Effects of Functionalized Iron Oxide Magnetic Nanoparticle Suspensions on Seed Morphology and Physiology in Yellow Maize and Chili Pepper
Abstract
1. Introduction
2. Materials and Methods
2.1. Precursor Reagents for the Synthesis of the Nanocomposites
2.2. Preparation of N-2-Hydroxy-Propyl-3-Trimethyl Ammonium Chitosan Chloride (HTCC)
2.3. Preparation of Aqueous Suspensions of Magnetic Nanocomposites Coated with Quaternized Chitosan
2.4. Binding of PO43− Ions to the Surface of Fe3O4-QC
2.5. Characterization of Magnetic Nanocomposites
2.6. Preparation of the Aqueous Suspensions of Nanocomposite
2.7. Seeds Preparation
2.8. In Vitro Phytotoxicity Assays
2.9. Greenhouse Assay
2.10. Total Oxidizable Organic Carbon Measurements
2.11. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the Nanocomposite
3.1.1. X-Ray Measurements
3.1.2. FTIR Measurements
3.1.3. Zeta Potential and Dynamic Light Scattering Measurements
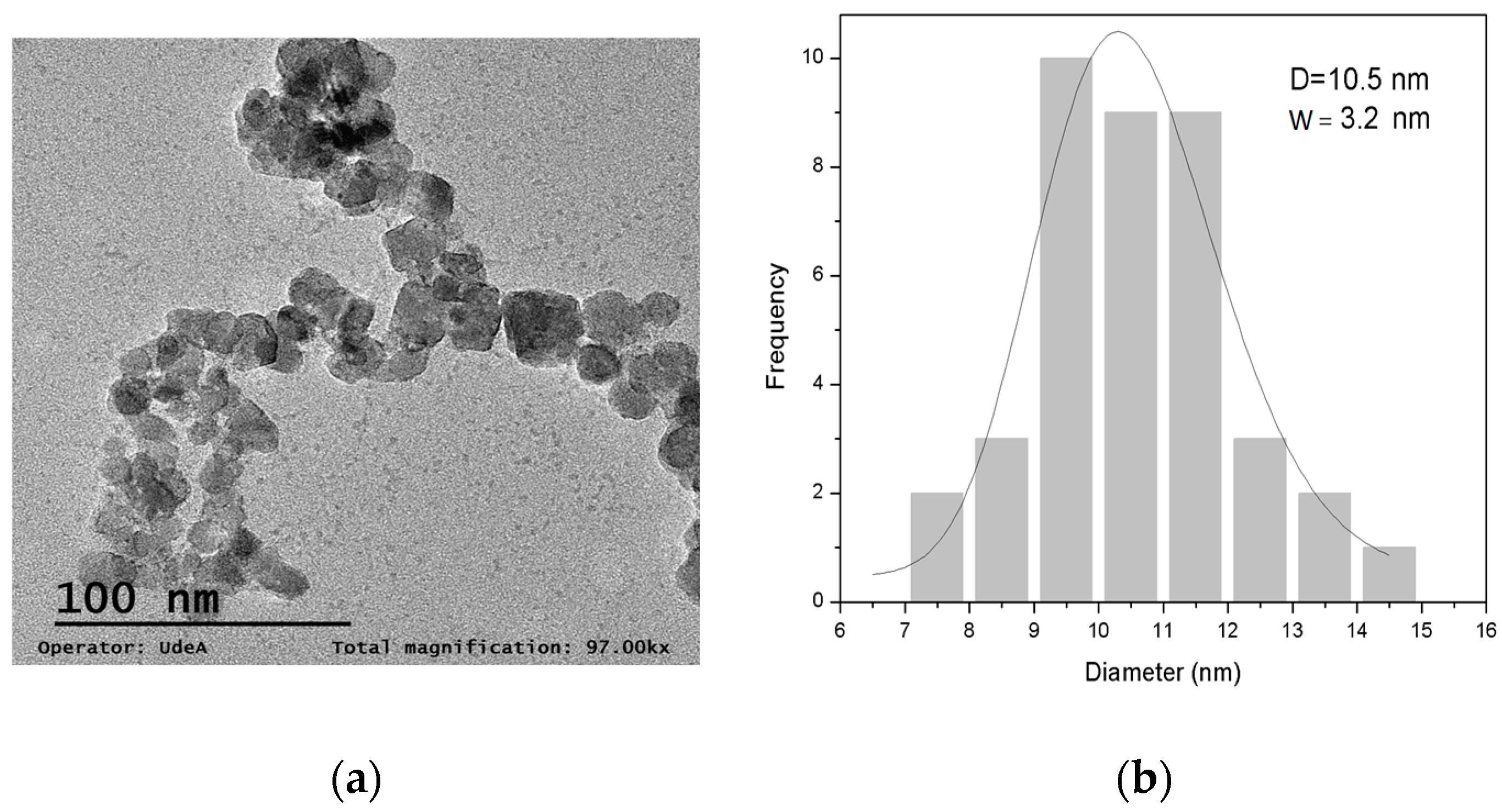
3.1.4. TEM Measurements
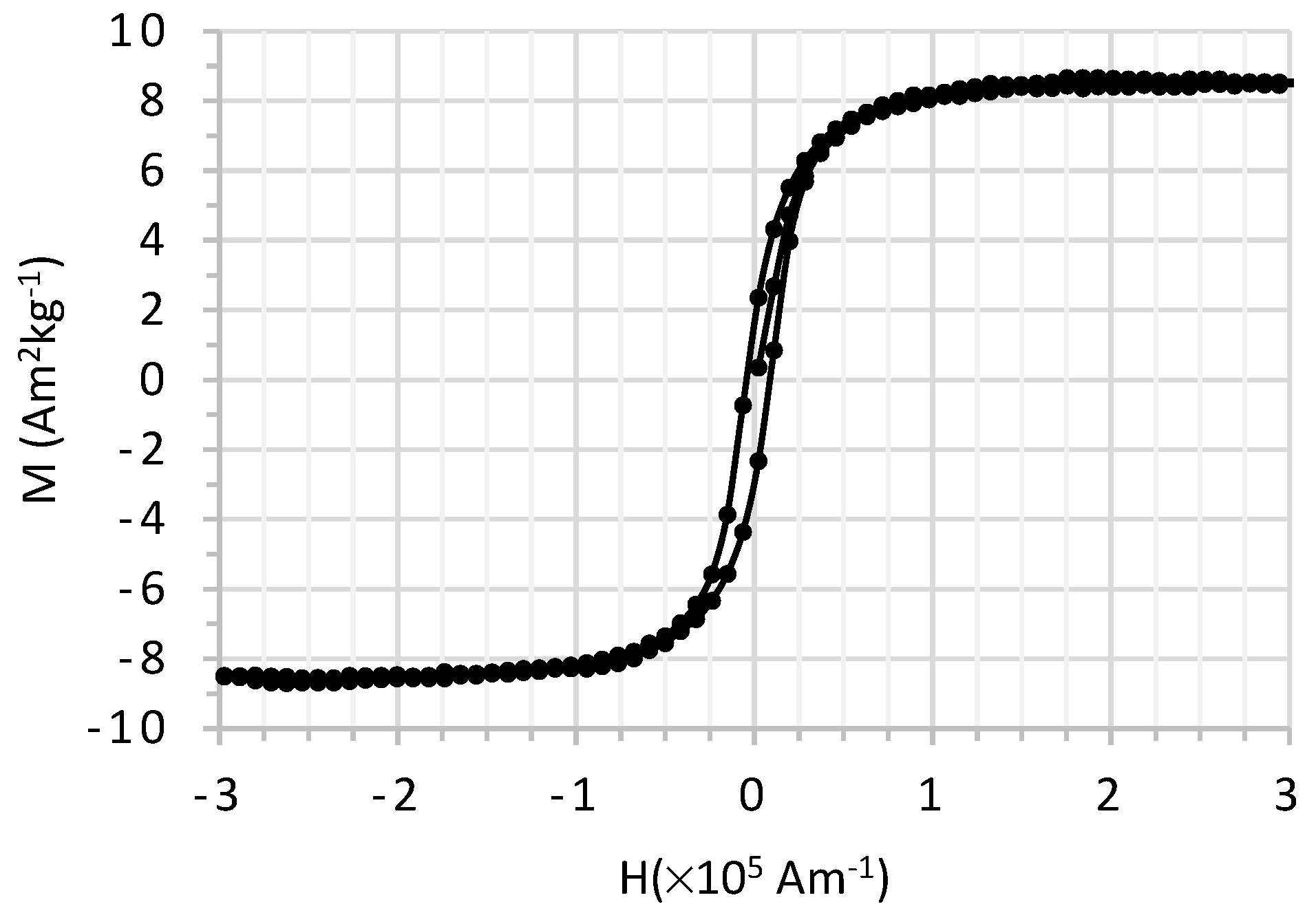
3.1.5. Magnetization Measurements
3.2. Application of the Nanocomposites in Maize and Chili Pepper Seeds
3.2.1. In Vitro Phytotoxicity Assay
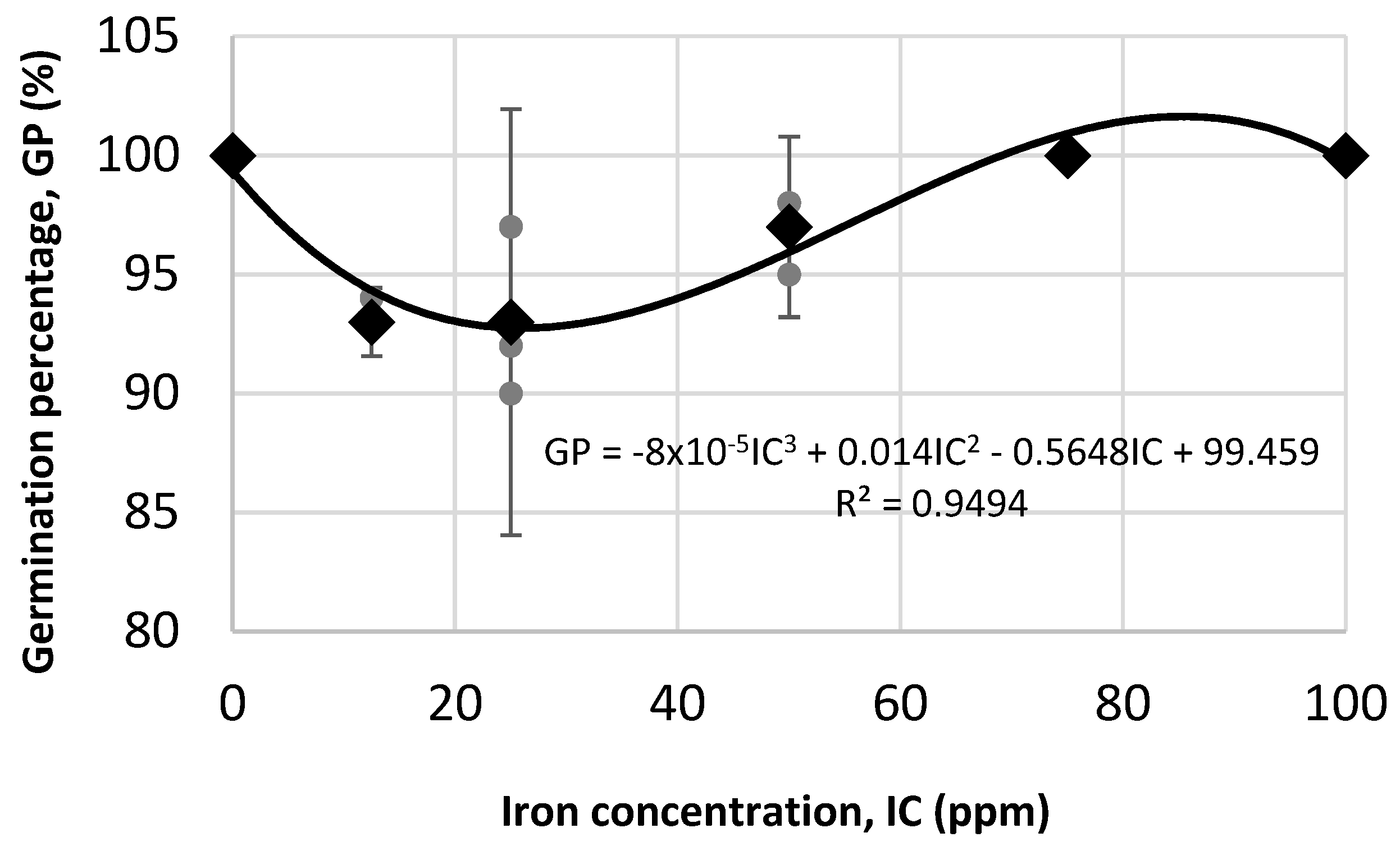
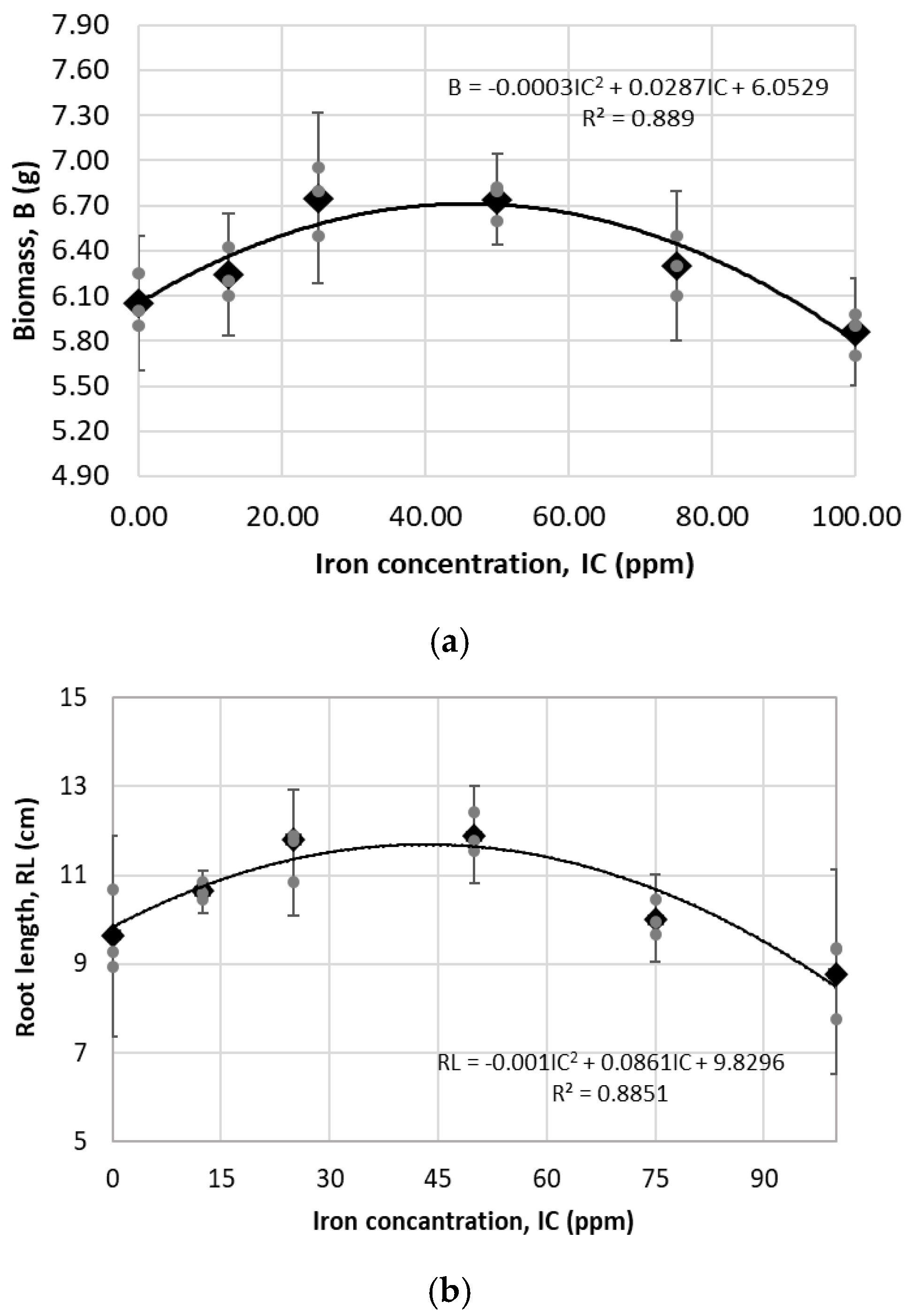
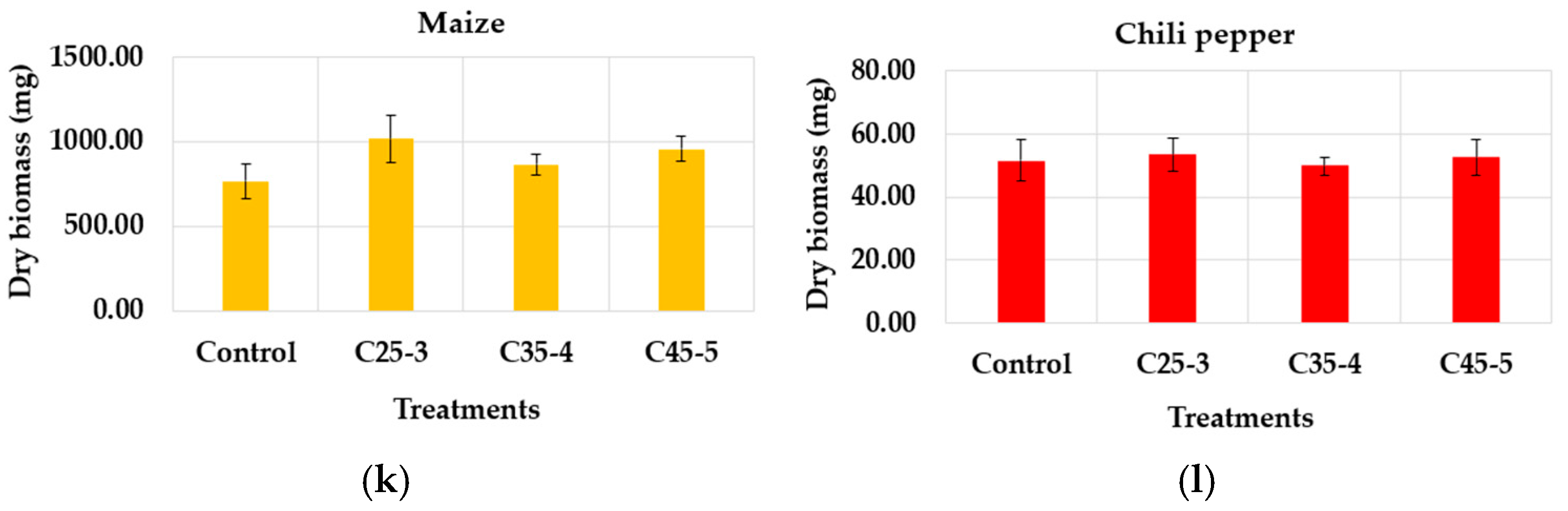
3.2.2. Greenhouse Assay
3.3. Total Oxidizable Organic Carbon Measurement (TOC)
4. Conclusions
4.1. On the Biocompatibility of Nanocomposite Magnetite–Maghemite Quaternized Chitosan
4.2. On the Enhancement of Morphological and Physiological Parameters in Maize and Chili Pepper Seeds and Seedlings Derived from Seeds Treated with Nanocomposites of Varying Iron–Phosphorus Concentrations
4.3. Future Works
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mangalampalli, B.; Naresh, D.; Paramjit, G. Allium cepa root tip assay in assessment of toxicity of magnesium oxide nanoparticles and microparticles. J. Environ. Sci. 2018, 66, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Roco, M.C. Broader Societal Issues of Nanotechnology. J. Nanopart. Res. 2003, 5, 181–189. [Google Scholar] [CrossRef]
