Significance of Morpho-Palynological Diversity in Melliferous Plants in the Anzer Region (Türkiye) with Regard to Honey Authentication
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area, Plant Collection, and Identification
2.2. Palynological Studies
2.3. Statistical Analysis
3. Results
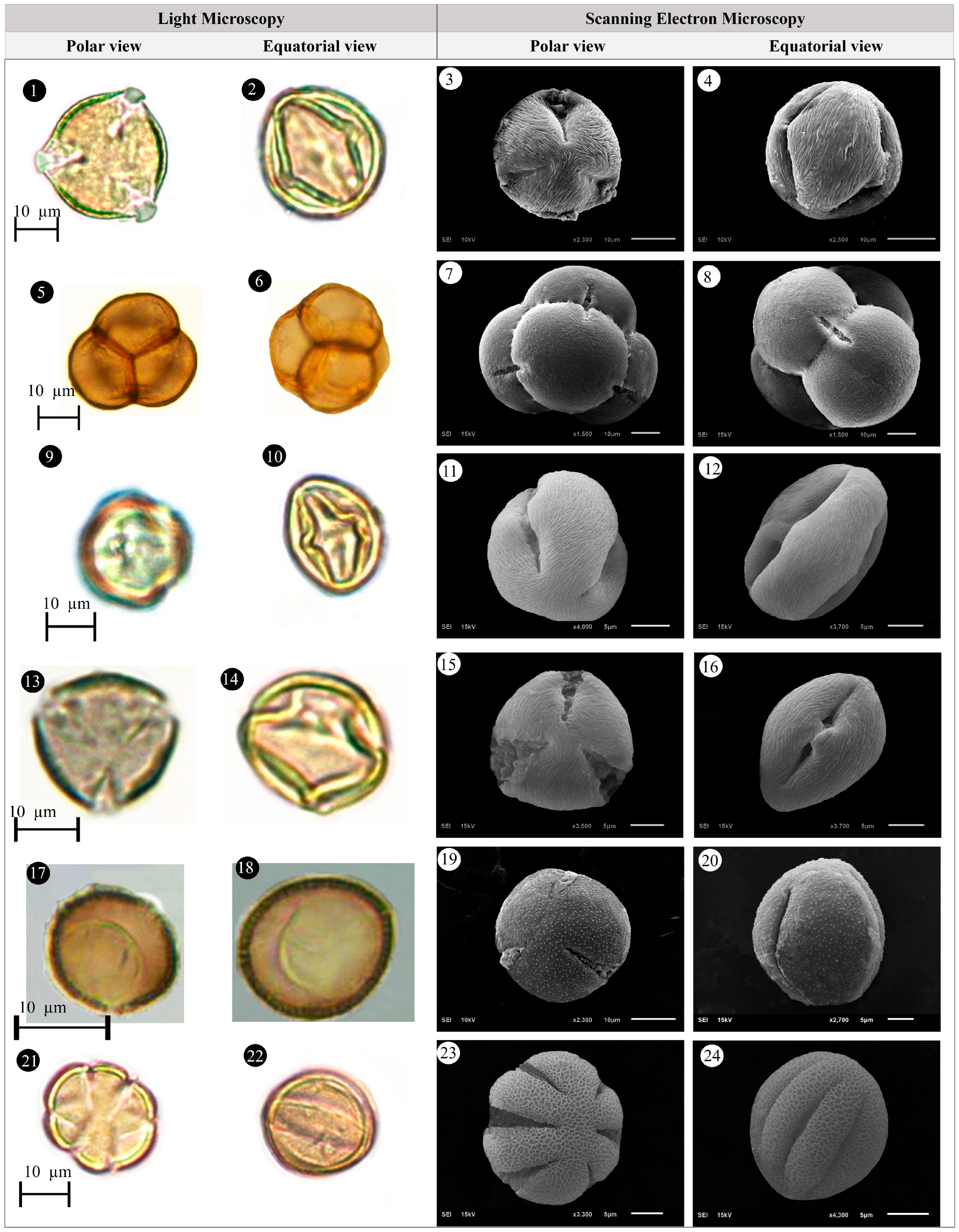
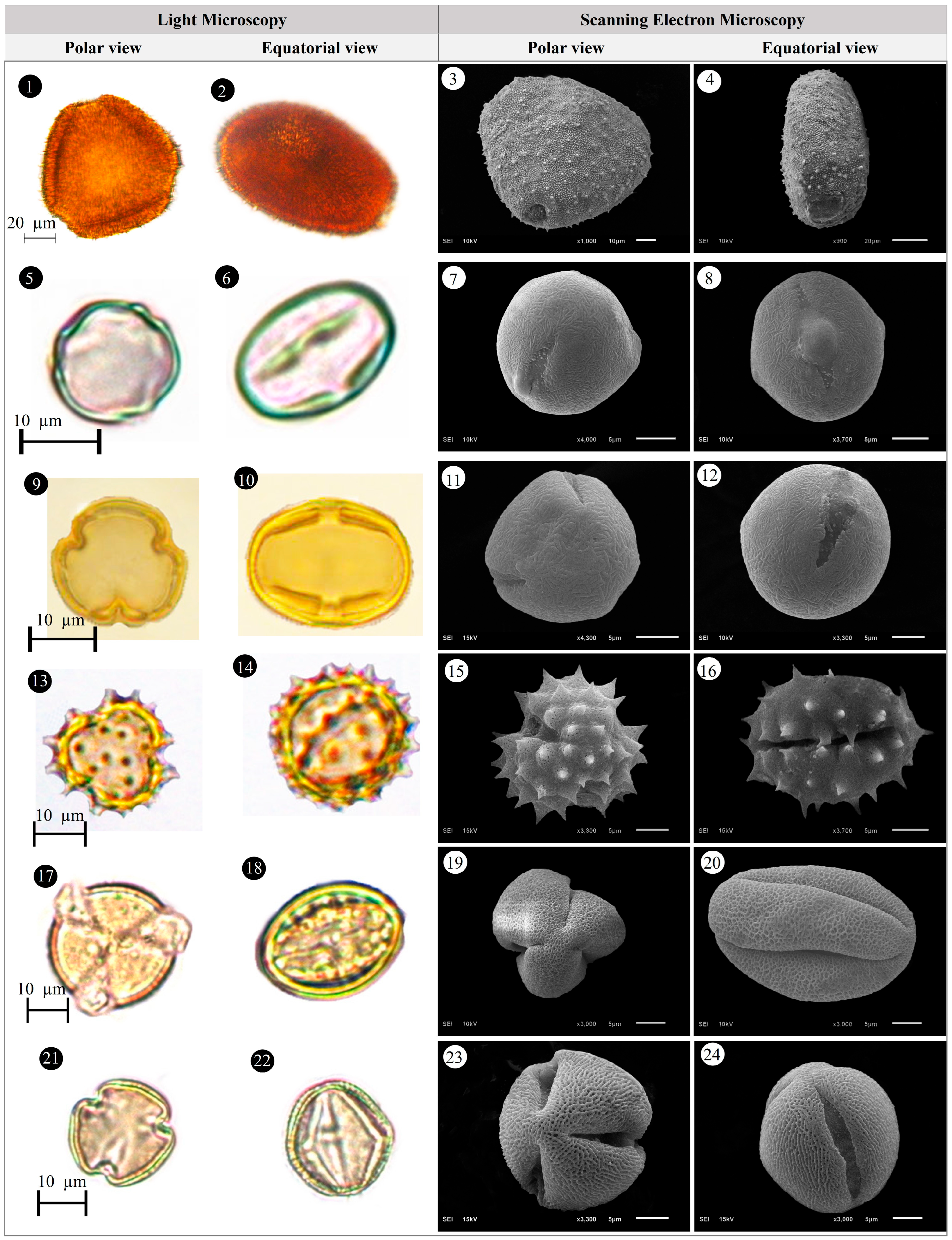
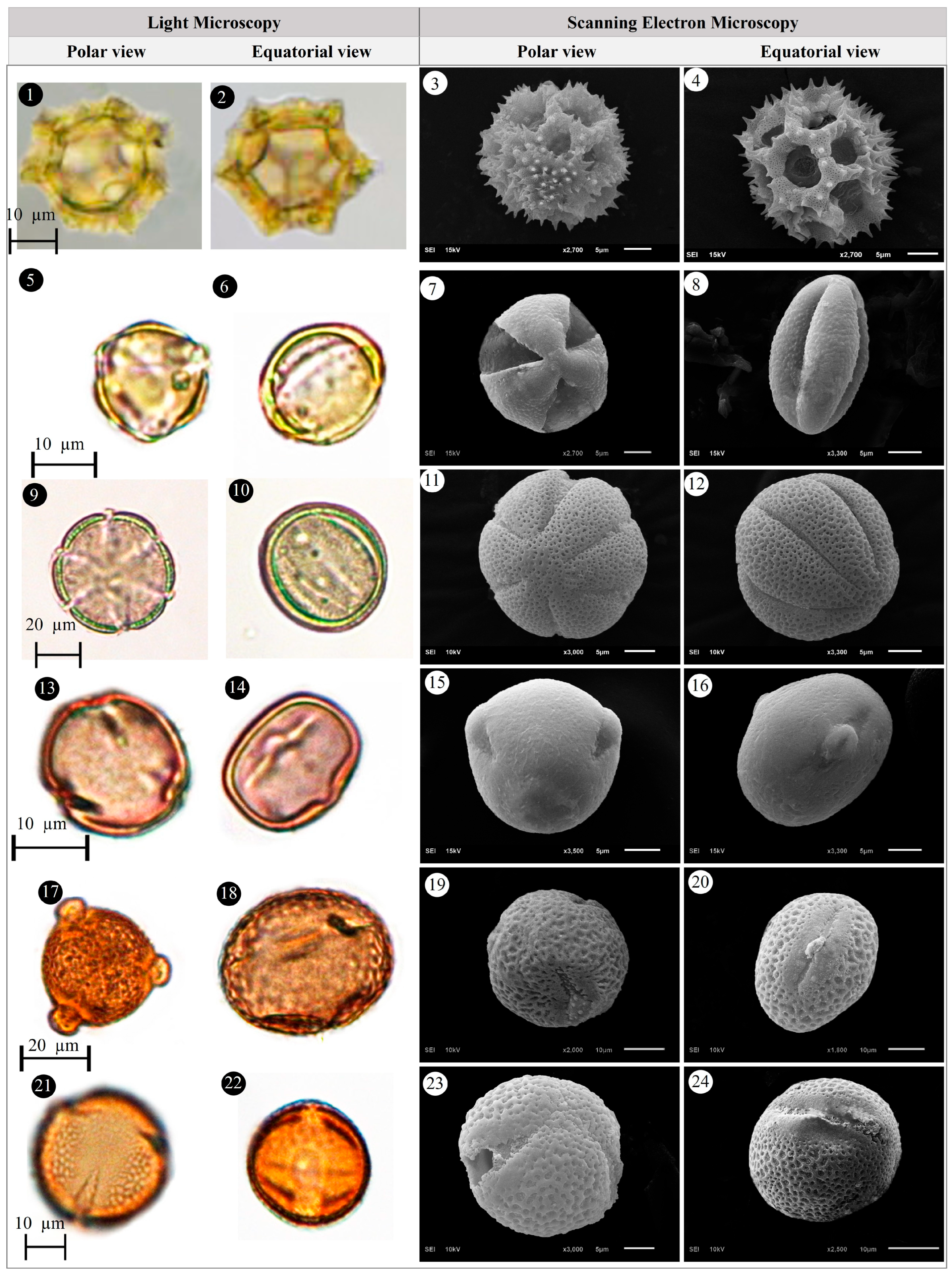
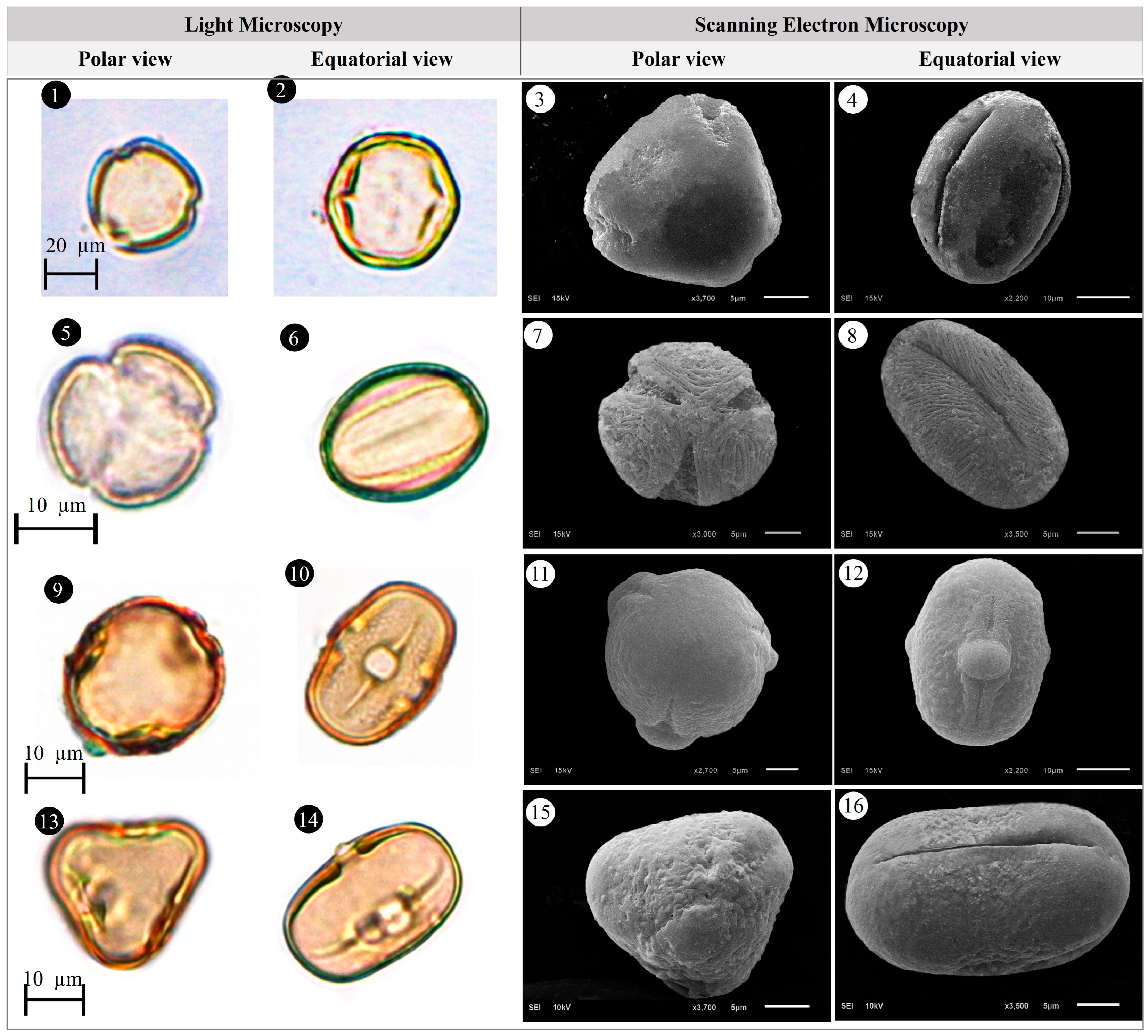
3.1. Evaluation of Floristic and Pollen Morphology Findings
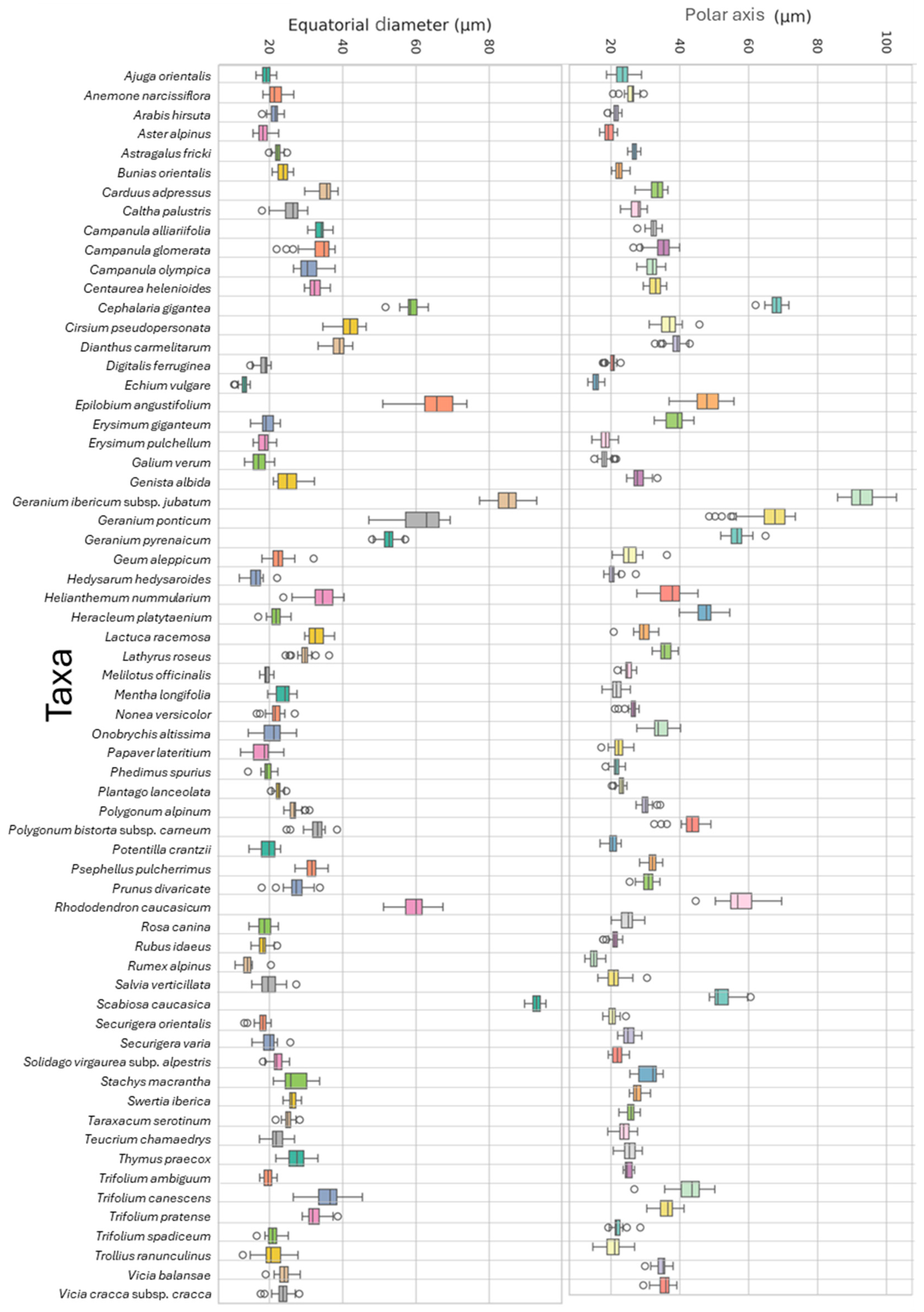
3.2. Polar Axis and Equatorial Diameter
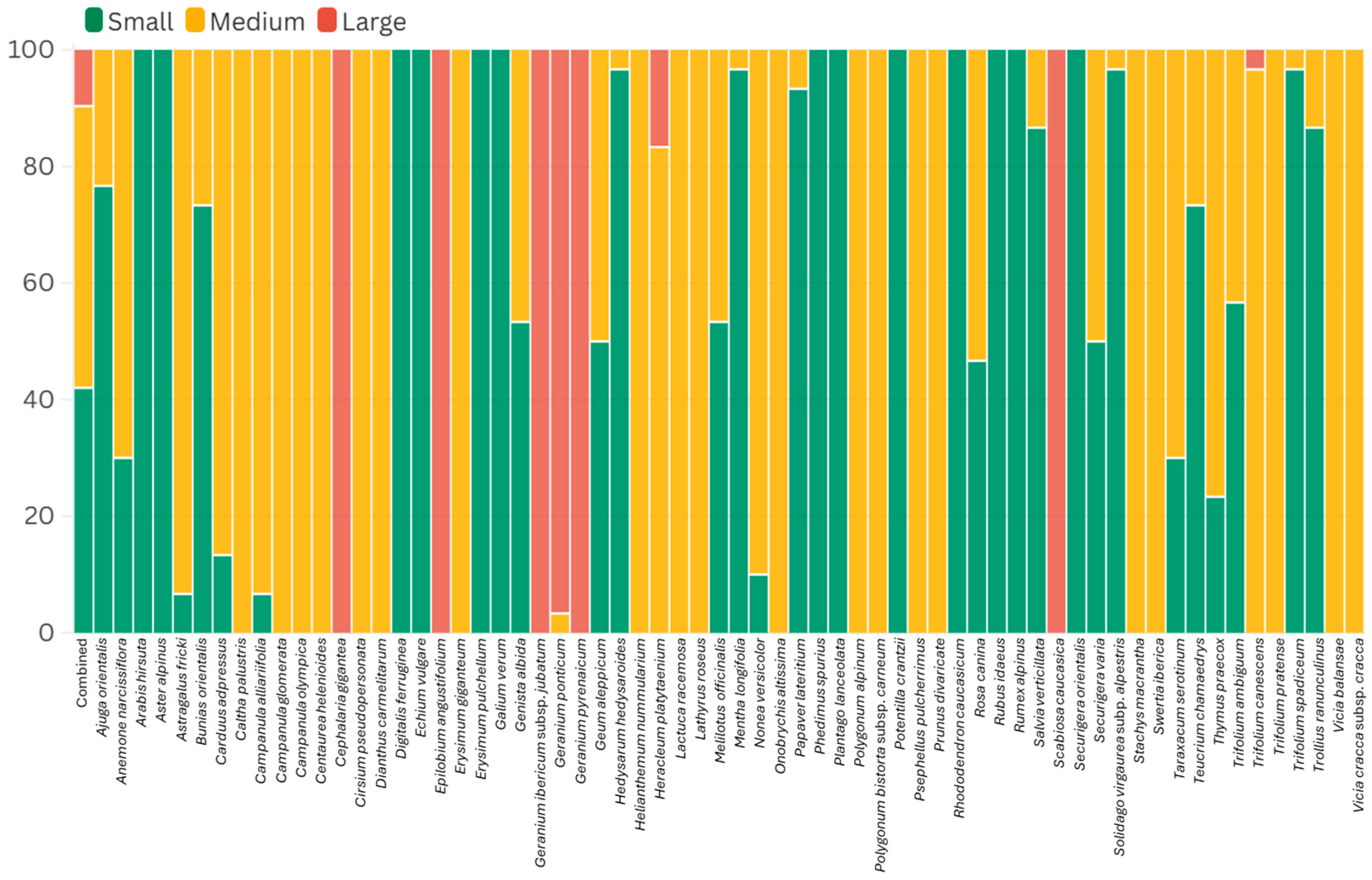
3.3. Pollen Size
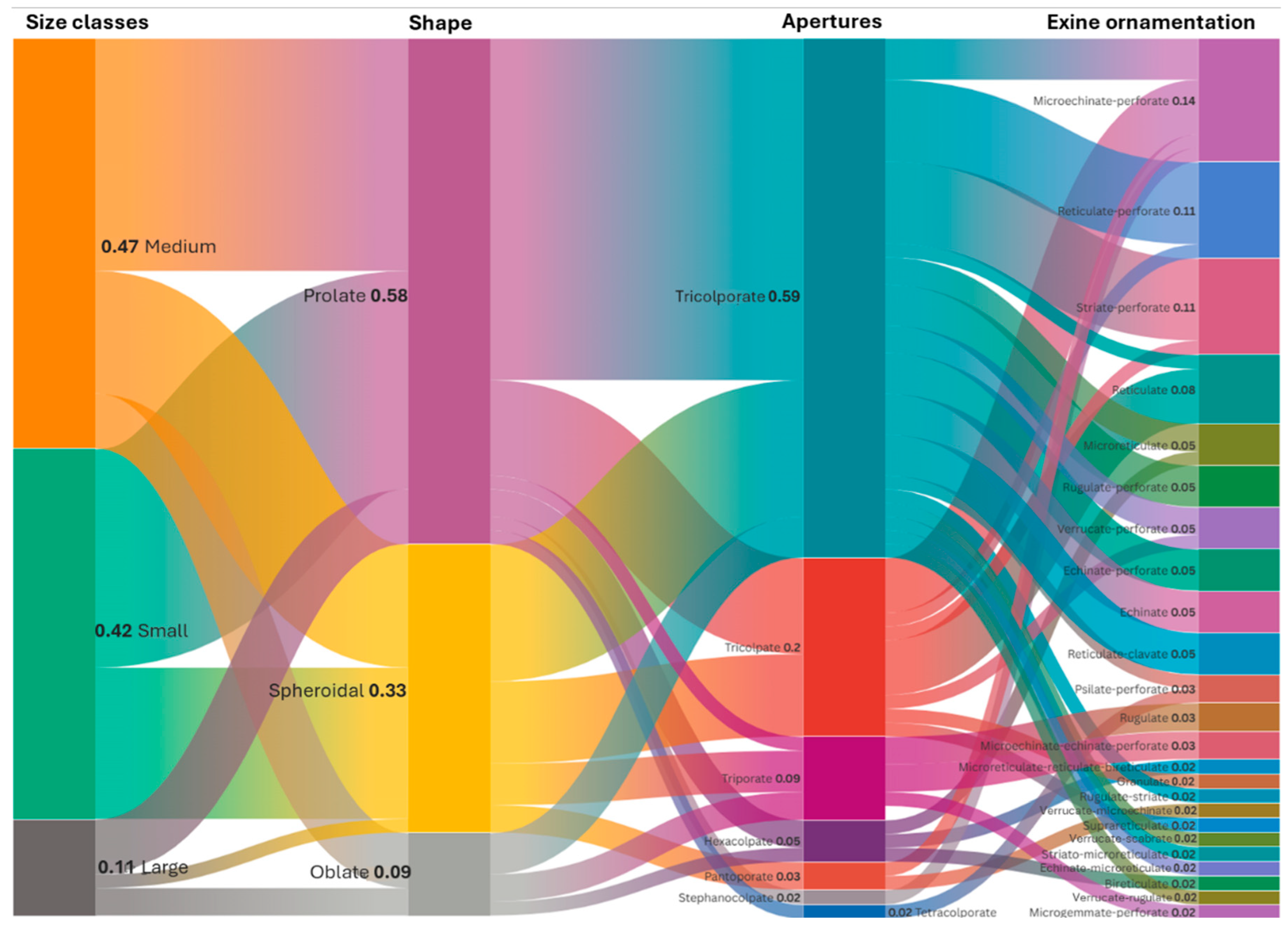
3.4. The Ratio of Polar Axis to Equatorial Diameter and Pollen Shape
3.5. Pollen Type and Exine Ornamentation
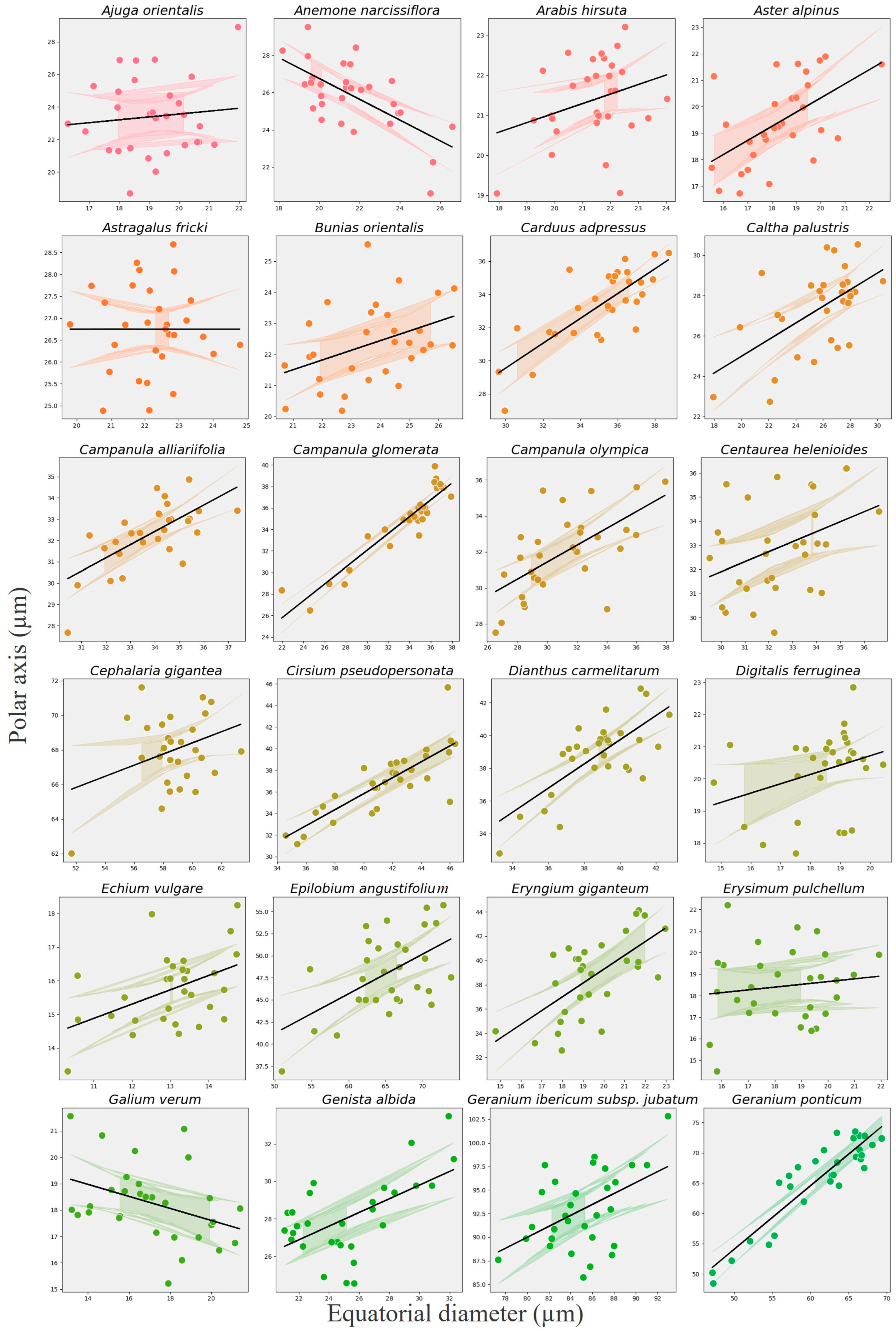
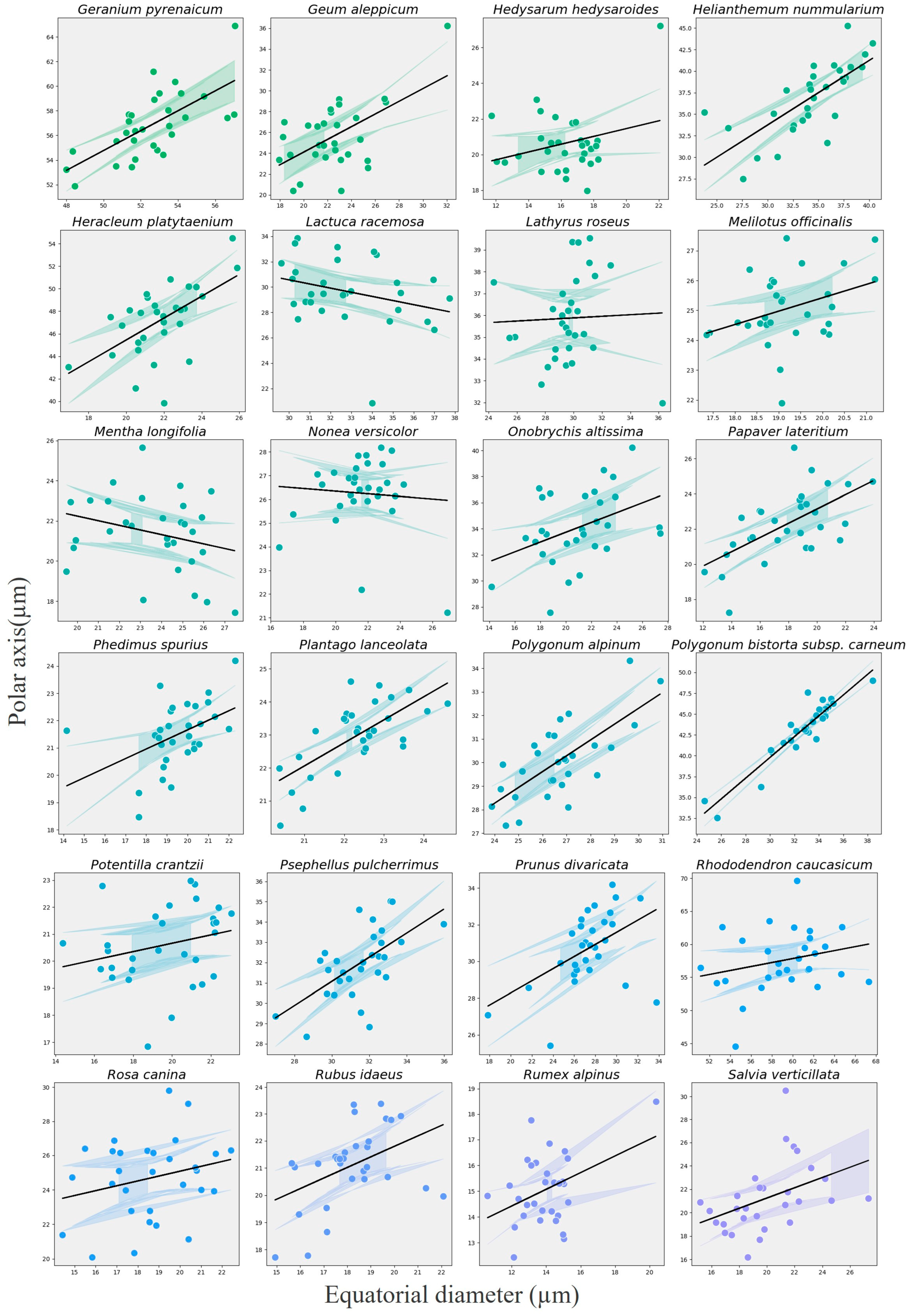
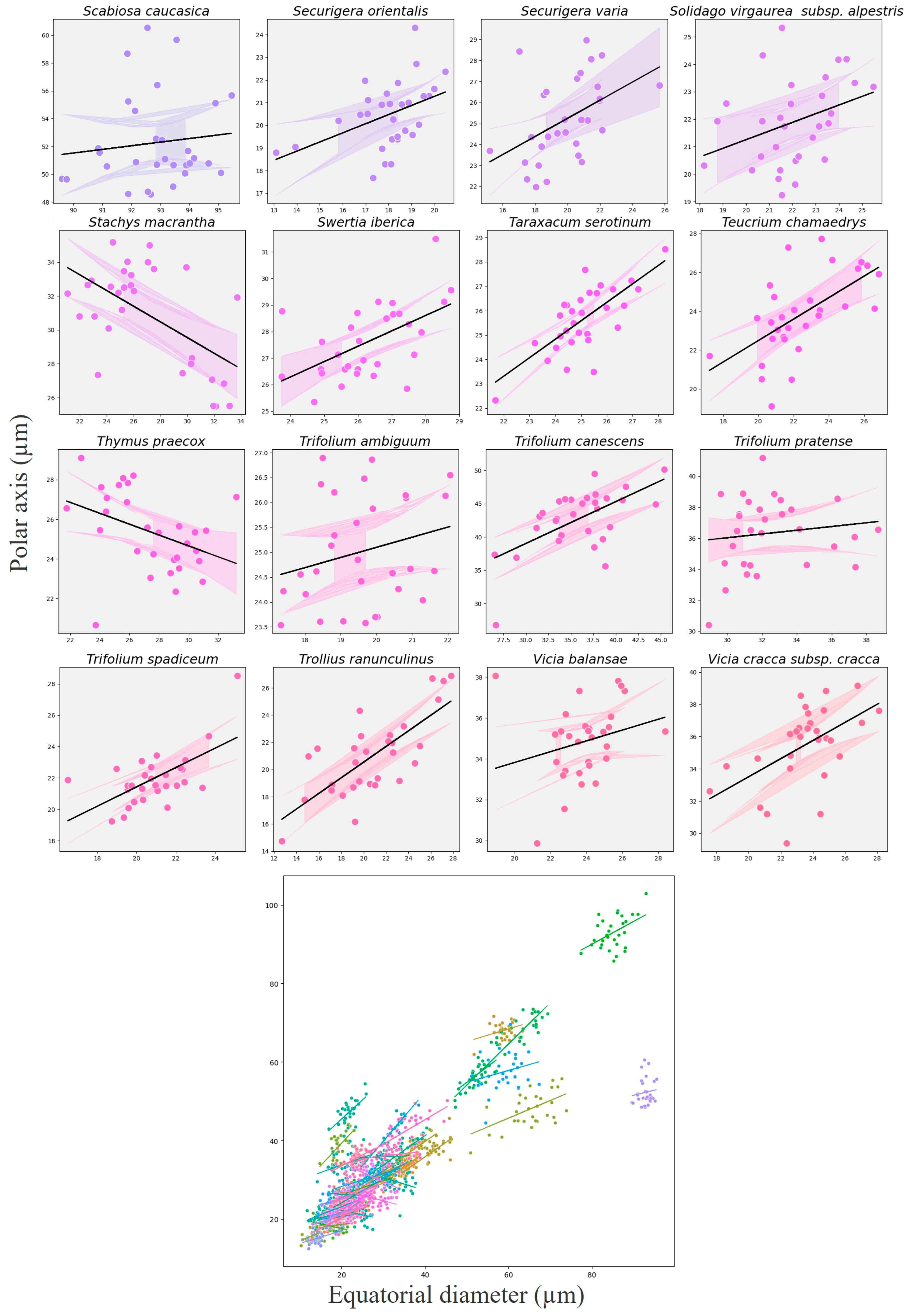
3.6. Relationship Between Polar Axis and Equatorial Diameter
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, R.K.; Reybroeck, W.; Van Veen, J.W.; Gupta, A. Beekeeping for Poverty Alleviation and Livelihood Security: Vol. 1: Technological Aspects of Beekeeping; Springer: Dordrecht, The Netherlands, 2014; ISBN 9789401791991. [Google Scholar]
- Bhalchandra, W.; Baviskar, R.K.; Nikam, T.B. Diversity of Nectariferous and Polleniferous Bee Flora at Anjaneri and Dugarwadi Hills of Western Ghats of Nasik District (M. S.) India. J. Entomol. Zool. Stud. 2014, 2, 244–249. [Google Scholar]
