Mineral Composition of Olea europaea L. Leaves and Tisanes
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Olive Leaves Collection and Analysis
3.2. Tisane’s Preparation and Analysis
3.3. Statistical Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Loumou, A.; Giourga, C. Olive Groves: “The Life and Identity of the Mediterranean.”. Agric. Hum. Values 2003, 20, 87–95. [Google Scholar] [CrossRef]
- Malik, N.; Bradford, J. Recovery and Stability of Oleuropein and Other Phenolic Compounds during Extraction and Processing of Olive (Olea europaea L.) Leaves. J. Food Agric. Environ. 2008, 6, 122–125. [Google Scholar]
- Boskou, D. Olive Oil: Chemistry and Technology, 2nd ed.; AOCS Press: Champaign, IL, USA, 2006. [Google Scholar] [CrossRef]
- Silvestrini, A.; Giordani, C.; Bonacci, S.; Giuliani, A.; Ramini, D.; Matacchione, G.; Sabbatinelli, J.; Di Valerio, S.; Pacetti, D.; Procopio, A.D.; et al. Anti-Inflammatory Effects of Olive Leaf Extract and Its Bioactive Compounds Oleacin and Oleuropein-Aglycone on Senescent Endothelial and Small Airway Epithelial Cells. Antioxidants 2023, 12, 1509. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, N.M.; Machado, J.; Chéu, M.H.; Lopes, L.; Barroso, M.F.; Silva, A.; Sousa, S.; Domingues, V.F.; Grosso, C. Potential Therapeutic Properties of Olea Europaea Leaves from Selected Cultivars Based on Their Mineral and Organic Profiles. Pharmaceuticals 2024, 17, 274. [Google Scholar] [CrossRef]
- Abdel-Aziz, S.M.; Abdel-Aziz, M.S.; Garg, N. Health Benefits of Trace Elements in Human Diseases. In Microbes in Food and Health; Springer: Berlin/Heidelberg, Germany, 2016; pp. 117–142. [Google Scholar] [CrossRef]
- Baccouri, B.; Rajhi, I.; Theresa, S.; Najjar, Y.; Mohamed, S.N.; Willenberg, I. The Potential of Wild Olive Leaves (Olea europaea L. Subsp. Oleaster) Addition as a Functional Additive in Olive Oil Production: The Effects on Bioactive and Nutraceutical Compounds Using LC-ESI-QTOF/MS. Eur. Food Res. Technol. 2022, 248, 2809–2823. [Google Scholar] [CrossRef]
- Szymczycha-Madeja, A.; Welna, M.; Pohl, P. Elemental Analysis of Teas and Their Infusions by Spectrometric Methods. TrAC Trends Anal. Chem. 2012, 35, 165–181. [Google Scholar] [CrossRef]
- Chizzola, R. Metallic Mineral Elements and Heavy Metals in Medicinal Plants. Med. Aromat. Plant Sci. Biotechnol. 2012, 6, 39–53. [Google Scholar]
- Cavalheiro, C.V.; Picoloto, R.S.; Cichoski, A.J.; Wagner, R.; de Menezes, C.R.; Zepka, L.Q.; Da Croce, D.M.; Barin, J.S. Olive Leaves Offer More than Phenolic Compounds—Fatty Acids and Mineral Composition of Varieties from Southern Brazil. Ind. Crops Prod. 2015, 71, 122–127. [Google Scholar] [CrossRef]
- de Oliveira, N.M.; Lopes, L.; Chéu, M.H.; Soares, E.; Meireles, D.; Machado, J. Updated Mineral Composition and Potential Therapeutic Properties of Different Varieties of Olive Leaves from Olea europaea. Plants 2023, 12, 916. [Google Scholar] [CrossRef]
- Cetinkaya, H.; Koc, M.; Kulak, M. Monitoring of Mineral and Polyphenol Content in Olive Leaves under Drought Conditions: Application Chemometric Techniques. Ind. Crops Prod. 2016, 88, 78–84. [Google Scholar] [CrossRef]
- Bouhafa, K.; Moughli, L.; Bouabid, R.; Douaik, A.; Taarabt, Y. Dynamics of Macronutrients in Olive Leaves. J. Plant Nutr. 2018, 41, 956–968. [Google Scholar] [CrossRef]
