Evaluating the Role of Tobacco Stalk Biochar in Wheat Growth Under Microplastic Exposure
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Design
2.3. Analysis of Soil Physicochemical Properties
2.4. Analysis of Growth and Physiological Traits in Wheat Seedlings
2.5. Statistical Analysis
3. Results
3.1. Effects of TSB on Soil Physicochemical Properties Under PE-MP Contamination
3.2. TSB Amendment Modulates Wheat Physiological Traits Under PE-MP Stress
3.3. Effects of TSB on Growth of Wheat Under PE-MP Contamination
3.4. Biotic and Abiotic Drivers of Wheat Phenotypic Traits
4. Discussion
4.1. TSB Ameliorates Soil Properties Under PE-MP Stress
4.2. TSB Modulates Wheat Physiological Responses to PE-MPs
4.3. An Integrated Hypothetical Pathway: Linking Soil Improvement to Growth Recovery
4.4. Limitations and Future Perspectives
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, Y.; Liu, Q.; Jia, W.; Yan, C.; Wang, J. Agricultural plastic mulching as a source of microplastics in the terrestrial environment. Environ. Pollut. 2020, 260, 114096. [Google Scholar] [CrossRef]
- Wang, F.; Wong, C.S.; Chen, D.; Lu, X.; Wang, F.; Zeng, E.Y. Interaction of toxic chemicals with microplastics: A critical review. Water Res. 2018, 139, 208–219. [Google Scholar] [CrossRef]
- de Souza Machado, A.A.; Kloas, W.; Zarfl, C.; Hempel, S.; Rillig, M.C. Microplastics as an emerging threat to terrestrial ecosystems. Glob. Change Biol. 2018, 24, 1405–1416. [Google Scholar] [CrossRef]
- Rillig, M.C.; Lehmann, A. Microplastic in terrestrial ecosystems. Science 2020, 368, 1430–1431. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Yang, X.; Pelaez, A.M.; Huerta Lwanga, E.; Beriot, N.; Gertsen, H.; Garbeva, P.; Geissen, V. Macro- and micro- plastics in soil-plant system: Effects of plastic mulch film residues on wheat (Triticum aestivum) growth. Sci. Total Environ. 2018, 645, 1048–1056. [Google Scholar] [CrossRef]