- Ahmad, S.; Zafar, M.; Ahmad, M.; Lubna; Yaseen, G.; Sultana, S. Microscopic Investigation of Palyno-Morphological Features of Melliferous Flora of Lakki Marwat District, Khyber Pakhtunkhwa, Pakistan. Microsc. Res. Tech. 2019, 82, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Zafar, M.; Ahmad, M.; Ozdemir, A.F.; Lubna; Yaseen, G.; Sultana, S.; Kutlu, A.M. Palynological Studies of Winter Weeds Melliferous Flora of District Bannu, Khyber Pakhtunkhwa, Pakistan. Ann. Bot. 2020, 10, 77–86. [Google Scholar] [CrossRef]
- Zafar, A.; Zafar, M.; Ahmad, M.; Khan, A.M.; Mahmood, T.; Kilic, O.; Fatima, A.; Habib, D.; Sultana, S.; Majeed, S.; et al. Microscopic (LM and SEM) Visualization of Pollen Ultrastructure among Honeybee Flora from Lower Margalla Hills and Allied Areas. Microsc. Res. Tech. 2022, 85, 3325–3338. [Google Scholar] [CrossRef]
- Rasoloarijao, T.M.; Ramavovololona, P.; Ramamonjisoa, R.; Clemencet, J.; Lebreton, G.; Delatte, H. Pollen Morphology of Melliferous Plants for Apis mellifera Unicolor in the Tropical Rainforest of Ranomafana National Park, Madagascar. Palynology 2019, 43, 292–320. [Google Scholar] [CrossRef]
- Santos do Nascimento, A.; Carlos Marchini, L.; Alfredo Lopes de Carvalho, C.; Feliciano Dias Araújo, D.; Antonia da Silveira, T. Pollen Spectrum of Stingless Bees Honey (Hymenoptera: Apidae), Paraná State, Brazil. J. Entomol. Zool. Stud. 2015, 3, 290–296. [Google Scholar]
- Hesse, M.; Halbritter, H.; Zetter, R.; Weber, M.; Buchner, R.; Frosch-Radivo, A.; Ulrich, S. Pollen Terminology. An Illustrated Handbook; Oxford Academic: Oxford, UK, 2009; Volume 105. [Google Scholar]