- Zipori, I.; Yermiyahu, U.; Dag, A.; Erel, R.; Ben-Gal, A.; Quan, L.; Kerem, Z. Effect of Macronutrient Fertilization on Olive Oil Composition and Quality under Irrigated, Intensive Cultivation Management. J. Sci. Food Agric. 2022, 103, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Toplu, C.; Uygur, V.; Yildiz, E. Leaf Mineral Composition of Olive Varieties and Their Relation to Yield and Adaptation Ability. J. Plant Nutr. 2009, 32, 1560–1573. [Google Scholar] [CrossRef]
- Maathuis, F.J. Physiological Functions of Mineral Macronutrients. Curr. Opin. Plant Biol. 2009, 12, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Assunção, A.G.L.; Cakmak, I.; Clemens, S.; González-Guerrero, M.; Nawrocki, A.; Thomine, S. Micronutrient Homeostasis in Plants for More Sustainable Agriculture and Healthier Human Nutrition. J. Exp. Bot. 2022, 73, 1789–1799. [Google Scholar] [CrossRef]
- Romanova, E.V.; Kalugina, O.V.; Zaitseva, L.V. Geochemical Features of Soils of the Southern Coast of Crimea and Their Influence on the Accumulation of Trace Elements in Plants. Geokhimiia 2019, 64, 987–996. [Google Scholar] [CrossRef]
- Korsakova, S.P.; Korsakov, P.B. Climatic Characteristics of the 2020 Seasons on the Southern Coast of Crimea. Nauchnye Zap. Prir. Zapov. Mys Martian 2021, 15, 6–27. [Google Scholar] [CrossRef]
- Pashtetskaya, A.V.; Bakova, N.N.; Paliy, A.E.; Karpova, A.N. Organoleptic and Physico-Chemical Characteristics of Olive Oil Obtained in the Conditions of the Southern Coast of the Crimea. Plant Biol. Hortic. Theory Innov. 2021, 1, 83–93. [Google Scholar] [CrossRef]
- Lavrinenko, Y.; Plieva, A.; Zinicovscaia, I.; Hristozova, G.; Frontasyeva, M.; Tkachenko, K.; Dogadkin, D.; Gromyak, I.; Kolotov, V. Elemental Composition of Infusions of Herbs (Tisanes) of North Ossetia (the Caucasus). Agriculture 2021, 11, 841. [Google Scholar] [CrossRef]
- Samolińska, W.; Kiczorowska, B.; Kwiecień, M.; Rusinek-Prystupa, E. Determination of Minerals in Herbal Infusions Promoting Weight Loss. Biol. Trace Elem. Res. 2016, 175, 495–502. [Google Scholar] [CrossRef]
- Pohl, P.; Dzimitrowicz, A.; Jedryczko, D.; Szymczycha-Madeja, A.; Welna, M.; Jamroz, P. The Determination of Elements in Herbal Teas and Medicinal Plant Formulations and Their Tisanes. J. Pharm. Biomed. Anal. 2016, 130, 326–335. [Google Scholar] [CrossRef]
- Паштецкая, А.В.; Бакoва, Н.Н.; Пехoва, О.А.; Данилoва, И.Л.; Карпoва, А.Н. ОЦЕНКА КАЧЕСТВА ПРОДУКТОВ ПЕРЕРАБОТКИ ПЛОДОВ OLEA EUROPAEA L., ПРОИЗРАСТАЮЩЕЙ В УСЛОВИЯХ ЮЖНОГО БЕРЕГА КРЫМА. Бюллетень Гoсударственнoгo Никитскoгo бoтаническoгo сада 2023, 147, 72–82. [Google Scholar] [CrossRef]
- Tarchoune, I.; Sgherri, C.; Eddouzi, J.; Zinnai, A.; Quartacci, M.F.; Zarrouk, M. Olive Leaf Addition Increases Olive Oil Nutraceutical Properties. Molecules 2019, 24, 545. [Google Scholar] [CrossRef] [PubMed]
- Stateras, D.C.; Moustakas, N.K. Seasonal Changes of Macro- and Micro-Nutrients Concentration in Olive Leaves. J. Plant Nutr. 2018, 41, 186–196. [Google Scholar] [CrossRef]
- Markert, B.; Fränzle, S.; Wünschmann, S. Chemical Evolution: The Biological System of the Elements; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Lukić, I.; Pasković, I.; Žurga, P.; Germek, V.M.; Brkljača, M.; Marcelić, Š.; Ban, D.; Grozić, K.; Lukić, M.; Užila, Z.; et al. Determination of the Variability of Biophenols and Mineral Nutrients in Olive Leaves with Respect to Cultivar, Collection Period and Geographical Location for Their Targeted and Well-Timed Exploitation. Plants 2020, 9, 1667. [Google Scholar] [CrossRef]