- Lau, C.H.T.; Carvalho, L.B.; Pereira, A.E.S.; Montanha, G.S.; Corrêa, C.G.; Carvalho, H.W.P.; Ganin, A.Y.; Fraceto, L.F.; Yiu, H.H.P. Localization of Coated Iron Oxide (Fe3O4) Nanoparticles on Tomato Seeds and Their Effects on Growth. ACS Appl. Bio Mater. 2020, 3, 4109–4117. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Cheng, J.; Dou, R.; Gao, X.; Mao, C.; Wang, L. Assessment of the Phytotoxicity of Metal Oxide Nanoparticles on Two Crop Plants, Maize (Zea mays L.) and Rice (Oryza sativa L.). Int. J. Environ. Res. Public Health 2015, 12, 15100–15109. [Google Scholar] [CrossRef]
- Ruttkay, B.; Krystofova, O.; Nejdl, L.; Adam, V. Nanoparticles Based on Essential Metals and Their Phytotoxicity. J. Nanobiotechnol. 2017, 15, 33. [Google Scholar] [CrossRef]
- Gogos, A.; Knauer, K.; Bucheli, T.D. Nanomaterials in Plant Protection and Fertilization: Current State, Foreseen Applications, and Research Priorities. J. Agric. Food Chem. 2012, 60, 9781–9792. [Google Scholar] [CrossRef]
- Sundaria, N.; Singh, M.; Upreti, P.; Chauhan, R.; Jaiswal, J.P.; Kumar, A. Seed Priming with Iron Oxide Nanoparticles Triggers Iron Acquisition and Biofortification in Wheat (Triticum aestivum L.) Grains. J. Plant Growth Regul. 2018, 38, 122–131. [Google Scholar] [CrossRef]
- Rui, M.; Ma, C.; Hao, Y.; Guo, J.; Yukui, R.; Tang, X.; Zhao, Q.; Fan, X.; Zhang, Z.; Hou, T.; et al. Iron Oxide Nanoparticles as a Potential Iron Fertilizer for Peanut (Arachis hypogaea). Front. Plant. Sci. 2016, 7, 815. [Google Scholar] [CrossRef]
- Kanjana, D. Foliar study on effect of iron oxide nanoparticles as an alternate source of iron fertilizer to cotton. Int. J. Chem. Stud. 2019, 7, 4374–4379. [Google Scholar]
- Khalid, U.; Sher, F.; Noreen, S.; Lima, E.C.; Rasheed, T.; Sehar, S.; Amami, R. Comparative effects of conventional and nano-enabled fertilizers on morphological and physiological attributes of Caesalpinia bonducella plants. J. Saudi Soc. Agric. Sci. 2022, 21, 61–72. [Google Scholar] [CrossRef]
- Alkhatib, R.; Alkhatib, B.; Abdo, N.; AL-Eitan, L.; Creamer, R. Physio-biochemical and ultrastructural impact of (Fe3O4) nanoparticles on tobacco. Plants 2019, 8, 2–12. [Google Scholar] [CrossRef]
