Anatomical Markers for Identification and Standardization of Crataegus mexicana, Commercially Marketed as “Raíz de Tejocote”
Abstract
1. Introduction
2. Results and Discussion
2.1. External Morphology of C. mexicana Plant Parts and Marketed Tejocote Root
2.2. Microscopic Description of C. mexicana and Market Sample Characteristics
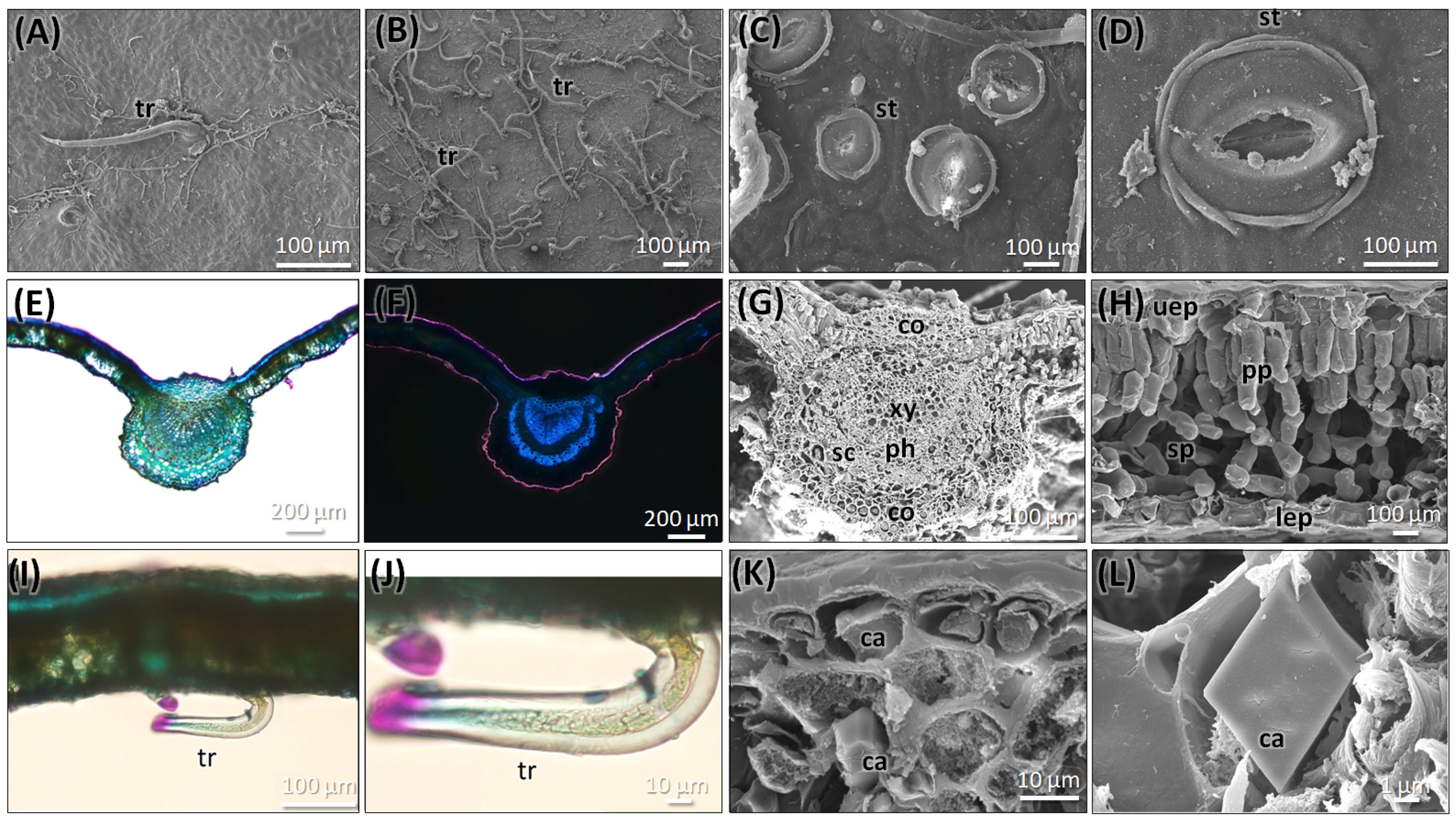
2.2.1. Leaf Anatomy of C. mexicana
2.2.2. Stem Anatomy of C. mexicana
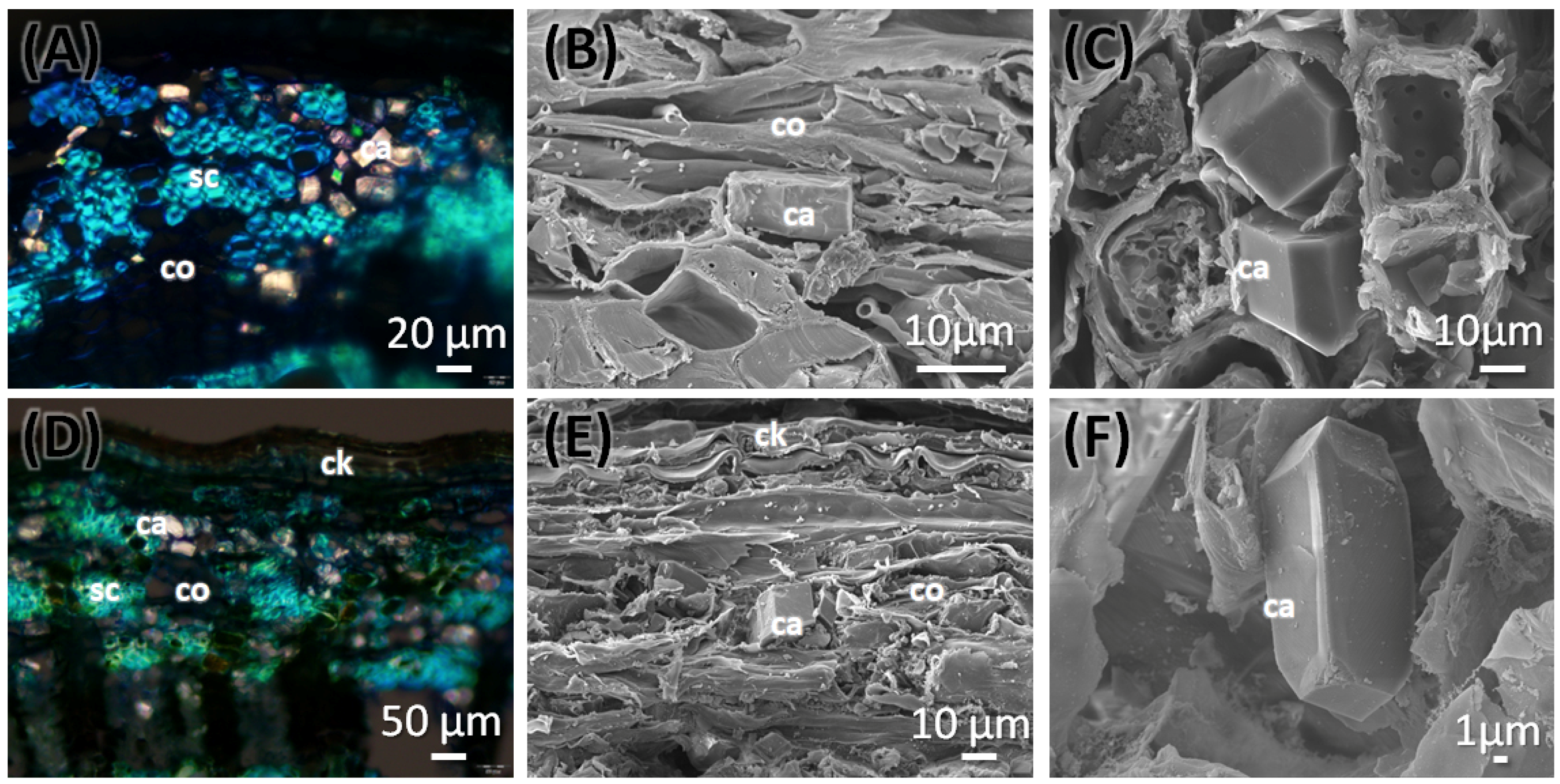
2.2.3. Root Anatomy of C. mexicana
2.2.4. Anatomy of the Market Sample
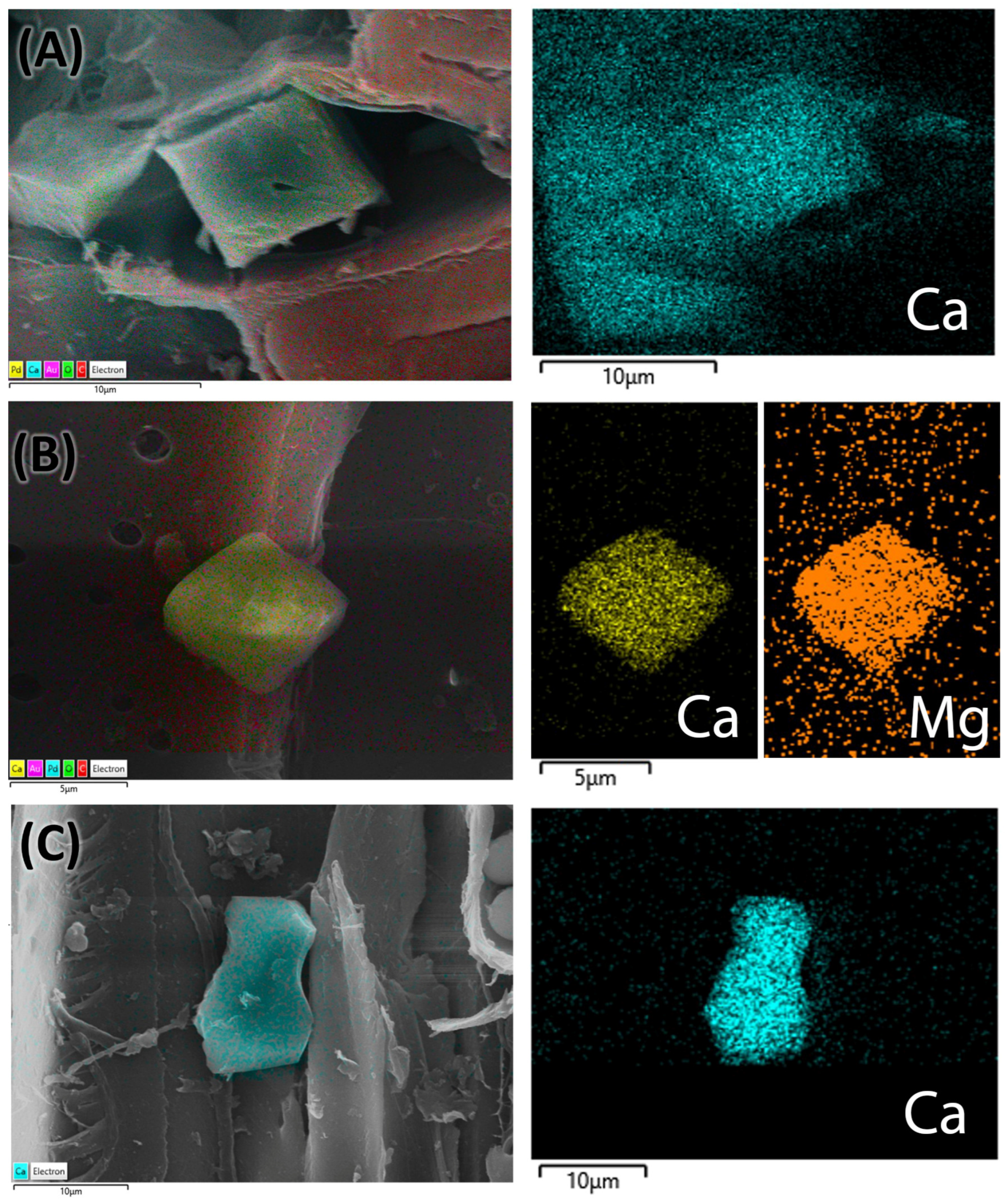
2.2.5. Energy-Dispersive X-Ray Spectroscopy (EDS) Analysis
3. Discussion
4. Materials and Methods
4.1. Botanical Materials
4.2. Preparation of Samples for Macro- and Microscopic Studies
4.3. Preparation of Samples for Scanning Electron Microscopy (SEM) Observation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Phipps, J.B. Studies in Mespilus, Crataegus, and ×Crataemespilus (Rosaceae), I. Differentiation of Mespilus and Crataegus, expansion of ×Crataemespilus, with supplementary observations on differences between the Crataegus and Amelanchier clades. Phytotaxa 2016, 257, 201–229. [Google Scholar] [CrossRef]
- Antonio, B.-T.J.; Margarita, C.-R.; Daniel, M.-I. Biological properties and antioxidant activity of hawthorn Crataegus mexicana. J. Pharmacogenomics Pharmacoproteomics 2015, 6, 1. [Google Scholar] [CrossRef]