- Pasković, I.; Lukić, I.; Žurga, P.; Germek, V.M.; Brkljača, M.; Koprivnjak, O.; Major, N.; Grozić, K.; Franić, M.; Ban, D.; et al. Temporal Variation of Phenolic and Mineral Composition in Olive Leaves Is Cultivar Dependent. Plants 2020, 9, 1099. [Google Scholar] [CrossRef]
- Alhaithloul, H.A.S.; Awad, N.S.; Qari, S.H.; El-Homosy, R.F.; Qaoud, E.S.M.; Alqahtani, M.M.; Ghanem, K.Z.; Alasmari, A.; Alzuaibr, F.M.; Ghazzawy, H.S.; et al. Genetic Diversity, Chemical Constituents and Anatomical Analysis of Eight Popular Olive (Olea Europaea L.) Cultivars in Al-Jouf Region, Saudi Arabia. Sci. Rep. 2024, 14, 14860. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Food and Nutrition Board; Committee to Review the Dietary Reference Intakes for Sodium and Potassium; Oria, M.; Harrison, M.; Stallings, V.A. Potassium: Dietary Reference Intakes for Adequacy. In Dietary Reference Intakes for Sodium and Potassium; The National Academies Press: Washington, DC, USA, 2019. [Google Scholar] [CrossRef]
- Phosphorus—Health Professional Fact Sheet. Available online: https://ods.od.nih.gov/factsheets/Phosphorus-HealthProfessional/ (accessed on 8 November 2025).
- Plantz, M.A.; Bittar, K. Dietary Calcium and Supplementation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Fatima, G.; Dzupina, A.; Alhmadi, H.B.; Magomedova, A.; Siddiqui, Z.; Mehdi, A.; Hadi, N. Magnesium Matters: A Comprehensive Review of Its Vital Role in Health and Diseases. Cureus 2024, 16, e71392. [Google Scholar] [CrossRef]
- Durán-Cabral, M.; Estévez-Santiago, R.; Winter-Matos, A.; García-Estrella, K.; Olmedilla-Alonso, B.; García-Lithgow, C.H. Assessment of Dietary Sodium, Potassium and Sodium-Potassium Ratio Intake by 72 h Dietary Recall and Comparison with a 24 h Urinary Sodium and Potassium Excretion in Dominican Adults. Nutrients 2025, 17, 434. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; The National Academies Press: Washington, DC, USA, 1997. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; The National Academies Press: Washington, DC, USA, 2001. [Google Scholar] [CrossRef]
- Capozzi, A.; Scambia, G.; Lello, S. Calcium, Vitamin D, Vitamin K2, and Magnesium Supplementation and Skeletal Health. Maturitas 2020, 140, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Ross, A.C.; Taylor, C.L.; Yaktine, A.L.; Valle, H.B. Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Miriyala, S.; Spasojevic, I.; Tovmasyan, A.; Salvemini, D.; Vujaskovic, Z.; St. Clair, D.; Batinic-Haberle, I. Manganese Superoxide Dismutase, MnSOD and Its Mimics. Biochim. Biophys. Acta 2011, 1822, 794–814. [Google Scholar] [CrossRef] [PubMed]
- Plugatar, Y.V.; Korsakova, S.P.; Ilnitsky, O.A. Ecological Monitoring of the Southern Coast of Crimea; IT “ARIAL”: Simferopol, Crimea, 2015. [Google Scholar]
- Kostenko, I.V.; Dunaevskaya, E.V. Microelements and Heavy Metals in the Soils of the Arboretum of the Nikitsky Botanical Gardens. Plant Biol. Hortic. Theory Innov. 2021, 3, 38–49. [Google Scholar] [CrossRef]
- Chupina, V.I. Soils of the Nikitsky Botanical Garden and Their Geochemical Properties. Vestn. Mosk. Univ. Ser. 5 Geogr. 2020, 75, 35–41. [Google Scholar]
- ISO 3103:2019; Tea-Preparation of Liquor for Use in Sensory Tests. International Organization for Standardization: Geneva, Switzerland, 2019.