- Bombin, S.; LeFebvre, M.; Sherwood, J.; Xu, Y.; Bao, Y.; Ramonell, K.M. Developmental and Reproductive Effects of Iron Oxide Nanoparticles in Arabidopsis thaliana. Int. J. Mol. Sci. 2015, 16, 24174–24193. [Google Scholar] [CrossRef]
- Lu, K.; Shen, D.; Liu, X.; Dong, S.; Jing, X.; Wu, W.; Tong, Y.; Gao, S.; Mao, L. Uptake of iron oxide nanoparticles inhibits the photosynthesis of wheat after foliar exposure. Chemosphere 2020, 259, 127445. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.R.R.; Bernardes, L.E.; Barbetta, M.F.S.; da Veiga, M.A.M.S. Iron oxide nanoparticle phytotoxicity to the aquatic plant Lemna minor: Effect on reactive oxygen species (ROS) production and chlorophyll a/chlorophyll b ratio. Environ. Sci. Pollut. Res. 2019, 26, 24121–24131. [Google Scholar] [CrossRef] [PubMed]
- Zuverza, N.; Martínez, D.; Du, W.; Hernandez, J.; Bonilla, N.; López, M.; Komárek, M.; Peralta, J.; Gardea, J. Exposure of engineered nanomaterials to plants: Insights into the physiological and biochemical responses—A review. Plant Physiol. Biochem. 2017, 110, 236–264. [Google Scholar] [CrossRef]
- Fatima, F.; Hashim, A.; Anees, S. Efficacy of nanoparticles as nanofertilizer production: A review. Environ. Sci. Pollut. Res. 2021, 28, 1292–1303. [Google Scholar] [CrossRef]
- López Valdez, F.; Miranda Arámbula, M.; Cortés, A.M.; Fernández Luqueño, F.; de-la-Luz, V. Nanofertilizers and Their Controlled Delivery of Nutrients. In Agricultural Nanobiotechnology; López-Valdez, F., Fernández-Luqueño, F., Eds.; Springer: Cham, Switzerland, 2018; pp. 35–48. [Google Scholar] [CrossRef]
- Frank, L.A.; Onzi, G.R.; Morawski, A.S.; Pohlmann, A.R.; Guterres, S.S.; Contri, R.V. Chitosan as a coating material for nanoparticles intended for biomedical applications. React. Funct. Polym. 2020, 147, 104459. [Google Scholar] [CrossRef]
- Shukla, S.; Jadaun, A.; Arora, V.; Sinha, R.K.; Biyani, N.; Jain, V.K. In vitro toxicity assessment of chitosan oligosaccharide coated iron oxide nanoparticles. Toxicol. Rep. 2015, 27–39. [Google Scholar] [CrossRef]