- Edwards, J.E.; Brown, P.N.; Talent, N.; Dickinson, T.A.; Shipley, P.R. A review of the chemistry of the genus Crataegus. Phytochemistry 2012, 79, 5–26. [Google Scholar] [CrossRef]
- Villaseñor, J.L. Checklist of the native vascular plants of Mexico. Rev. Mex. Biodivers. 2016, 87, 559–902. [Google Scholar] [CrossRef]
- Sánchez-Herrera, S.; Rodríguez-Martínez, N.; Rodríguez-Ortega, A.; Ponce-Lira, B. Physico-chemical evaluation of three varieties of tejocote (Crataegus mexicana). J. Nat. Agric. Sci. 2019, 6, 31–38. [Google Scholar] [CrossRef]
- Moerman, D.E. Native American Ethnobotany; Timber Press: Portland, OR, USA, 1998; Volume 879. [Google Scholar]
- Turner, N.J. Thompson Ethnobotany: Knowledge and Usage of Plants by the Thompson Indians of British Columbia; Royal British Columbia Museum: Victoria, BC, Canada, 1990. [Google Scholar]
- Núñez-Colín, C.; Sánchez-Vidaña, D. Ethnobotanical, cultural, and agricultural uses of tejocote (Crataegus species) in Mexico. In Proceedings of the XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010): III International Symposium on 918, Lisbon, Portugal, 22 August 2010; pp. 901–910. [Google Scholar]
- Robles-Botero, M.V.; Ronquillo-de Jesús, E.; Quiroz-Reyes, C.N.; Aguilar-Méndez, M.A. Characterization and identification of bioactive compounds with antioxidant activity from peel, pulp and seed of tejocote fruit (Crataegus mexicana). TIP Rev. Espec. En Cienc. Químico-Biológicas 2020, 23, 1–10. [Google Scholar]