- Vuong, Q.V.; Pham, H.N.T.; Negus, C. From Herbal Teabag to Infusion—Impact of Brewing on Polyphenols and Antioxidant Capacity. Beverages 2022, 8, 81. [Google Scholar] [CrossRef]
- Długaszek, M.; Mierczyk, J. Elemental Composition of Green Tea Infusions Depending on the Method of Their Brewing. Eur. Food Res. Technol. 2024, 250, 301–309. [Google Scholar] [CrossRef]
- Durmus, Y.; Atasoy, A.D.; Atasoy, A.F. Mathematical Optimization of Multilinear and Artificial Neural Network Regressions for Mineral Composition of Different Tea Types Infusions. Sci. Rep. 2024, 14, 14860. [Google Scholar] [CrossRef] [PubMed]
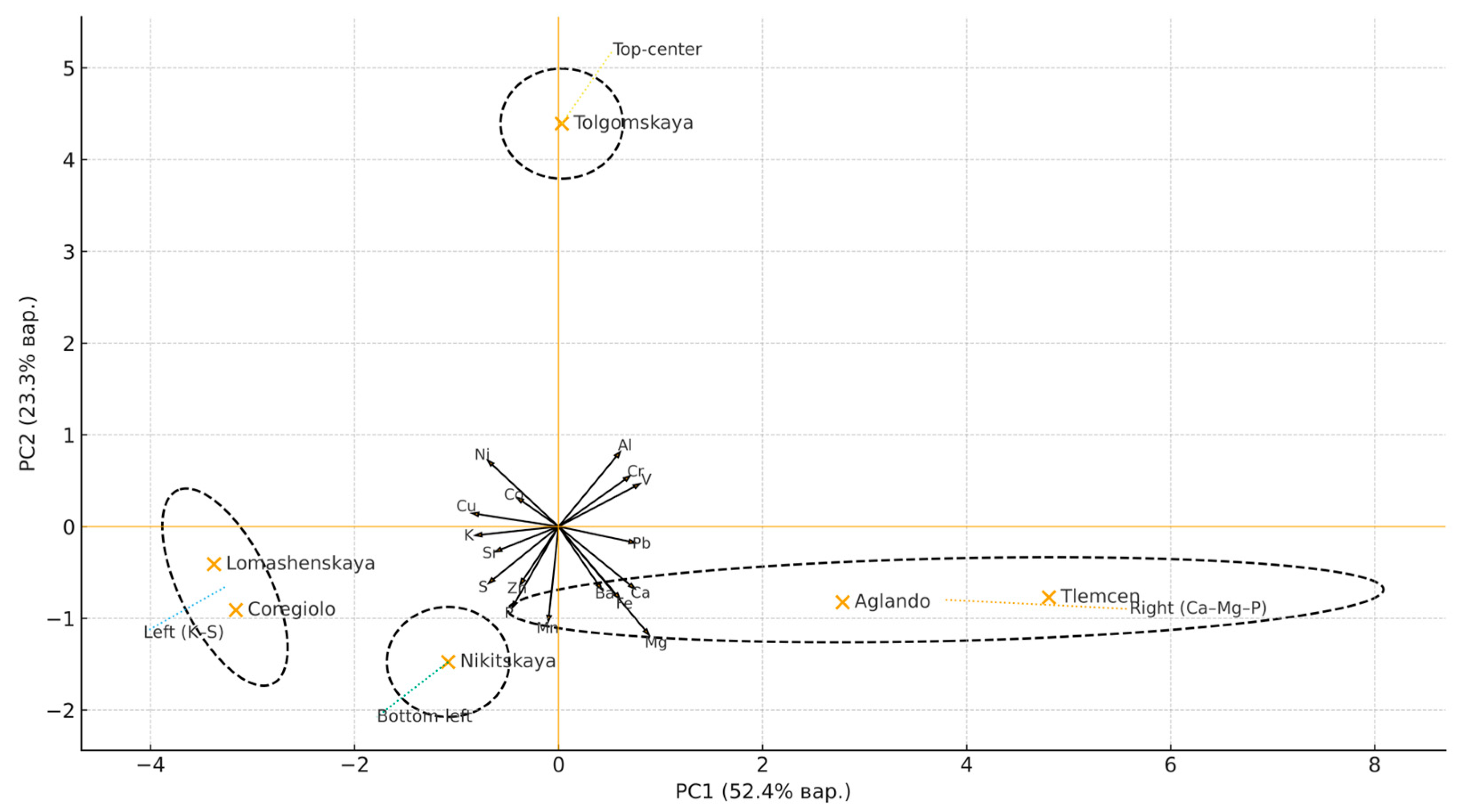

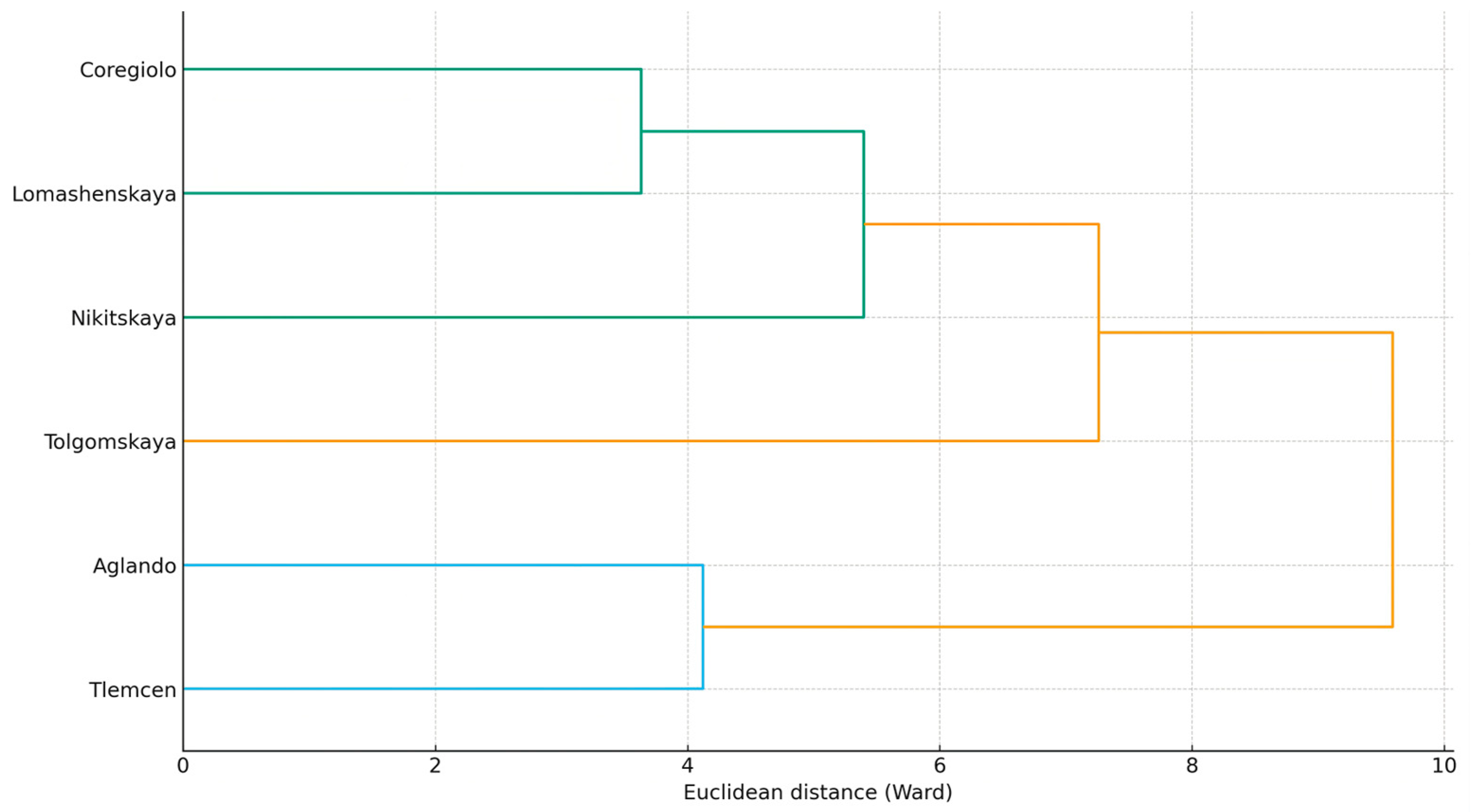
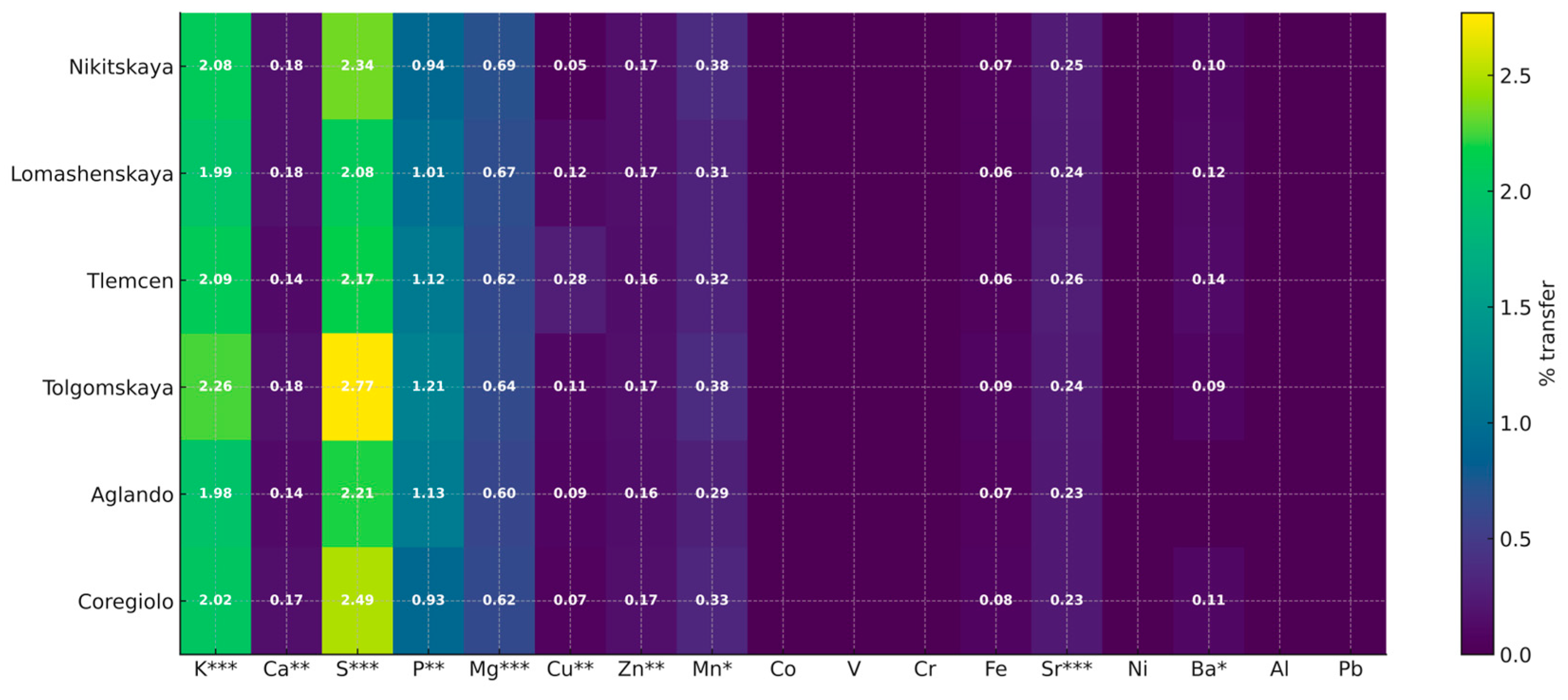
| Element | p-Value | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ‘Nikitskaya’ | ‘Tlemcen’ | ‘Aglando’ | ‘Coregiolo’ | ‘Lomashenskaya’ | ‘Tolgomskaya’ | Leaf | Tisane | |||||||
| Leaf | Tisane | Leaf | Tisane | Leaf | Tisane | Leaf | Tisane | Leaf | Tisane | Leaf | Tisane | |||
| Macro-elements, g/kg for leaves and mg/L for tisanes | ||||||||||||||
| K | 15.6 a | 325 a | 11.7 c | 244 c | 12.4 c | 244 c | 15.4 a | 310 ab | 14.9 ab | 298 b | 13.9 b | 314 ab | <0.001 | <0.001 |
| Ca | 14.8 b | 26 a | 18.6 a | 26 a | 17.2 b | 23 ab | 13.1 bc | 22 ab | 12.7 c | 22 b | 11.8 c | 21 b | <0.001 | 0.004 |