- Halbritter, H.; Ulrich, S.; BGrímsson, F.; Weber, M.; Zetter, R.; Hesse, M.; Buchner, R.; Svojtka, M.; Frosch-Radivo, A. Illustrated Pollen Terminology; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–483. [Google Scholar]
- Kaya, Y.; Erez, M.E.; Karabacak, O.; Kayci, L.; Fidan, M. An Automatic Identification Method for the Comparison of Plant and Honey Pollen Based on GLCM Texture Features and Artificial Neural Network. Grana 2013, 52, 71–77. [Google Scholar] [CrossRef]
- Nabila; Ahmad, M.; Zafar, M.; Bahadur, S.; Sultana, S.; Taj, S.; Celep, F.; Majeed, S.; Rozina. Palynomorphological Diversity among the Asteraceous Honeybee Flora: An Aid to the Correct Taxonomic Identification Using Multiple Microscopic Techniques. Microsc. Res. Tech. 2022, 85, 570–590. [Google Scholar] [CrossRef]
- Attique, R.; Zafar, M.; Ahmad, M.; Zafar, S.; Ghufran, M.A.; Mustafa, M.R.U.; Yaseen, G.; Ahmad, L.; Sultana, S.; Nabila; et al. Pollen Morphology of Selected Melliferous Plants and Its Taxonomic Implications Using Microscopy. Microsc. Res. Tech. 2022, 85, 2361–2380. [Google Scholar] [CrossRef]
- Güner, A.; Vural, M.; Sorkun, K. Rize Florası, Vejetasyonu ve Yöre Ballarının Polen Analizi; TÜBİTAK: Ankara, Turkey, 1987. [Google Scholar]
- Terzioğlu, S. Of-İkizdere-Anzer Vadisi Florası; Karadeniz Teknik Üniversitesi: Trabzon, Turkey, 1994. [Google Scholar]
- Rize İl Tarım ve Orman Müdürlüğü Anzer Balı 2021. Available online: https://ci.turkpatent.gov.tr/Files/GeographicalSigns/d032bdaf-ec73-4769-a8e3-cac9a0cfcacc.pdf (accessed on 17 November 2025).
