Histological Features Detected for Separation of the Edible Leaves of Allium ursinum L. from the Poisonous Leaves of Convallaria majalis L. and Colchicum autumnale L.
Abstract
1. Introduction
2. Results
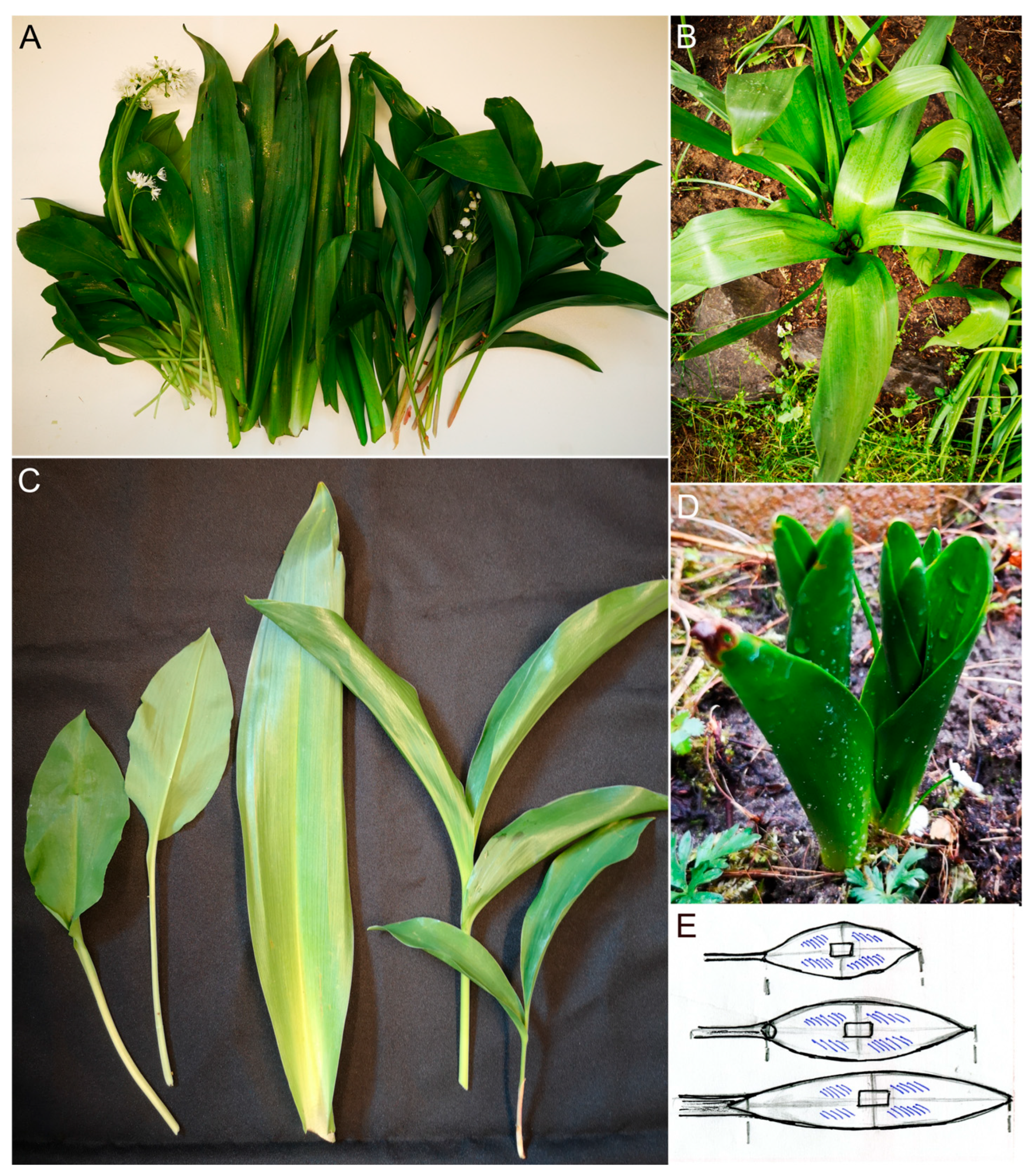
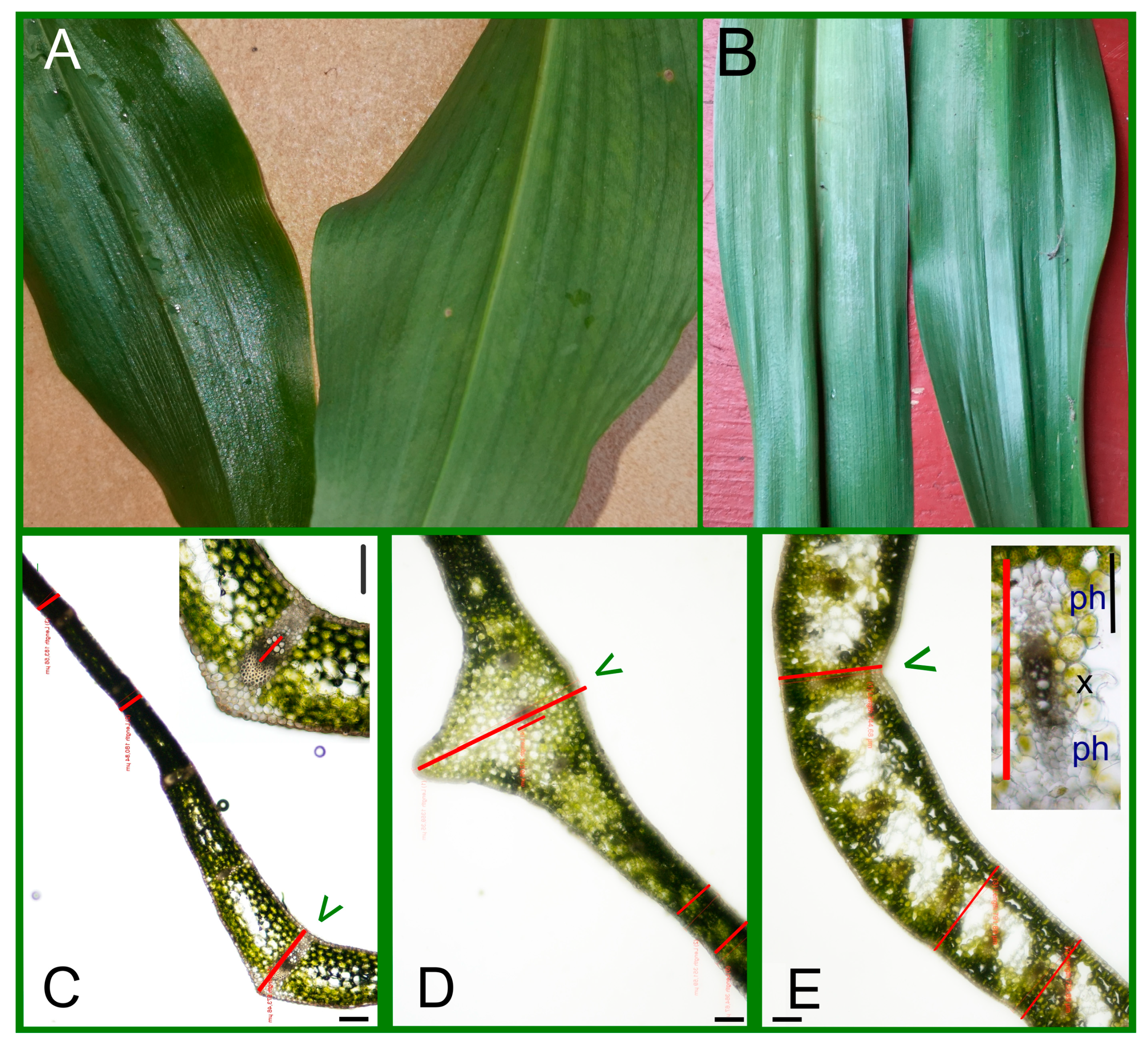
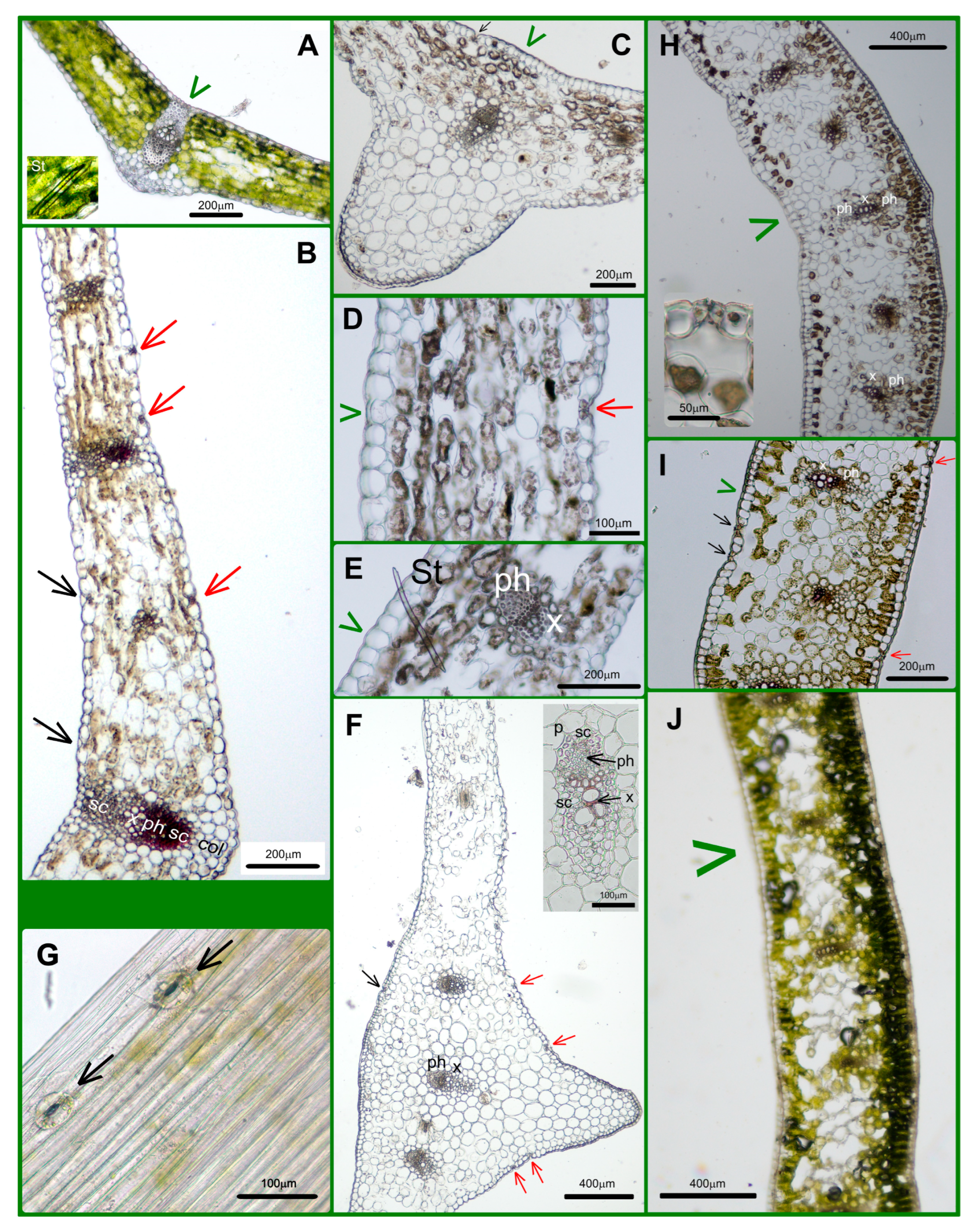
2.1. The Anatomical Characters of Leaves
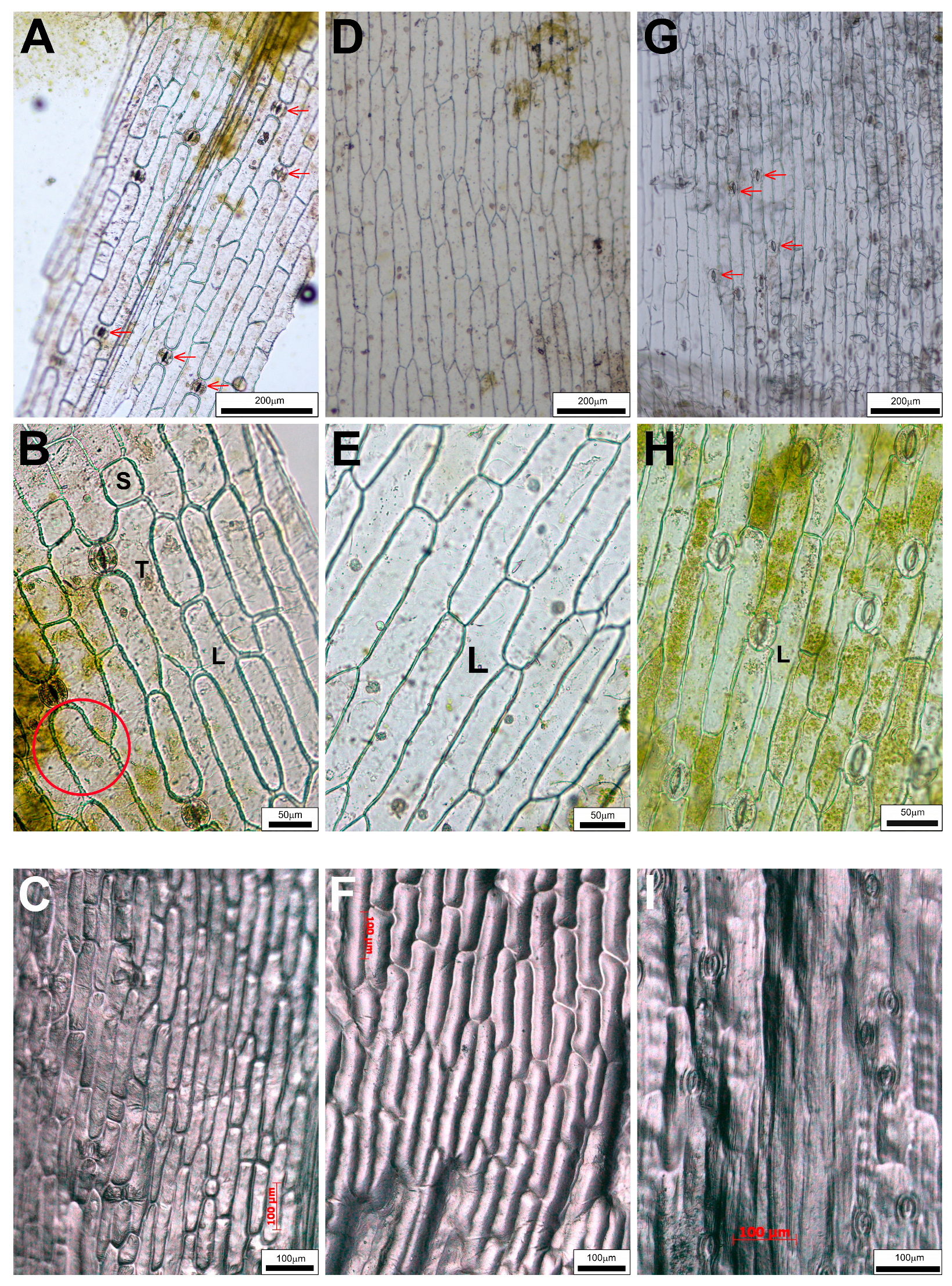
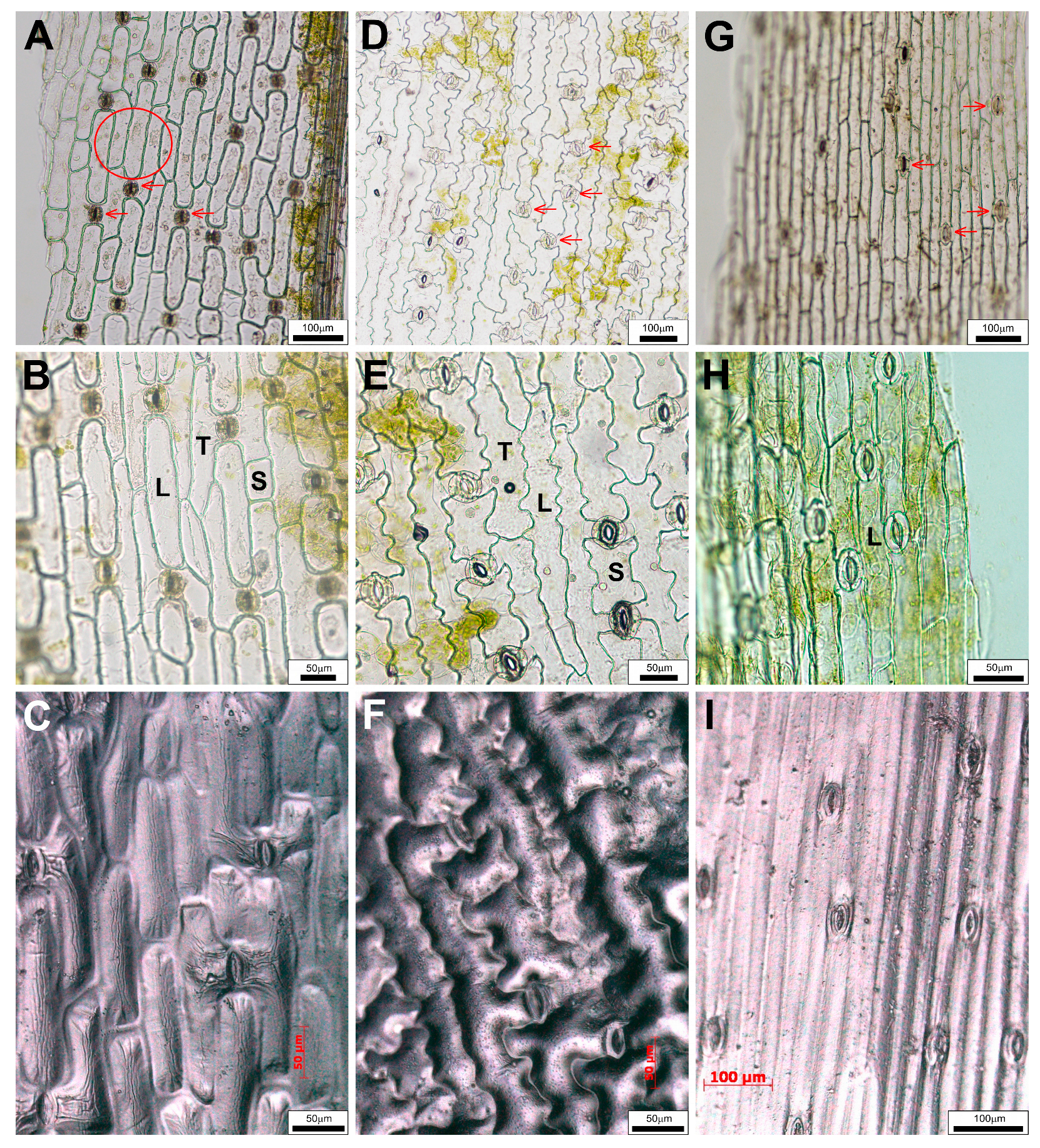
2.2. Differences in Epidermal Peels
2.3. Differences Between the Parameters in 2022 and 2023
2.4. Impressions of Leaf Surfaces
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Anatomical and Histological Investigations of Leaves
4.3. Epidermal Peels and Impressions
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Błażewicz-Woźniak, M.; Michowska, A. The growth, flowering and chemical composition of leaves of three ecotypes of Allium ursinum L. Acta Agrobot. 2011, 64, 171–180. [Google Scholar] [CrossRef]
- Dénes, A.; Papp, N.; Babai, D.; Czúcz, B.; Molnár, Z.S. Wild plants used for food by Hungarian ethnic groups living in the Carpathian Basin. Acta Soc. Bot. Pol. 2012, 81, 381–396. [Google Scholar] [CrossRef]
- Guarrera, P.M.; Savo, V. Perceived health properties of wild and cultivated food plants in local and popular traditions of Italy: A review. J. Ethnopharmacol. 2013, 146, 659–680. [Google Scholar] [CrossRef]