| P | 1.97 a | 18 b | 1.82 bc | 20 a | 1.73 bc | 20 ab | 1.87 ab | 17 b | 1.92 ab | 19 ab | 1.7 c | 21 a | <0.001 | 0.006 |
| S | 2.05 a | 48 a | 1.73 b | 37 c | 1.83 b | 40 bc | 1.94 ab | 48 a | 2.05 a | 43 b | 1.75 b | 49 a | <0.001 | <0.001 |
| Mg | 1.3 b | 8.9 a | 1.46 a | 8.9 a | 1.36 ab | 8.2 b | 1.29 b | 7.9 b | 1.18 c | 7.9 b | 1.14 c | 7.3 c | <0.001 | <0.001 |
| Micro-elements | ||||||||||||||
| Essential, mg/kg for leaves and mg/L for tisanes | ||||||||||||||
| Cu | 5.7 b | 0.001 b | 4.5 c | 0.01 a | 4.8 c | 0.001 b | 6.9 a | 0.01 b | 6.6 b | 0.01 a | 5.90 b | 0.01 b | 0.002 | 0.002 |
| Zn | 17.8 b | 0.03 b | 18.9 ab | 0.03 b | 18.3 ab | 0.03 b | 19.4 ab | 0.03 ab | 21.3 a | 0.04 a | 16.9 b | 0.03 b | 0.001 | 0.008 |
| Mn | 21.3 ab | 0.08 a | 20.7 ab | 0.07 b | 21.8 ab | 0.06 b | 22.4 a | 0.07 ab | 20.5 b | 0.06 b | 19.3 b | 0.07 ab | 0.012 | 0.012 |
| Co | 0.02 b | <DL | 0.03 ab | <DL | 0.01 b | <DL | 0.03 ab | <DL | 0.04 a | <DL | 0.03 ab | <DL | 0.008 | - |
| V | 0.18 b | <DL | 0.26 a | <DL | 0.21 ab | <DL | 0.15 b | <DL | 0.15 b | <DL | 0.23 ab | <DL | 0.004 | - |
| Cr | 0.12 a | <DL | 0.14 a | <DL | 0.12 a | <DL | 0.08 b | <DL | 0.08 b | <DL | 0.14 a | <DL | 0.005 | - |
| Fe | 86.0 b | 0.06 a | 111 a | 0.07 a | 95.1 ab | 0.06 a | 69.7 bc | 0.06 a | 86.7 b | 0.05 a | 63.2 c | 0.06 a | <0.001 | NS |
| Conditionally essential, mg/kg for leaves and mg/L for tisanes | ||||||||||||||
