Sex-Related Differences in Physiological and Biochemical Responses of Populus nigra to Bifunctionalized Silver Nanoparticles and Silver Ions Exposure In Vitro
Abstract
1. Introduction
2. Results
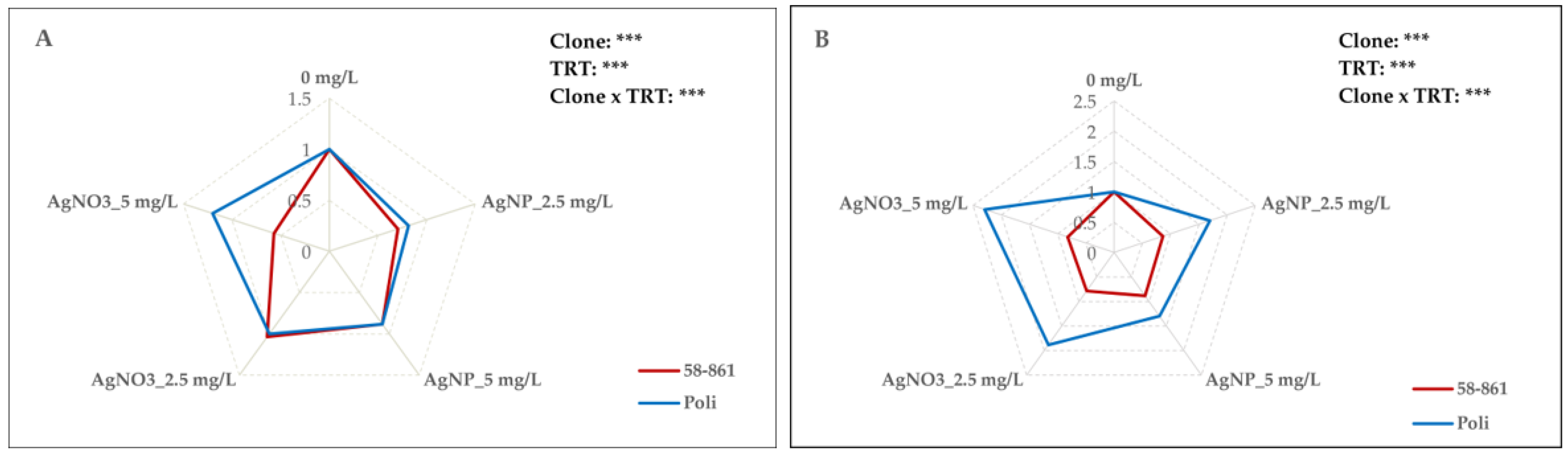
2.1. AgNPs-cit-GSH and AgNO3 Effect on Poplar Calli Growth
2.2. Oxidative Damage Determination by Lipid Peroxidation
2.3. Effects of AgNPs-cit-GSH and AgNO3 on Protein Content
2.4. AgNPs-cit-GSH and AgNO3 Effect on Antioxidant Enzyme Activities
2.5. AgNPs-cit-GSH and AgNO3 Effect on Plasma Membrane H+-ATPase Activity
2.6. Silver (Ag) Accumulation
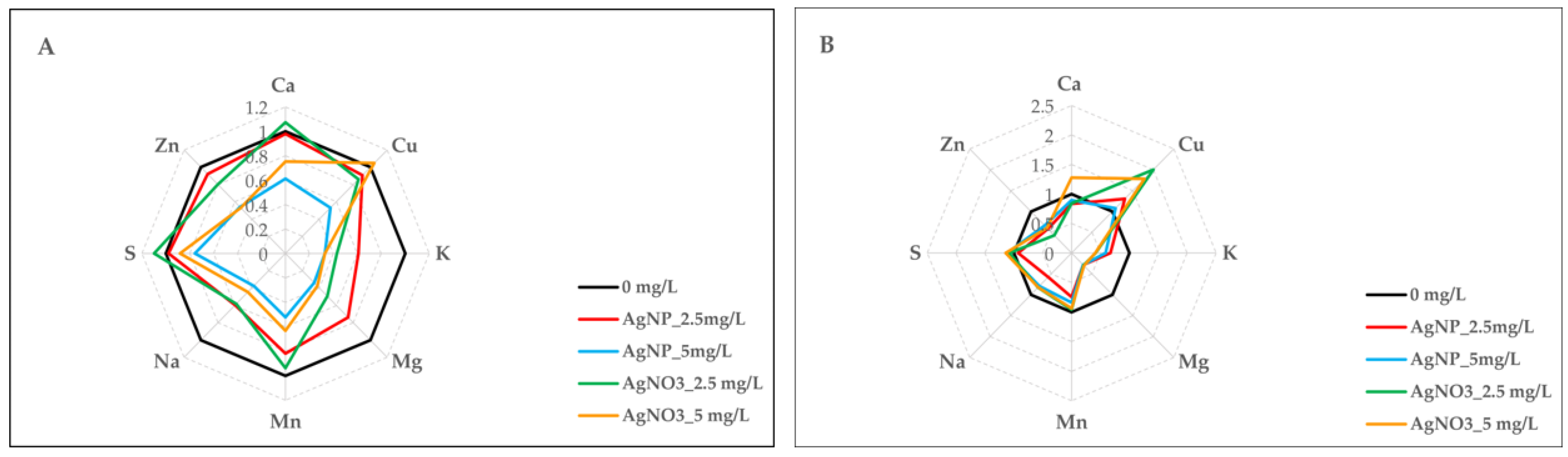
2.7. AgNPs-cit-GSH and AgNO3 Effect on Nutrient Uptake
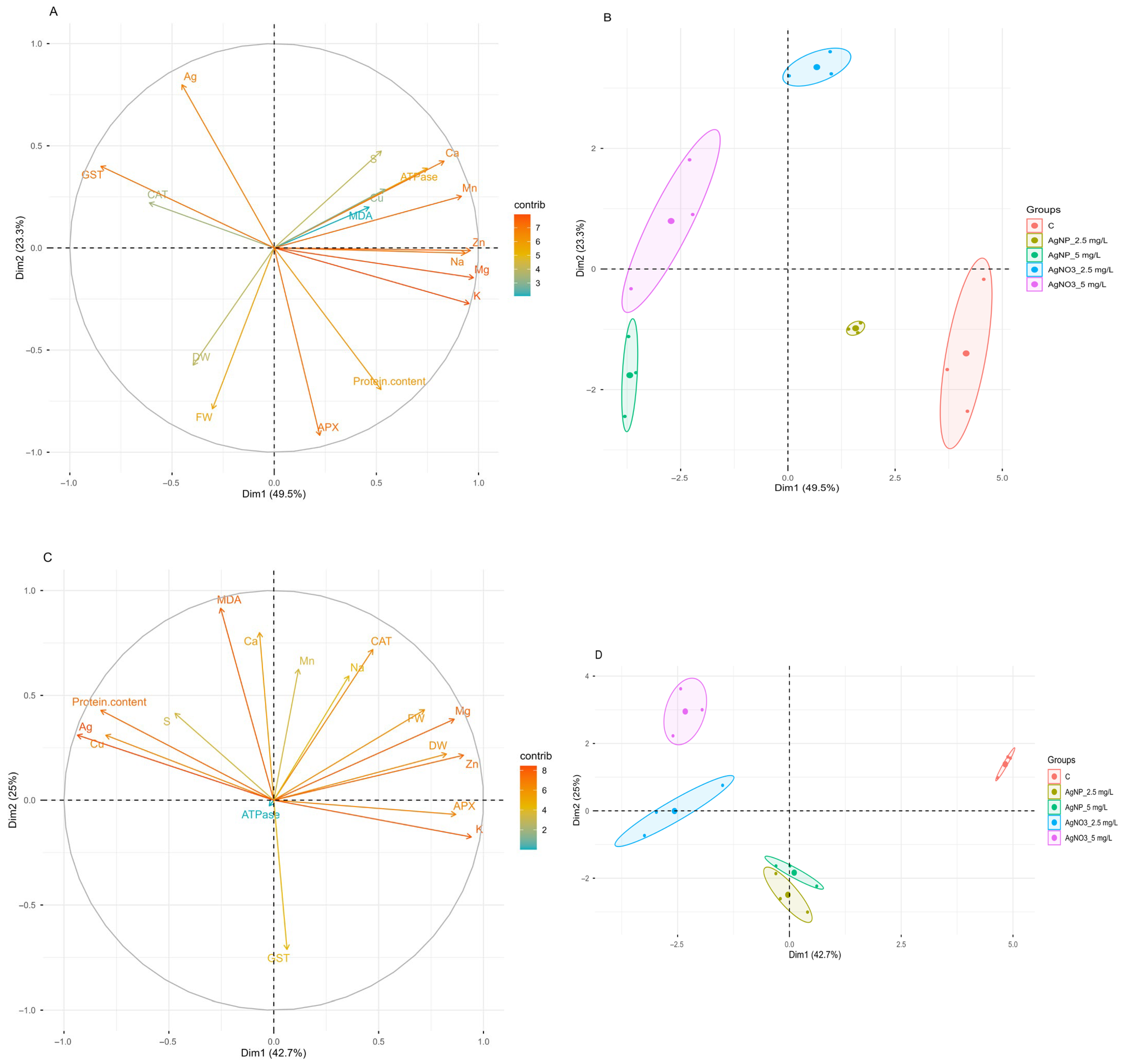
2.8. Principal Component Analysis (PCA)
3. Discussion
4. Materials and Methods
4.1. Synthesis and Characterization of AgNPs-cit-GSH
4.2. Plant Material and Experimental Setup
4.3. Determination of Malondialdehyde (MDA) Content and Antioxidant Enzymatic Activities
4.4. Purification of Plasma Membranes and H+-ATPase Activity
4.5. Analysis of Ag and Nutrient Contents
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biswas, P.; Wu, C.Y. Nanoparticles and the environment. J. Air Waste Manag. Assoc. 2005, 55, 708–746. [Google Scholar] [CrossRef]
- Ihtisham, M.; Noori, A.; Yadav, S.; Sarraf, M.; Kumari, P.; Brestic, M.; Imran, M.; Jiang, F.; Yan, X.; Rastogi, A. Silver nanoparticle’s toxicological effects and phytoremediation. Nanomaterials 2021, 11, 2164. [Google Scholar] [CrossRef]
- Singh, R.P.; Handa, R.; Manchanda, G. Nanoparticles in sustainable agriculture: An emerging opportunity. J. Control. Release 2021, 329, 1234–1248. [Google Scholar] [CrossRef]
- Yan, A.; Chen, Z. Impacts of silver nanoparticles on plants: A focus on the phytotoxicity and underlying mechanism. Int. J. Mol. Sci. 2019, 20, 1003. [Google Scholar] [CrossRef]