- Sobolewska, D.; Podolak, I.; Makowska-Wąs, J. Allium ursinum: Botanical, phytochemical and pharmacological overview. Phytochem. Rev. 2015, 14, 81–97. [Google Scholar] [CrossRef]
- Wagner, C.S.; De Gezelle, J.; Robertson, M.; Robertson, K.; Wilson, M.; Komarnytsky, S. Antibacterial activity of medicinal plants from The Physicians of Myddvai, a 14th century Welsh medical manuscript. J. Ethnopharmacol. 2017, 203, 171–181. [Google Scholar] [CrossRef]
- Matejić, J.S.; Stefanović, N.; Ivković, M.; Živanović, N.; Marin, P.D.; Džamić, A.M. Traditional uses of autochthonous medicinal and ritual plants and other remedies for health in Eastern and South-Eastern Serbia. J. Ethnopharmacol. 2020, 261, 113186. [Google Scholar] [CrossRef]
- Ginko, E.; Alajmovic Demirović, E.; Šarić-Kundalić, B. Ethnobotanical study of traditionally used plants in the municipality of Zavidović, BiH. J. Ethnopharmacol. 2023, 302, 115888. [Google Scholar] [CrossRef]
- Gordanić, S.V.; Kostić, A.Ž.; Krstić, Đ.; Vuković, S.; Kilibarda, S.; Marković, T.; Moravčević, Đ. A detailed survey of agroecological status of Allium ursinum across the republic of Serbia: Mineral composition and bioaccumulation potential. Heliyon 2023, 9, e22134. [Google Scholar] [CrossRef]
- Taleghani, A.; Ayati, Z.; Eghbali, S.; Emami, S.A.; Tayarani-Najaran, Z. Health benefits of Allium spp. in metabolic syndrome: A review. Afr. J. Bot. 2024, 167, 217–255. [Google Scholar] [CrossRef]