- Çil, E. A Functional Food: Anzer Honey. In Versatile Approaches to Engineering and Applied Sciences: Materials and Methods; Özgür Yayınları: Gaziantep, Turkey, 2023; pp. 99–105. [Google Scholar]
- Ulusoy, E.; Kolayli, S.; Sarikaya, A.O. Antioxidant and Antimicrobial Activity of Different Floral Origin Honeys from Türkiye. J. Food Biochem. 2010, 34, 321–335. [Google Scholar] [CrossRef]
- Duyum, S.; Friedman, S. The Turkish Beekeeping and Honey Sector; USDA Foreign Agricultural Service: Ankara, Turkey, 2015.
- Sorkun, K.; Doğan, C. Pollen Analysis of Rize-Anzer (Turkish) Honey. Apiacta/Int. Tech. Mag. Apic. Econ. Inf. 1995, 30, 75–82. [Google Scholar]
- Cavdar, S.; Yıldız, O.; Şahin, H.; Karahalil, F.; Kolaylı, S. Comparison of Physical and Biochemical Characteristics of Different Quality of Turkish Honey. Uludağ Arıcılık Dergisi 2013, 13, 55. [Google Scholar]
- Ulusoy, E.; Kolayli, S. Phenolic Composition and Antioxidant Properties of Anzer Bee Pollen. J. Food Biochem. 2014, 38, 73–82. [Google Scholar] [CrossRef]
- Hepsağ, F. Determination of Total Phenolic Compounds and Antioxidant Capacity of Anzer Honey Produced in Rize, Turkey. Gıda J. Food 2019, 44, 641–653. [Google Scholar] [CrossRef]
- Malkoç, M.; Çakir, H.; Kara, Y.; Can, Z.; Kolayli, S. Phenolic Composition and Antioxidant Properties of Anzer Honey from Black Sea Region of Turkey. DergiPark 2019, 19, 143–151. [Google Scholar] [CrossRef]
- Türker, Z.; Coşkunçelebï, K.; Güzel, M.E.; Makbul, S. Improving Floral Authentication of Geographically Labeled Anzer Honey through ITS-Based Metabarcoding and Traditional Melissopalynology. J. Food Compos. Anal. 2025, 148, 108478. [Google Scholar] [CrossRef]
- Tezcan, F.; Kolaylı, S.; Sahin, H.; Ulusoy, E.; Erim, F.B. Evaluation of Organic Acid, Saccharide Composition and Antioxidant Properties of Some Authentic Turkish Honeys. J. Food Nutr. Res. 2011, 50, 33–40. [Google Scholar]
- Özbalci, B.; Boyaci, I.H.; Topcu, A.; Kadilar, C.; Tamer, U. Rapid Analysis of Sugars in Honey by Processing Raman Spectrum Using Chemometric Methods and Artificial Neural Networks. Food Chem. 2013, 136, 1444–1452. [Google Scholar] [CrossRef]
- Hotaman, H.E. Anzer Bal ve Poleninin Bazı Biyoaktif Özelliklerinin in Vitro Olarak Incelenmesi; Recep Tayyip Erdoğan Üniversitesi: Rize, Turkey, 2015. [Google Scholar]
- Sorkun, K.; Doğan, C. The Importance of the Total Number of Pollen Types in 10 Gr of Honey in Distinguishing Natural Honey and Artificial Honey Produced in Turkey. Mellifera 2002, 2, 34. [Google Scholar]
- Özkök, A.; Akel Bilgiç, H.; Kosukcu, C.; Arık, G.; Canlı, D.; Yet, İ.; Karaaslan, C. Comparing the Melissopalynological and next Generation Sequencing (NGS) Methods for the Determining of Botanical Origin of Honey. Food Control 2023, 148, 109630. [Google Scholar] [CrossRef]
- Akman, Y. İklim ve Biyoiklim (Biyoiklim ve Türkiye İklimleri); Kariyer Matbaacılık, Palme Yayınları: Ankara, Turkey, 1999. [Google Scholar]
- Güner, A.; Özhatay, N.; Ekim, T.; Başer, H.C. Flora of Turkey and the East Islands; Edinburgh University Press: Edinburgh, UK, 2000; Volume 11. [Google Scholar]
- Davis, P.H. Flora of Turkey and the East Aegean Islands; Edinburgh University Press: Edinburgh, UK, 1965; Volume 1–10. [Google Scholar]
- WFO. The WFO Plant List. Available online: https://wfoplantlist.org/ (accessed on 17 March 2025).
- GBIF. Available online: https://www.gbif.org/ (accessed on 17 March 2025).
- Güner, A.; Aslan, S.; Ekim, T.; Vural, M.; Babaç, M.T. Türkiye Bitkileri Listesi (Damarlı Bitkiler); Nezahat Gökyiğit Botanik Bahçesi ve Flora Araştırmaları Derneği Yayını: İstanbul, Turkey, 2012. [Google Scholar]
- Erdtman, G. Pollen Morphology and Plant Taxonomy; Almqvist and Wiksells: Uppsala, The Switzerland, 1952. [Google Scholar]
- Kwak, S.G.; Kim, J.H. Central Limit Theorem: The Cornerstone of Modern Statistics. Korean J. Anesthesiol. 2017, 70, 144–156. [Google Scholar] [CrossRef]
- Orme, D. Pyrealm: Ecosystem Models in Python. 2021. Available online: https://pypi.org/project/pyrealm/0.3.0/ (accessed on 17 November 2025).
- Nakagawa, S.; Schielzeth, H. A General and Simple Method for Obtaining R2 from Generalized Linear Mixed-Effects Models. Methods Ecol. Evol. 2013, 4, 133–142. [Google Scholar] [CrossRef]
- Anonymous Ulusal Biyolojik Çeşitlilik Envanter ve İzleme Proje Raporları; Doğa Koruma ve Milli Parklar Genel Müdürlüğü: Ankara, Turkey, 2014.
- Ghosh, S.; Jeon, H.; Jung, C. Foraging Behaviour and Preference of Pollen Sources by Honey Bee (Apis mellifera) Relative to Protein Contents. J. Ecol. Environ. 2020, 44, 4. [Google Scholar] [CrossRef]
- Vaudo, A.D.; Tooker, J.F.; Patch, H.M.; Biddinger, D.J.; Coccia, M.; Crone, M.K.; Fiely, M.; Francis, J.S.; Hines, H.M.; Hodges, M.; et al. Pollen Protein: Lipid Macronutrient Ratios May Guide Broad Patterns of Bee Species Floral Preferences. Insects 2020, 11, 132. [Google Scholar] [CrossRef]
- Özgen, U.; Mavi, A.; Terzi, Z.; Yildirim, A.; Coşkun, M.; Houghton, P.J. Antioxidant Properties of Some Medicinal Lamiaceae (Labiatae) Species. Pharm. Biol. 2006, 44, 107–112. [Google Scholar] [CrossRef]
- Khan, K.; Malik, K.; Ahmad, M.; Qureshi, R.; Aziz, M.A.; Gul, S.; Al-Qahtani, W.H.; Khan, R. Diversity of Melliferous Flora (Apiaries) in Honey and Microscopic Authentication Using LM and SEM Techniques. Flora 2024, 312, 152477. [Google Scholar] [CrossRef]