- Lian, J.; Wu, J.; Xiong, H.; Zeb, A.; Yang, T.; Su, X.; Su, L.; Liu, W. Impact of polystyrene nanoplastics (PSNPs) on seed germination and seedling growth of wheat (Triticum aestivum L.). J. Hazard. Mater. 2020, 385, 121620. [Google Scholar] [CrossRef]
- Dong, Y.; Gao, M.; Qiu, W.; Song, Z. Effect of microplastics and arsenic on nutrients and microorganisms in rice rhizosphere soil. Ecotoxicol. Environ. Saf. 2021, 211, 111899. [Google Scholar] [CrossRef]
- Kerner, P.; Struhs, E.; Mirkouei, A.; Aho, K.; Lohse, K.A.; Dungan, R.S.; You, Y. Microbial responses to biochar soil amendment and influential factors: A three-level meta-analysis. Environ. Sci. Technol. 2023, 57, 19838–19848. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.; Chi, J. Impact of biochar coexistence with polar/nonpolar microplastics on phenanthrene sorption in soil. J. Hazard. Mater. 2023, 447, 130761. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Cowie, A.; Masiello, C.A.; Kammann, C.; Woolf, D.; Amonette, J.E.; Cayuela, M.L.; Camps-Arbestain, M.; Whitman, T. Biochar in climate change mitigation. Nat. Geosci. 2021, 14, 883–892. [Google Scholar] [CrossRef]
- Manikandan, S.; Vickram, S.; Subbaiya, R.; Karmegam, N.; Woong Chang, S.; Ravindran, B.; Kumar Awasthi, M. Comprehensive review on recent production trends and applications of biochar for greener environment. Bioresour. Technol. 2023, 388, 129725. [Google Scholar] [CrossRef]
- Campion, L.; Bekchanova, M.; Malina, R.; Kuppens, T. The costs and benefits of biochar production and use: A systematic review. J. Clean. Prod. 2023, 408, 137138. [Google Scholar] [CrossRef]
- Bolan, S.; Sharma, S.; Mukherjee, S.; Kumar, M.; Rao, C.S.; Nataraj, K.C.; Singh, G.; Vinu, A.; Bhowmik, A.; Sharma, H.; et al. Biochar modulating soil biological health: A review. Sci. Total Environ. 2024, 914, 169585. [Google Scholar] [CrossRef]