- Álvarez Cortés, A.B. Análisis Fitoquímico de la Raíz de Tejocote Crataegus mexicana; Universidad Nacional Autónoma de México, UNAM: Mexico City, Mexico, 2019. [Google Scholar]
- Bolaños López, P. Estudio del Potencial Inhibitorio de la Raíz de Crataegus mexicana Moc. & Sesse ex DC. (Rosaceae) Sobre las Enzimas Amilasa y Lipasa Porcinas; Universidad Nacional Autónoma de México, UNAM: Mexico City, Mexico, 2025. [Google Scholar]
- González-Jiménez, F.E.; Salazar-Montoya, J.A.; Calva-Calva, G.; Ramos-Ramírez, E.G. Phytochemical Characterization, In Vitro Antioxidant Activity, and Quantitative Analysis by Micellar Electrokinetic Chromatography of Hawthorn (Crataegus pubescens) Fruit. J. Food Qual. 2018, 2018, 2154893. [Google Scholar] [CrossRef]
- Nazhand, A.; Lucarini, M.; Durazzo, A.; Zaccardelli, M.; Cristarella, S.; Souto, S.B.; Silva, A.M.; Severino, P.; Souto, E.B.; Santini, A. Hawthorn (Crataegus spp.): An Updated Overview on Its Beneficial Properties. Forests 2020, 11, 564. [Google Scholar] [CrossRef]
- Woerdenbag, H.J.; Ursidae, M.; Ekhart, C.; Schmidt, M.; Vitalone, A.; van Hunsel, F.P.A.M. Analysis of Adverse Reactions Associated with the Use of Crataegus-Containing Herbal Products. Pharmaceuticals 2024, 17, 1490. [Google Scholar] [CrossRef]