- Montiel Schneider, M.G.; Martín, M.J.; Otarola, J.; Vakarelska, E.; Simeonov, V.; Lassalle, V.; Nedyalkova, M. Biomedical Applications of Iron Oxide Nanoparticles: Current Insights Progress and Perspectives. Pharmaceutics 2022, 14, 204. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and Surface Engineering of Iron Oxide Nanoparticles for Biomedical Applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.-H.; Lee, S.-Y.; Park, S.A.; Kim, J.H.; Hwang, D.; Kim, K.A.; Kim, H.S. Antioxidant Iron Oxide Nanoparticles: Their Biocompatibility and Bioactive Properties. Int. J. Mol. Sci. 2023, 24, 15901. [Google Scholar] [CrossRef]
- Ruihua, H.; Bingchao, Y.; Zheng, D.; Wang, B. Preparation and characterization of a quaternized chitosan. J. Mater. Sci. 2012, 47, 845–851. [Google Scholar] [CrossRef]
- Velásquez, A.A.; Trujillo, J.M.; Morales, A.L.; Tobón, J.E.; Reyes, L.; Gancedo, J.R. Design and Construction of an Autonomous Control System for Mössbauer Spectrometry. Hyp. Interact. 2005, 161, 139–145. [Google Scholar] [CrossRef]
- Hu, Z.; Ding, Z.; Al-Yasi, H.M.; Ali, E.F.; Eissa, M.A.; Abou-Elwafa, S.F.; Sayed, M.A.; Said, M.T.; Said, A.A.; Ibrahim, K.A.M.; et al. Modeling of Phosphorus Nutrition to Obtain Maximum Yield, High P Use Efficiency and Low P-Loss Risk for Wheat Grown in Sandy Calcareous Soils. Agronomy 2021, 11, 1950. [Google Scholar] [CrossRef]
- Centro Internacional de Agricultura Tropical (CIAT). Maíz de alta calidad de proteína. AgroSalud. 2010. Available online: https://hdl.handle.net/10568/70521 (accessed on 11 July 2025).
- Olatunji, T.L.; Afolayan, A.J. The suitability of chili pepper (Capsicum annum L.) for alleviating human micronutrient dietary deficiencies: A review. Food Sci. Nutr. 2018, 6, 2239–2251. [Google Scholar] [CrossRef]
- Mylavarapu, R. Walkley-Black method. In Soil Test Methods from the Southeastern United States; Southern Cooperative Series: Richmond, VA, USA, 2014; pp. 158–161. Available online: https://aesl.ces.uga.edu/sera6/PUB/MethodsManualFinalSERA6.pdf (accessed on 12 August 2025).
