Thymus apulus (T. sect. Hyphodromi, Lamiaceae), a New Species from Southern Italy
Abstract
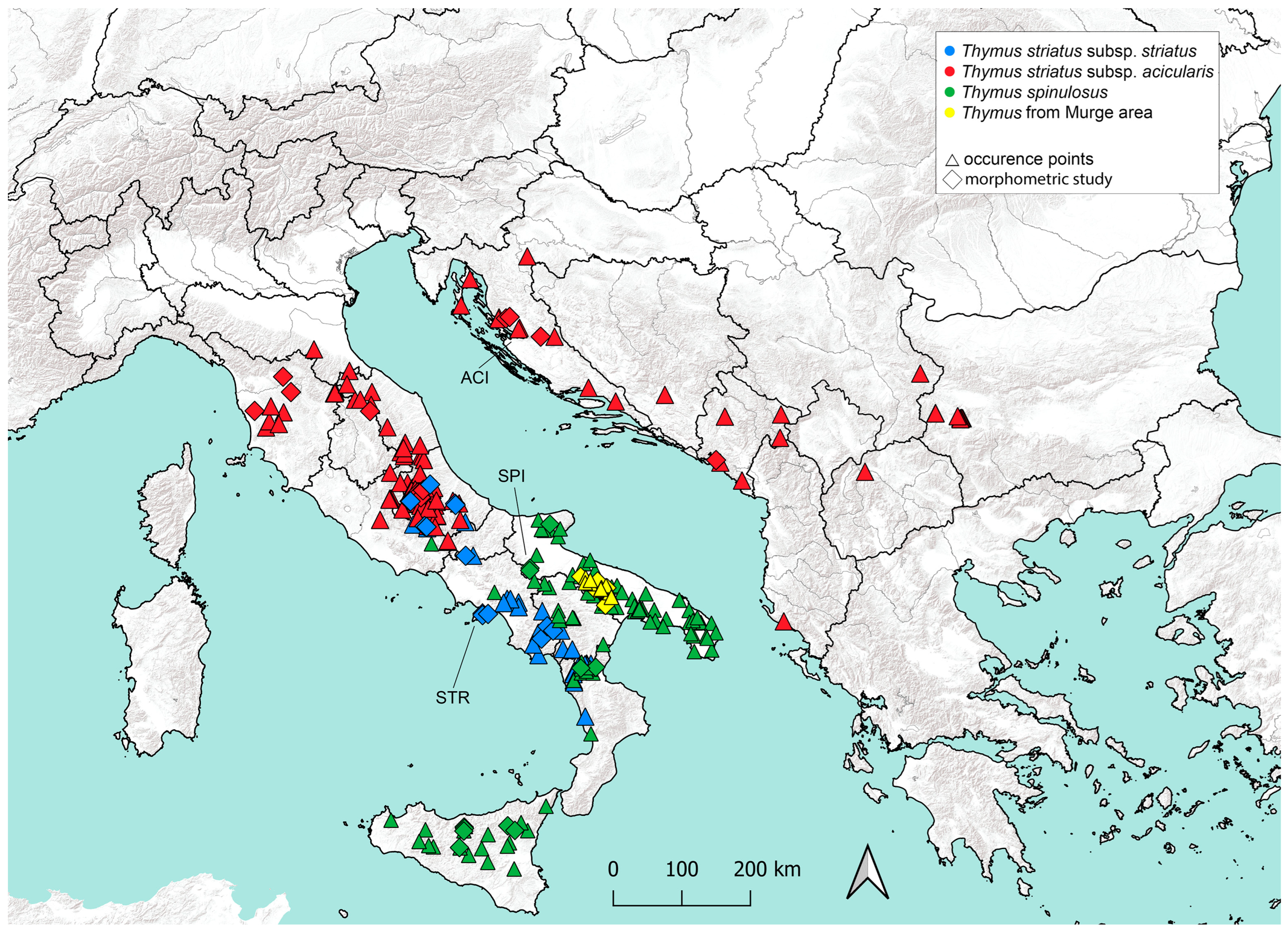
1. Introduction
2. Materials and Methods
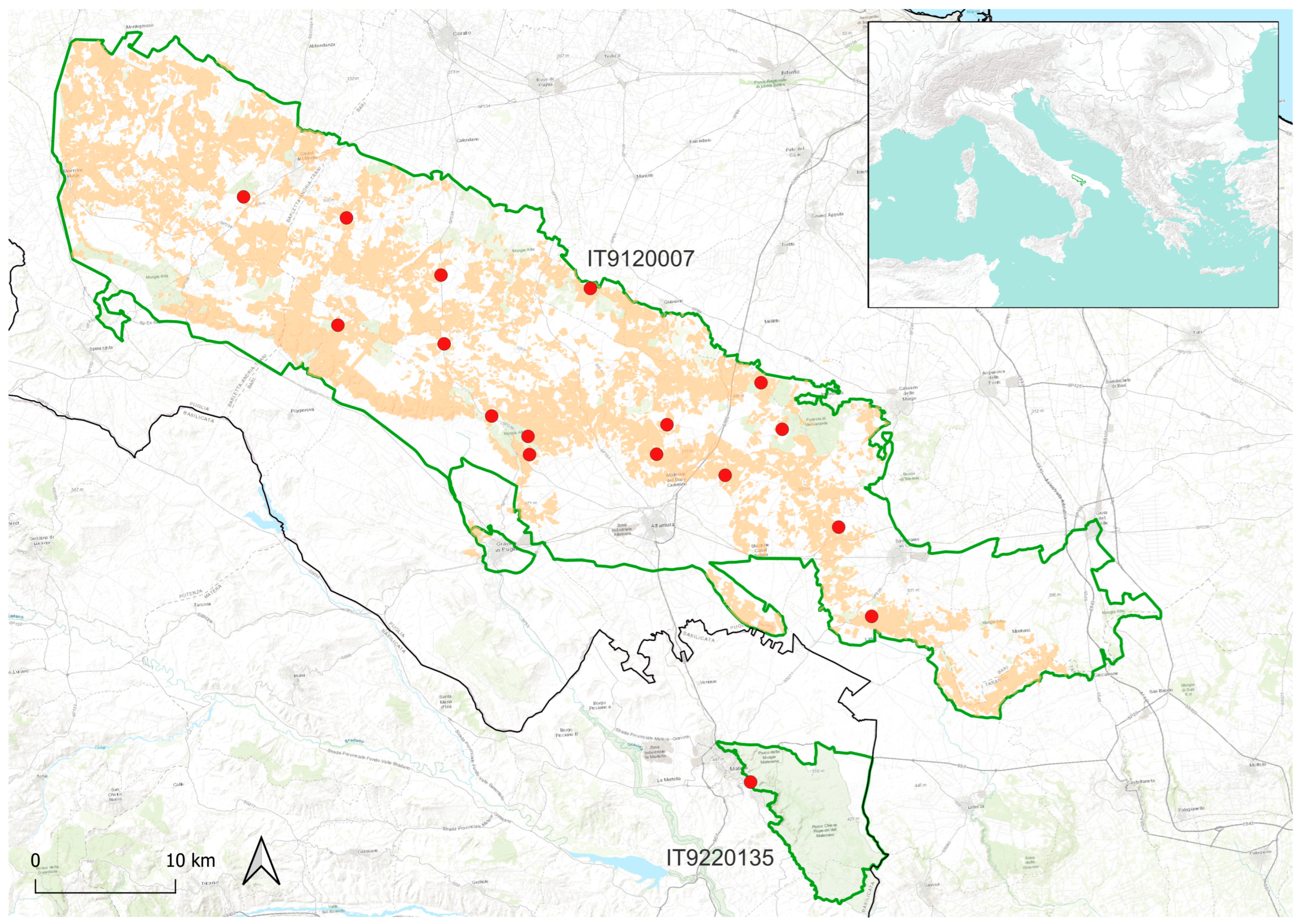
2.1. Sampling
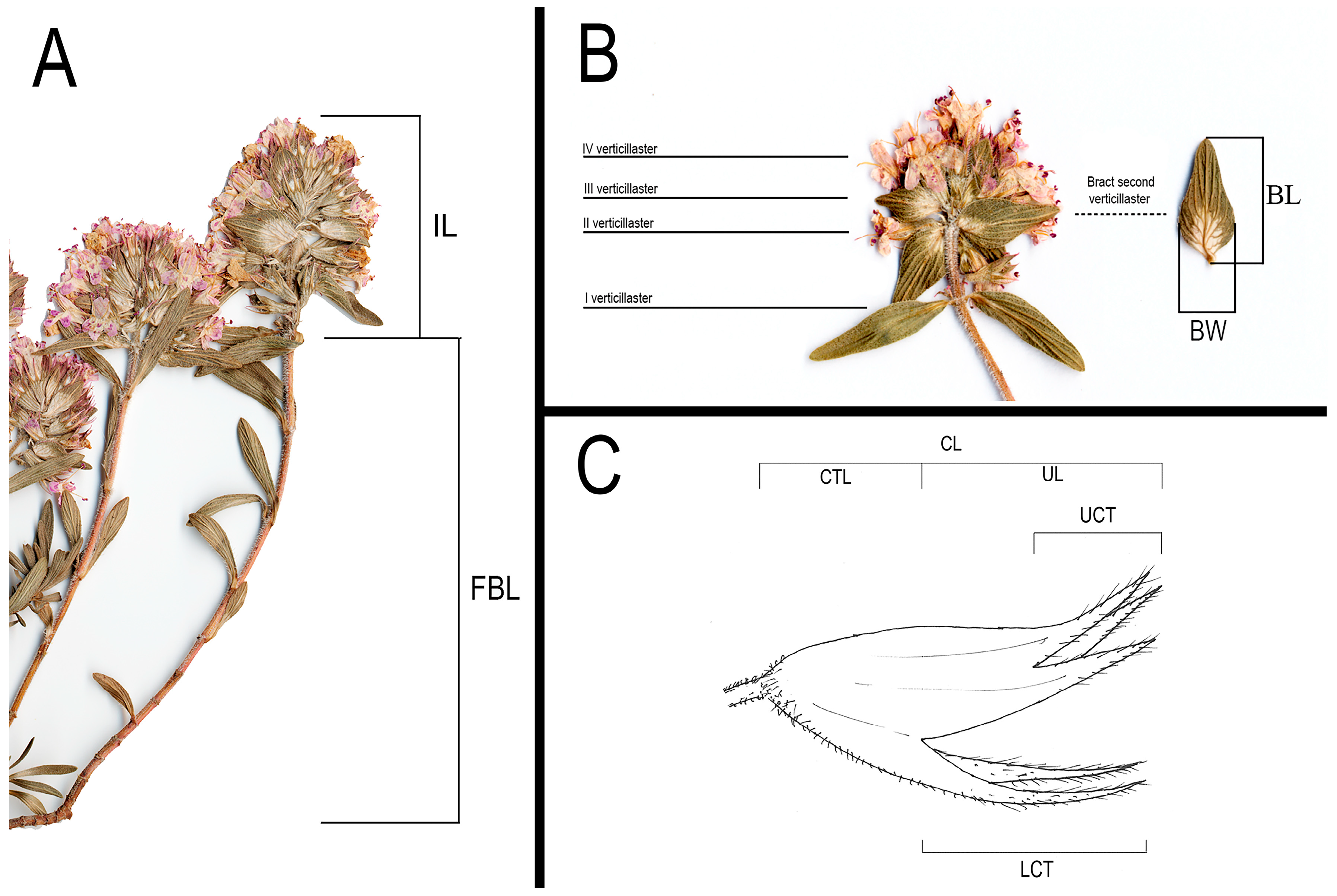
2.2. Morphometric Analyses
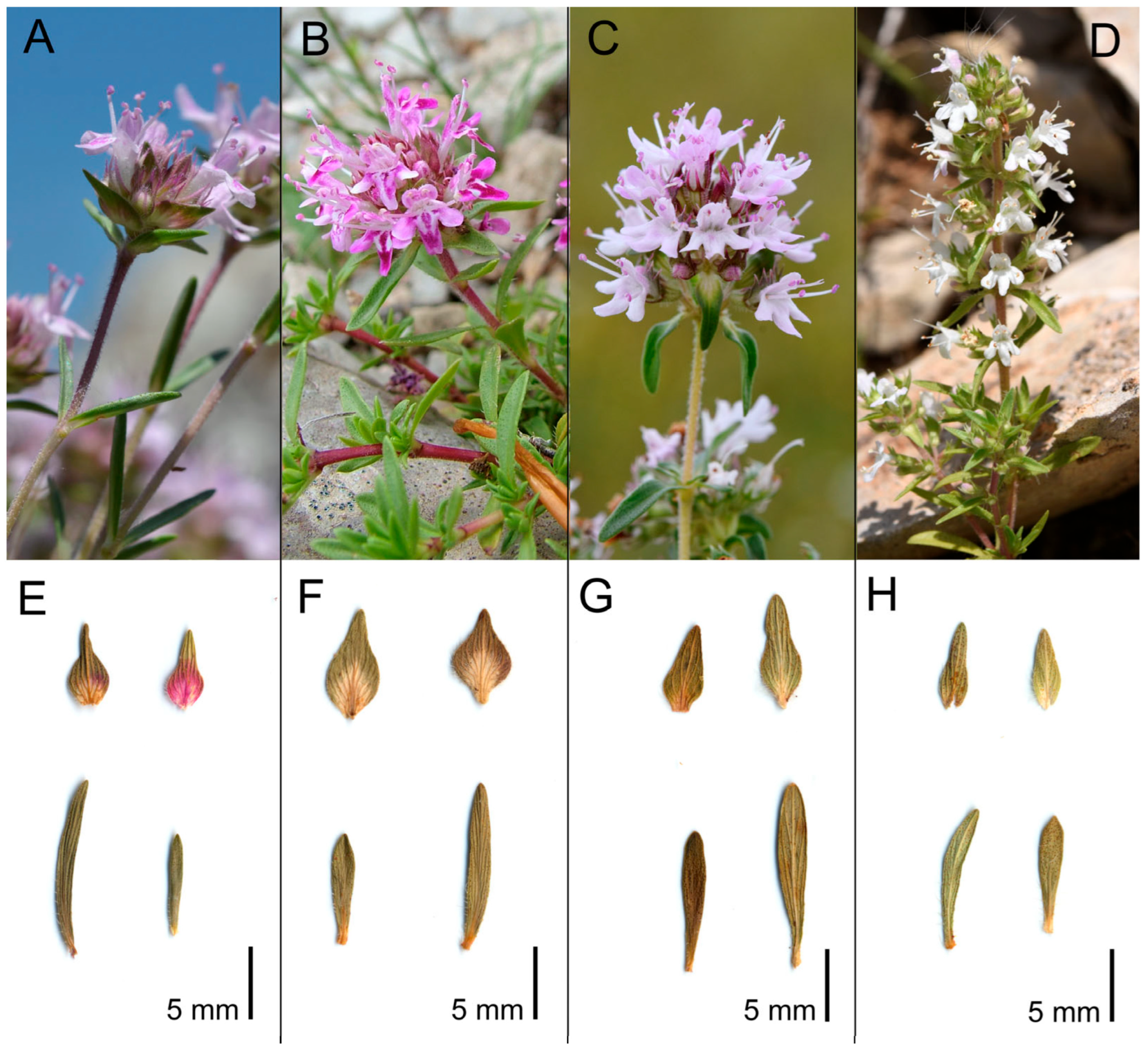
3. Results