- Adams, S.J.; Avula, B.; Katragunta, K.; Saroja, S.G.; Zhao, J.; Chittiboyina, A.G.; Khan, I.A. Microscopy, HPTLC, and LC-DAD-Q-ToF validation of nut-based weight-loss dietary supplements, Aleurites moluccanus (candlenut) and Bertholletia excelsa (Brazil nut). Food Addit. Contam. Part A 2024, 41, 468–478. [Google Scholar] [CrossRef]
- Palmer, K.G.; Lebin, J.A.; Cronin, M.T.; Mazor, S.S.; Burns, R.A. Crataegus mexicana (Tejocote) Exposure Associated with Cardiotoxicity and a Falsely Elevated Digoxin Level. J. Med. Toxicol. 2019, 15, 295–298. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Adiministration. FDA Issues Warning About Certain Supplements Substituted with Toxic Yellow Oleander (January 2024); 2024. Available online: https://www.fda.gov/food/alerts-advisories-safety-information/fda-issues-warning-about-certain-supplements-substituted-toxic-yellow-oleander-january-2024#:~:text=What%20is%20the%20problem?,are%20actually%20toxic%20yellow%20oleander (accessed on 7 August 2025).
- COFEPRIS. Sobre el Uso de los Suplementos Alimenticios Etiquetados con Raiz de Tejocote. 2025. Available online: https://www.gob.mx/cms/uploads/attachment/file/977378/Comunicado_Raiz_de_tejocote_10022025.pdf (accessed on 7 August 2025).
- RBG-KEW. The International Plant Names Index and World Checklist of Vascular Plants 2025. Available online: https://powo.science.kew.org/ (accessed on 7 August 2025).