- Jarić, S.; Popović, Z.; Mačukanović-Jocić, M.; Djurdjević, L.; Mijatović, M.; Karadžić, B.; Mitrović, M.; Pavlović, P. An ethnobotanical study on the usage of wild medicinal herbs from Kopaonik Mountain (Central Serbia). J. Ethnopharmacol. 2007, 111, 160–175. [Google Scholar] [CrossRef] [PubMed]
- Jarić, S.; Mačukanović-Jocić, M.; Djurdjević, L.; Mitrović, M.; Kostić, O.; Karadžić, B.; Pavlović, P. An ethnobotanical survey of traditionally used plants on Suva planina mountain (South-Eastern Serbia). J. Ethnopharmacol. 2015, 175, 93–108. [Google Scholar] [CrossRef]
- Djurdjevic, L.; Dinic, A.; Pavlović, P.; Mitrović, M.; Karadzic, B.; Tešević, V. Allelopathic potential of Allium ursinum L. Biochem. Syst. Ecol. 2004, 32, 533–544. [Google Scholar] [CrossRef]
- Kevey, B.K. Magyarország Erdőtársulásai. Tilia 2008, 14, 34–57. (In Hungarian) [Google Scholar]
- Oborny, B.; Botta-Dukat, Z.; Rudolf, K.; Morschhauser, T. Population ecology of Allium ursinum, a space-monopolizing clonal plant. Acta Bot. Hung. 2011, 53, 371–388. [Google Scholar] [CrossRef]
- Rola, K. Taxonomy and distribution of Allium ursinum (Liliaceae) in Poland and adjacent countries. Biologia 2012, 67, 1080–1087. [Google Scholar] [CrossRef]
- Bodó, A.; Farkas, Á.; Nagy, D.U.; Rudolf, K.; Hoffmann, R.; Kocsis, M.; Morschhauser, T. Soil Humus, Iron, Sulphate and Magnesium Content Affect Nectar Traits of Wild Garlic (Allium ursinum L.). Plants 2021, 10, 597. [Google Scholar] [CrossRef]