| Sr | 61.6 a | 0.15 a | 39.2 c | 0.10 c | 45.9 c | 0.11 bc | 53.9 b | 0.12 b | 49.9 bc | 0.12 b | 48.1 bc | 0.12 b | <0.001 | <0.001 |
| Ni | 0.51 ab | <DL | 0.38 b | <DL | 0.40 b | <DL | 0.55 ab | <DL | 0.52 ab | <DL | 0.59 a | <DL | 0.006 | - |
| Toxic and poorly studied, mg/kg for leaves and mg/L for tisanes | ||||||||||||||
| Ba | 3.3 a | <DL | 2.6 ab | 0.004 a | 2.7 ab | 0.003 a | 1.4 b | 0.002 ab | 1.7 b | 0.002 ab | 1.5 b | 0.001 b | 0.003 | 0.015 |
| Al | 119 b | <DL | 146 b | <DL | 146 b | <DL | 80.4 c | <DL | 111 b | <DL | 168 a | <DL | <0.001 | - |
| Pb | 0.36 a | <DL | 0.49 a | <DL | 0.38 a | <DL | 0.36 a | <DL | 0.30 a | <DL | 0.36 a | <DL | NS | - |
| Cd | <DL | <DL | <DL | <DL | <DL | <DL | <DL | <DL | <DL | <DL | <DL | <DL | - | - |
| Element | Daily Intake Norms (DRI) Russia [24] | Contribution of the Extract, % DRI | Daily Intake Norms (DRI) International | Contribution of the Extract, % DRI |
|---|---|---|---|---|
| K | 1–2 g | 3–7 | 3510 mg/day [35] | 1.4–1.8 |
| Ca | 800–1200 mg | 0.4–0.7 | 1000 mg/day [36] | 0.42–0.52 |
| P | 400–1200 mg | 0.3–0.9 | 700 mg/day [36] | 0.49–0.60 |
| S | 500–1000 mg | 1–2 | n/a | n/a |
| Mg | 300–400 mg | 0.5–0.6 | 400/320 mg/day [36] | 0.35–0.56 |
| Cu | 1–2 mg | 0.20 | 0.9 mg/day [37] | 0.22 |
| Zn | 10–20 mg | 0.03–0.08 | 11/8 mg/day [37] | 0.05–0.1 |
| Mn | 2–5 mg | 0.24–0.8 | 2.3/1.8 mg/day [37] | 0.52–0.89 |
| V | 20–30 mcg | <1.1–1.7 | n/a | n/a |