- Sagaradze, V.A.; Babaeva, E.Y.; Kalenikova, E.I.; Trusov, N.A.; Peshchanskaya, E.V. Quantitative Anatomical Characteristics of the Leaf Blades of the Several Species of Crataegus L. Drug Dev. Regist. 2021, 10, 138–146. [Google Scholar] [CrossRef]
- Tuyakova, A.T.; Imanbayeva, A.A.; Duysenova, N.I.; Ishmuratova, M.Y. Anatomical Structure Study of Aerial Organs of Crataegus ambigua C.A.Mey. Biotech. Res. Asia 2016, 13, 1. [Google Scholar] [CrossRef]
- Tichi, A.H.; Gholamiyan, H. Examining the Characteristics of Anatomy and Biometry of Crataegus azarolus. BioResources 2023, 18, 4657–4665. [Google Scholar] [CrossRef]
- Saribas, M.; Yaman, B. Wood anatomy of Crataegus tanacetifolia (lam.) Pers.(Rosaceae), endemic to Turkey. Int. J. Bot. 2005, 1, 158–162. [Google Scholar] [CrossRef]
- Fahn, A.; Werker, E.; Baas, P. Wood Anatomy and Identification of Trees and Shrubs from Israel and Adjacent Regions; Israel Academy of Sciences and Humanities: Jerusalem, Israel, 1986. [Google Scholar]
- Pérez Olvera, C.d.l.P.; Mendoza Aguirre, M.; Ceja Romero, J.; Pacheco, L. Wood anatomy of five specie of the Rosaceae family. Madera Y Bosques 2008, 14, 81–105. [Google Scholar]
- InsideWood. Published on the Internet. 2004-Onwards. Available online: https://insidewood.lib.ncsu.edu/search (accessed on 11 October 2025).
- Wheeler, E.A. InsideWood—A Web Resource for Hardwood Anatomy. IAWA J. 2011, 32, 199–211. [Google Scholar] [CrossRef]
- Meier, E. The Wood Database. 2008. Available online: https://www.wood-database.com/ (accessed on 31 August 2025).
- O’Brien, T.; Feder, N.M.; McCully, M.E. Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 1964, 59, 368–373. [Google Scholar] [CrossRef]
- Drnovšek, T.; Perdih, A. Selective staining as a tool for wood fibre characterization. Dye. Pigment. 2005, 67, 197–206. [Google Scholar] [CrossRef]