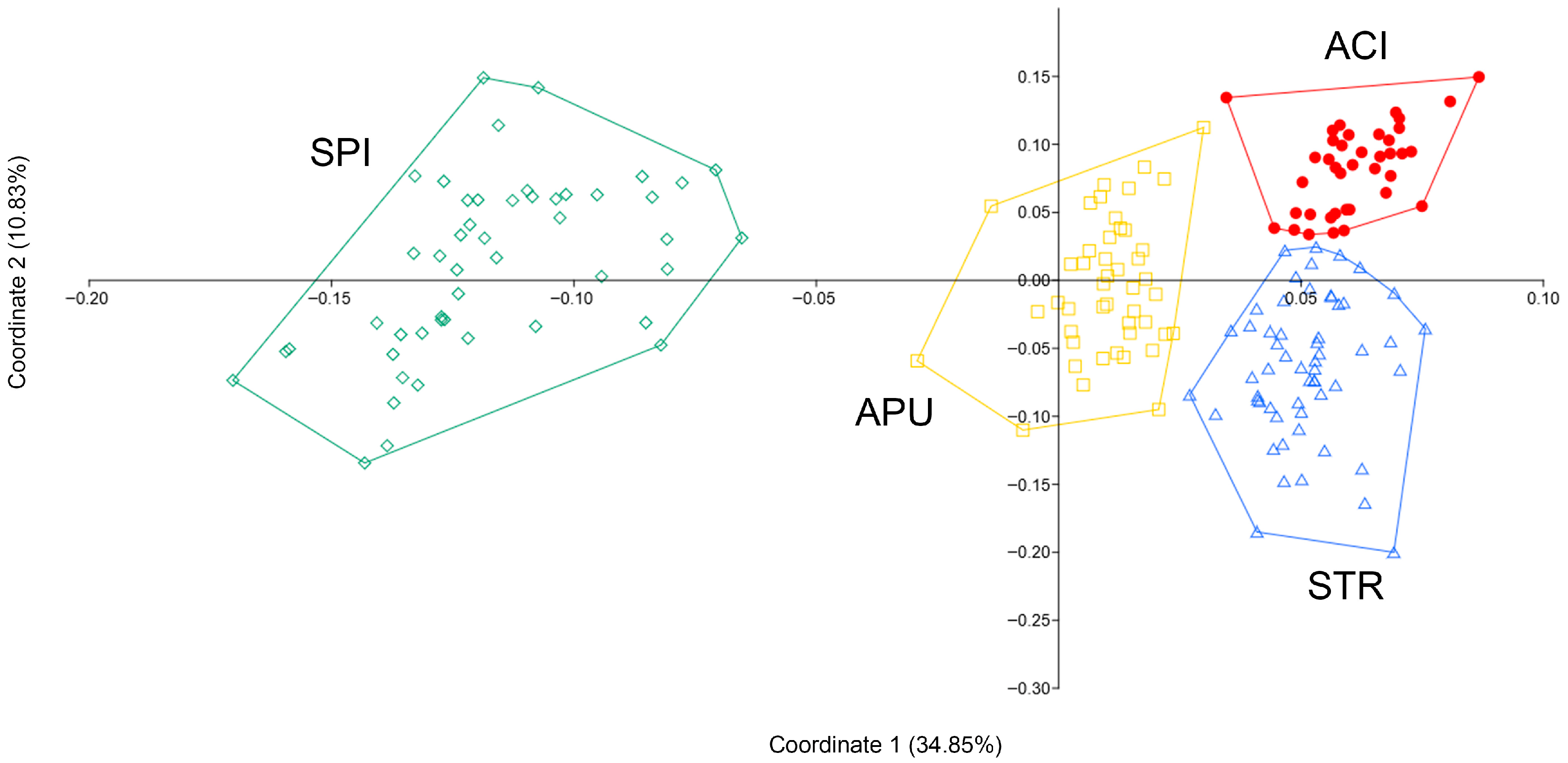
Morphometric Analyses
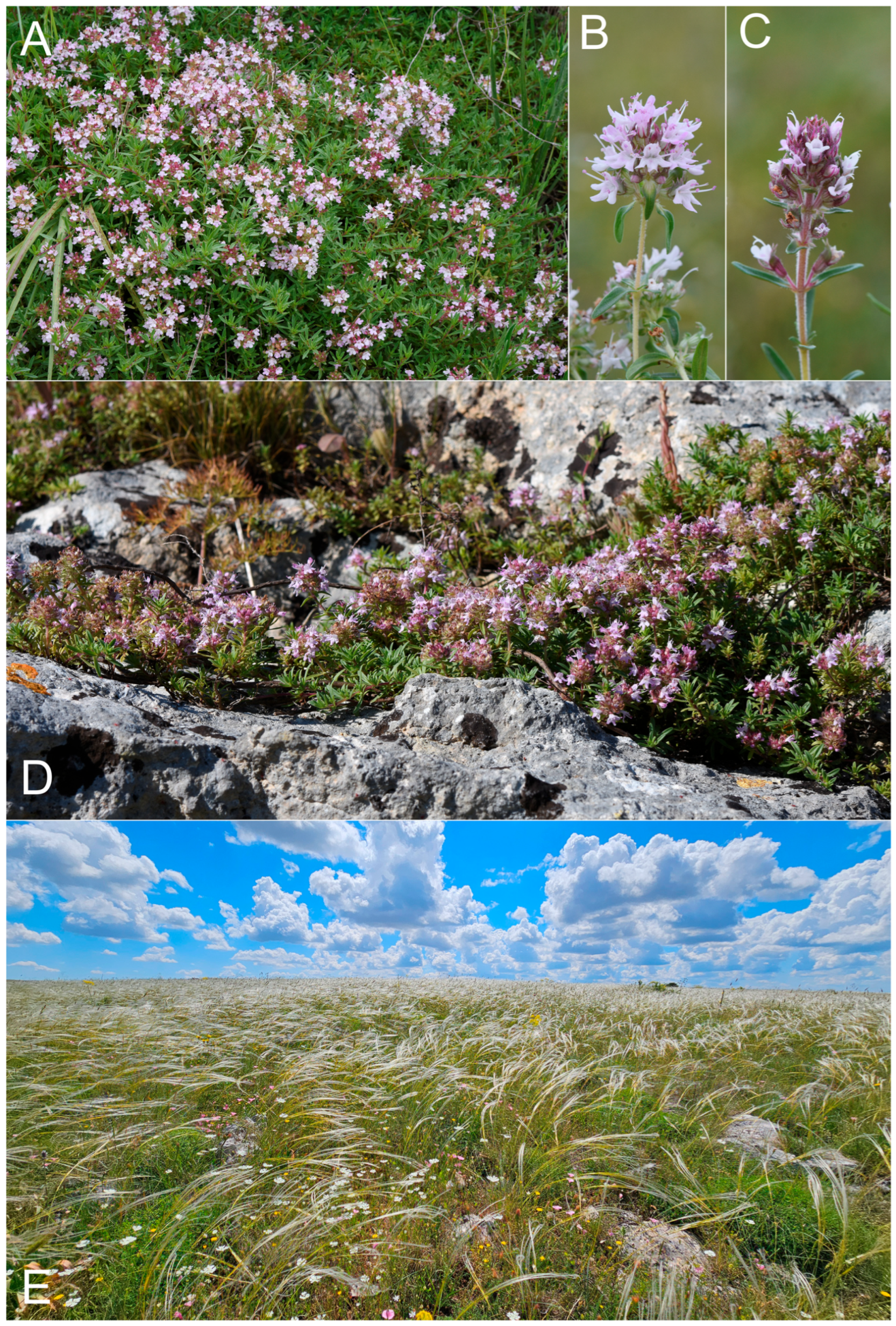
4. Taxonomic Treatment
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Federici, S.; Galimberti, A.; Bartolucci, F.; Bruni, I.; De Mattia, F.; Cortis, P.; Labra, M. DNA barcoding to analyse taxonomically complex groups in plants: The case of Thymus (Lamiaceae). Bot. J. Linn. Soc. 2013, 171, 687–699. [Google Scholar] [CrossRef]
- Karbstein, K.; Kösters, L.; Hodač, L.; Hofmann, M.; Hörandl, E.; Tomasello, S.; Wagner, N.D.; Emerson, B.C.; Albach, D.C.; Scheu, S.; et al. Species delimitation 4.0: Integrative taxonomy meets artificial intelligence. Trends Ecol. Evol. 2024, 39, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Bartolucci, F.; Nachychko, V. The Euro+Med treatment of Thymus (Lamiaceae)—Nomenclature, typification of names and distribution updates. Willdenowia 2025, 55, 295–304. [Google Scholar] [CrossRef]
- POWO. Plants of the World Online, Facilitated by the Royal Botanic Gardens, Kew. Available online: http://www.plantsoftheworldonline.org/ (accessed on 1 September 2025).