- Khan, S.; Zahoor, M.; Khan, R.S.; Ikram, M.; Islam, N.U. The impact of silver nanoparticles on the growth of plants: The agriculture applications. Heliyon 2023, 9, e16928. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Mahra, S.; Sharma, S.; Mathew, S.; Sharma, S. Interaction of silver nanoparticles with plants: A focus on the phytotoxicity, underlying mechanism, and alleviation strategies. Plant Nano Biol. 2024, 9, 100082. [Google Scholar] [CrossRef]
- Courtois, P.; Rorat, A.; Lemiere, S.; Guyoneaud, R.; Attard, E.; Levard, C.; Vandenbulcke, F. Ecotoxicology of silver nanoparticles and their derivatives introduced in soil with or without sewage sludge: A review of effects on microorganisms, plants and animals. Environ. Pollut. 2019, 253, 578–598. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Dang, F.; Huang, Y.; Chen, N.; Zhou, D. Uptake, translocation, and transformation of silver nanoparticles in plants. Environ. Sci. Nano 2022, 9, 12–39. [Google Scholar] [CrossRef]
- Noori, A.; Hasanuzzaman, M.; Roychowdhury, R.; Sarraf, M.; Afzal, S.; Das, S.; Rastogi, A. Silver nanoparticles in plant health: Physiological response to phytotoxicity and oxidative stress. Plant Physiol. Biochem. 2024, 209, 108538. [Google Scholar] [CrossRef]
- Bellingeri, A.; Scattoni, M.; Venditti, I.; Battocchio, C.; Protano, G.; Corsi, I. Ecologically based methods for promoting safer nanosilver for environmental applications. J. Hazard. Mat. 2022, 438, 129523. [Google Scholar] [CrossRef] [PubMed]
- Waktole, G. Toxicity and molecular mechanisms of actions of silver nanoparticles. J. Biomater. Nanobiotechnol. 2023, 14, 53–70. [Google Scholar] [CrossRef]
- Bellingeri, A.; Bono, N.; Venditti, I.; Bertelà, F.; Burratti, L.; Faleri, C.; Protano, G.; Paccagnini, E.; Lupetti, P.; Candiani, G.; et al. Capping drives the behavior, dissolution and (eco)toxicity of silver nanoparticles towards microorganisms and mammalian cells. Environ. Sci. Nano 2024, 11, 2049–2060. [Google Scholar] [CrossRef]