- Adams, S.J.; Kumar, T.S.; Muthuraman, G.; Majeed, A. Distribution, morphology, anatomy and histochemistry of Crepidium acuminatum. Mod. Phytomorphology 2018, 12, 15–32. [Google Scholar] [CrossRef]
- Ruzin, S.E. Plant Microtechnique and Microscopy; Oxford University Press: New York, NY, USA, 1999; Volume 198. [Google Scholar]
- Lersten, N.R.; Horner, H.T. Unique calcium oxalate “duplex” and “concretion” idioblasts in leaves of tribe Naucleeae (Rubiaceae). Am. J. Bot. 2011, 98, 1–11. [Google Scholar] [CrossRef] [PubMed]

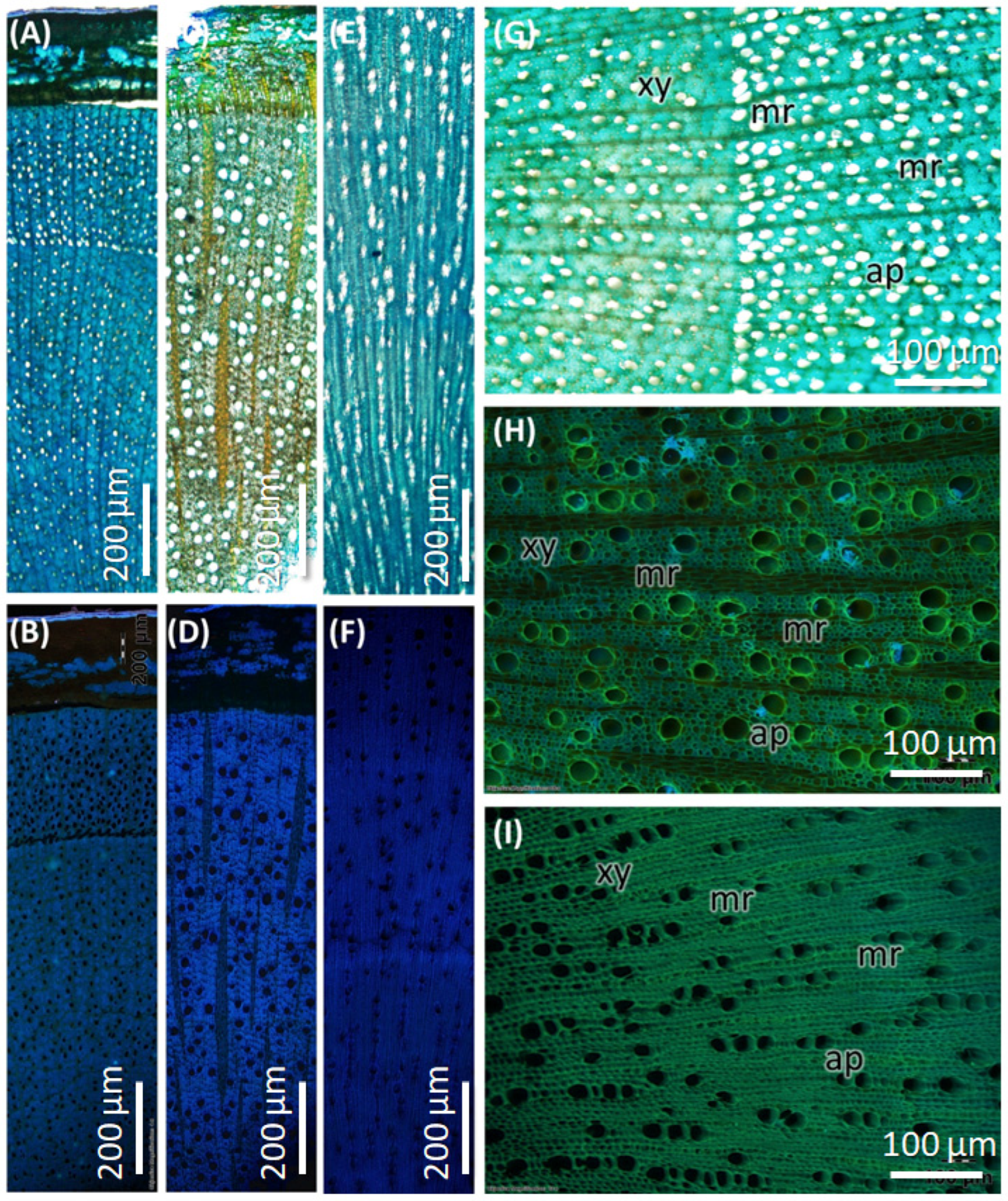
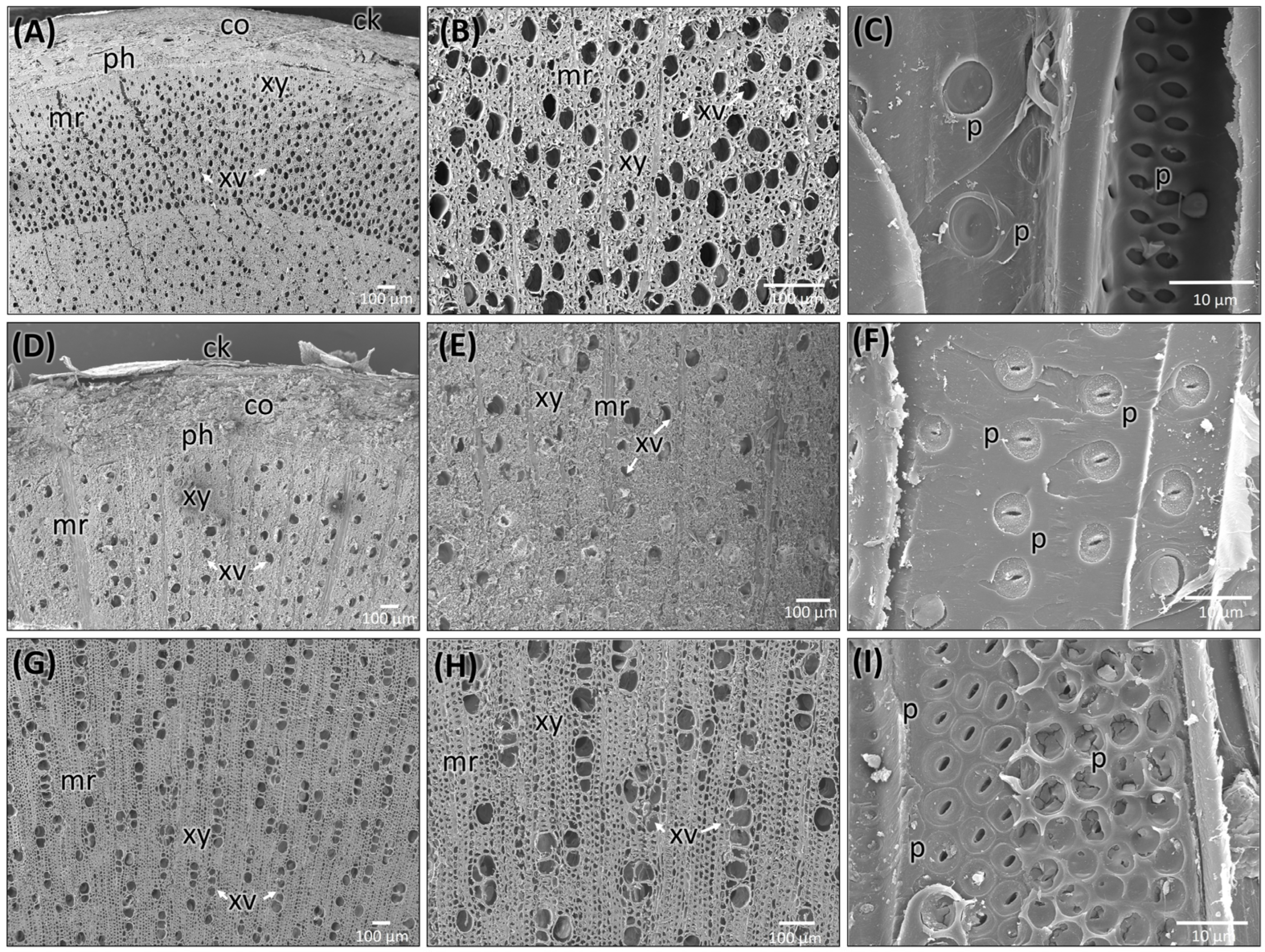
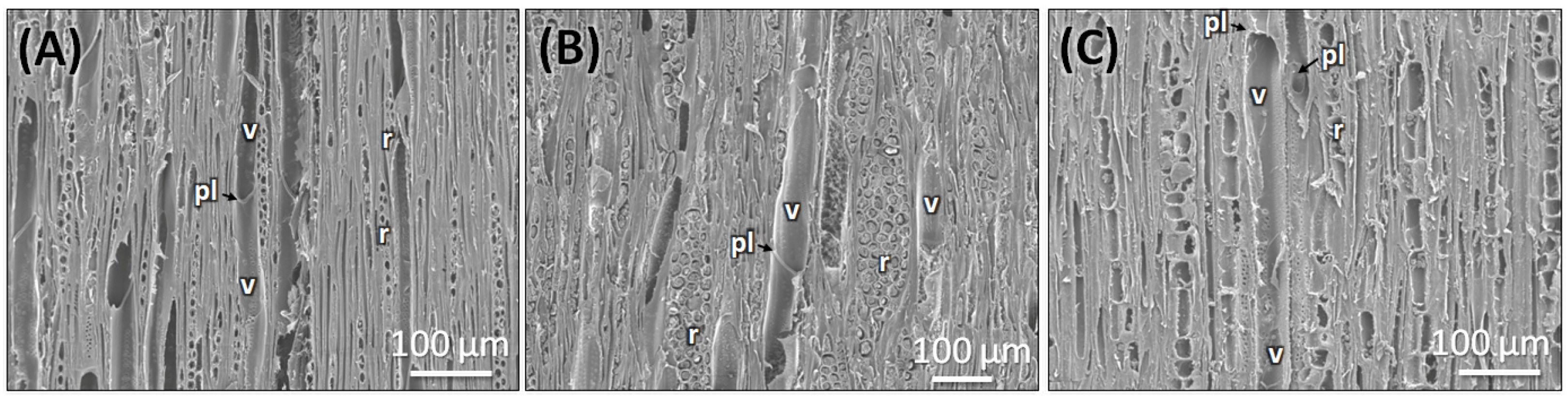
| Features | Stem (Figure 5A–C) | Root (Figure 5D–F) | Market Sample (Figure 5G–I) |
|---|---|---|---|
| Cork | Present, 4–6 layers thick 50 ± 15 µm (mean + SD) | Present, 2–3 layers thick 80 ± 15 µm (mean + SD) | Not found |
| Cortex | 10–15 cells thick,150 ± 20 µm (mean + SD) | 10–15 cells thick, 200 ± 20 µm (mean + SD) | Not found |
| Sclerenchyma in Cortex | Present, in grouped patches | Present, in grouped patches | Not found |
| Cork Cambium (CC) | 2–3 cells thick | 2–3 cells thick | Not found |
| Vascular Cambium (VC) | 2–3 cells thick | 2–3 cells thick | Not found |
| Wood Type | Semi-ring porous (Figure 5A) | Diffuse porous | Diffuse porous |
| Growth Ring Boundaries | Present | Indistinct or absent (Figure 5D) | Indistinct or absent (Figure 5G) |
| Earlywood vs. Latewood Vessels | Difference in vessel density and size | Not much difference | The difference is only in size |
| Vessel Arrangement | Semi-ring porous; vessels in the earlywood are distinctly | Diffuse, solitary, close-by, uneven diameter | Solitary, diffuse, Radial pattern with multiples of 2- and 3- adjacent vessels |
| larger than those in the latewood of the previous growth ring | |||
| Vessel size | 30 ± 20 µm (mean + SD) in earlywood and 20 ± 10 µm (mean + SD) in latewood | 50 ± 10 µm (mean + SD) | 55 ± 20 µm (mean + SD) |