- WFO. World Flora Online. Available online: http://www.worldfloraonline.org (accessed on 1 September 2025).
- Sáez, L.; Bogunić, F.; Cambria, S.; Riera, J.; Bogdanović, S. On the identity of Thymus humifusus var. aureopunctatus (Lamiaceae) and taxonomic notes on the Th. richardii complex. PhytoKeys 2021, 186, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Bartolucci, F.; Peruzzi, L.; Galasso, G.; Alessandrini, A.; Ardenghi, N.M.G.; Bacchetta, G.; Banfi, E.; Barberis, G.; Bernardo, L.; Bouvet, D.; et al. A second update to the checklist of the vascular flora native to Italy. Plant Biosyst. 2024, 158, 219–296. [Google Scholar] [CrossRef]
- Bartolucci, F. Thymus L. In Flora d’Italia, 2nd ed.; Pignatti, S., Guarino, R., La Rosa, M., Eds.; New Business Media: Milano, Italy, 2018; Volume 3, pp. 278–291. [Google Scholar]
- Barina, Z.; Somogyi, G.; Pifkó, D.; Rakaj, M. Checklist of vascular plants of Albania. Phytotaxa 2018, 378, 1–339. [Google Scholar] [CrossRef]
- Baden, C. Thymus L. In Mountain Flora of Greece; Strid, A., Tan, K., Eds.; Edinburgh University Press: Edinburgh, UK, 1991; Volume 2, pp. 139–165. [Google Scholar]
- Jalas, J. Thymus L. In Flora of Turkey and the East Aegean Islands; Davis, P.H., Ed.; Edinburgh University Press: Edinburgh, UK, 1982; Volume 7, pp. 349–382. [Google Scholar]
- Ronniger, K. Thymus L. In Prodromus Florae Peninsulae Balcanicae 2; Hayek, A.v., Ed.; Repertorium Specierum Novarum Regni Vegetabilis; Beihefte; Verlag des Repertoriums: Dahlem, Germany, 1930; Volume 30, pp. 337–382. [Google Scholar]
- Matevski, V. Thymus L. In The Flora of the Rn Macedonia; Matevski, V., Ed.; Macedonian Academy of Sciences and Arts: Skopje, Macedonia, 2021; Volume 2, pp. 353–386. [Google Scholar]
- Dimopoulos, P.; Raus, T.; Strid, A. Flora of Greece Web. Vascular Plants of Greece. An Annotated Checklist. Version VI (September 2024). Available online: http://portal.cybertaxonomy.org/flora-greece (accessed on 1 October 2025).
- Markova, M. Thymus L. In Flora Reipublicae Popularis Bulgaricae; Velčev, V., Ed.; Aedibus Academiae Scientiarum Bulgaricae: Serdicae, Bulgaria, 1989; Volume 9, pp. 288–331. [Google Scholar]
- Guşuleac, M. Thymus L. In Flora Republicii Socialiste România; Săvulescu, T., Ed.; Editura Academiei Republicii Populare Române: Bucuresti, Romania, 1961; Volume 8, pp. 301–334. [Google Scholar]
- Nikolić, T. Flora Croatica—Vaskularna Flora Republike Hrvatske; ALFA d.d.: Zagreb, Croatia, 2020; Volume 3, p. 794. [Google Scholar]
- Diklic, N. Thymus L. In Flora SR Srbije; Josifovic, M., Ed.; Serbian Academy of Scienceand Art: Belgrade, Serbia, 1974; Volume 6, pp. 475–509. [Google Scholar]
- Pavela, R.; Bartolucci, F.; Desneux, N.; Lavoir, A.V.; Canale, A.; Maggi, F.; Benelli, G. Chemical profiles and insecticidal efficacy of the essential oils from four Thymus taxa growing in central-southern Italy. Ind. Crops Prod. 2019, 138, 111460. [Google Scholar] [CrossRef]
- Morales, R. The history, botany and taxonomy of the genus Thymus. In Thyme: The Genus Thymus; Stahl-Biskup, E., Sáez, F., Eds.; Taylor & Francis: London, UK, 2002; pp. 1–43. [Google Scholar]
- Bartolucci, F.; Peruzzi, L. Thymus paronychioides (Lamiaceae), a Neglected Species from Sicily Belonging to Thymus sect. Hyphodromi. Folia Geobot. 2014, 49, 83–106. [Google Scholar] [CrossRef]
- Bartolucci, F.; Domina, G. The genus Thymus (Lamiaceae) in Sicily. Plant Biosyst. 2015, 149, 710–719. [Google Scholar] [CrossRef]
- Stoyanov, S.; Marinov, Y. Thymus jalasianus (Lamiaceae), a New Species from the Serpentine Area of the Eastern Rhodope Mountains, Bulgaria. Ann. Bot. Fenn. 2020, 57, 163–172. [Google Scholar] [CrossRef]
- El Mokni, R.; Domina, G.; Barone, G. Taxonomic, nomenclatural, and conservation notes to the Algerian-Tunisian endemic Thymus numidicus (Nepetoideae, Lamiaceae). Feddes Repert. 2023, 134, 289–297. [Google Scholar] [CrossRef]