- Lutterotti, L.; Matthies, S.; Wenk, H.R. MAUD: A friendly Java program for material analysis using diffraction. IUCr Newsl. 1999, 21, 14–15. [Google Scholar]
- Smith, A.; Jones, B.; Doe, C. Physicochemical Properties of Iron-Oxide Based Nanoparticles for Diagnostics and Drug Delivery. Nanotheranostics 2023, 7, 424–439. [Google Scholar]
- Lowry, G.V.; Hill, R.J.; Harper, S.; Rawle, A.F.; Hendren, C.O.; Klaessig, F.; Nobbmann, U.; Sayre, P.; Rumble, J. Guidance to Improve the Scientific Value of Zeta-Potential Measurements in NanoEHS. Environ. Sci. Nano 2016, 3, 953–965. [Google Scholar] [CrossRef]
- Asadi-Kavan, Z.; Khavari-Nejad, R.A.; Iranbakhsh, A.; Najafi, F. Cooperative effects of iron oxide nanoparticle (α-Fe2O3) and citrate on germination and oxidative system of evening primrose (Oenthera biennis L.). J. Plant Interact. 2020, 15, 166–179. [Google Scholar] [CrossRef]
- Tawfik, M.M.; Mohamed, M.H.; Sadak, M.; Thalooth, A.T. Iron oxide nanoparticles effect on growth, physiological traits and nutritional contents of Moringa oleifera grown in saline environments. Bull. Natl. Res. Cent. 2021, 45, 177. [Google Scholar] [CrossRef]
- Malhotra, H.; Vandana; Sharma, S.; Pandey, R. Phosphorus Nutrition: Plant Growth in Response to Deficiency and Excess. In Plant Nutrients and Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B., Eds.; Springer Nature: Singapore, 2018; pp. 171–190. [Google Scholar] [CrossRef]