- Kovacs, J.A. Data to vegetation biology and coenological relations of Allium ursinum L. stands in Eastern Transylvania. Kanitzia 2007, 15, 63–76. [Google Scholar]
- Herden, T.; Neuffer, B.; Friese, N. Allium ursinum L. in Germany—Surprisingly low genetic variability. Feddes Reper. 2013, 123, 81–95. [Google Scholar] [CrossRef]
- Stajner, D.; Varga Sz, I. An evaluation of the antioxidant abilities of Allium species. Acta Biol. Szeged. 2003, 47, 103–106. [Google Scholar]
- Amagova, Z.; Matsadze, V.; Kavarnakaeva, Z.; Golubkina, N.; Antoshkina, M.; Sekara, A.; Tallarita, A.; Caruso, G. Joint Cultivation of Allium ursinum and Armoracia rusticana under Foliar Sodium Selenate Supply. Plants 2022, 11, 2778. [Google Scholar] [CrossRef]
- Burton, G.P.; Prescott, T.A.K.; Fang, R.; Lee, M.A. Regional variation in the antibacterial activity of a wild plant, wild garlic (Allium ursinum L.). Plant Physiol. Biochem. 2023, 202, 107959. [Google Scholar] [CrossRef]
- Sendl, A. Allium sativum and Allium ursinum: Part 1. Chemistry, analysis, history, botany. Phytomedicine 1995, 1, 323–339. [Google Scholar] [CrossRef]
- Lanzotti, V.; Scala, F.; Bonanomi, G. Compounds from Allium species with cytotoxic and antimicrobial activity. Phytochem. Rev. 2014, 13, 769–791. [Google Scholar] [CrossRef]