- Dobignard, A.; Chatelain, C. Index Synonymique de la Flore d’Afrique du Nord; Conservatoire et Jardin Botaniques: Genève, Switzerland, 2012; Volume 4. [Google Scholar]
- Morales, R. El género Thymus L. (Labiatae) en África. An. Jardín Botánico Madr. 1994, 51, 205–236. [Google Scholar]
- Morales, R. Taxonomía de los géneros Thymus (excluida la sección SerpyIlum) y Thymbra en la Península Ibérica. Ruizia 1986, 3, 1–324. [Google Scholar]
- Menitsky, Y. Survey of the genus Thymus L. (Lamiaceae) species in the flora of Caucasus. Novit. Syst. Plant. Vasc. 1986, 23, 117–142. [Google Scholar]
- Ciocârlan, V. Flora Illustrata a României: Pteridophyta et Spermatophyta [Romanian Illustrated Flora: Pteridophyta et Spermatophyta], 3rd ed.; Ceres: Bucureşti, Romania, 2009; p. 1141. [Google Scholar]
- Jalas, J. Notes on Thymus L. (Labiatae). I. Supraspecific classification and nomenclature. Bot. J. Linn. Soc. 1971, 64, 199–215. [Google Scholar]
- Jalas, J. Turkish taxa of Thymus (Labiatae) described as new or revised. Ann. Bot. Fenn. 1980, 17, 315–324. [Google Scholar]
- Bartolucci, F.; Walter, J. Typification of names in the Genus Thymus (Lamiaceae). Phytotaxa 2015, 221, 137–147. [Google Scholar] [CrossRef]
- Stoyanov, S.; Marinov, Y. Thymus aznavourii Velen. (Lamiaceae): First Records for Bulgarian and Greek Flora. Comptes Rendus L’acade’mie Bulg. Sci. 2021, 74, 352–362. [Google Scholar] [CrossRef]
- Palanza, A. Flora della Terra di Bari. In La Terra di Bari; Jatta, A., Ed.; Tipografia dell’Editore V. Vecchi: Trani, Italy, 1900; Volume III, pp. 1–90. [Google Scholar]
- Messeri, A. Erborizzazione durante una gita al Pulo di Altamura. Nuovo G. Bot. Ital. ns 1948, 55, 578–579. [Google Scholar]
- Terzi, M.; Forte, L.; Cavallaro, V.; Lattanzi, A.; Macchia, F. Ecological factors of biodiversity for the Mediterranean steppic grassland of Murgia (Apulia Italy). In Italo-Albanian Cooperation for the Enhancement of Plant Biodiversity; Ricciardi, L., Myrta, A., Castro, F., Eds.; CIHEAM: Bari, Italy, 2001; Volume 47, pp. 73–90. [Google Scholar]
- Forte, L.; Perrino, E.V.; Terzi, M. Le praterie a Stipa austroitalica Martinovsky ssp. austroitalica dell’Alta Murgia (Puglia) e della Murgia Materana (Basilicata). Fitosociologia 2005, 42, 83–103. [Google Scholar]
- Scaramuzzi, F. La Vegetazioine della Murgia di S. Elio. G. Bot. Ital. ns 1952, 59, 361–367. [Google Scholar] [CrossRef]
- Tomaselli, V.; Silletti, G.; Forte, L. A new association of Satureja montana L. subsp. montana dominated garrigues in Puglia (SE Italy). Plant Sociol. 2021, 58, 1–14. [Google Scholar] [CrossRef]
- Perrino, E.V.; Wagensommer, R.P. Habitats of Directive 92/43/EEC in the National Park of Alta Murgia (Apulia—Southern Italy): Threat, Action and Relationships with Plant Communities. J. Environ. Sci. Eng. 2013, 2, 229–235. [Google Scholar]
- Perrino, E.V.; Valerio, F.; Jallali, S.; Trani, A.; Mezzapesa, G.N. Ecological and Biological Properties of Satureja cuneifolia Ten. and Thymus spinulosus Ten.: Two Wild Officinal Species of Conservation Concern in Apulia (Italy). A Preliminary Survey. Plants 2021, 10, 1952. [Google Scholar] [CrossRef]
- Bianco, P. Flora e vegetazione delle Murge di Nord-Ovest. Ann. Della Fac. Agrar. Dell’università Bari 1962, 16, 459–640. [Google Scholar]
- Terzi, M.; Di Pietro, R.; D’Amico, F.S. Application of Indicator Species Analysis to communities with Stipa austroitalica Martinovsky and relevant syntaxonomic problems. Fitosociologia 2010, 47, 3–29. [Google Scholar]
- Di Pietro, R.; Silletti, G.; Wagensommer, R.P.; Misano, G. Juncus valvatus (Juncaceae) new for the Italian Flora. Flora Mediterr. 2009, 19, 241–250. [Google Scholar]
- Bartolucci, F.; Oriolo, G.; Casolo, V. Typification and taxonomy of Thymus carstiensis (Lamiaceae). Plant Biosyst. 2017, 151, 737–744. [Google Scholar] [CrossRef]
- Thiers, B.M. Index Herbariorum. Available online: https://sweetgum.nybg.org/science/ih (accessed on 1 April 2025).