- Meng, X.; Chen, W.; Wang, Y.; Huang, Z.; Ye, X.; Chen, L.; Yang, L. Effects of phosphorus deficiency on the absorption of mineral nutrients, photosynthetic system performance and antioxidant metabolism in Citrus grandis. PLoS ONE 2021, 16, 1–20. [Google Scholar] [CrossRef]
- Steil, A. How to Store Seeds and Test Germination Rates. In Culture and Home Pest News; IOWA State University: Ames, IA, USA, 2023; Available online: https://hortnews.extension.iastate.edu/how-store-seeds-and-test-germination-rates#:~:text=For%20most%20species%2C%20a%20germination,naturally%20have%20lower%20germination%20rates (accessed on 24 July 2025).
- Wawrzyniak, M.K.; Michalak, M.; Chmielarz, P. Effect of different conditions of storage on seed viability and seedling growth of six European wild fruit woody plants. Ann. Forest Sci. 2020, 77, 58. [Google Scholar] [CrossRef]
- Bareke, T. Biology of seed development and germination physiology. Adv. Plants Agric. Res. 2018, 8, 336–346. [Google Scholar] [CrossRef]
- Medrano, H.; Galmés, J.; Flexas, J. Fijación del dióxido de carbono y biosíntesis de fotoasimilados. In Fundamentos de Fisiología Vegetal; Azcón-Bieto, J., Talón, M., Eds.; Mc Graw Hill: Columbus, OH, USA, 2008; pp. 211–225. [Google Scholar]