- Luo, L.; Wang, J.; Lv, J.; Liu, Z.; Sun, T.; Yang, Y.; Zhu, Y. Carbon sequestration strategies in soil using biochar: Advances, challenges, and opportunities. Environ. Sci. Technol. 2023, 57, 11357–11372. [Google Scholar] [CrossRef]
- Sun, F.; Deng, X.; Jiao, Y.; Li, J.; Li, J.; Xing, H.; Tong, W.; Ma, E.; Zhang, Y.; Xiong, Z. Biochar-based fertilizer enhances carbon neutrality and net ecosystem economic benefit in tobacco (Nicotiana tabacum L) production. J. Environ. Manag. 2025, 387, 125862. [Google Scholar] [CrossRef]
- Yu, X.; Zhou, H.; Ye, X.; Wang, H. From hazardous agriculture waste to hazardous metal scavenger: Tobacco stalk biochar-mediated sequestration of Cd leads to enhanced tobacco productivity. J. Hazard. Mater. 2021, 413, 125303. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Wang, J.; Pan, Y.; Shen, B.; Wu, C. Review of biochar for the management of contaminated soil: Preparation, application and prospect. Sci. Total Environ. 2019, 659, 473–490. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhou, Y.; Wang, G.; Dai, K.; Li, J.; Song, X.; Xu, Y.; Cui, Y.; Yang, X. Biochar-based organic fertilizer application promotes the alleviation of tobacco (Nicotiana tabacum L.) continuous cropping obstacles by improving soil chemical properties and microbial community structure. BMC Plant Biol. 2025, 25, 271. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Zhan, W.; Zheng, K.; Wang, J.; Zhang, C.; Chen, R. Stabilization of heavy metal-contaminated soils by biochar: Challenges and recommendations. Sci. Total Environ. 2020, 729, 139060. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, Z.; Xu, L.; Buyong, F.; Chay, T.C.; Li, Z.; Cai, Y.; Hu, B.; Zhu, Y.; Wang, X. Modified biochar: Synthesis and mechanism for removal of environmental heavy metals. Carbon Res. 2022, 1, 8. [Google Scholar] [CrossRef]