- Darwin, C. The Different Forms of Flowers on Plants of the Same Species; John Murray: London, UK, 1877. [Google Scholar]
- Thompson, J.D.; Rolland, A.G.; Prugnolle, F. Genetic variation for sexual dimorphism in flower size within and between populations of gynodioecious Thymus vulgaris. J. Evol. Biol. 2002, 15, 362–372. [Google Scholar] [CrossRef]
- Stakelienė, V.; Ložienė, K. Gynodioecy in Thymus pulegioides L., T. serpyllum L., and their hybrid T. × oblongifolius Opiz (Lamiaceae): Flower size dimorphism, female frequency, and effect of environmental factors. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2014, 148, 49–57. [Google Scholar] [CrossRef]
- Gordeeva, N.I.; Talovskaya, E.B. Breeding System of Thymus mongolicus (Lamiaceae). Rastit. Resur. 2023, 59, 129–136. [Google Scholar] [CrossRef]
- Nachychko, V.O.; Sosnovsky, Y.V.; Honcharenko, V.I. First record of Balkan Thymus jankae (Lamiaceae) from Ukraine, with taxonomic remarks on the species. Bot. Lett. 2019, 166, 41–50. [Google Scholar] [CrossRef]
- IBM. IBM SPSS Statistics for Windows, Version 25.0; IBM Corp.: Armonk, NY, USA, 2017.
- Gower, J.C. A general coefficient of similarity and some of its properties. Biometrics 1971, 27, 857–874. [Google Scholar] [CrossRef]
- Podani, J. Extending Gower’s general coefficient of similarity to ordinal characters. Taxon 1999, 48, 331–340. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Tomaselli, V.; Sciandrello, S.; Minissale, P.; Forte, L.; Costanzo, E.; Giusso del Galdo, G.; Carruggio, F.; Pazienza, G.; Brullo, S. Syntaxonomical Remarks on the Garrigues from Apulia (S Italy) and Neighboring Territories. Plants 2024, 13, 1800. [Google Scholar] [CrossRef]
- Bachman, S.; Moat, J.; Hill, A.W.; de la Torre, J.; Scott, B. Supporting Red List threat assessments with GeoCAT: Geospatial conservation assessment tool. ZooKeys 2011, 150, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Angelini, P.; Casella, L.; Carli, E. IV report direttiva habitat: Habitat. In Rapporti Direttive Natura (2013–2018). Sintesi Dello Stato di Conservazione Delle Specie e Degli Habitat di Interesse Comunitario e Delle Azioni di Contrasto Alle Specie Esotiche di Rilevanza Unionale in Italia; Ercole, S., Angelini, P., Carnevali, L., Casella, L., Giacanelli, V., Grignetti, A., La Mesa, G., Nardelli, R., Serra, L., Stoch, F., et al., Eds.; ISPRA: Roma, Italy, 2021. [Google Scholar]
- Labadessa, R.; Ancillotto, L.; Adamo, M.P.; Forte, L.; Vicario, S.; Zollo, L.; Tarantino, C. Echoes of the past: Agricultural legacies shape the successional dynamics of protected semi-natural dry grasslands. Sci. Total Environ. 2023, 905, 166990. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, C.; Aquilino, M.; Labadessa, R.; Adamo, M. Time Series of Land Cover Mappings Can Allow the Evaluation of Grassland Protection Actions Estimated by Sustainable Development Goal 15.1.2 Indicator: The Case of Murgia Alta Protected Area. Remote Sens. 2023, 15, 505. [Google Scholar] [CrossRef]
- Regione Puglia. Rete Natura 2000. Distribuzione di Habitat e Specie Animali e Vegetali Presenti nel Territorio Della Regione Puglia. Available online: https://pugliacon.regione.puglia.it/web/sit-puglia-sit/habitat-e-specie-animali-e-vegetali1 (accessed on 24 October 2025).
- IUCN. IUCN Guidelines for Using the IUCN Red List Categories and Criteria. Version 16. Available online: https://www.iucnredlist.org/documents/RedListGuidelines.pdf (accessed on 30 March 2024).
- Peruzzi, L.; Conti, F.; Bartolucci, F. An inventory of vascular plants endemic to Italy. Phytotaxa 2014, 168, 1–75. [Google Scholar] [CrossRef]
- Boschin, M.; Schönswetter, P.; Frajman, B. Genetic and morphological differentiation within Euphorbia japygica (Euphorbiaceae) suggests divergence of populations from the south-eastern Apennine Peninsula. Bot. J. Linn. Soc. 2024, 205, 38–54. [Google Scholar] [CrossRef]

| Characters Code | Description of the Characters [Unit of Measure or Characters States] | Type |
|---|---|---|
| CC | Corolla color: 0 = white; 1 = pink to purplish | Qualitative |
| EH | Eglandular trichomes on the adaxial, middle cauline leaf, surface: 0 = absent; 1 = present | Qualitative |
| OD | Sessile oil glands (leaves, calyx, and corolla): 0 = rare; 1 = abundant | Qualitative |
| LV | Lateral veins of leaves (best seen in dried specimens): 0 = parallel; 1 = non-parallel | Qualitative |