- Torrent, L.; Iglesias, M.; Marguí, E.; Hidalgo, M.; Verdaguer, D.; Llorens, L.; Kodre, A.; Kavčič, A.; Vogel-Mikuš, K. Uptake, translocation and ligand of silver in Lactuca sativa exposed to silver nanoparticles of different size, coatings and concentration. J. Hazard. Mat. 2020, 384, 121201. [Google Scholar] [CrossRef]
- Nie, P.; Zhao, Y.; Xu, H. Synthesis, applications, toxicity and toxicity mechanisms of silver nanoparticles: A review. Ecotoxicol. Environ. Saf. 2023, 253, 114636. [Google Scholar] [CrossRef]
- Cvjetko, P.; Milošić, A.; Domijan, A.-M.; Vrček, I.V.; Tolić, S.; Štefanić, P.P.; Letofsky-Papst, I.; Tkalec, M.; Balen, B. Toxicity of silver ions and differently coated silver nanoparticles in Allium cepa roots. Ecotoxicol. Environ. Saf. 2017, 137, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Budhani, S.; Egboluche, N.P.; Arslan, Z.; Yu, H.; Deng, H. Phytotoxic effect of silver nanoparticles on seed germination and growth of terrestrial plants. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2019, 37, 330–355. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Koo, Y.; Alexander, A.; Yang, Y.; Westerhof, S.; Zhang, Q.; Schnoor, J.L.; Colvin, V.L.; Braam, J.; Alvarez, P.J.J. Phytostimulation of poplars and Arabidopsis exposed to silver nanoparticles and Ag+ at sublethal concentrations. Environ. Sci. Technol. 2013, 47, 5442–5449. [Google Scholar] [CrossRef]
- Aleksandrowicz-Trzcińska, M.; Bederska-Blaszczyk, M.; Szaniawski, A.; Olchowik, J.; Studnicki, M. The effects of copper and silver nanoparticles on container-grown Scots pine (Pinus sylvestris L.) and pedunculated oak (Quercus robur L.) seedlings. Forests 2019, 10, 269. [Google Scholar] [CrossRef]
- Cocozza, C.; Perone, A.; Giordano, C.; Salvatici, M.C.; Pignattelli, S.; Raio, A.; Schaub, M.; Sever, K.; Innes, J.L.; Tognetti, R.; et al. Silver nanoparticles enter the tree faster through leaves than through roots. Tree Physiol. 2019, 39, 1251–1261. [Google Scholar] [CrossRef]