- Preuss, H.G.; Clouatre, D.; Mohamadi, A.; Jarrell, S.T. Wild garlic has a greater effect than regular garlic on blood pressure and blood chemistries of rats. Int. Urol. Nephrol. 2001, 32, 525–530. [Google Scholar] [CrossRef]
- Sabha, D.; Hiyasat, B.; Grotzinger, K.; Hennig, L.; Schlegel, F.; Mohr, F.W.; Rauwald, H.W.; Dhein, S. Allium ursinum L.: Bioassay-guided isolation and identification of a galactolipid and a phytosterol exerting antiaggregatory effects. Pharmacology 2012, 89, 260–269. [Google Scholar] [CrossRef]
- Bombicz, M.; Priksz, D.; Varga, B.; Gesztelyi, R.; Kertesz, A.; Lengyel, P.; Balogh, P.; Csupor, D.; Hohmann, J.; Bhattoa, H.P.; et al. Anti-atherogenic properties of Allium ursinum liophylisate: Impact on lipoprotein homeostasis and cardiac biomarkers in hypercholesterolemic rabbits. Int. J. Mol. Sci. 2016, 17, 1284. [Google Scholar] [CrossRef] [PubMed]
- Bombicz, M.; Priksz, D.; Varga, B.; Kurucz, A.; Kertész, A.; Takacs, A.; Posa, A.; Kiss, R.; Szilvassy, Z.; Juhasz, B. A Novel Therapeutic Approach in the Treatment of Pulmonary Arterial Hypertension: Allium ursinum Liophylisate Alleviates Symptoms Comparably to Sildenafil. Int. J. Mol. Sci. 2017, 18, 1436. [Google Scholar] [CrossRef]
- Council of Europe. Ph.Eur.9.0. In Eur. Pharmacop, 9th ed.; Council of Europe: Strasbourg, France, 2016. [Google Scholar]
- Ph. Hg. VIII. Magyar Gyógyszerkönyv (Hungarian Pharmacopoeia 8th Edition). Available online: https://ogyei.gov.hu/gyogyszerkonyv (accessed on 25 January 2008).