| IS | Inflorescence shape: 0 = capituliform to elongated (basal verticillaster remote); 1 = interrupted (2–3 verticillasters remote below oblong terminal head) | Qualitative |
| FBL | Flowering branches length (without inflorescence) (mm) | Quantitative continuous |
| LW | Middle cauline leaf width (mm) | Quantitative continuous |
| LL | Middle cauline leaf length (mm) | Quantitative continuous |
| IL | Inflorescence length (mm) | Quantitative continuous |
| FL | Corolla length (mm) | Quantitative continuous |
| PL | Pedicel length (mm) | Quantitative continuous |
| BPL | Bracteole of the pedicel length (mm) | Quantitative continuous |
| CL | Calyx length (mm) | Quantitative continuous |
| CTL | Calyx tube length (mm) | Quantitative continuous |
| UCT | Upper calyx teeth length (mm) | Quantitative continuous |
| LCT | Lower calyx teeth length (mm) | Quantitative continuous |
| UL | Upper limb length (mm) | Quantitative continuous |
| BL | Bract length (second verticillaster of the inflorescence) (mm) | Quantitative continuous |
| BW | Bract width (second verticillaster of the inflorescence) (mm) | Quantitative continuous |
| FN | Number of flowers (single inflorescence) | Quantitative discrete |
| VN | Number of verticillasters (single inflorescence) | Quantitative discrete |
| LL/LW | Middle cauline leaf length/Middle cauline leaf width | Ratio |
| CTL/CL | Calyx tube length/calyx length | Ratio |
| LCT/UL | Lower calyx teeth length/upper limb length | Ratio |
| BW/LW | Bract width/Middle cauline leaf width | Ratio |
| BL/BW | Bract length/bract width | Ratio |
| ACI | APU | SPI | STR | Total | |
|---|---|---|---|---|---|
| ACI | 39 | 0 | 0 | 0 | 39 |
| APU | 0 | 40 | 4 | 2 | 46 |
| SPI | 2 | 4 | 41 | 2 | 49 |
| STR | 3 | 3 | 2 | 48 | 56 |
| Total | 44 | 47 | 47 | 52 | 190 |
| Character Code | Thymus from Murge Area (APU) | T. spinulosus (SPI) | T. striatus subsp. striatus (STR) | T. acicularis subsp. acicularis (ACI) |
|---|---|---|---|---|
| CC | pink | white | pink to purplish | pink to purplish |
| EH | absent or present | present | absent | absent |
| OD | rare | abundant | rare | rare |
| LV | non-parallel | non-parallel | parallel | parallel |
| IS | capituliform to elongated (rarely interrupted) | elongated to interrupted | capituliform (rarely elongated) | capituliform (rarely elongated) |
| FBL | 46.80 ± 19.15 | 46 ± 21.82 | 51.93 ± 18.33 | 36.00 ± 15.5 |
| LW | 1.77 ± 0.28 | 1.76 ± 0.38 | 1.89 ± 0.36 | 1.14 ± 0.21 |
| LL | 9.57 ± 1.92 | 10.1 ± 1.92 | 9.99 ± 1.76 | 8.29 ± 1.98 |
| IL | 15.58 ± 7.97 | 26.18 ± 13.35 | 15.82 ± 6.28 | 8.23 ± 3.34 |
| FL | 5.91 ± 0.58 | 5.61 ± 0.48 | 5.75 ± 0.46 | 4.83 ± 0.54 |
| PL | 2.04 ± 0.55 | 2.11 ± 0.64 | 1.61 ± 0.48 | 1.26 ± 0.36 |
| BPL | 1.17 ± 0.33 | 1.4 ± 0.37 | 1.41 ± 0.40 | 1.05 ± 0.35 |
| CL | 4.71 ± 0.45 | 4.55 ± 0.42 | 4.86 ± 0.46 | 3.87 ± 0.33 |
| CTL | 1.80 ± 0.26 | 1.81 ± 0.24 | 1.88 ± 0.24 | 1.44 ± 0.18 |
| UCT | 1.14 ± 0.23 | 1.09 ± 0.15 | 1.44 ± 0.21 | 1.13 ± 0.21 |
| LCT | 2.37 ± 0.39 | 2.21 ± 0.24 | 2.58 ± 0.31 | 2.02 ± 0.20 |
| UL | 2.89 ± 0.33 | 2.76 ± 0.31 | 2.97 ± 0.30 | 2.44 ± 0.29 |
| BL | 5.57 ± 1.04 | 6.44 ± 0.89 | 7.44 ± 1.45 | 5.39 ± 0.76 |
| BW | 2.32 ± 0.43 | 2.22 ± 0.38 | 3.67 ± 0.59 | 2.43 ± 0.43 |
| VN | 3–7 | 4–9 | 3–6 | 2–4 |
| FN | 10–60 | 20–70 | 16–68 | 8–36 |
| LL/LW | 5.45 ± 0.92 | 5.94 ± 1.32 | 5.37 ± 0.95 | 7.5 ± 2.29 |
| CTL/CL | 0.31 ± 0.08 | 0.49 ± 0.15 | 0.39 ± 0.03 | 0.37 ± 0.04 |
| LCT/UL | 0.82 ± 0.10 | 0.81 ± 0.09 | 0.87 ± 0.08 | 0.84 ± 0.09 |
| BW/LW | 1.33 ± 0.27 | 1.31 ± 0.32 | 2.00 ± 0.45 | 2.19 ± 0.47 |
| BL/BW | 2.45 ± 0.57 | 2.97 ± 0.63 | 2.07 ± 0.51 | 2.26 ± 0.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartolucci, F.; Conti, F. Thymus apulus (T. sect. Hyphodromi, Lamiaceae), a New Species from Southern Italy. Plants 2025, 14, 3584. https://doi.org/10.3390/plants14233584
Bartolucci F, Conti F. Thymus apulus (T. sect. Hyphodromi, Lamiaceae), a New Species from Southern Italy. Plants. 2025; 14(23):3584. https://doi.org/10.3390/plants14233584
Chicago/Turabian StyleBartolucci, Fabrizio, and Fabio Conti. 2025. "Thymus apulus (T. sect. Hyphodromi, Lamiaceae), a New Species from Southern Italy" Plants 14, no. 23: 3584. https://doi.org/10.3390/plants14233584
APA StyleBartolucci, F., & Conti, F. (2025). Thymus apulus (T. sect. Hyphodromi, Lamiaceae), a New Species from Southern Italy. Plants, 14(23), 3584. https://doi.org/10.3390/plants14233584