- Xiang, L.; Harindintwali, J.D.; Wang, F.; Redmile-Gordon, M.; Chang, S.X.; Fu, Y.; He, C.; Muhoza, B.; Brahushi, F.; Bolan, N.; et al. Integrating biochar, bacteria, and plants for sustainable remediation of soils contaminated with organic pollutants. Environ. Sci. Technol. 2022, 56, 16546–16566. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Meng, Q.; Peng, W.; Shi, J.; Dong, Z.; Li, Z.; Hu, Q.; Zhang, G.; Wang, L.; Ma, S.; et al. Application of functionalized biochar for adsorption of organic pollutants from environmental media: Synthesis strategies, removal mechanisms and outlook. J. Clean. Prod. 2023, 423, 138690. [Google Scholar] [CrossRef]
- Miao, J.; Chen, Y.; Zhang, E.; Yang, Y.; Sun, K.; Gao, B. Effects of microplastics and biochar on soil cadmium availability and wheat plant performance. GCB Bioenergy 2023, 15, 1046–1057. [Google Scholar] [CrossRef]
- Du, L.; Wu, D.; Yang, X.; Xu, L.; Tian, X.; Li, Y.; Huang, L.; Liu, Y. Joint toxicity of cadmium (II) and microplastic leachates on wheat seed germination and seedling growth. Environ. Geochem. Health 2024, 46, 166. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhong, H.; Liu, G.; Dai, Z.; Brookes, P.C.; Xu, J. Remediation of heavy metal contaminated soils by biochar: Mechanisms, potential risks and applications in China. Environ. Pollut. 2019, 252, 846–855. [Google Scholar] [CrossRef]
- Wu, C.; Wang, Y.; Clarke, J.L.; Su, H.; Wang, L.; Glazunova, O.A.; Moiseenko, K.V.; Zhang, L.; Mao, L.; Zhu, L.; et al. Biochar enhances the sorption and degradation of fluridone and its main metabolite in soil: Insights into biodegradation potential and remediation of microbial communities. Biochar 2025, 7, 81. [Google Scholar] [CrossRef]
- Dai, H.; Liang, T.; Li, C.; Luo, Z.; Guan, L.; Zhong, S.; Zhai, Z.; Bian, W.; Huang, W.; Zhang, Y. Effects of tobacco-stalk biochar returning on soil nutrients, the structure and functioning of bacterial communities in tobacco-planting yellow-brown fields. J. South. Agric. 2023, 54, 476–487, (In Chinese with English abstract). [Google Scholar]