- Iori, V.; Muzzini, V.G.; Venditti, I.; Casentini, B.; Iannelli, M.A. Phytotoxic impact of bifunzionalized silver nanoparticles (AgNPs-Cit-L-Cys) and silver nitrate (AgNO3) on chronically exposed callus cultures of Populus nigra L. Environ. Sci. Pollut. Res. Int. 2023, 30, 116175–116185. [Google Scholar] [CrossRef]
- Douglas, C.J. Populus as a Model Tree. In Comparative and Evolutionary Genomics of Angiosperm Trees. Plant Genetics and Genomics: Crops and Models; Groover, A., Cronk, Q., Eds.; Springer: Cham, Switzerland, 2017; Volume 21. [Google Scholar] [CrossRef]
- Juvany, M.; Munné-Bosch, S. Sex-related differences in stress tolerance in dioecious plants: A critical appraisal in a physiological context. J. Exp. Bot. 2015, 20, 6083–6092. [Google Scholar] [CrossRef]
- Melnikova, N.V.; Borkhert, E.V.; Snezhvina, A.V.; Kudryavtseva, A.V.; Dmitriev, A.A. Sex-specific response to stress in Populus. Front. Plant Sci. 2017, 8, 1827. [Google Scholar] [CrossRef]
- Liu, M.; Liu, X.; Kang, J.; Korpelainen, H.; Li, C. Are males and females of Populus cathayana differentially sensitive to Cd stress? J. Hazard. Mater. 2020, 393, 122411. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhao, Y.; Wang, Y.; Korpelainen, H.; Li, C. Stem xylem traits and wood formation affect sex-specific responses to drought and rewatering in Populus cathayana. Tree Physiol. 2022, 42, 1350–1363. [Google Scholar] [CrossRef]
- Regier, N.; Streb, S.; Cocozza, C.; Schaub, M.; Cherubini, P.; Zeeman, S.C.; Frey, B. Drought tolerance of two black poplar (Populus nigra L.) clones: Contribution of carbohydrates and oxidative stress defence. Plant Cell Environ. 2009, 32, 1724–1736. [Google Scholar] [CrossRef]
- Cocozza, C.; Cherubini, P.; Regier, N.; Saurer, M.; Frey, B.; Tognetti, R. Early effects of water deficit on two parental clones of Populus nigra L. grown under different environmental conditions. Funct. Plant Biol. 2010, 37, 244–254. [Google Scholar] [CrossRef]