- Aumeeruddy, M.Z.; Mahomoodally, M.F. Traditional herbal therapies for hypertension: A systematic review of global ethnobotanical field studies. S. Afr. J. Bot. 2020, 135, 451–464. [Google Scholar] [CrossRef]
- Stupar, A.; Vidović, S.; Vladić, J.; Radusin, T.; Mišan, A. A Sustainable Approach for Enhancing Stability and Bioactivity of Allium ursinum Extract for Food Additive Applications. Separations 2024, 11, 81. [Google Scholar] [CrossRef]
- Guarrera, P.M.; Savo, V. Wild food plants used in traditional vegetable mixtures in Italy. J. Ethnopharmacol. 2016, 185, 202–234. [Google Scholar] [CrossRef]
- Colombo, L.M.; Assisi, F.; Puppa, T.D.; Moro, P.; Sesana, F.M.; Bissoli, M.; Borghini, R.; Perego, S.; Galasso, G.; Banfi, E.; et al. Exposures and Intoxications after herb-induced poisoning: A retrospective hospital-based study. J. Pharm. Sci. Res. 2009, 2, 123–136. [Google Scholar]
- Danilović, M.; Isailović, J.; Aleksić, I.; Džambas, J.; Marinković, N. Accidental colchicine poisoning with fatal outcome after ingestion of meadow saffron (Colchicum autumnale L.). Vojnosanit. Pregl. 2020, 77, 1104–1108. [Google Scholar] [CrossRef]
- Davanzo, F.; Miaglia, S.; Perego, S.; Assisi, F.; Bissoli, M.; Borghini, R.; Cassetti, F.; Puppa, T.D.; Dimasi, V.; Falciola, C.; et al. Plant poisoning: Increasing relevance, a problem of public health and education. North-western Italy, Piedmont region. J. Pharm. Sci. Res. 2011, 3, 1338–1343. [Google Scholar]
- Fuchs, J.; Rauber-Lüthy, C.; Kupferschmidt, H.; Kupper, J.; Kullak-Ublick, G.A.; Ceschi, A. Acute plant poisoning: Analysis of clinical features and circumstances of exposure. Clin. Toxicol. 2011, 49, 671–680. [Google Scholar] [CrossRef]
- Hermanns-Clausen, M.; Koch, I.; Pietsch, J.; Andresen-Streichert, I.; Begemann, K. Akzidentelle Vergiftungen mit Gartenpflanzen und Pflanzen in der freien Natur. Daten aus zwei deutschen Giftinformationszentren. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2009, 62, 73–83. [Google Scholar] [CrossRef]
- Wollersen, H.; Erdmann, F.; Risse, M.; Dettmeyer, R. Accidental fatal ingestion of colchicine-containing leaves—Toxicological and histological findings. Leg. Med. 2009, 11, 498–499. [Google Scholar] [CrossRef] [PubMed]
- Peters, F.T.; Beyer, J.; Ewald, A.H.; Maurer, H.H. Colchicine Poisoning after Mix-up of Ramsons (Allium ursinum L.) and Meadow Saffron (Colchicum autumnale L.) Case report from the “Clinical Toxicology” committee of the GTFCh. Toxicol. Krim. 2004, 71, 156. [Google Scholar]
- Brnčić, N.; Višković, I.; Perić, R.; Ðirlić, A.; Vitezić, D.; Cuculić, D. Accidental Plant Poisoning with Colchicum autumnale: Report of Two Cases. Croat. Med. J. 2001, 42, 673–675. [Google Scholar]
- Klintschar, M.; Beham-Schmidt, C.; Radner, H.; Henning, G.; Roll, P. Colchicine poisoning by accidental ingestion of meadow saffron (Colchicum autumnale): Pathological and medicolegal aspects. Forensic Sci. Int. 1999, 106, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Gabrscek, L.; Lesnicar, G.; Krivec, B.; Voga, G.; Sibanc, B.; Blatnik, J.; Jagodic, B. Accidental poisoning with Autumn crocus. J. Toxicol. Clin. Toxicol. 2004, 42, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Area Maps: Hermann Meusel und Eckehart Jäger: Vergleichende Chorologie der Zentraleuropäischen Flora. Available online: http://chorologie.biologie.uni-halle.de/choro/ (accessed on 1 July 2024).
- Kerchner, A.; Farkas, Á. Worldwide poisoning potential of Brugmansia and Datura. Forensic Toxicol. 2020, 38, 30–41. [Google Scholar] [CrossRef]
- Vončina, M.; Baričevič, D.; Brvar, M. Adverse effects and intoxications related to medicinal/harmful plants. Acta Agric. Slov. 2014, 103, 263–270. [Google Scholar] [CrossRef]
- Romm, A.; Hardy, M.L.; Mills, S. Botanical Medicine for Women’s Health; Churchill Livingstone: London, UK, 2010. [Google Scholar] [CrossRef]
- Spillum, B.J.; Muan, B. Human plant poisoning in the Nordic countries—Experiences from the Poisons Information Centres. In Bioactive Compounds in Plants—Benefits and Risks for Man and Animals; Bernhoft, A., Ed.; The Norwegian Academy of Science and Letters: Oslo, Norway, 2010; pp. 44–51. [Google Scholar]
- Alexandre, J.; Foucault, A.; Coutance, G.; Scanu, P.; Milliez, P. Digitalis Intoxication Induced by an Acute Accidental Poisoning by Lily of the Valley. Circulation 2012, 125, 1053–1055. [Google Scholar] [CrossRef]
- Schneider, N.F.Z.; Silva, I.T.; Persich, L.; de Carvalho, A.; Rocha, S.C.; Marostica, L.; Ramos, A.C.P.; Taranto, A.G.; Pádua, R.M.; Kreis, W.; et al. Cytotoxic effects of the cardenolide convallatoxin and its Na, K-ATPase regulation. Mol. Cell. Biochem. 2017, 428, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Welsh, K.J.; Huang, R.S.; Actor, J.K.; Dasgupta, A. Rapid detection of the active cardiac glycoside convallatoxin of lily of the valley using LOCI digoxin assay. Am. J. Clin. Pathol. 2014, 142, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, M.; Tatsumi, K.; Yuui, K.; Terazawa, I.; Kudo, R.; Kasuda, S. Convallatoxin, the primary cardiac glycoside in lily of the valley (Convallaria majalis), induces tissue factor expression in endothelial cells. Vet. Med. Sci 2021, 7, 2440–2444. [Google Scholar] [CrossRef] [PubMed]
- JAMA. Therapeutics. JAMA. 1 April 1911. Available online: http://jamanetwork.com (accessed on 13 June 2015).