- Bao, S.T. Soil Agrochemical Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2008. [Google Scholar]
- Yang, Y.; Tu, L.; Liao, Y.; Zhao, D.; Ye, S.; Luo, H.; Li, A.; Deng, H.; Hu, L. Phosphorus-modified biochar regulates CO2 emissions and bacterial communities in an incubation study of manganese-contaminated soils. Biomass Bioenergy 2025, 197, 107823. [Google Scholar] [CrossRef]
- Yue, L.; Liu, Y.; Li, G.; Deng, H.; Zhao, Y.; Feng, D.; Sun, H.; Yu, H.; Ge, C.; Chen, H. Convergence of soil bacterial communities with time and reduction of bacterial interaction complexity in response to meso- and microplastic stress. J. Environ. Chem. Eng. 2023, 11, 110447. [Google Scholar] [CrossRef]
- Zhang, N.; Xing, J.; Wei, L.; Liu, C.; Zhao, W.; Liu, Z.; Wang, Y.; Liu, E.; Ren, X.; Jia, Z.; et al. The potential of biochar to mitigate soil acidification: A global meta-analysis. Biochar 2025, 7, 49. [Google Scholar] [CrossRef]
- Liu, S.; Cen, B.; Yu, Z.; Qiu, R.; Gao, T.; Long, X. The key role of biochar in amending acidic soil: Reducing soil acidity and improving soil acid buffering capacity. Biochar 2025, 7, 52. [Google Scholar] [CrossRef]
- Arwenyo, B.; Varco, J.J.; Dygert, A.; Brown, S.; Pittman, C.U.; Mlsna, T. Contribution of modified P-enriched biochar on pH buffering capacity of acidic soil. J. Environ. Manag. 2023, 339, 117863. [Google Scholar] [CrossRef]
- Dey, S.; Purakayastha, T.J.; Sarkar, B.; Rinklebe, J.; Kumar, S.; Chakraborty, R.; Datta, A.; Lal, K.; Shivay, Y.S. Enhancing cation and anion exchange capacity of rice straw biochar by chemical modification for increased plant nutrient retention. Sci. Total Environ. 2023, 886, 163681. [Google Scholar] [CrossRef]
- Sun, P.; Chen, Y.; Liu, J.; Lu, S.; Guo, J.; Zhang, Z.; Zheng, X. Quantitative evaluation of the synergistic effect of biochar and plants on immobilization of Pb. J. Environ. Manag. 2022, 316, 115200. [Google Scholar] [CrossRef]