| Cr | - | - | 35/25 μg/day [37] | 0.17–0.24 |
| Fe | 10–20 mg | 0.05–0.14 | 8/18 mg/day [37] | 0.06–0.18 |
| Element | SRM | Measured Values, mg/kg | Certified Values, mg/kg | Recovery, % |
|---|---|---|---|---|
| Al | 1547 | 252 ± 0.03 | 249 ± 6.5 | 101 |
| 1575a | 579 ± 1.1 | 580 ± 30 | 100 | |
| Ba | 1547 | 122 ± 1.55 | 124 ± 5.5 | 99 |
| 1575a | 4.92 ± 0.01 | 6.0 ± 0.2 | 82 | |
| Pb | 1547 | 0.91 ± 0.0002 | 0.87 ± 0.02 | 104 |
| Zn | 1547 | 18.18 ± 0.05 | 18.0 ± 0.53 | 102 |
| 1575a | 37.5 ± 0.16 | 38 ± 2 | 99 | |
| V | 1547 | 0.33 ± 0.01 | 0.37 ± 0.04 | 88 |
| Mn | 1547 | 97.8 ± 0.16 | 97.8 ± 1.8 | 100 |
| Cr | 1547 | 0.72 ± 0.02 | 1 * | 72 |
| Ni | 1547 | 0.62 ± 0.001 | 0.69 ± 0.10 | 90 |
| Sr | 1547 | 61.7 ± 0.38 | 53.0 ± 5.0 | 116 |
| P | 1547 | 1525 ± 7 | 1371 ± 82 | 111 |
| 1575a | 1157 ± 5.1 | 1070 ± 80 | 108 | |
| Ca | 1575a | 2620 ± 2.25 | 2500 ± 100 | 105 |
| Mg | 1575a | 1031 ± 0.27 | 1060 ± 170 * | 97 |
| S | 1547 | 1634 ± 15 | 2000 * | 82 |
| Fe | 1547 | 204 ± 3 | 220 ± 6.8 | 94 |
| K | 1575a | 4391 ± 2.33 | 4170 ± 70 | 105 |
| Element | Measured Values, mg/L | Certified Values, mg/L | Recoveries, % | Detection Limit, mg/L |
|---|---|---|---|---|
| Al | 0.05 ± 0.0001 | 0.05 | 99 | 0.004 |
| Ba | 0.04 ± 0.0001 | 0.05 | 86 | 0.003 |
| Cd | 0.02 ± 0.0007 | 0.02 | 116 | 0.0003 |
| Co | 0.03 ± 0.0001 | 0.03 | 112 | 0.0004 |
| Zn | 0.05 ± 0.0004 | 0.05 | 101 | 0.009 |