- Laallam, H.; Rouidja, S.; Bergoug, S.; Tlili, R.; Chenchouni, H. Every Pollen Grain Tells a Story: A Palynological Analysis of Selected Melliferous Plant Species Native to the Sahara Desert with Implications for Honey Origin Determination. Curr. Plant Biol. 2024, 38, 100348. [Google Scholar] [CrossRef]
- García-García, M.C.; Ortiz, P.L.; Díez Dapena, M.J. Variations in the Weights of Pollen Loads Collected by Apis mellifera L. Grana 2004, 43, 183–192. [Google Scholar] [CrossRef]
- Hao, K.; Tian, Z.X.; Wang, Z.C.; Huang, S.Q. Pollen Grain Size Associated with Pollinator Feeding Strategy. Proc. R. Soc. B 2020, 287, 20201191. [Google Scholar] [CrossRef] [PubMed]
- Ejsmond, M.J.; Wrońska-Pilarek, D.; Ejsmond, A.; Dragosz-Kluska, D.; Karpińska-Kołaczek, M.; Kołaczek, P.; Kozłowski, J.A.N. Does Climate Affect Pollen Morphology? Optimal Size and Shape of Pollen Grains under Various Desiccation Intensity. Ecosphere 2011, 2, 1–15. [Google Scholar] [CrossRef]
- Lu, X.; Ye, X.; Liu, J. Morphological Differences between Anemophilous and Entomophilous Pollen. Microsc. Res. Tech. 2022, 85, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Modro, A.F.H.; Message, D.; Da Luz, C.F.P.; Neto, J.A.A.M. Nutritional Composition and Quality of Bee Pollen Collected in Minas Gerais, Brazil. Pesqui. Agropecu. Bras. 2007, 42, 1057–1065. [Google Scholar] [CrossRef]
- González-Suárez, M.; Mora-Olivo, A.; Villanueva-Gutiérrez, R.; Lara-Villalón, M.; Vanoye-Eligio, V.; Guerra-Pérez, A.; González-Suárez, M.; Mora-Olivo, A.; Villanueva-Gutiérrez, R.; Lara-Villalón, M.; et al. Diversity of Melliferous Flora in the State of Tamaulipas, Mexico. Rev. Mex. Cienc. Pecu. 2020, 11, 914–932. [Google Scholar] [CrossRef]
- Amina, H.; Ahmad, M.; Bhatti, G.R.; Zafar, M.; Sultana, S.; Butt, M.A.; Bahadur, S.; Haq, I.U.; Ghufran, M.A.; Lubna; et al. Microscopic Investigation of Pollen Morphology of Brassicaceae from Central Punjab-Pakistan. Microsc. Res. Tech. 2020, 83, 446–454. [Google Scholar] [CrossRef]
- Noor, M.J.; Ahmad, M. Scanning Electron Imaging of Mellitophilous and Allergenic Pollen Grain of Arid and Northern Irrigated Agroecological Zones of Pakistan. Microsc. Res. Tech. 2021, 84, 1834–1861. [Google Scholar] [CrossRef]
- Coh-Martínez, M.E.; Cetzal-Ix, W.; Martínez-Puc, J.F.; Basu, S.K.; Noguera-Savelli, E.; Cuevas, M.J. Perceptions of the Local Beekeepers on the Diversity and Flowering Phenology of the Melliferous Flora in the Community of Xmabén, Hopelchén, Campeche, Mexico. J. Ethnobiol. Ethnomed. 2019, 15, 16. [Google Scholar] [CrossRef]
- Faegri, K.; Van Der Pijl, L. Principles of Pollination Ecology, 3rd ed.; Pergamon Press: Oxford, UK, 2013; Volume 1, ISBN 0-08-021338-3. [Google Scholar]
- Dar, S.A.; Gh Hassan, K.-I.I.; Bilal Padder, K.-I.A.; Ab Wani, K.-I.R.; Sajad Parey, K.-I.H.; Hassan, G.I.; Padder, B.A.; Wani, A.R.; Parey, S.H. Pollination and Evolution of Plant and Insect Interaction. J. Pharmacogn. Phytochem. 2017, 6, 304–311. [Google Scholar]
- Potts, S.G.; Vulliamy, B.; Dafni, A.; Ne’eman, G.; Willmer, P. Linking Bees and Flowers: How Do Floral Communities Structure Pollinator Communities? Ecology 2003, 84, 2628–2642. [Google Scholar] [CrossRef]
- Hegland, S.J.; Nielsen, A.; Lázaro, A.; Bjerknes, A.L.; Totland, Ø. How Does Climate Warming Affect Plant-Pollinator Interactions? Ecol. Lett. 2009, 12, 184–195. [Google Scholar] [CrossRef] [PubMed]
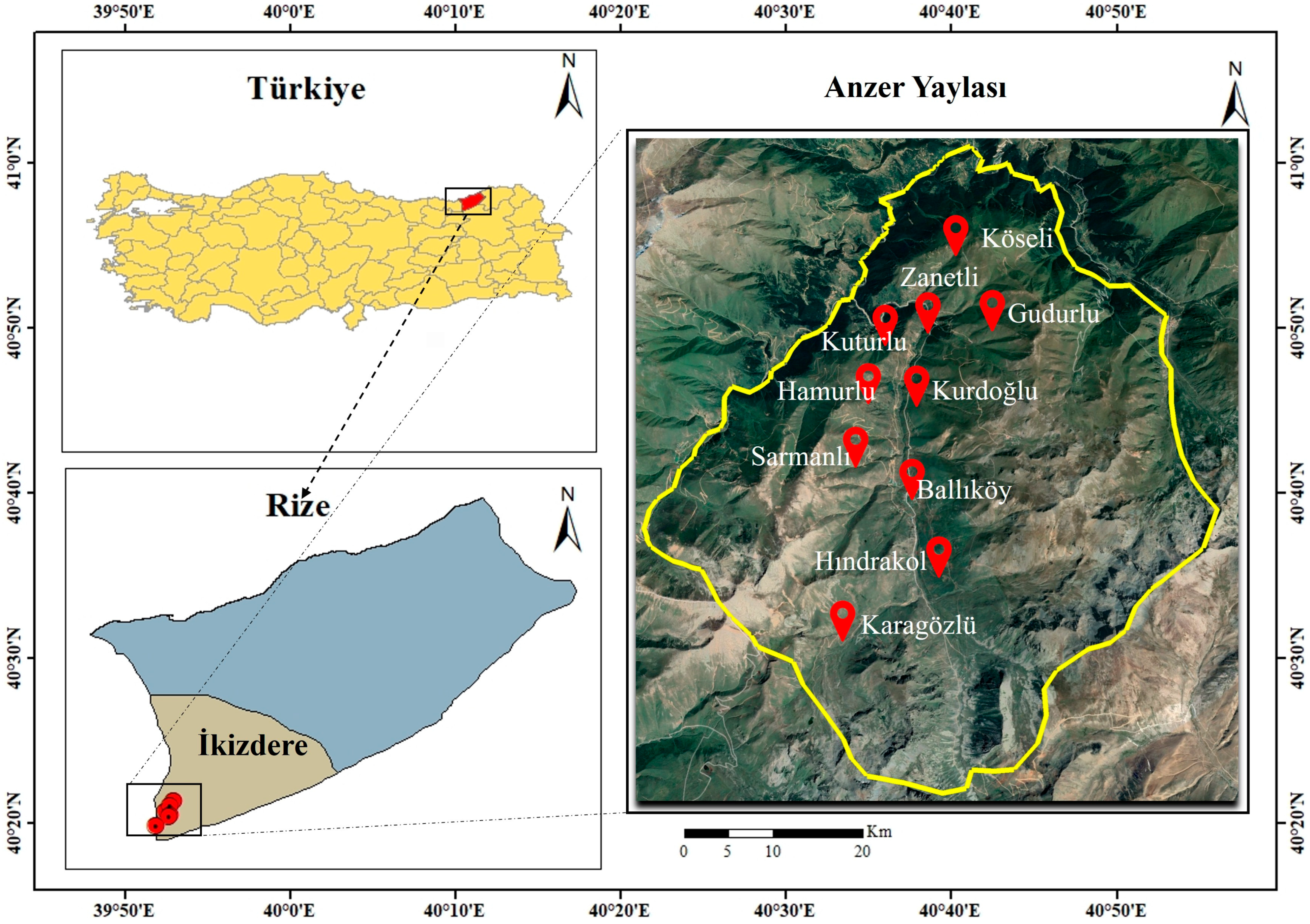



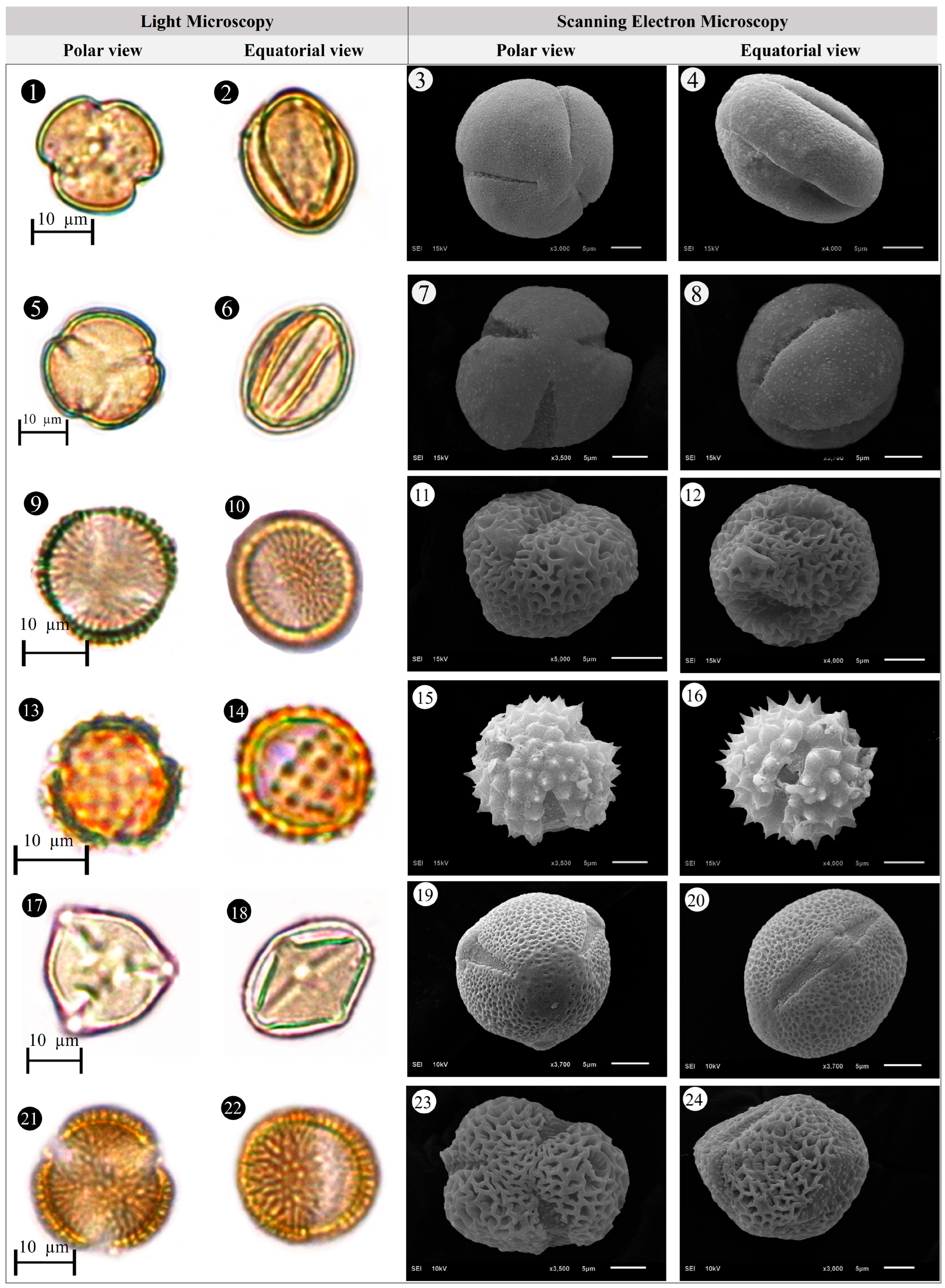
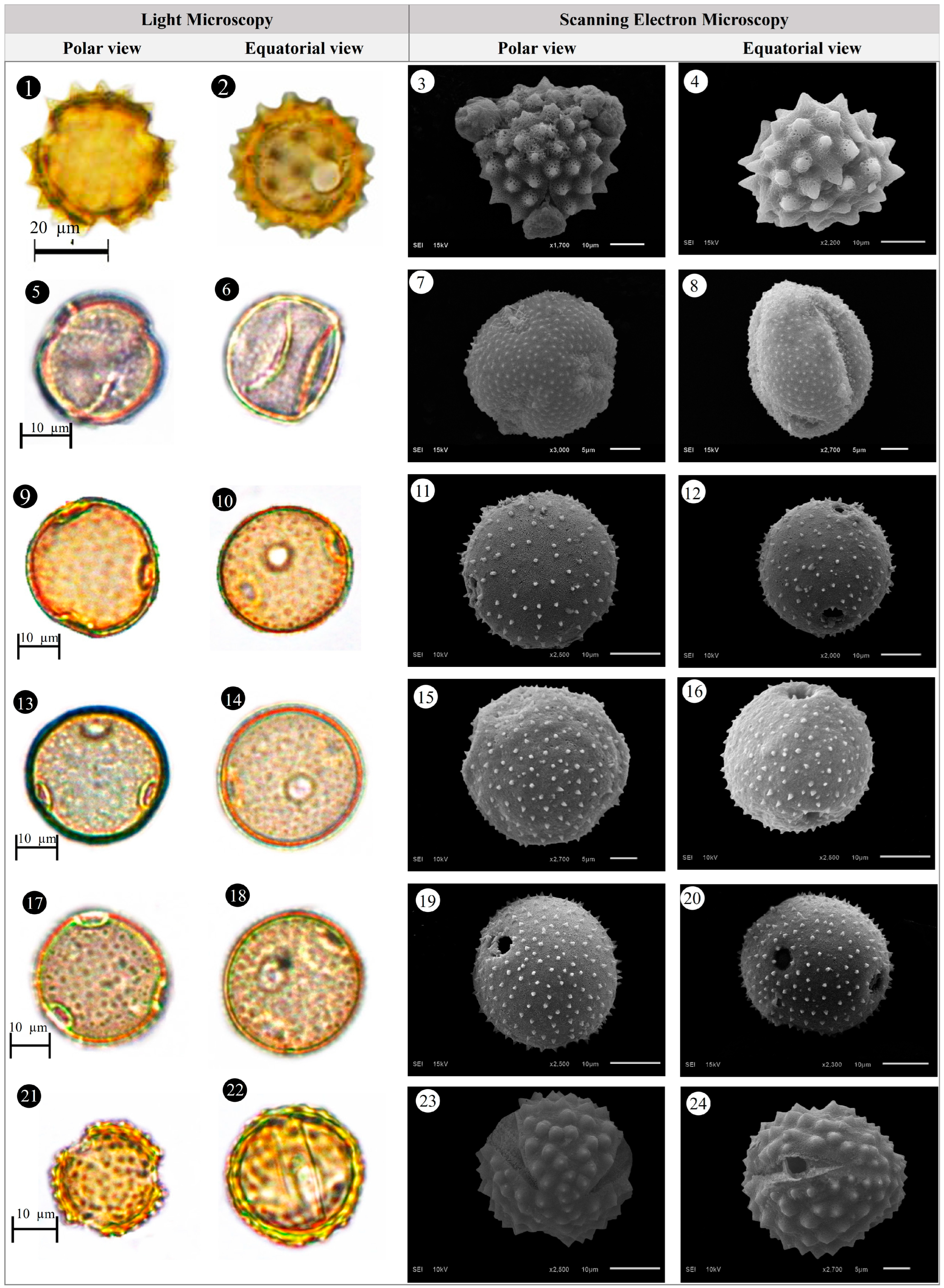
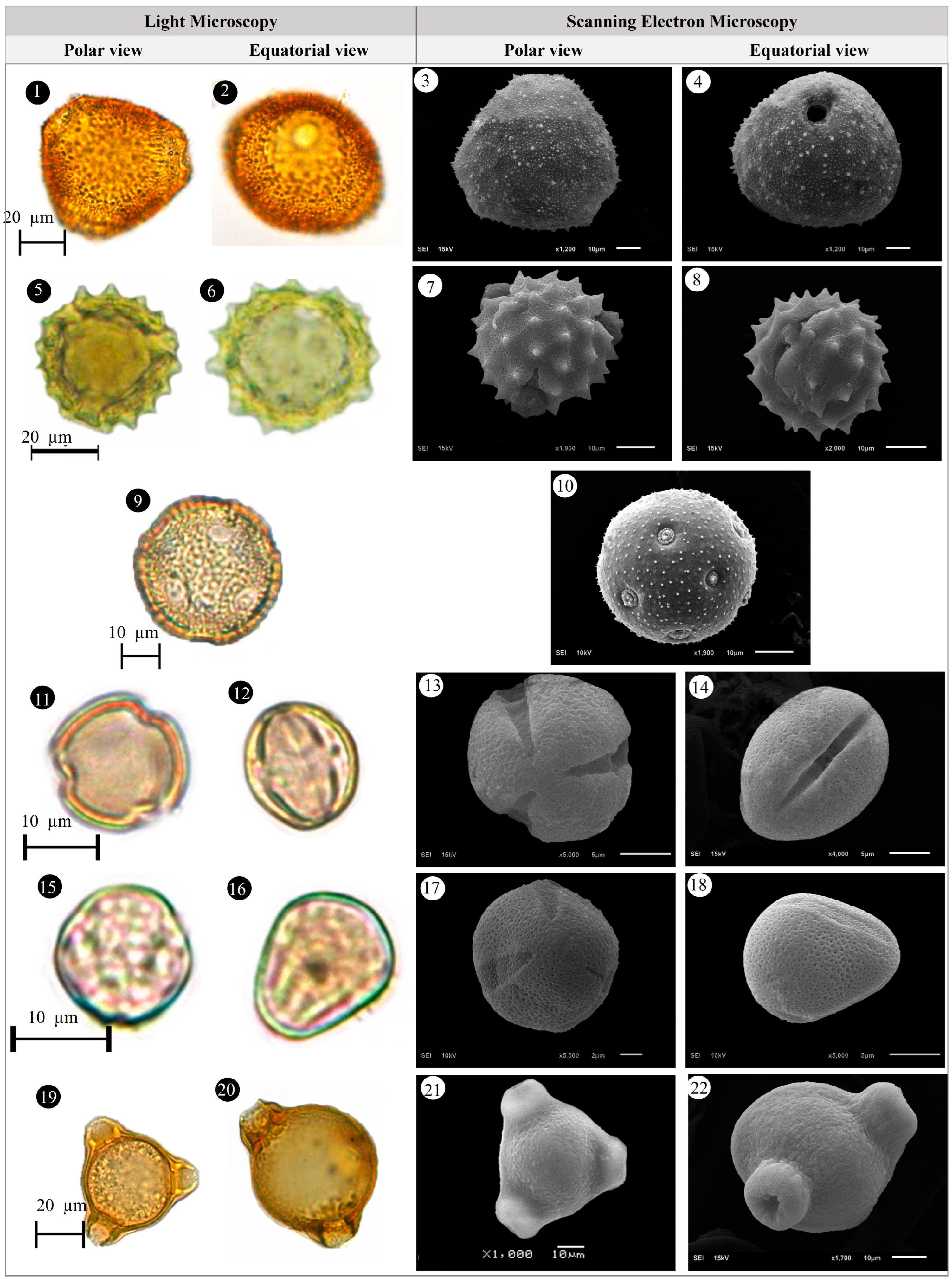
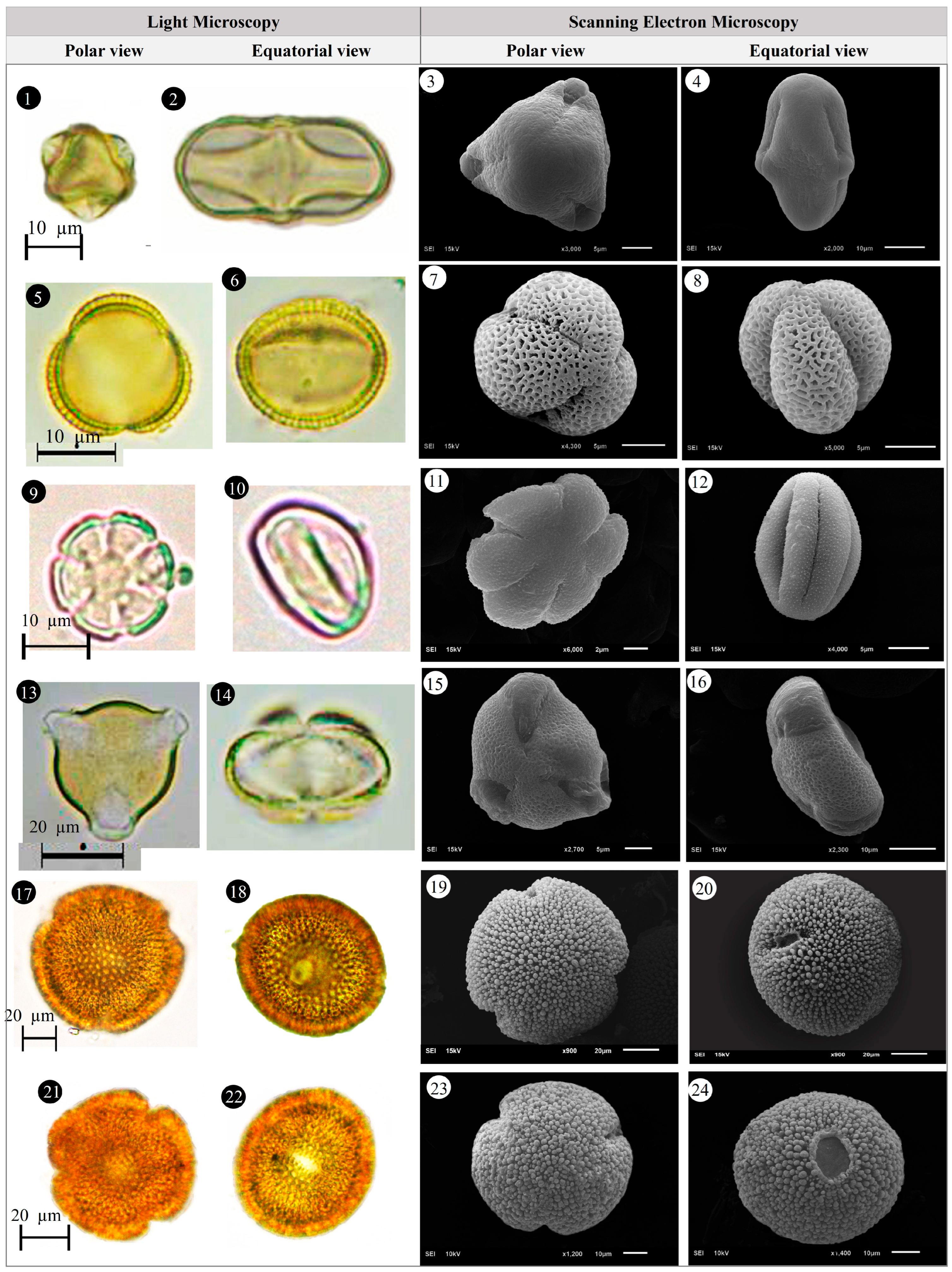
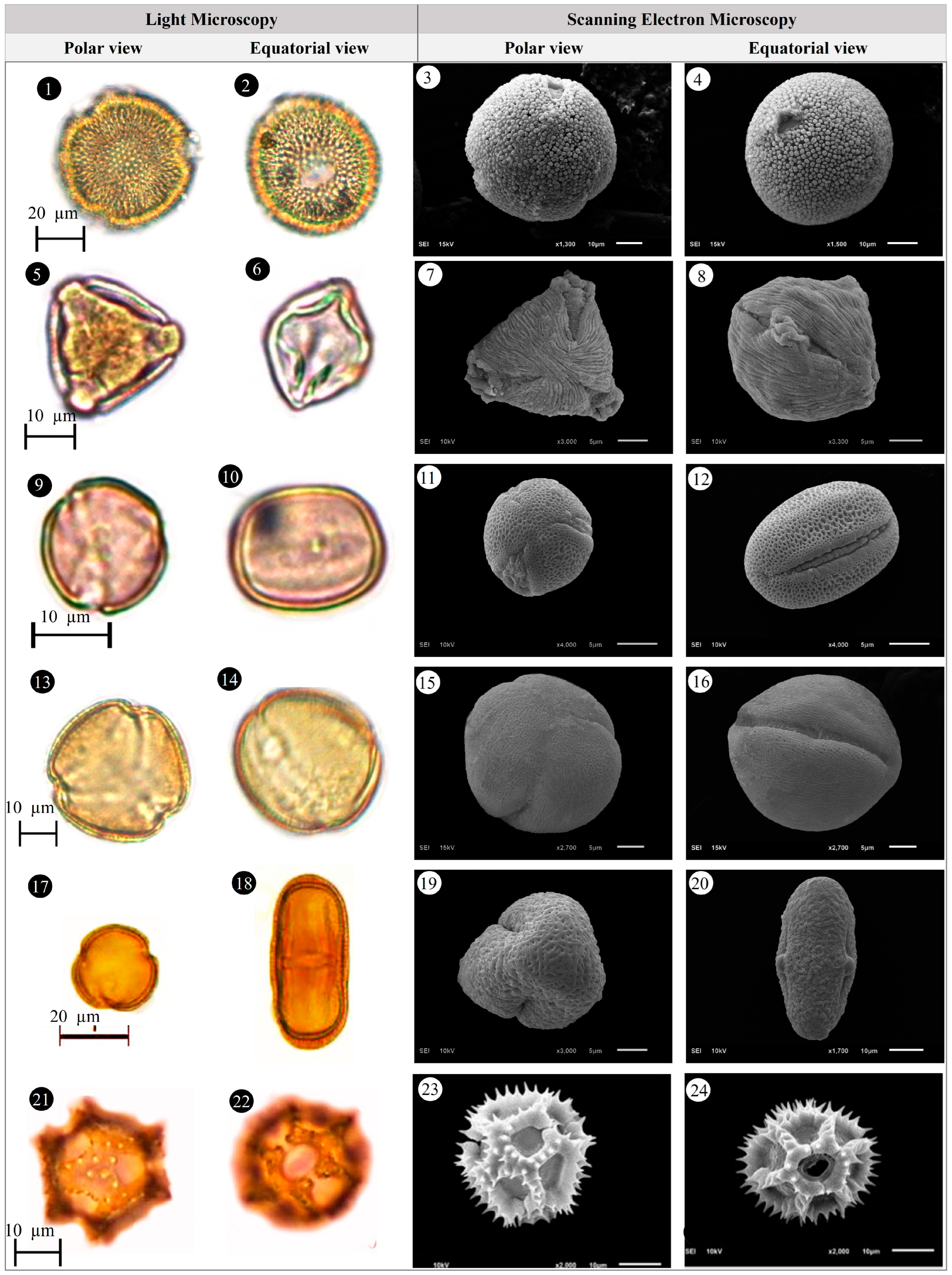
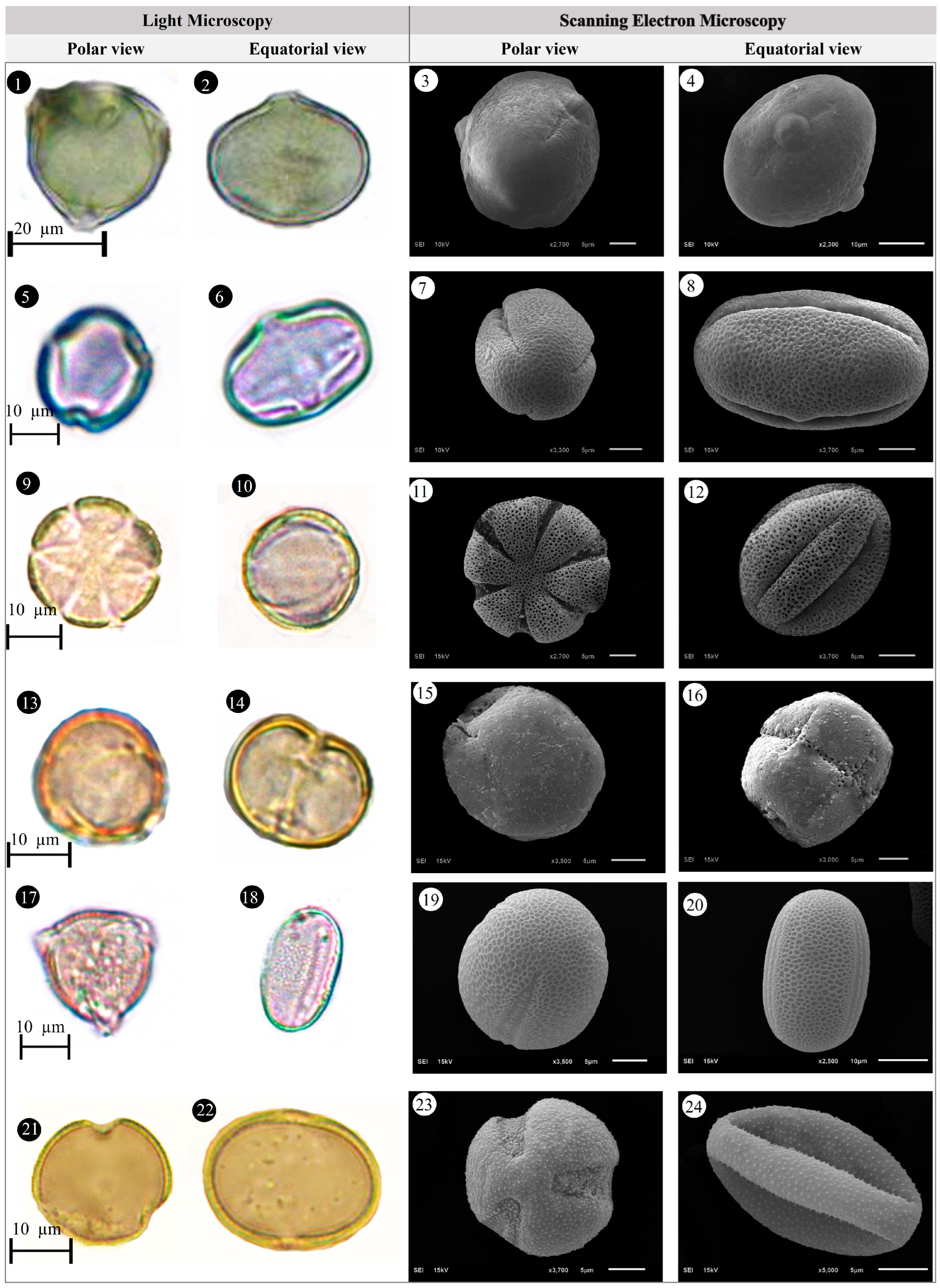
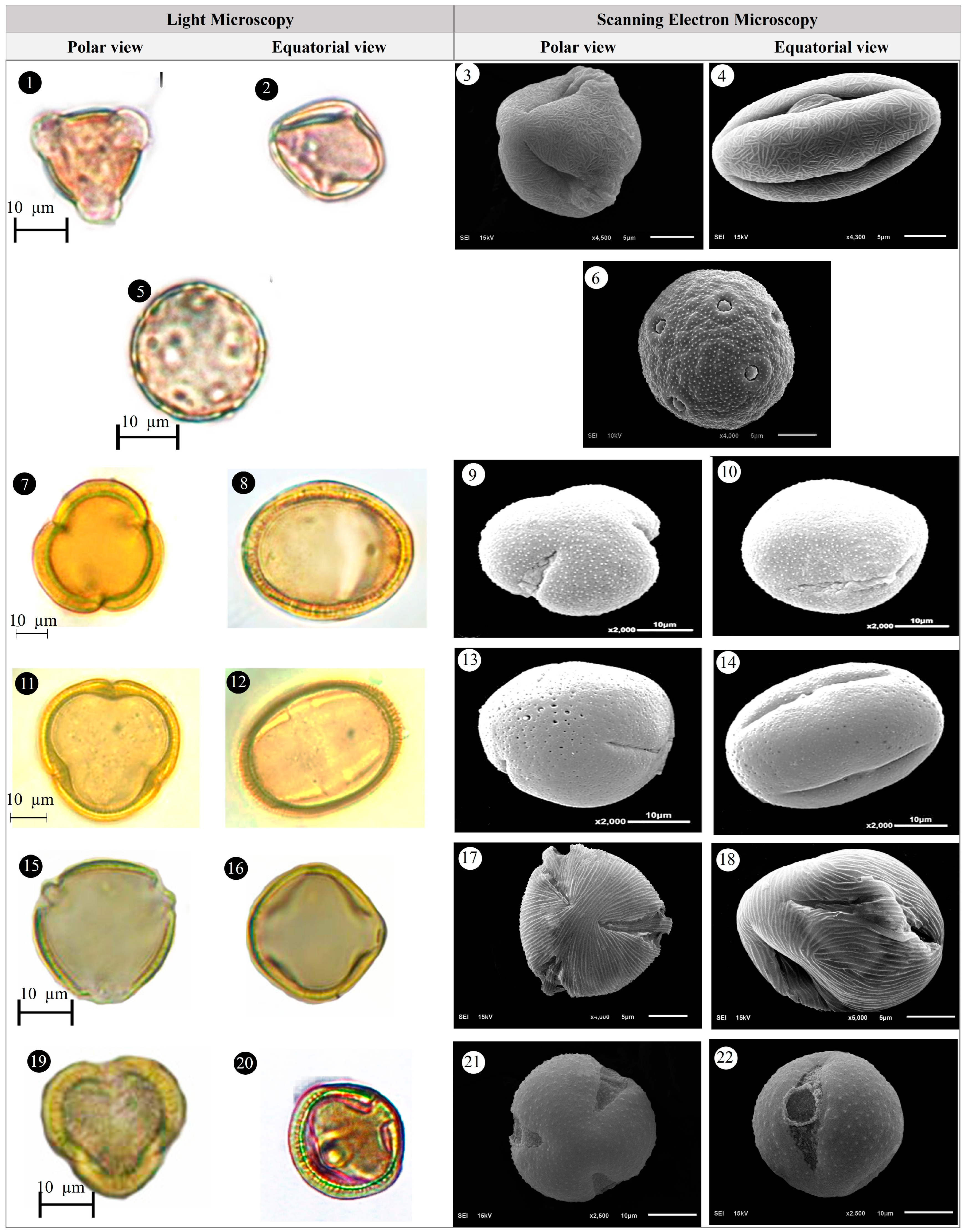
| Family | Taxa | Locality | Altitude (m) | Voucher Number |
|---|---|---|---|---|
| Apiaceae | Eryngium giganteum M.Bieb. | İkizdere, Anzer, Ballıköy | 2169 | Gültepe & Z.Türker 285 (KTUB) |
| Apiaceae | Heracleum platytaenium Boiss. | İkizdere, Anzer, Hındrakol | 2265 | Coşkunçelebi & Z.Türker 81 (KTUB) |
| Asteraceae | Aster alpinus L. | İkizdere, Anzer, Ballıköy | 2706 | Gültepe & Z.Türker 267 (KTUB) |
| Asteraceae | Carduus adpressus C.A.Mey. | İkizdere, Anzer, Hındrakol | 2190 | Coşkunçelebi & Z.Türker 109 (KTUB) |
| Asteraceae | Centaurea helenioides Boiss. | İkizdere, Anzer, Kurdoğlu | 2487 | Gültepe & Z.Türker 258 (KTUB) |
| Asteraceae | Cirsium pseudopersonata Boiss. & Balansa | İkizdere, Anzer, Hındrakol | 2416 | Coşkunçelebi & Z.Türker 151 (KTUB) |
| Asteraceae | Lactuca racemosa Willd. | İkizdere, Anzer, Ballıköy | 2136 | Coşkunçelebi & Z.Türker 158 (KTUB) |
| Asteraceae | Psephellus pulcherrimus (Willd.) Wagenitz | İkizdere, Anzer, Hındrakol | 2190 | Coşkunçelebi & Z.Türker 114 (KTUB) |
| Asteraceae | Solidago virgaurea subsp. alpestris (Waldst. & Kit.) Gaudin | İkizdere, Anzer, Hındrakol | 2416 | Coşkunçelebi & Z.Türker 140 (KTUB) |
| Asteraceae | Taraxacum serotinum (Waldst. & Kit.) Poir. | İkizdere, Anzer, Hındrakol | 2221 | Coşkunçelebi & Z.Türker 13 (KTUB) |
| Boraginaceae | Echium vulgare L. | İkizdere, Anzer, Hındrakol | 2238 | Coşkunçelebi & Z.Türker 186 (KTUB) |
| Boraginaceae | Nonea versicolor (Steven) Sweet | İkizdere, Anzer, Hındrakol | 2265 | Coşkunçelebi & Z.Türker 73 (KTUB) |
| Brassicaceae | Arabis hirsuta (L.) Scop. | İkizdere, Anzer, Hındrakol | 2265 | Coşkunçelebi & Z.Türker 38 (KTUB) |
| Brassicaceae | Bunias orientalis L. | İkizdere, Anzer, Karagözlü | 2460 | Coşkunçelebi & Z.Türker 131 (KTUB) |