- Mallén-Ponce, M.J.; Pérez-Pérez, M.E.; Crespo, J.L. Analyzing the impact of autotrophic and heterotrophic metabolism on the nutrient regulation of TOR. New Phytol. 2022, 236, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.; Dolores, M.; Robert, N.; Fernández, J. Biomass Resources. In The Role of Bioenergy in the Emerging Bioeconomy: Resources, Technologies, Sustainability and Policy; Lago, C., Caldés, N., Lechón, Y., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 25–111. [Google Scholar] [CrossRef]
- Bonilla, I. Introducción a la nutrición mineral de las plantas: Los elementos minerales. In Fundamentos de Fisiología Vegetal; Azcón-Bieto, J., Talón, M., Eds.; Mc Graw Hill: Columbus, OH, USA, 2008; pp. 103–121. [Google Scholar]
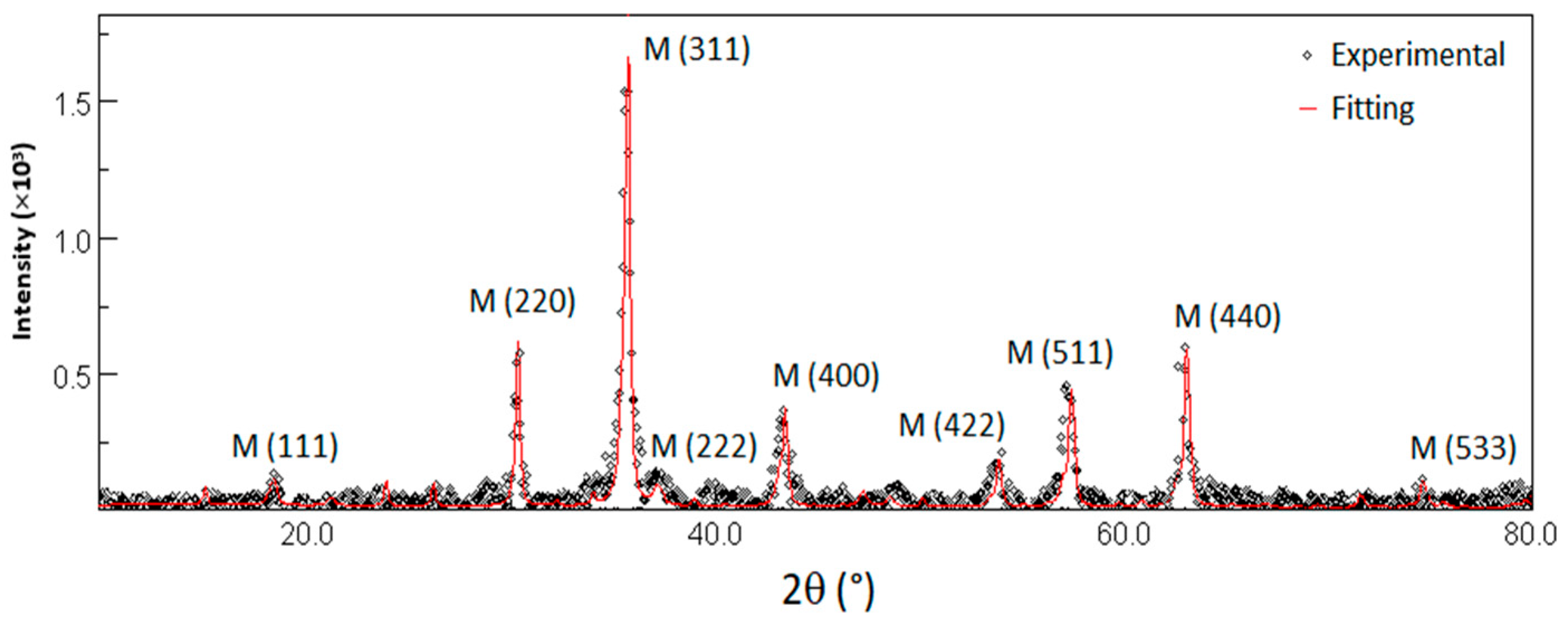
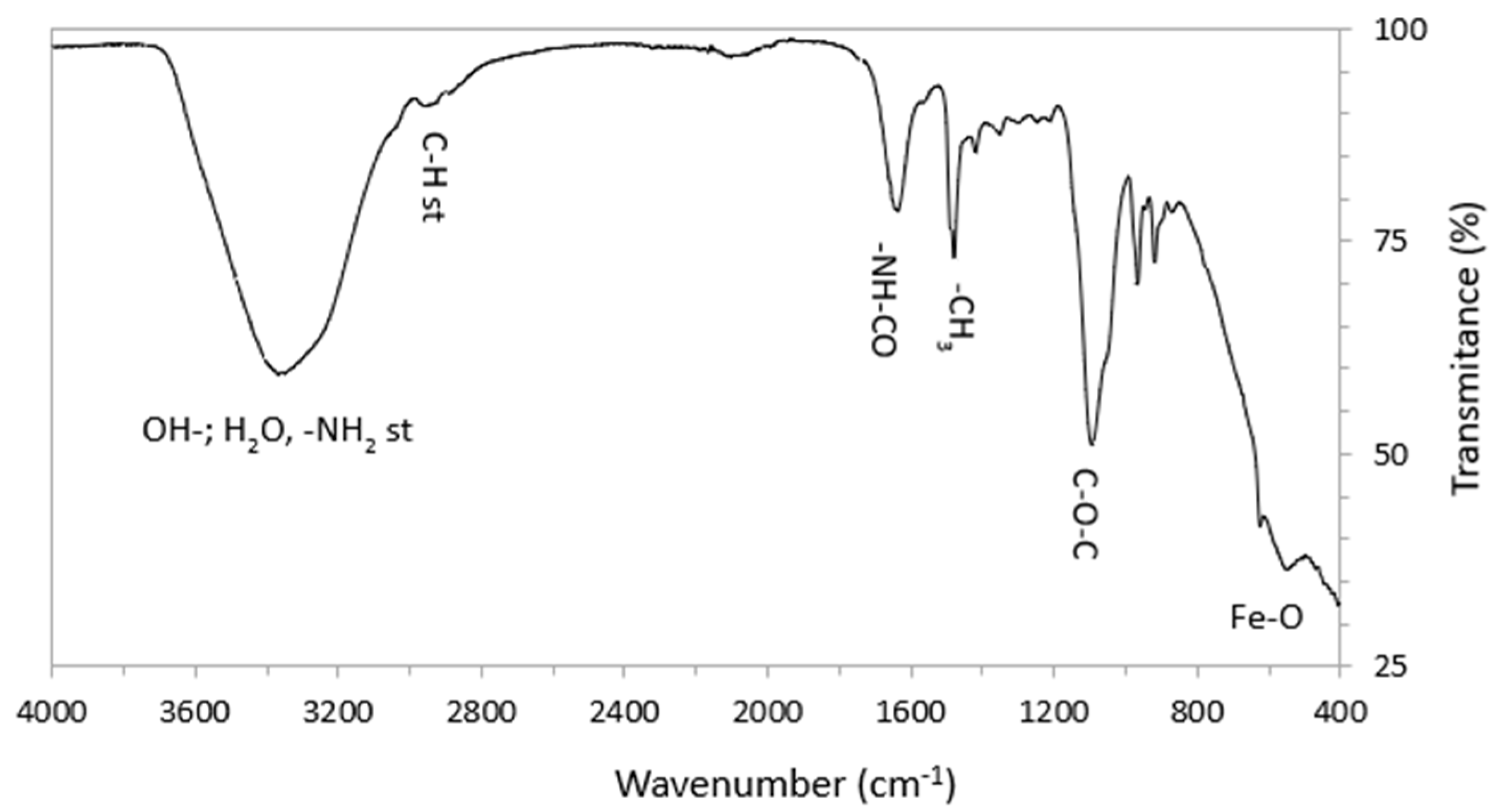
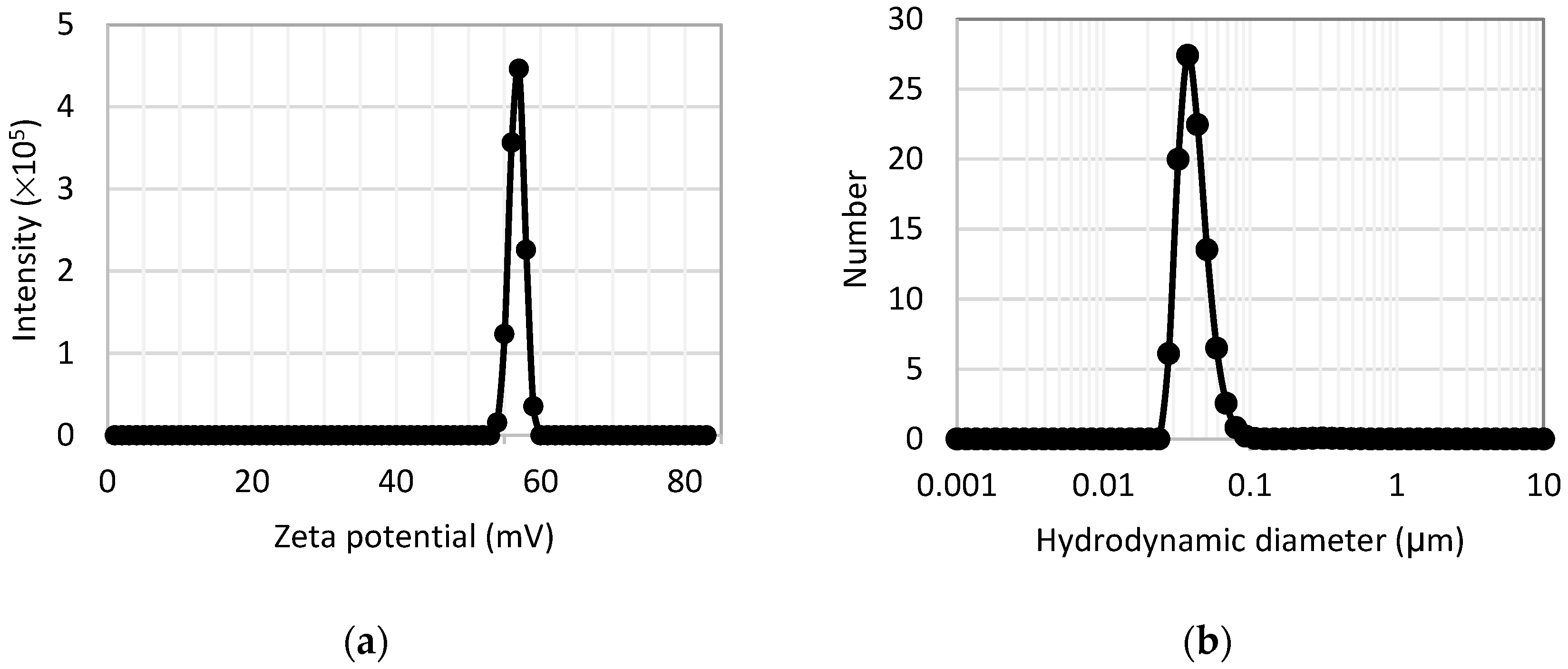
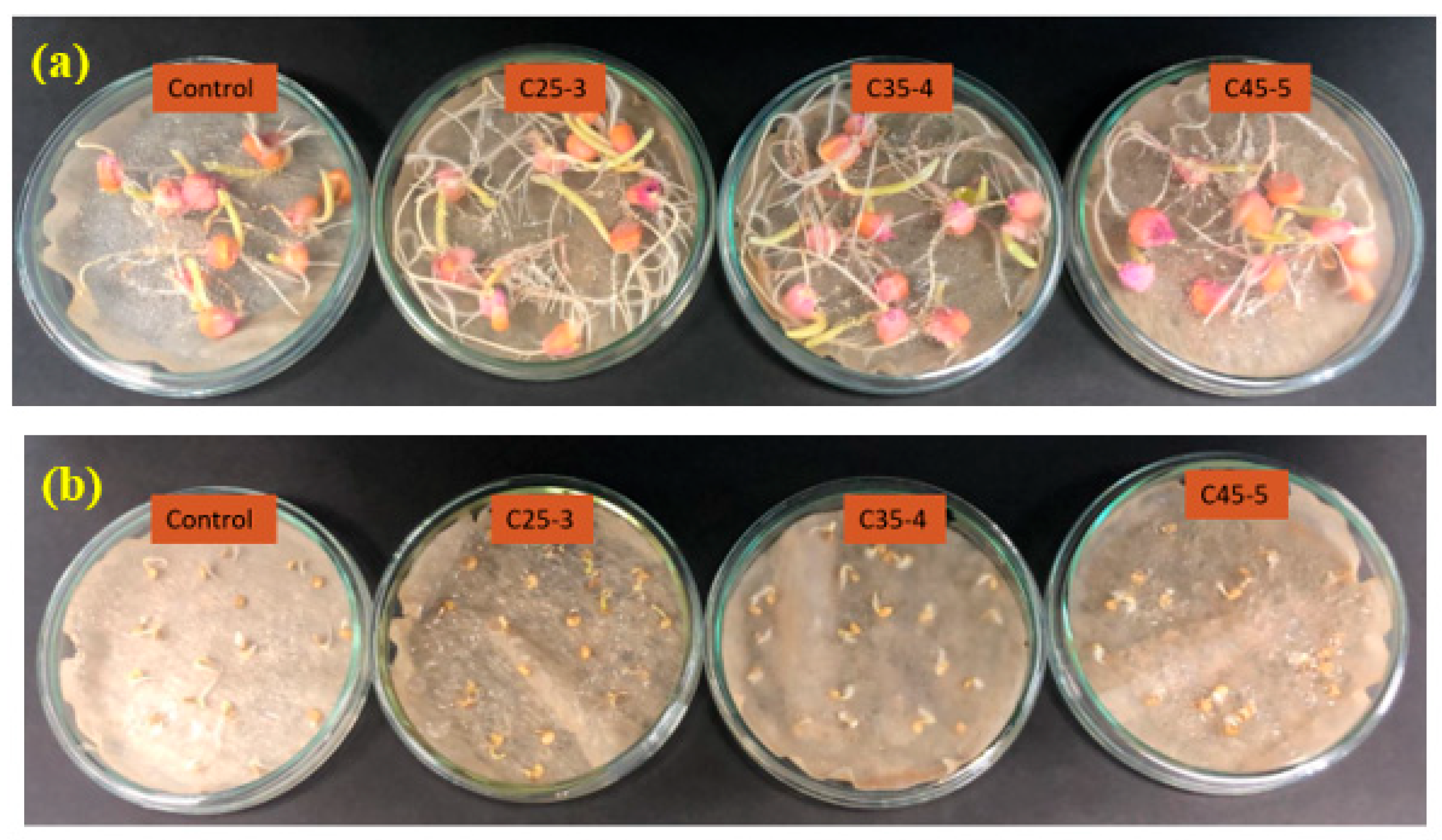
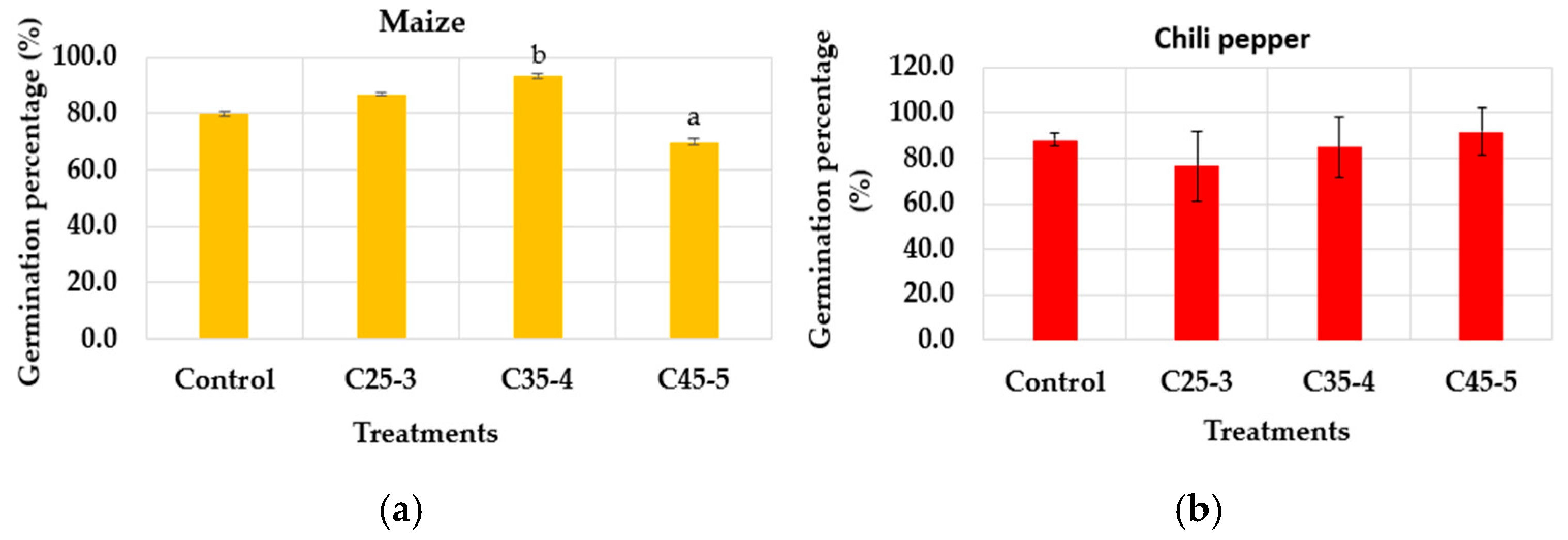
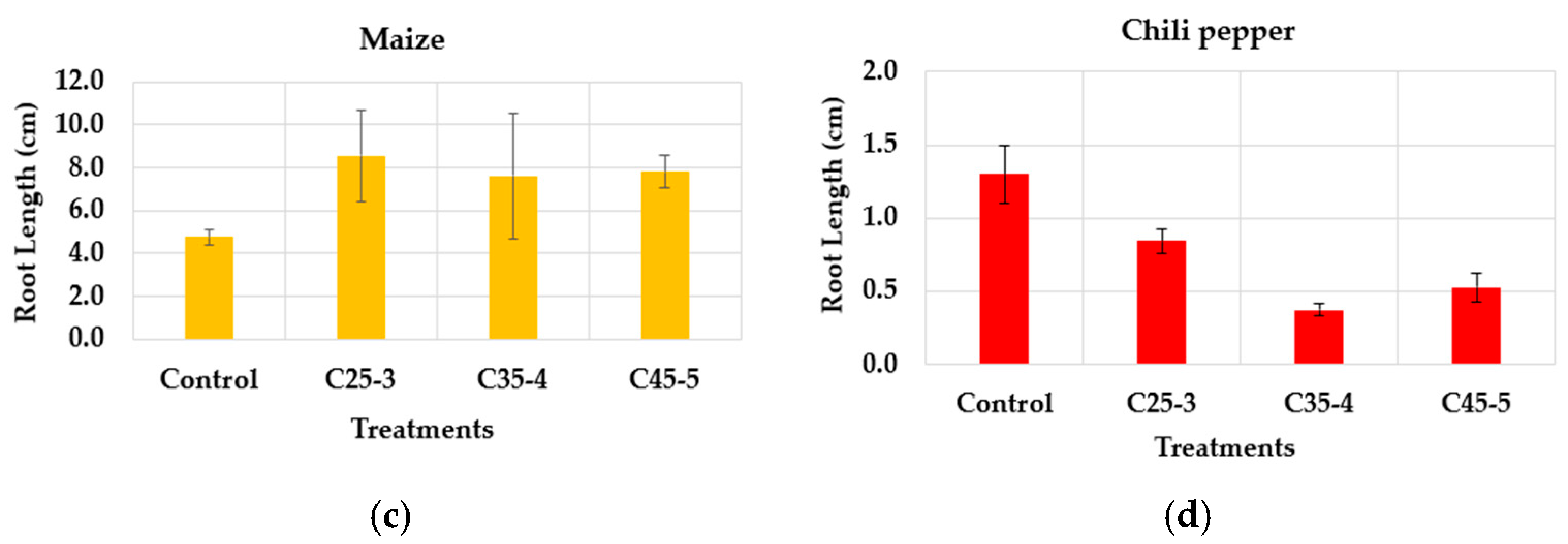
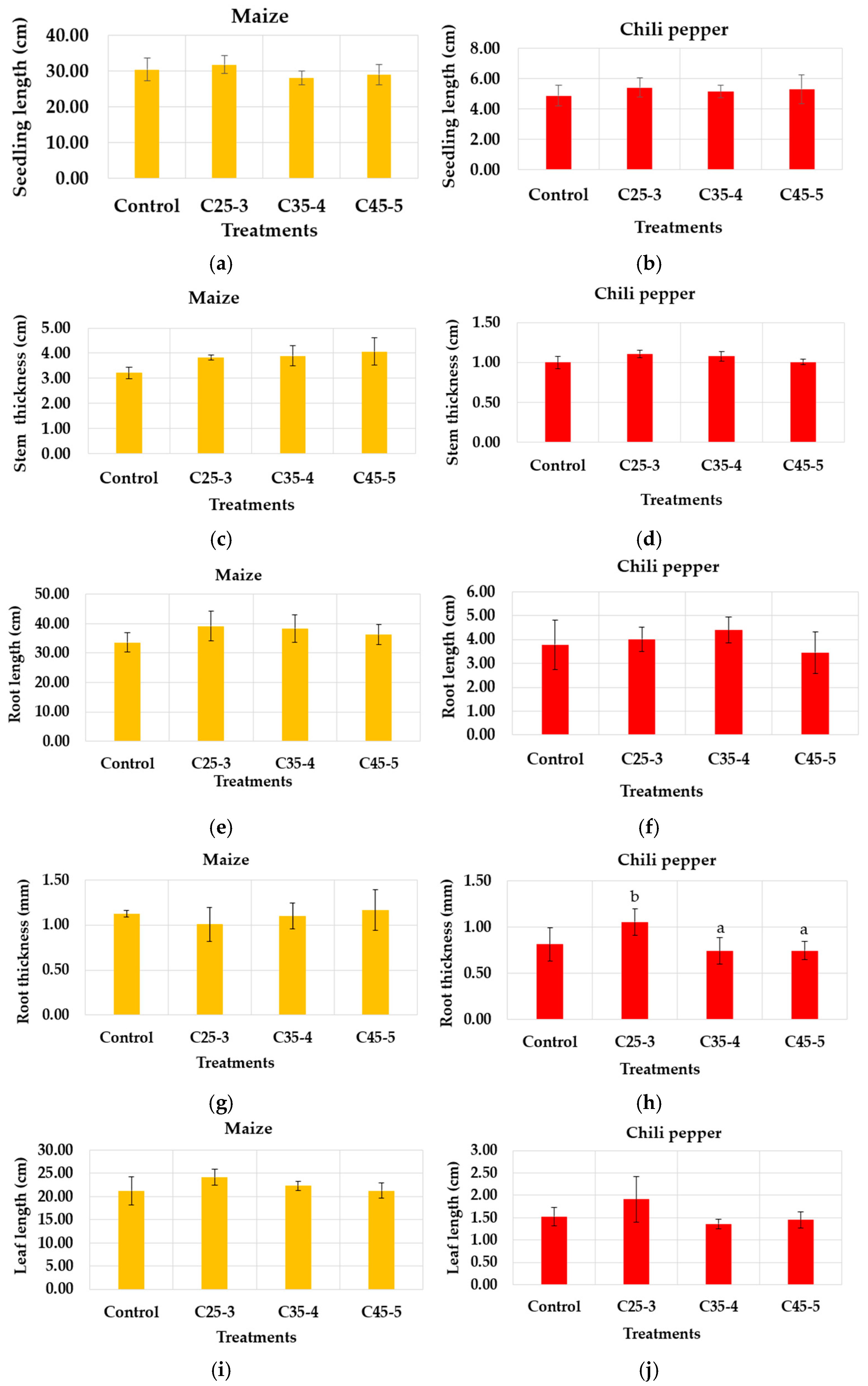

| Suspension Label | Iron Concentration (ppm) | Phosphorus Concentration (ppm) |
|---|---|---|
| C12.5-0 | 12.5 | 0 |
| C50-0 | 50 | 0 |
| C75-0 | 75 | 0 |
| C100-0 | 100 | 0 |
| C25-3 | 25 | 2.9 |
| C35-4 | 35 | 4 |
| C45-5 | 45 | 5.2 |
| Phase | Percentage (%) | a (Å) | D (nm) |
|---|---|---|---|
| Magnetite | 42 ± 13 | 8.377 ± 0.004 | 25 ± 5 |
| Maghemite | 58 ± 13 | 8.329 ± 0.002 | 18 ± 2 |
| Ms (A m2 kg−1) | Mr (A m2 kg−1) | Hc (kA m−1) |
|---|---|---|
| 8.48 ± 0.01 | 1.90 ± 0.01 | 8.3 ± 0.2 |