- Mushtaq, T.; Bano, A.; Ullah, A. Effects of rhizospheric microbes, growth regulators, and biochar in modulating antioxidant machinery of plants under stress. J. Plant Growth Regul. 2025, 44, 1846–1867. [Google Scholar] [CrossRef]
- Farhangi-Abriz, S.; Torabian, S. Biochar Increased Plant Growth-Promoting Hormones and Helped to Alleviates Salt Stress in Common Bean Seedlings. J. Plant Growth Regul. 2018, 37, 591–601. [Google Scholar] [CrossRef]
- Elbasiouny, H.; Mostafa, A.A.; Zedan, A.; Elbltagy, H.M.; Dawoud, S.F.M.; Elbanna, B.A.; El-Shazly, S.A.; El-Sadawy, A.A.; Sharaf-Eldin, A.M.; Darweesh, M.; et al. Potential effect of biochar on soil properties, microbial activity and Vicia faba properties affected by microplastics contamination. Agronomy 2023, 13, 149. [Google Scholar] [CrossRef]
- Anyanwu, I.N.; Alo, M.N.; Onyekwere, A.M.; Crosse, J.D.; Nworie, O.; Chamba, E.B. Influence of biochar aged in acidic soil on ecosystem engineers and two tropical agricultural plants. Ecotoxicol. Environ. Saf. 2018, 153, 116–126. [Google Scholar] [CrossRef]
- Joseph, S.; Cowie, A.L.; Van Zwieten, L.; Bolan, N.; Budai, A.; Buss, W.; Cayuela, M.L.; Graber, E.R.; Ippolito, J.A.; Kuzyakov, Y.; et al. How biochar works, and when it doesn’t: A review of mechanisms controlling soil and plant responses to biochar. GCB Bioenergy 2021, 13, 1731–1764. [Google Scholar] [CrossRef]
- Dinh, V.M.; Nguyen, H.T.; Nguyen, A.M.; Nguyen, T.T.; Nguyen, T.-L.; Uteau, D.; Nguyen, N.H.; Tran, T.M.; Dultz, S.; Nguyen, M.N. Pelletized rice-straw biochar as a slow-release delivery medium: Potential routes for storing and serving of phosphorus and potassium. J. Environ. Chem. Eng. 2022, 10, 107237. [Google Scholar] [CrossRef]
- Su, J.; Zhu, Y.; Chen, X.; Lu, X.; Yan, J.; Yan, L.; Zou, W. Biochar influences polyethylene microplastic-contaminated soil properties and enzyme activities. Agronomy 2024, 14, 2919. [Google Scholar] [CrossRef]