| Vessel Pits | Bordered pits, alternate arrangement (Figure 5C) | Bordered pits, scattered (Figure 5F) | Bordered pits, clustered arrangement (Figure 5I) |
| Axial Parenchyma | Diffuse, aggregated apotracheal | Diffuse, apotracheal | Extremely rare or absent |
| Medullary Rays | 1–3 cells thick | Alternating 5–6 cells and two thick cells | Uniseriate |
| Pith | Present | Absent | Present |
| Wood Characteristics | Crataegus mexicana | Crataegus azarolus L. | Crataegus douglasii Lindl | Crataegus germanica (L.) Kuntze | Crataegus hupehensis Sarg. | Crataegus laevigata (Poir.) DC. | Crataegusmonogyna | Crataegus tanacetifolia (Poir.) Pers. |
|---|---|---|---|---|---|---|---|---|
| Growth Ring | Distinct | Boundaries are indistinct or absent. | Boundaries are indistinct or absent. | Boundaries are indistinct or absent. | Boundaries are indistinct or absent. | Boundaries are indistinct or absent. | Distinct | Boundaries are indistinct or absent. |
| Wood porosity | Semi-ring porous in the stem, diffuse porous in the root | Diffuse porous | Diffuse porous | Semi-ring porous | Diffuse porous | Diffuse porous | Diffuse porous | Diffuse porous |
| Vessel arrangement | Exclusively solitary | Exclusively solitary | May varies | Exclusively solitary | May varies | May varies | May varies | Exclusively solitary |
| Perforation plates | Simple | Simple | Scalariform | Simple | Scalariform | Simple | Simple | Simple |
| vessel pit | Inter-vessel pits alternate | Inter-vessel pits alternate | Inter-vessel pits alternate | Inter-vessel pits alternate | Inter-vessel pits alternate | Inter-vessel pits alternate | Inter-vessel pits alternate | Inter-vessel pits alternate |
| pit size range | Minute to 4 µm | Minute to 4 µm | 4–7 µm | 4–7 µm | Medium: 7–10 µm | Medium: 7–10 µm | Minute to 4 µm | 4–7 µm |
| Gums and deposits | Present | Absent | Absent | Absent | Absent | Present | Absent | Absent |
