 ab | 9.9 ± 0.8 abc |
| R/FR = 0.4 | 10.3 ± 0.6 bcd | 8.6 ± 0.9 a | 9.1 ± 0.8 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagamova, V.M.; Bizyakina, D.O.; Blinkov, A.O.; Minkova, Y.V.; Svistunova, N.Y.; Radzeniece, S.; Yanovsky, A.S.; Kocheshkova, A.A.; Divashuk, M.G. A Low Red/Far-Red Light Ratio Promotes a Reduction in Time from Sowing to Flowering in Wheat Under Speed Breeding Conditions. Plants 2025, 14, 3614. https://doi.org/10.3390/plants14233614
Nagamova VM, Bizyakina DO, Blinkov AO, Minkova YV, Svistunova NY, Radzeniece S, Yanovsky AS, Kocheshkova AA, Divashuk MG. A Low Red/Far-Red Light Ratio Promotes a Reduction in Time from Sowing to Flowering in Wheat Under Speed Breeding Conditions. Plants. 2025; 14(23):3614. https://doi.org/10.3390/plants14233614
Chicago/Turabian StyleNagamova, Valeriya M., Daria O. Bizyakina, Andrey O. Blinkov, Yana V. Minkova, Nataliya Yu. Svistunova, Svetlana Radzeniece, Aleksey S. Yanovsky, Alina A. Kocheshkova, and Mikhail G. Divashuk. 2025. "A Low Red/Far-Red Light Ratio Promotes a Reduction in Time from Sowing to Flowering in Wheat Under Speed Breeding Conditions" Plants 14, no. 23: 3614. https://doi.org/10.3390/plants14233614
APA StyleNagamova, V. M., Bizyakina, D. O., Blinkov, A. O., Minkova, Y. V., Svistunova, N. Y., Radzeniece, S., Yanovsky, A. S., Kocheshkova, A. A., & Divashuk, M. G. (2025). A Low Red/Far-Red Light Ratio Promotes a Reduction in Time from Sowing to Flowering in Wheat Under Speed Breeding Conditions. Plants, 14(23), 3614. https://doi.org/10.3390/plants14233614