- Li, Y.; Ding, B.H.; Geng, X. Effect of biochar on microplastics penetration treatment within soil porous medium under the wetting-drying cycles and optimisation of soil-biochar mixing format. Sci. Total Environ. 2024, 935, 173194. [Google Scholar] [CrossRef] [PubMed]
- Chai, B.; Xiao, T.; Xiao, E.; Du, S.; Yang, S.; Yin, H.; Dang, Z.; Pan, K. Enhancing microplastics removal from soils using wheat straw and cow dung-derived biochars. J. Clean. Prod. 2024, 470, 143288. [Google Scholar] [CrossRef]
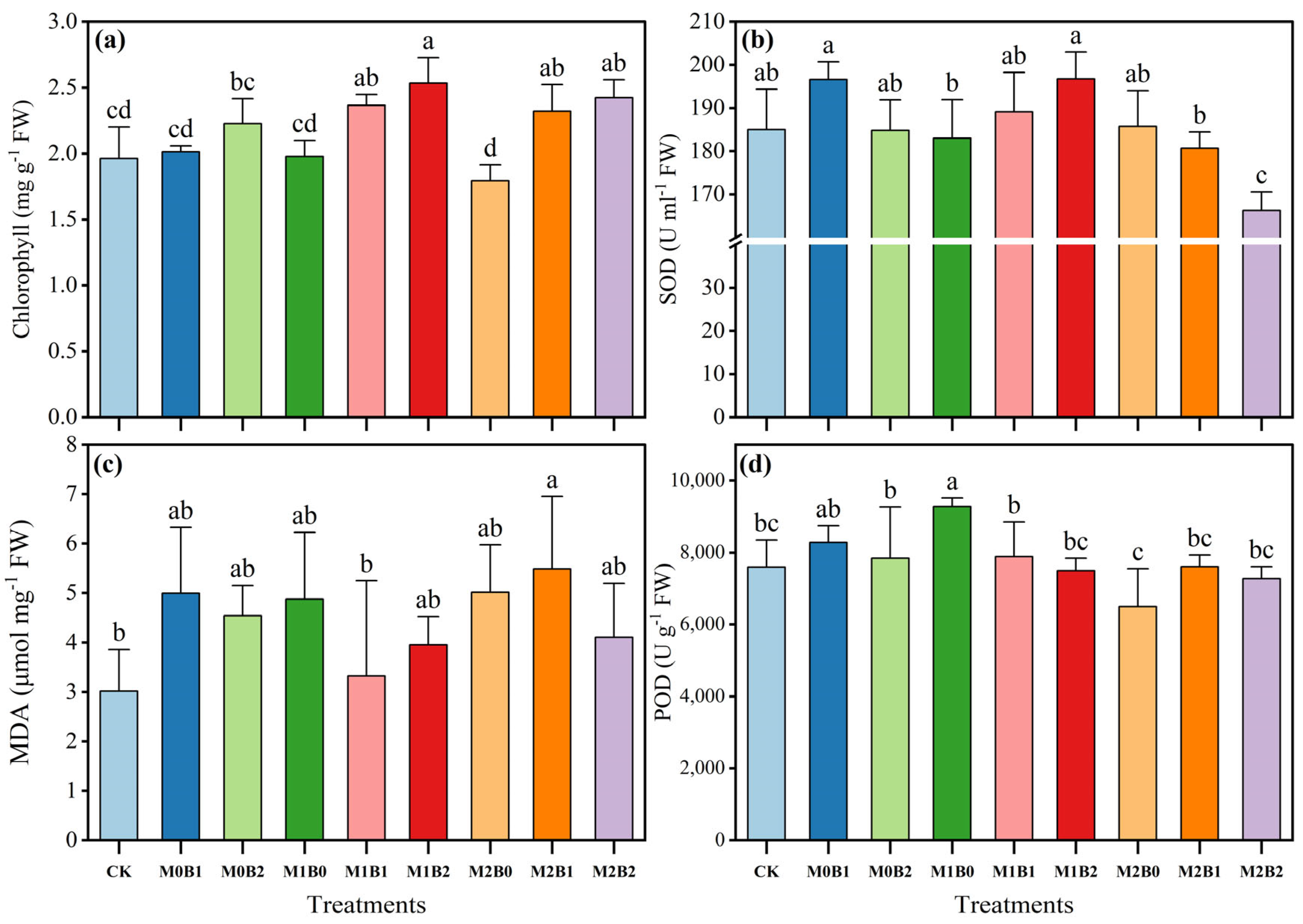
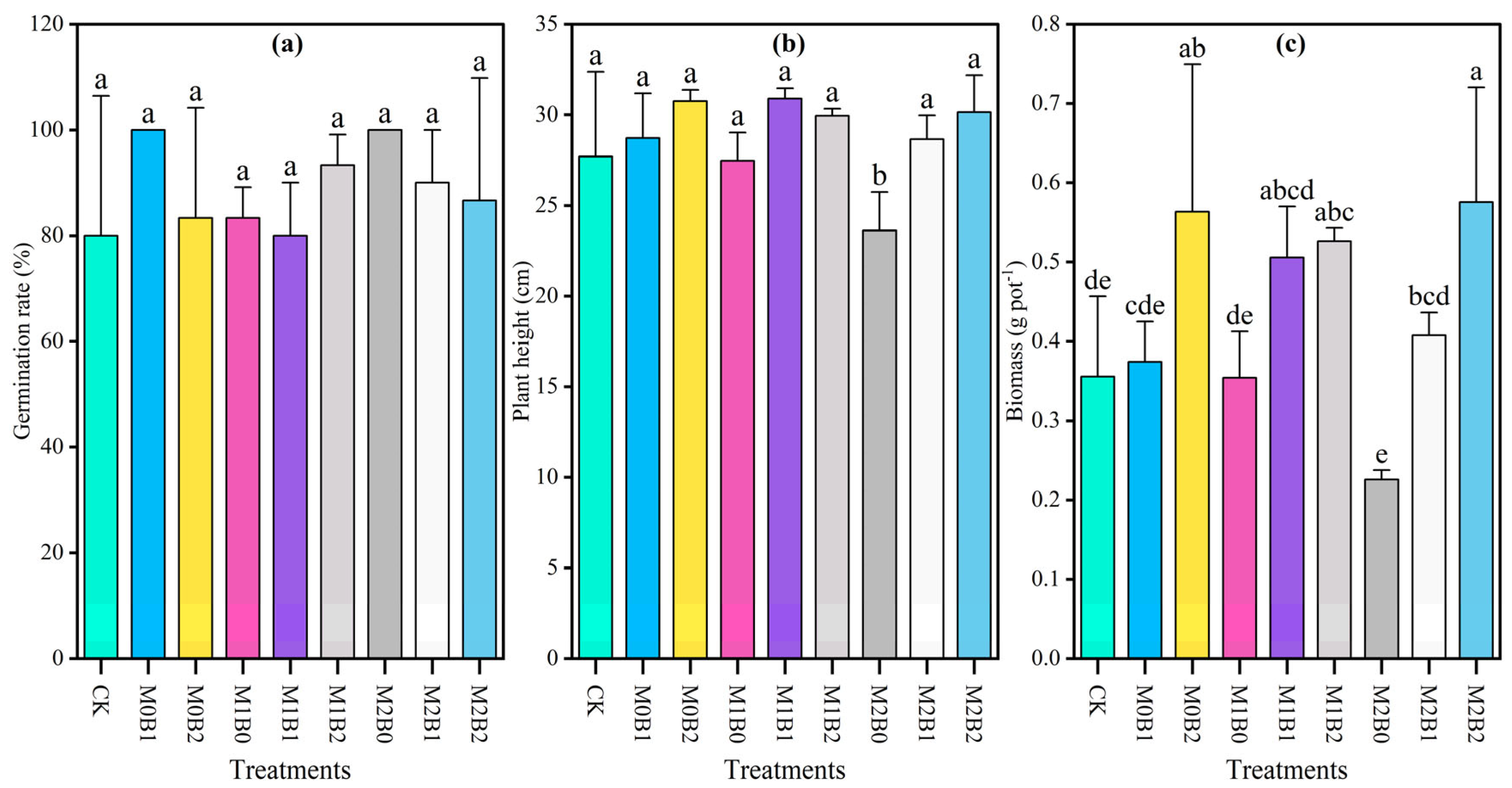
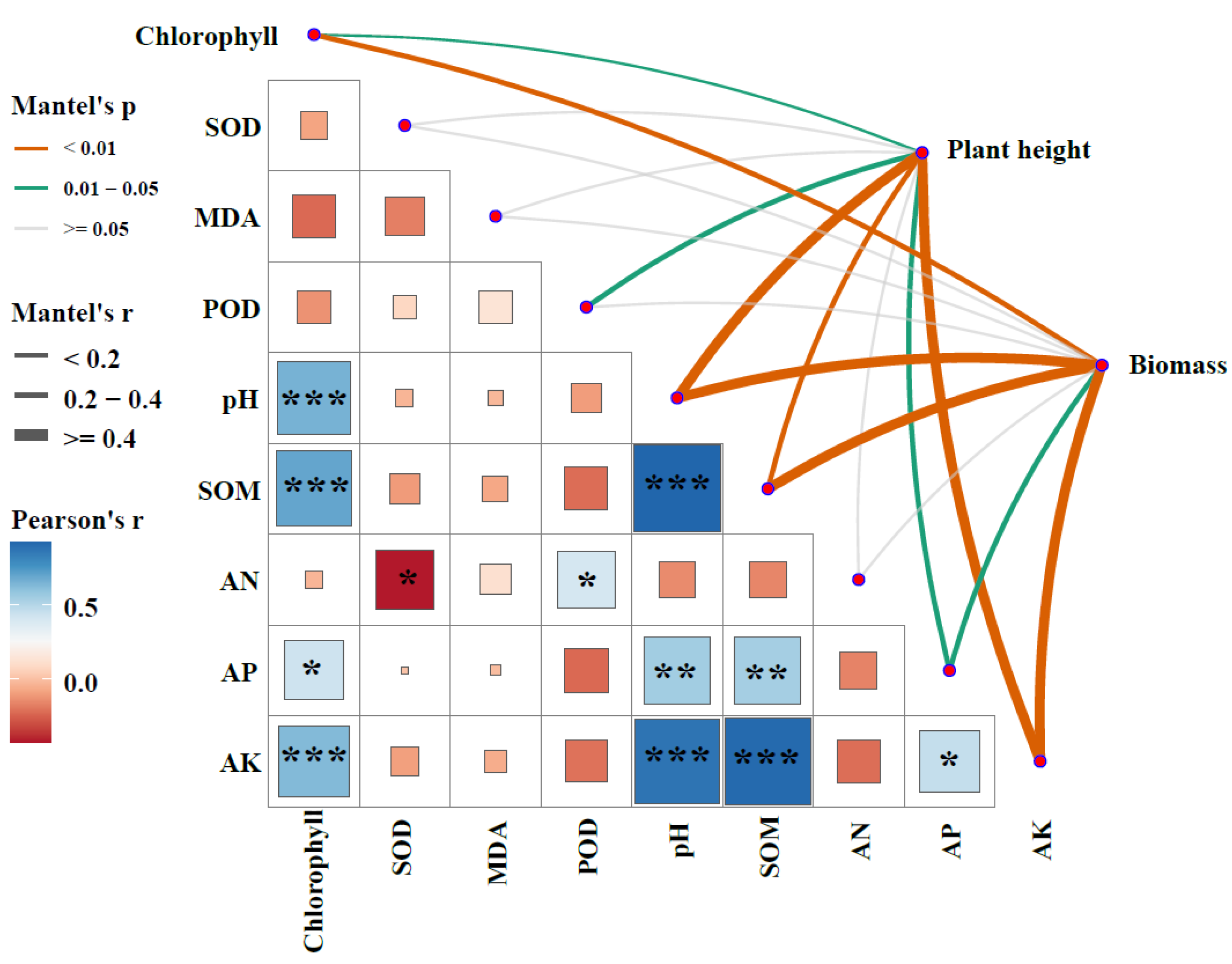
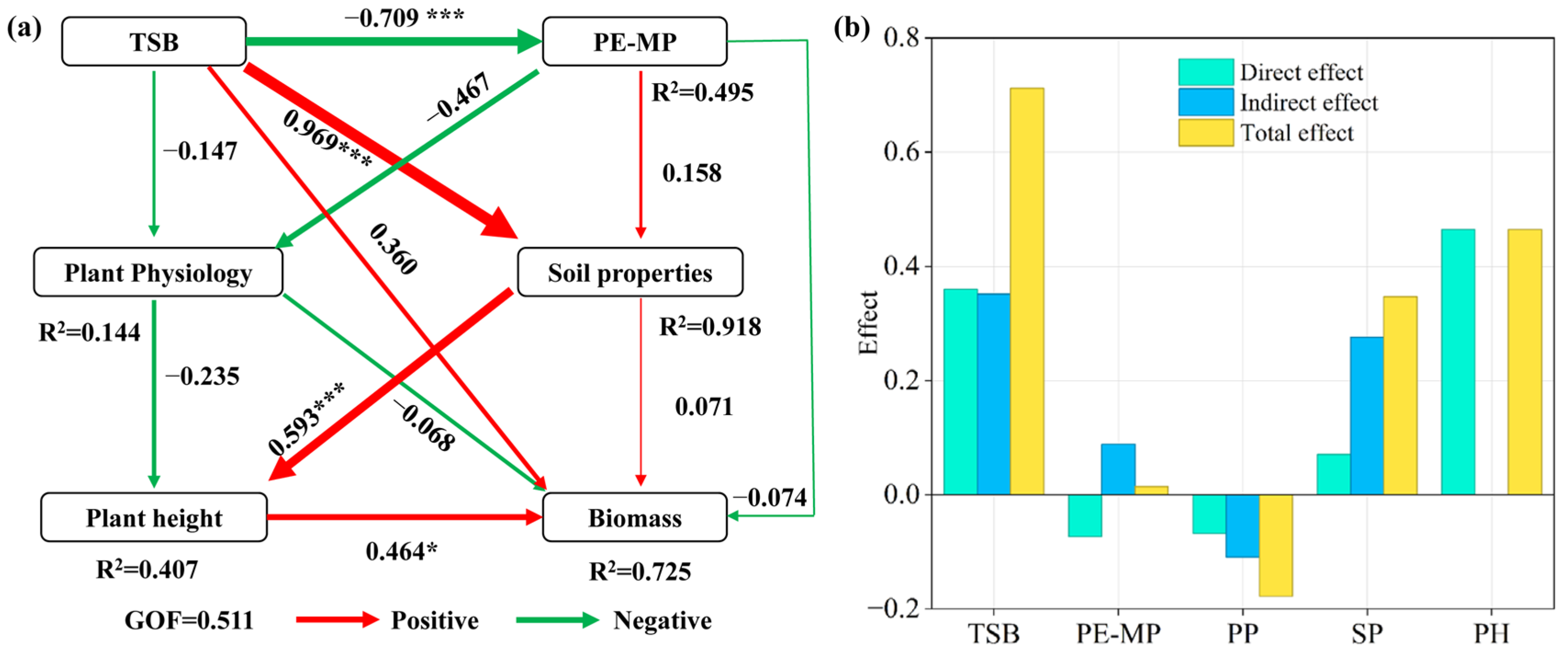
| Treatments | pH | SOM (g kg−1) | AN (mg kg−1) | AP (mg kg−1) | AK (mg kg−1) |
|---|---|---|---|---|---|
| CK | 4.87 ± 0.08 d | 17.94 ± 1.17 g | 34.65 ± 11.49 b | 20.10 ± 9.66 d | 61.33 ± 2.29 e |
| M0B1 | 5.73 ± 0.13 b | 46.83 ± 1.92 f | 36.06 ± 7.90 b | 24.29 ± 2.50 bcd | 95.31 ± 2.86 cd |
| M0B2 | 6.25 ± 0.10 a | 89.82 ± 2.52 c | 23.52 ± 5.64 b | 29.09 ± 2.16 abcd | 105.76 ± 5.85 abc |
| M1B0 | 5.17 ± 0.09 c | 18.09 ± 0.46 g | 75.04 ± 3.50 b | 21.40 ± 3.77 cd | 55.47 ± 12.63 e |
| M1B1 | 5.76 ± 0.08 b | 82.70 ± 2.37 d | 36.07 ± 2.34 b | 26.67 ± 2.94 abcd | 89.56 ± 6.12 d |
| M1B2 | 6.29 ± 0.06 a | 112.95 ± 1.66 b | 23.67 ± 5.58 b | 36.31 ± 6.88 a | 106.51 ± 4.61 ab |
| M2B0 | 5.03 ± 0.05 c | 18.45 ± 0.40 g | 32.25 ± 9.38 b | 30.00 ± 10.12 abcd | 53.23 ± 6.20 e |
| M2B1 | 5.75 ± 0.07 b | 74.33 ± 1.18 e | 33.48 ± 13.05 b | 34.91 ± 3.85 ab | 96.49 ± 8.16 bcd |
| M2B2 | 6.30 ± 0.10 a | 124.04 ± 3.11 a | 65.53 ± 12.09 a | 32.75 ± 11.27 abc | 114.15 ± 0.54 a |
| Variables | PE-MPs | TSB | PE-MPs × TSB | |||
|---|---|---|---|---|---|---|
| F | P | F | P | F | P | |
| pH | 4.810 | 0.021 | 493.765 | 0.000 | 2.462 | 0.082 |
| SOM | 355.021 | 0.000 | 5367.650 | 0.000 | 106.988 | 0.000 |