- Stansbury, J.; Saunders, P.; Winston, D.; Zampieron, E.R. The use of Convallaria and Crataegus in the Treatment of Cardiac Dysfunction. J. Restor. Med. 2012, 1, 107–111. [Google Scholar] [CrossRef][Green Version]
- Stapczynski, J.S.; Rothstein, R.J.; Gaye, W.A.; Niemann, J.T. Colchicine overdose: Report of two cases and review of the literature. Ann. Emerg. Med. 1981, 10, 364–369. [Google Scholar] [CrossRef]
- Akram, M.; Alam, O.; Usmanghani, K.; Akhter, N.; Asif, H.M. Colchicum autumnale: A review. J. Med. Plants Res. 2012, 6, 1489–1491. [Google Scholar] [CrossRef]
- Király, G. (Ed.) Új Magyar Fűvészkönyv; Aggteleki Nemzeti Park Igazgatóság: Jósvafő, Hungary, 2009; p. 472. (In Hungarian) [Google Scholar]
- Lewis, W.H.; Memory Elvin-Lewis, P.F. Medical Botany: Plants Affecting Human Health, 2nd ed.; John Wiley & Sons: New York, NJ, USA, 2003. [Google Scholar]
- Sundov, Z.; Nincevic, Z.; Definis-Gojanovic, M.; Glavina-Durdov, M.; Jukic, I.; Hulina, N.; Tonkic, A. Fatal colchicine poisoning by accidental ingestion of meadow saffron-case report. Forensic Sci. Int. 2005, 149, 253–256. [Google Scholar] [CrossRef]
- Stemmermann, G.N.; Hayashi, T. Colchicine intoxication. A reappraisal of its pathology bases on study of three fatal cases. Hum. Pathol. 1971, 2, 321–332. [Google Scholar] [CrossRef]
- Ng, W.Y.; Hung, L.Y.; Lam, Y.H.; Chan, S.S.; Pang, K.S.; Chong, Y.K.; Ching, C.K.; Mak, T.W.L. Poisoning by toxic plants in Hong Kong: A 15-year review. Hong Kong Med. J. 2019, 25, 102–112. [Google Scholar] [CrossRef]
- Upton, R.; Graff, A.; Jollifle, G.; Länger, R.; Williamson, E. (Eds.) American Herbal Pharmacopeia: Botanical Pharmacognosy-Microscopic Characterization of Botanical Medicines; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: New York, NY, USA, 2011; pp. 72–727. ISBN 978-1-4200-7326-3. [Google Scholar]
- Stegelmeier, B.L.; Davis, T.Z.; Clayton, M.J.; Gardner, D.R. Identifying plant poisoning in livestock in North America. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Mashayekhi, S.; Columbus, J.T. Evolution of leaf blade anatomy in Allium (Amaryllidaceae) subgenus Amerallium with a focus on the North American species. Am. J. Bot. 2014, 101, 63–85. [Google Scholar] [CrossRef]
- Climate and Precipitation Data of 2021, 2022 and 2023 Are from HungaroMet. Available online: https://www.metnet.hu/ (accessed on 6 November 2021).
- Nepi, M.; Stpiczyńska, M. The Complexity of Nectar: Secretion and Resorption Dynamically Regulate Nectar Features. Naturwissenschaften 2008, 95, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Jandl, R.; Kopeszki, H.; Glatzel, G. Effect of dense Allium ursinum (L.) ground cover on nutrient dynamics and mesofauna of a Fagus sylvatica (L.) woodland. Plant Soil 1997, 189, 245–255. [Google Scholar] [CrossRef]
- Brvar, M.; Koźelj, G.; Moźina, M.; Bunc, M. Acute poisoning with autumn crocus (Colchicum autumnale L.). Case report. Wien. Klin. Wochenschr. 2004, 116, 205–208. [Google Scholar] [CrossRef]
- Wehner, F.; Mußhoff, F.; Schulz, M.M.; Martin, D.D.; Wehner, H.-D. Detection of Colchicine by Means of LC-MS/MS After Mistaking Meadow Saffron for Bear’s Garlic. Forensic Sci. Med. Pathol. 2006, 2, 193–198. [Google Scholar] [CrossRef]
- Mezzasalma, V.; Ganopoulos, I.; Galimberti, A.; Cornara, L.; Ferri, E.; Labra, M. Poisonous or non-poisonous plants? DNA-based tools and applications for accurate identification. Int. J. Legal. Med. 2017, 131, 119. [Google Scholar] [CrossRef]
- Hack, J. Toxicology Answer: The Lovely Lily of the Valley. ACEP Now, 21 March 2022. [Google Scholar]
- Beyer, D.; Surányi, G.; Vasas, G.; Roszik, J.; Erdődi, F.; M-Hamvas, M.; Bácsi, I.; Bátori, R.; Serfőző, Z.; Szigeti, Z.M.; et al. Cylindrospermopsin induces alterations of root histology and microtubule organization in common reed (Phragmites australis) plantlets cultured in vitro. Toxicon 2009, 54, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Máthé, C.; Beyer, D.; Erdődi, F.; Serfőző, Z.; Székvölgyi, L.; Vasas, G.; M-Hamvas, M.; Jámbrik, K.; Gonda, S.; Kiss, A.; et al. Microcystin-LR induces abnormal root development by altering its microtubule organization in tissue-cultured common reed (Phragmites australis) plantlets. Aquat. Toxicol. 2009, 92, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Sárkány, S.; Szalai, I. Növényszervezettani Gyakorlatok; Tankönyvkiadó: Budapest, Hungary, 1964; pp. 124, 547–557. (In Hungarian) [Google Scholar]