| Treatment | Maize (%) | Chili Pepper (%) |
|---|---|---|
| Control | 36.7 ± 0.1 | 30.8 ± 0.1 |
| C25-3 | 35.6 ± 0.1 | 32.7 ± 0.1 |
| C35-4 | 35.3 ± 0.1 | 31.3 ± 0.1 |
| C45-5 | 37.5 ± 0.1 | 30.5 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velásquez, Á.; Urquijo, J.; Montoya, Y.; Susunaga, D.; Villanueva, D. Effects of Functionalized Iron Oxide Magnetic Nanoparticle Suspensions on Seed Morphology and Physiology in Yellow Maize and Chili Pepper. Plants 2025, 14, 3592. https://doi.org/10.3390/plants14233592
Velásquez Á, Urquijo J, Montoya Y, Susunaga D, Villanueva D. Effects of Functionalized Iron Oxide Magnetic Nanoparticle Suspensions on Seed Morphology and Physiology in Yellow Maize and Chili Pepper. Plants. 2025; 14(23):3592. https://doi.org/10.3390/plants14233592
Chicago/Turabian StyleVelásquez, Álvaro, Jeaneth Urquijo, Yessica Montoya, Danna Susunaga, and Diego Villanueva. 2025. "Effects of Functionalized Iron Oxide Magnetic Nanoparticle Suspensions on Seed Morphology and Physiology in Yellow Maize and Chili Pepper" Plants 14, no. 23: 3592. https://doi.org/10.3390/plants14233592
APA StyleVelásquez, Á., Urquijo, J., Montoya, Y., Susunaga, D., & Villanueva, D. (2025). Effects of Functionalized Iron Oxide Magnetic Nanoparticle Suspensions on Seed Morphology and Physiology in Yellow Maize and Chili Pepper. Plants, 14(23), 3592. https://doi.org/10.3390/plants14233592