| AN | 6.686 | 0.007 | 4.912 | 0.020 | 19.835 | 0.000 |
| AP | 3.153 | 0.067 | 3.822 | 0.041 | 0.779 | 0.553 |
| AK | 1.100 | 0.354 | 157.083 | 0.000 | 1.371 | 0.283 |
| Variables | PE-MPs | TSB | PE-MPs × TSB | |||
|---|---|---|---|---|---|---|
| F | P | F | P | F | P | |
| Chlorophyll | 4.490 | 0.026 | 21.467 | 0.000 | 1.990 | 0.139 |
| SOD | 8.043 | 0.003 | 1.776 | 0.198 | 4.865 | 0.008 |
| MDA | 1.205 | 0.323 | 0.273 | 0.764 | 2.134 | 0.118 |
| POD | 4.906 | 0.020 | 0.602 | 0.558 | 3.092 | 0.042 |
| Variables | PE-MPs | TSB | PE-MPs × TSB | |||
|---|---|---|---|---|---|---|
| F | P | F | P | F | P | |
| Germination rate | 0.483 | 0.625 | 0.069 | 0.934 | 1.440 | 0.262 |
| Plant height | 2.119 | 0.149 | 8.726 | 0.002 | 1.236 | 0.331 |
| Biomass | 0.909 | 0.421 | 15.543 | 0.000 | 1.444 | 0.260 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Yang, Q.; Jiang, L.; Yao, J.; Luo, Y.; Ma, R.; Liu, J.; Ren, J.; Xiang, Y.; Liu, Y. Evaluating the Role of Tobacco Stalk Biochar in Wheat Growth Under Microplastic Exposure. Plants 2025, 14, 3578. https://doi.org/10.3390/plants14233578
Li S, Yang Q, Jiang L, Yao J, Luo Y, Ma R, Liu J, Ren J, Xiang Y, Liu Y. Evaluating the Role of Tobacco Stalk Biochar in Wheat Growth Under Microplastic Exposure. Plants. 2025; 14(23):3578. https://doi.org/10.3390/plants14233578
Chicago/Turabian StyleLi, Suhang, Qiong Yang, Longcheng Jiang, Jiaxin Yao, Yang Luo, Rou Ma, Jiaojiao Liu, Jun Ren, Yangzhou Xiang, and Ying Liu. 2025. "Evaluating the Role of Tobacco Stalk Biochar in Wheat Growth Under Microplastic Exposure" Plants 14, no. 23: 3578. https://doi.org/10.3390/plants14233578
APA StyleLi, S., Yang, Q., Jiang, L., Yao, J., Luo, Y., Ma, R., Liu, J., Ren, J., Xiang, Y., & Liu, Y. (2025). Evaluating the Role of Tobacco Stalk Biochar in Wheat Growth Under Microplastic Exposure. Plants, 14(23), 3578. https://doi.org/10.3390/plants14233578