| Brassicaceae | Erysimum pulchellum (Willd.) J.Gay | İkizdere, Anzer, Hındrakol | 2265 | Coşkunçelebi & Z.Türker 39 (KTUB) |
| Campanulaceae | Campanula alliariifolia Willd. | İkizdere, Anzer, Hamurlu | 2455 | Coşkunçelebi & Z.Türker 137 (KTUB) |
| Campanulaceae | Campanula glomerata L. | İkizdere, Anzer, Hamurlu | 2455 | Coşkunçelebi & Z.Türker 138 (KTUB) |
| Campanulaceae | Campanula olympica Boiss. | İkizdere, Anzer, Köseli | 1933 | Coşkunçelebi & Z.Türker 129 (KTUB) |
| Caprifoliaceae | Cephalaria gigantea (Ledeb.) Bobrov | İkizdere, Anzer, Sarmanlı | 2518 | Gültepe & Z.Türker 246 (KTUB) |
| Caprifoliaceae | Scabiosa caucasica M.Bieb. | İkizdere, Anzer, Ballıköy | 2136 | Coşkunçelebi & Z.Türker 154 (KTUB) |
| Caryophyllaceae | Dianthus carmelitarum Reut. ex Boiss. | İkizdere, Anzer, Karagözlü | 2460 | Coşkunçelebi & Z.Türker 132 (KTUB) |
| Cistaceae | Helianthemum nummularium (L.) Mill. | İkizdere, Anzer, Hındrakol | 2280 | Gültepe & Z.Türker 253 (KTUB) |
| Crassulaceae | Phedimus spurius (M.Bieb.) ’t Hart | İkizdere, Anzer, Hındrakol | 2587 | Gültepe & Z.Türker 230 (KTUB) |
| Ericaceae | Rhododendron caucasicum Pall | İkizdere, Anzer, Hındrakol | 2238 | Coşkunçelebi & Z.Türker 185 (KTUB) |
| Fabaceae | Astragalus fricki Bunge | İkizdere, Anzer, Kuturlu | 2108 | Coşkunçelebi & Z.Türker 19 (KTUB) |
| Fabaceae | Genista albida Willd. | İkizdere, Anzer, Ballıköy | 2148 | Gültepe & Z.Türker 284 (KTUB) |
| Fabaceae | Hedysarum hedysaroides (L.) Schinz & Thell. | İkizdere, Anzer, Ballıköy | 2650 | Gültepe & Z.Türker 264 (KTUB) |
| Fabaceae | Lathyrus roseus Steven | İkizdere, Anzer, Ballıköy | 2156 | Gültepe & Z.Türker 282 (KTUB) |
| Fabaceae | Melilotus officinalis (L.) Desr. | İkizdere, Anzer, Ballıköy | 2156 | Gültepe & Z.Türker 283 (KTUB) |
| Fabaceae | Onobrychis altissima Grossh. | İkizdere, Anzer, Hındrakol | 2341 | Gültepe & Z.Türker 273 (KTUB) |
| Fabaceae | Securigera orientalis (Mill.) Lassen | İkizdere, Anzer, Kurdoğlu | 2487 | Gültepe & Z.Türker 259 (KTUB) |
| Fabaceae | Securigera varia (L.) Lassen | İkizdere, Anzer, Sarmanlı | 2416 | Coşkunçelebi & Z.Türker 170 (KTUB) |
| Fabaceae | Trifolium ambiguum M.Bieb. | İkizdere, Anzer, Hındrakol | 2341 | Gültepe & Z.Türker 275 (KTUB) |
| Fabaceae | Trifolium canescens Willd. | İkizdere, Anzer, Sarmanlı | 2518 | Gültepe & Z.Türker 244 (KTUB) |
| Fabaceae | Trifolium pratense L. | İkizdere, Anzer, Sarmanlı | 2518 | Gültepe & Z.Türker 248 (KTUB) |
| Fabaceae | Trifolium spadiceum L. | İkizdere, Anzer, Hındrakol | 2190 | Coşkunçelebi & Z.Türker 110 (KTUB) |
| Fabaceae | Vicia balansae Boiss. | İkizdere, Anzer, Hındrakol | 2265 | Coşkunçelebi & Z.Türker 54 (KTUB) |
| Fabaceae | Vicia cracca subsp. cracca L. | İkizdere, Anzer, Hındrakol | 2115 | Coşkunçelebi & Z.Türker 187 (KTUB) |
| Gentianaceae | Swertia iberica Fisch. ex C.A.Mey. | İkizdere, Anzer, Hındrakol | 2239 | Coşkunçelebi & Z.Türker 203 (KTUB) |
| Geraniaceae | Geranium ibericum subsp. jubatum (Hand.-Mazz.) P.H.Davis | İkizdere, Anzer, Hındrakol | 2265 | Coşkunçelebi & Z.Türker 77 (KTUB) |
| Geraniaceae | Geranium ponticum (P.H.Davis & J.Roberts) Aedo | İkizdere, Anzer, Hındrakol | 2626 | Coşkunçelebi & Z.Türker 87 (KTUB) |
| Geraniaceae | Geranium pyrenaicum Burm.f. | İkizdere, Anzer, Hamurlu | 2455 | Coşkunçelebi & Z.Türker 125 (KTUB) |
| Lamiaceae | Ajuga orientalis L. | İkizdere, Anzer, Ballıköy | 2567 | Gültepe & Z.Türker 270 (KTUB) |
| Lamiaceae | Mentha longifolia (L.) L. | İkizdere, Anzer, Hındrakol | 2238 | Coşkunçelebi & Z.Türker 180 (KTUB) |
| Lamiaceae | Salvia verticillata L. | İkizdere, Anzer, Sarmanlı | 2518 | Gültepe & Z.Türker 249 (KTUB) |
| Lamiaceae | Stachys macrantha (K.Koch) Stearn | İkizdere, Anzer, Kurdoğlu | 2096 | Coşkunçelebi & Z.Türker 96 (KTUB) |
| Lamiaceae | Teucrium chamaedrys L. | İkizdere, Anzer, Hındrakol | 2238 | Coşkunçelebi & Z.Türker 182 (KTUB) |
| Lamiaceae | Thymus praecox Opiz | İkizdere, Anzer, Hındrakol | 2190 | Coşkunçelebi & Z.Türker 105 (KTUB) |
| Onagraceae | Epilobium angustifolium L. | İkizdere, Anzer, Hındrakol | 2238 | Coşkunçelebi & Z.Türker 179 (KTUB) |
| Papaveraceae | Papaver lateritium K.Koch | İkizdere, Anzer, Ballıköy | 2536 | Gültepe & Z.Türker 272 (KTUB) |
| Plantaginaceae | Digitalis ferruginea L. | İkizdere, Anzer, Karagözlü | 2460 | Coşkunçelebi & Z.Türker 135 (KTUB) |
| Plantaginaceae | Plantago lanceolata L. | İkizdere, Anzer, Sarmanlı | 2518 | Gültepe & Z.Türker 243 (KTUB) |
| Polygonaceae | Polygonum alpinum All. | İkizdere, Anzer, Hındrakol | 2265 | Coşkunçelebi & Z.Türker 50 (KTUB) |
| Polygonaceae | Polygonum bistorta subsp. carneum (K.Koch) Coode & Cullen | İkizdere, Anzer, Hındrakol | 2265 | Coşkunçelebi & Z.Türker 52 (KTUB) |
| Polygonaceae | Rumex alpinus L. | İkizdere, Anzer, Kurdoğlu | 2096 | Coşkunçelebi & Z.Türker 94 (KTUB) |
| Ranumculaceae | Anemone narcissiflora L. | İkizdere, Anzer, Kuturlu | 2108 | Coşkunçelebi & Z.Türker 22 (KTUB) |
| Ranumculaceae | Caltha palustris L. | İkizdere, Anzer, Kuturlu | 2108 | Coşkunçelebi & Z.Türker 21 (KTUB) |
| Ranumculaceae | Trollius ranunculinus (Sm.) Stearn | İkizdere, Anzer, Hındrakol | 2265 | Coşkunçelebi & Z.Türker 58 (KTUB) |
| Rosaceae | Geum aleppicum Jacq. | İkizdere, Anzer, Hındrakol | 2248 | Gültepe & Z.Türker 281 (KTUB) |
| Rosaceae | Potentilla crantzii (Crantz) Fritsch | İkizdere, Anzer, Karagöz | 2412 | Coşkunçelebi & Z.Türker 06 (KTUB) |
| Rosaceae | Prunus divaricata Ledeb. | İkizdere, Anzer, Kuturlu | 2108 | Coşkunçelebi & Z.Türker 20 (KTUB) |