- Peterson, R.L.; Peterson, C.A.; Melville, L.H. Teaching Plant Anatomy Through Creative Laboratory Exercises; NRC Press: Ottawa, ON, Canada, 2008; p. 164. ISBN 978-0-660-19798-2. [Google Scholar]
- Gáspár, A.; Bácsi, I. Forced flow paper chromatography: A simple tool for separations in short time. Microchem. J. 2009, 92, 83–86. [Google Scholar] [CrossRef]
- Takács, A.; Nagy, T.; Fekete, R.; Lovas-Kiss, Á.; Ljubka, T.; Löki, V.; Lisztes-Szabó, Z.S.; Molnár, V.A. A Debreceni Egyetem Herbáriuma (DE) I.: A Soó Rezső Herbárium”. Kitaibelia 2014, 19, 142–155. [Google Scholar]
| Year | Convallaria majalis | Allium ursinum | Colchicum autumnale | p | |
|---|---|---|---|---|---|
| average leaf lamina length (cm) | 2022 | 18.55 ± 3.33 b | 13.30 ± 2.07 c | 38.10 ± 3.52 a | <0.001 |
| 2023 | 16.37 ± 3.44 b | 13.46 ± 3.98 c | 29.19 ± 3.64 a | <0.05 | |
| 2022 ↔ 2023 | * p = 0.026 | NS | * p = 0.001 | ||
| average of the max. leaf widths (cm) | 2022 | 5.40 ± 1.06 | 4.836 ± 1.16 | 5.370 ± 1.347 | NS |
| 2023 | 5.63 ± 1.43 a | 4.39 ± 1.68 b | 6.04 ± 0.78 a | <0.05 | |
| 2022 ↔ 2023 | NS | NS | * p = 0.037 | ||
| the thickness of leaves at midveins (µm) | 2022 | 480.610 ± 61.77 c | 1330.17 ± 234.08 a | 742.880 ± 92.36 b | <0.005 |
| 2023 | 444.20 ± 80.40 c | 1813.50 ± 336.40 a | 839.91 ± 76.15 b | <0.05 | |
| 2022 ↔ 2023 | NS | * p = 0.002 | * p = 0.020 | ||
| average thickness of leaves (µm) | 2022 | 190.32 ± 19.89 c | 429.40 ± 46.55 b | 726.73 ± 123.27 a | <0.005 |
| 2023 | 195.90 ± 17.99 c | 447.90 ± 50.44 b | 803.07 ± 93.16 a | <0.05 | |
| 2022 ↔ 2023 | NS | NS | NS | ||
| diameter of midvein (µm) | 2022 | 145.30 ± 18.88 c | 291.2 ± 50.6 b | 400.30 ± 62.0 a | <0.001 |
| 2023 | 152.00 ± 34.89 c | 287.60 ± 46.42 b | 460.69 ± 72.91 a | <0.05 | |
| 2022 ↔ 2023 | NS | NS | NS | ||
| Area µm2 Year | Convallaria majalis | Allium ursinum | Colchicum autumnale | |||||
|---|---|---|---|---|---|---|---|---|
| Cell Types | Short | T | Long | Short | T | Long | Long | |
| upper epider-mis | 2022 | 1763.3 ± 490 c | 5755.9 ± 1401 b,d | 6347.2 ± 1693 b | - | - | 12,489.5 ± 2705 a | 7221.9 ± 1635 b,d |
| 2023 | 2658.1 ± 784 c | 7081.6 ± 1028 b,c | 8238.6 ± 1465 a,b | - | - | 11,040.9 ± 2534 a | 9859.2 ± 3588 a,b | |
| 2022 ↔ 2023 | * p = 0.003 | * p = 0.015 | * p = 0.01 | NS | * p = 0.01 | |||
| lower epider-mis | 2022 | 2255.6 ± 990 a | 5971.7 ± 1253 b | 5942 ± 1495 b,c | 5366 ± 794 b | 11,726.7 ± 5669 a.c | 14,118.5 ± 6335 a | 7512.2 ± 1739 a,b |
| 2023 | 2425.1 ± 718 b | 6532.8 ± 991 d | 7297.5 ± 1499 a,c,d | 5487.9 ± 1862 c | 14,128.7 ± 5295 a | 14,202.8 ± 3796 a | 7930.1 ± 1357 a,c,d | |
| 2022 ↔ 2023 | NS | NS | * p = 0.047 | NS | NS | NS | NS | |
| Pc/mm2 | Convallaria majalis | Allium ursinum | Colchicum autumnale | |
|---|---|---|---|---|
| upper epidermis | 2022 | 266.67 ± 70.08 b | 0 c | 488.00 ± 41.31 a |
| 2023 | 314.29 ± 53.81 b | 0 c | 460.00 ± 52.57 a | |
| lower epidermis | 2022 | 486.67 ± 127.54 a | 430.00 ± 18.52 a | 280.00 ± 116.237 b |
| 2023 | 400.00 ± 74.07 b | 500.00 ± 64.140 a* | 340.00 ± 79.09 b | |
| 2022 ↔ 2023 | NS | * p = 0.005 | NS | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
M-Hamvas, M.; Tótik, A.; Freytag, C.; Gáspár, A.; Nouar, A.; Garda, T.; Máthé, C. Histological Features Detected for Separation of the Edible Leaves of Allium ursinum L. from the Poisonous Leaves of Convallaria majalis L. and Colchicum autumnale L. Plants 2025, 14, 2377. https://doi.org/10.3390/plants14152377
M-Hamvas M, Tótik A, Freytag C, Gáspár A, Nouar A, Garda T, Máthé C. Histological Features Detected for Separation of the Edible Leaves of Allium ursinum L. from the Poisonous Leaves of Convallaria majalis L. and Colchicum autumnale L. Plants. 2025; 14(15):2377. https://doi.org/10.3390/plants14152377
Chicago/Turabian StyleM-Hamvas, Márta, Angéla Tótik, Csongor Freytag, Attila Gáspár, Amina Nouar, Tamás Garda, and Csaba Máthé. 2025. "Histological Features Detected for Separation of the Edible Leaves of Allium ursinum L. from the Poisonous Leaves of Convallaria majalis L. and Colchicum autumnale L." Plants 14, no. 15: 2377. https://doi.org/10.3390/plants14152377
APA StyleM-Hamvas, M., Tótik, A., Freytag, C., Gáspár, A., Nouar, A., Garda, T., & Máthé, C. (2025). Histological Features Detected for Separation of the Edible Leaves of Allium ursinum L. from the Poisonous Leaves of Convallaria majalis L. and Colchicum autumnale L. Plants, 14(15), 2377. https://doi.org/10.3390/plants14152377