| V | 0.05 ± 0.0007 | 0.05 | 100 | 0.0017 |
| Mn | 0.03 ± 0.0001 | 0.03 | 91 | 0.006 |
| Cr | 0.02 ± 0.0006 | 0.02 | 106 | 0.0003 |
| Ni | 0.05 ± 0.0001 | 0.05 | 96 | 0.0004 |
| Sr | 0.11 ± 0.0001 | 0.10 | 110 | 0.003 |
| Fe | 0.10 ± 0.0003 | 0.10 | 103 | 0.002 |
| Ca | 37.4 ± 0.28 | 35.0 | 107 | 0.43 |
| Mg | 13.1 ± 0.05 | 15.0 | 87 | 0.07 |
| K | 3.26 ± 0.02 | 3.00 | 109 | 0.02 |
| Cu | 0.02 ± 0.0001 | 0.02 | 105 | 0.0004 |
| Pb | 0.03 ± 0.0008 | 0.03 | 98 | 0.0003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pashtetskaia, A.; Kravtsova, A.; Peshkova, A.; Zinicovscaia, I.; Shevchuk, O. Mineral Composition of Olea europaea L. Leaves and Tisanes. Plants 2025, 14, 3566. https://doi.org/10.3390/plants14233566
Pashtetskaia A, Kravtsova A, Peshkova A, Zinicovscaia I, Shevchuk O. Mineral Composition of Olea europaea L. Leaves and Tisanes. Plants. 2025; 14(23):3566. https://doi.org/10.3390/plants14233566
Chicago/Turabian StylePashtetskaia, Aleksandra, Alexandra Kravtsova, Alexandra Peshkova, Inga Zinicovscaia, and Oksana Shevchuk. 2025. "Mineral Composition of Olea europaea L. Leaves and Tisanes" Plants 14, no. 23: 3566. https://doi.org/10.3390/plants14233566
APA StylePashtetskaia, A., Kravtsova, A., Peshkova, A., Zinicovscaia, I., & Shevchuk, O. (2025). Mineral Composition of Olea europaea L. Leaves and Tisanes. Plants, 14(23), 3566. https://doi.org/10.3390/plants14233566