| Rosaceae | Rosa canina L. | İkizdere, Anzer, Hındrakol | 2190 | Coşkunçelebi & Z.Türker 102 (KTUB) |
| Rosaceae | Rubus idaeus L. | İkizdere, Anzer, Hındrakol | 2190 | Coşkunçelebi & Z.Türker 101 (KTUB) |
| Rubiaceae | Galium verum L. | İkizdere, Anzer, Hındrakol | 2238 | Coşkunçelebi & Z.Türker 175 (KTUB) |
| Family | Taxa | P ± SD (μm) | E ± SD (μm) | P/E | Shape | Pollen Size | Aperture | Exine Ornamentation |
|---|---|---|---|---|---|---|---|---|
| Apiaceae | Eryngium giganteum M.Bieb. * | 38.89 ± 3.72 | 19.40 ± 2.08 | 2 | Prolate | Medium | Tricolporate | Verrucate–scabrate |
| Apiaceae | Heracleum platytaenium Boiss. | 47.02 ± 3.63 | 21.98 ± 2.07 | 2.14 | Prolate | Medium | Tricolporate | Rugulate–perforate |
| Asteraceae | Aster alpinus L. | 19.42 ± 1.58 | 18.30 ± 1.62 | 1.06 | Spheroidal | Small | Tricolporate | Echinate-perforate |
| Asteraceae | Carduus adpressus C.A.Mey. | 33.32 ± 2.27 | 35.04 ± 2.39 | 0.95 | Spheroidal | Medium | Tricolporate | Echinate |
| Asteraceae | Centaurea helenioides Boiss. *† | 33.05 ± 2.05 | 32.59 ± 1.94 | 1.01 | Spheroidal | Medium | Tricolporate | Echinate |
| Asteraceae | Cirsium pseudopersonata Boiss. & Balansa † | 36.91 ± 3.11 | 41.47 ± 3.43 | 0.89 | Oblate | Medium | Tricolporate | Echinate |
| Asteraceae | Lactuca racemosa Willd. | 29.91 ± 3.55 | 32.93 ± 3.68 | 0.91 | Oblate | Medium | Tricolporate | Echinate–microreticulate |
| Asteraceae | Psephellus pulcherrimus (Willd.) Wagenitz * | 31.95 ± 1.72 | 31.47 ± 1.75 | 1.02 | Spheroidal | Medium | Tricolporate | Microechinate-perforate |
| Asteraceae | Solidago virgaurea subsp. alpestris (Waldst. & Kit.) Gaudin * | 21.89 ± 1.57 | 22.11 ± 1.74 | 0.99 | Spheroidal | Small | Tricolporate | Echinate–perforate |
| Asteraceae | Taraxacum serotinum (Waldst. & Kit.) Poir. | 24.86 ± 1.33 | 24.84 ± 1.26 | 1 | Spheroidal | Small | Tricolporate | Echinate–perforate |
| Boraginaceae | Echium vulgare L. | 15.76 ± 1.11 | 13.05 ± 1.19 | 1.21 | Prolate | Small | Tricolporate | Reticulate–perforate |
| Boraginaceae | Nonea versicolor (Steven) Sweet * | 26.26 ± 1.55 | 22.53 ± 2.79 | 1.17 | Prolate | Medium | Tetracolporate | Psilate–perforate |
| Brassicaceae | Arabis hirsuta (L.) Scop. | 21.38 ± 1.04 | 21.37 ± 1.30 | 1 | Spheroidal | Small | Tricolpate | Reticulate |
| Brassicaceae | Bunias orientalis L. | 22.35 ± 1.29 | 23.71 ± 1.66 | 0.94 | Spheroidal | Small | Tricolpate | Reticulate |
| Brassicaceae | Erysimum pulchellum (Willd.) J.Gay * | 18.43 ± 1.73 | 18.33 ± 1.74 | 1.01 | Spheroidal | Small | Tricolpate | Reticulate |
| Campanulaceae | Campanula alliariifolia Willd. * | 32.41 ± 1.61 | 33.96 ± 1.58 | 0.95 | Spheroidal | Medium | Triporate | Rugulate |
| Campanulaceae | Campanula glomerata L. | 33.55 ± 3.90 | 34.78 ± 3.32 | 0.96 | Spheroidal | Medium | Triporate | Rugulate |
| Campanulaceae | Campanula olympica Boiss. | 31.11 ± 3.04 | 31.95 ± 2.30 | 0.97 | Spheroidal | Medium | Triporate | Rugulate |
| Caprifoliaceae | Cephalaria gigantea (Ledeb.) Bobrov * | 68.02 ± 2.12 | 58.79 ± 2.17 | 1.16 | Prolate | Large | Triporate | Microechinate–echinate–perforate |
| Caprifoliaceae | Scabiosa caucasica M.Bieb. | 51.27 ± 1.62 | 92.84 ± 1.12 | 0.55 | Oblate | Large | Triporate | Microechinate–echinate–perforate |
| Caryophyllaceae | Dianthus carmelitarum Reut. ex Boiss. † | 38.92 ± 2.31 | 38.83 ± 2.22 | 1 | Spheroidal | Medium | Pantoporate | Microechinate–perforate |
| Cistaceae | Helianthemum nummularium (L.) Mill. * | 36.94 ± 4.13 | 34.7 ± 3.92 | 1.08 | Prolate | Medium | Tricolporate | Striate–perforate |
| Crassulaceae | Phedimus spurius (M.Bieb.) ’t Hart | 25.29 ± 1.32 | 19.18 ± 1.54 | 1.32 | Prolate | Small | Tricolporate | Rugulate–striate |
| Ericaceae | Rhododendron caucasicum Pall * | 57.59 ± 5.64 | 59.10 ± 4.51 | 0.97 | Spheroidal | Large | Tricolporate | Verrucate–Rugulate |
| Fabaceae | Astragalus fricki Bunge * | 26.86 ± 1.12 | 22.29 ± 1.15 | 1.21 | Prolate | Medium | Tricolporate | Reticulate |
| Fabaceae | Genista albida Willd. | 25.09 ± 3.99 | 28.83 ± 1.88 | 0.87 | Oblate | Medium | Tricolporate | Microreticulate |
| Fabaceae | Hedysarum hedysaroides (L.) Schinz & Thell. | 20.62 ± 1.71 | 16.22 ± 2.16 | 1.27 | Prolate | Small | Tricolpate | Reticulate |
| Fabaceae | Lathyrus roseus Steven * | 35.87 ± 1.97 | 29.60 ± 2.16 | 1.21 | Prolate | Medium | Tricolporate | Reticulate–perforate |
| Fabaceae | Melilotus officinalis (L.) Desr. | 25.06 ± 1.20 | 19.20 ± 0.94 | 1.31 | Prolate | Small | Tricolporate | Reticulate–perforate |
| Fabaceae | Onobrychis altissima Grossh. | 34.22 ± 2.79 | 20.75 ± 2.83 | 1.65 | Prolate | Medium | Tricolpate | Suprareticulate |
| Fabaceae | Securigera orientalis (Mill.) Lassen * | 20.48 ± 1.44 | 17.99 ± 1.57 | 1.14 | Prolate | Small | Tricolporate | Rugulate–perforate |
| Fabaceae | Securigera varia (L.) Lassen | 25.20 ±2.19 | 19.83 ± 2.36 | 1.27 | Prolate | Small | Tricolporate | Rugulate–perforate |
| Fabaceae | Trifolium ambiguum M.Bieb. * | 24.97 ± 1.11 | 19.60 ± 1.28 | 1.27 | Prolate | Small | Tricolporate | Reticulate–perforate |
| Fabaceae | Trifolium canescens Willd. * | 42.69 ± 4.66 | 35.83 ± 4.48 | 1.19 | Prolate | Medium | Tricolporate | Reticulate–perforate |
| Fabaceae | Trifolium pratense L. | 36.42 ± 3.52 | 32.41 ± 3.29 | 1.12 | Prolate | Medium | Tricolporate | Reticulate–perforate |
| Fabaceae | Trifolium spadiceum L. * | 21.74 ± 1.87 | 20.89 ± 1.68 | 1.04 | Spheroidal | Small | Tricolporate | Psilate–perforate |