| Tracheid and fibers | Simple libriform fiber | Simple libriform fiber | Simple libriform fiber | Simple libriform fiber | Simple libriform fiber | Simple libriform fiber | Simple libriform fiber | Simple libriform fiber |
| Axial parenchyma | Diffuse | Diffuse | Diffuse, in aggregates | Diffuse | Diffuse | Diffuse | Diffuse | Diffuse |
| Rays | 1–3 cells | 1–3 cells | 1–3 cells | 1–3 cells | 1–3 cells | 4–10 cells | 1–3 cells | 1–3 cells |
| Mineral inclusions | Prismatic crystals | Prismatic crystals | Prismatic crystals | Prismatic crystals | Prismatic crystals | Prismatic crystals | Prismatic crystals | Prismatic crystals |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adams, S.J.; Estupiñán-Pérez, L.; González-Anduaga, G.M.; Navarrete, A.; Khan, I.A. Anatomical Markers for Identification and Standardization of Crataegus mexicana, Commercially Marketed as “Raíz de Tejocote”. Plants 2025, 14, 3607. https://doi.org/10.3390/plants14233607
Adams SJ, Estupiñán-Pérez L, González-Anduaga GM, Navarrete A, Khan IA. Anatomical Markers for Identification and Standardization of Crataegus mexicana, Commercially Marketed as “Raíz de Tejocote”. Plants. 2025; 14(23):3607. https://doi.org/10.3390/plants14233607
Chicago/Turabian StyleAdams, Sebastian J., Laura Estupiñán-Pérez, Gloria Melisa González-Anduaga, Andrés Navarrete, and Ikhlas A. Khan. 2025. "Anatomical Markers for Identification and Standardization of Crataegus mexicana, Commercially Marketed as “Raíz de Tejocote”" Plants 14, no. 23: 3607. https://doi.org/10.3390/plants14233607
APA StyleAdams, S. J., Estupiñán-Pérez, L., González-Anduaga, G. M., Navarrete, A., & Khan, I. A. (2025). Anatomical Markers for Identification and Standardization of Crataegus mexicana, Commercially Marketed as “Raíz de Tejocote”. Plants, 14(23), 3607. https://doi.org/10.3390/plants14233607