| Fabaceae | Vicia balansae Boiss. * | 34.85 ± 1.86 | 23.90 ± 1.72 | 1.46 | Prolate | Medium | Tricolporate | Verrucate–perforate |
| Fabaceae | Vicia cracca subsp. cracca L. | 35.47 ± 2.40 | 23.50 ± 2.25 | 1.51 | Prolate | Medium | Tricolporate | Verrucate–perforate |
| Gentianaceae | Swertia iberica Fisch. ex C.A.Mey. * | 27.60 ± 1.36 | 26.24 ± 1.32 | 1.05 | Spheroidal | Medium | Tricolporate | Striato–microreticulate |
| Geraniaceae | Geranium ibericum subsp. jubatum (Hand.–Mazz.) P.H.Davis * † | 92.88 ± 4.18 | 84.79 ± 3.67 | 1.1 | Prolate | Large | Tricolporate | Reticulate–clavate |
| Geraniaceae | Geranium ponticum (P.H.Davis & J.Roberts) Aedo * † | 65.59 ± 7.19 | 60.99 ± 6.29 | 1.08 | Prolate | Large | Tricolporate | Reticulate–clavate |
| Geraniaceae | Geranium pyrenaicum Burm.f. | 56.72 ± 2.66 | 52.55 ± 2.19 | 1.08 | Prolate | Large | Tricolporate | Reticulate–clavate |
| Lamiaceae | Ajuga orientalis L. | 23.47 ± 2.32 | 19.04 ± 1.30 | 1.23 | Prolate | Small | Tricolpate | Granulate |
| Lamiaceae | Mentha longifolia (L.) L. | 23.68 ± 2.22 | 21.25 ± 1.87 | 1.11 | Prolate | Small | Hexacolpate | Microreticulate |
| Lamiaceae | Salvia verticillata L. | 21.26 ± 2.93 | 19.88 ± 2.71 | 1.07 | Prolate | Small | Hexacolpate | Microreticulate–reticulate–bireticulate |
| Lamiaceae | Stachys macrantha (K.Koch) Stearn * | 30.82 ± 3.32 | 27.17 ± 4.01 | 1.13 | Prolate | Medium | Tricolpate | Reticulate–perforate |
| Lamiaceae | Teucrium chamaedrys L. | 23.81 ± 2.14 | 23.81 ± 2.26 | 1 | Spheroidal | Small | Tricolpate | Verrucate–perforate |
| Lamiaceae | Thymus praecox Opiz | 25.37 ± 2.01 | 27.41 ± 2.86 | 0.93 | Oblate | Medium | Hexacolpate | Bireticulate |
| Onagraceae | Epilobium angustifolium L. | 48.65 ± 4.74 | 64.16 ± 4.03 | 0.76 | Oblate | Large | Triporate | Microgemmate–perforate |
| Papaveraceae | Papaver lateritium K.Koch *† | 22.46 ± 1.93 | 17.82 ± 3.00 | 1.26 | Prolate | Small | Tricolpate | Microechinate–perforate |
| Plantaginaceae | Digitalis ferruginea L. * | 20.14 ± 1.28 | 18.26 ± 1.40 | 1.1 | Prolate | Small | Tricolporate | Microreticulate |
| Plantaginaceae | Plantago lanceolata L. | 22.96 ± 1.03 | 22.38 ± 1.06 | 1.03 | Spheroidal | Small | Pantoporate | Verrucate–microechinate |
| Polygonaceae | Polygonum alpinum All. | 30.17 ± 1.77 | 26.90 ± 1.98 | 1.12 | Prolate | Medium | Tricolpate | Microechinate–perforate |
| Polygonaceae | Polygonum bistorta subsp. carneum (K.Koch) Coode & Cullen | 43.16 ± 4.37 | 32.70 ± 3.23 | 1.32 | Prolate | Medium | Tricolporate | Microechinate–perforate |
| Polygonaceae | Rumex alpinus L. | 15.06 ± 1.35 | 13.96 ± 1.69 | 1.08 | Prolate | Small | Tricolporate | Microechinate–perforate |
| Ranumculaceae | Anemone narcissiflora L. | 25.91 ± 1.83 | 21.70 ± 2.10 | 1.19 | Prolate | Medium | Tricolpate | Microechinate–perforate |
| Ranumculaceae | Caltha palustris L. | 27.34 ± 2.06 | 25.71 ± 2.84 | 1.06 | Spheroidal | Medium | Tricolpate | Microechinate–perforate |
| Ranumculaceae | Trollius ranunculinus (Sm.) Stearn | 20.98 ± 2.96 | 20.77 ± 3.79 | 1.01 | Spheroidal | Small | Tricolpate | Striate–perforate |
| Rosaceae | Geum aleppicum Jacq. * | 25.63 ± 3.21 | 22.50 ± 2.98 | 1.14 | Prolate | Medium | Tricolporate | Striate–perforate |
| Rosaceae | Potentilla crantzii (Crantz) Fritsch * | 20.60 ± 1.45 | 19.62 ± 2.34 | 1.05 | Spheroidal | Small | Tricolporate | Striate–perforate |
| Rosaceae | Prunus divaricata Ledeb. * | 30.69 ± 2.03 | 27.30 ± 2.97 | 1.12 | Prolate | Medium | Tricolporate | Striate–perforate |
| Rosaceae | Rosa canina L. | 24.68 ± 2.35 | 18.60 ± 2.08 | 1.33 | Prolate | Small | Tricolporate | Striate–perforate |
| Rosaceae | Rubus idaeus L. | 21.03 ± 1.46 | 18.10 ± 1.69 | 1.16 | Prolate | Small | Tricolporate | Striate–perforate |
| Rubiaceae | Galium verum L. | 18.81 ± 1.99 | 17.10 ± 2.36 | 1.1 | Prolate | Small | Stephanocolpate | Microechinate–perforate |
| Index | Coef (β) | SE | z | p-Value | 95% CI Lower | 95% CI Upper | Var (Species) | R2 Marginal | R2 Conditional |
|---|---|---|---|---|---|---|---|---|---|
| Intercept | 17.07 | 1.17 | 14.65 | <0.001 | 14.78 | 19.35 | 65.61 | 0.4641 | 0.9621 |
| Equatorial Diameter | 0.50 | 0.02 | 25.08 | <0.001 | 0.46 | 0.53 | 65.61 | 0.4641 | 0.9621 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Türker, Z.; Coşkunçelebi, K.; Demir Kanbur, E.; Gültepe, M. Significance of Morpho-Palynological Diversity in Melliferous Plants in the Anzer Region (Türkiye) with Regard to Honey Authentication. Plants 2025, 14, 3600. https://doi.org/10.3390/plants14233600
Türker Z, Coşkunçelebi K, Demir Kanbur E, Gültepe M. Significance of Morpho-Palynological Diversity in Melliferous Plants in the Anzer Region (Türkiye) with Regard to Honey Authentication. Plants. 2025; 14(23):3600. https://doi.org/10.3390/plants14233600
Chicago/Turabian StyleTürker, Zeynep, Kamil Coşkunçelebi, Esra Demir Kanbur, and Mutlu Gültepe. 2025. "Significance of Morpho-Palynological Diversity in Melliferous Plants in the Anzer Region (Türkiye) with Regard to Honey Authentication" Plants 14, no. 23: 3600. https://doi.org/10.3390/plants14233600
APA StyleTürker, Z., Coşkunçelebi, K., Demir Kanbur, E., & Gültepe, M. (2025). Significance of Morpho-Palynological Diversity in Melliferous Plants in the Anzer Region (Türkiye) with Regard to Honey Authentication. Plants, 14(23), 3600. https://doi.org/10.3390/plants14233600