- Iori, V.; Pietrini, F.; Massacci, A.; Zacchini, M. Induction of metal binding compounds and antioxidative defence in callus cultures of two black poplar (P. nigra) clones with different tolerance to cadmium. Plant Cell Tiss. Organ Cult. 2012, 108, 17–26. [Google Scholar] [CrossRef]
- Iori, V.; Giorgetti, L.; Casentini, B.; Muzzini, V.G.; Okan, B.S.; Melucci, M.; Iannelli, M.A. Graphene effects on Populus nigra: Assessment of sex-specific responses by in vitro culture. Plant Cell Tiss. Organ Cult. 2025, 163, 22. [Google Scholar] [CrossRef]
- Confalonieri, M.; Balestrazzi, A.; Bisoffi, S.; Carbonera, D. In vitro culture and genetic engineering of Populus spp.: Synergy for forest tree improvement. Plant Cell Tiss. Organ Cult. 2003, 73, 109–138. [Google Scholar] [CrossRef]
- Wijerathna-Yapa, A.; Hiti-Bandaralage, J. Tissue-culture-A sustainable approach to explore plant stresses. Life 2023, 13, 780. [Google Scholar] [CrossRef]
- Bellingeri, A.; Bertelà, F.; Burratti, L.; Calantropio, A.; Battocchio, C.; Lupetti, P.; Paccagnini, E.; Iucci, G.; Marsotto, M.; Prosposito, P.; et al. Detection of Fe(III) ion based on bifunctionalized silver nanoparticles: Sensitivity, selectivity and environmental safety. Mat. Chem. Phys. 2024, 313, 128671. [Google Scholar] [CrossRef]
- Alizadeh, M.; Musazade, E.; Qaderi, S.; Qarachal, J.F.; Siahpoush, S.; Abbod, M.; Siahpoush, S.; Ghasemi, H. The environmental and anthropogenic impacts of na-noparticles on forest trees. J. Nanopart. Res. 2025, 27, 143. [Google Scholar] [CrossRef]
- Yang, J.; Cao, W.; Rui, Y. Interaction between nanoparticles and plants: Phytotoxicity and defense mechanisms. J. Plant Interact. 2017, 12, 158–169. [Google Scholar] [CrossRef]
- Iqbal, M.; Raja Iqbal, N.; Ali, A.; Rashid, H.; Hussain, M.; Ejaz, M.; Iqbal, R.; Khan, A.U.; Shaheen, N.; Rauf, A.; et al. Silver nanoparticles and silver salt (AgNO3) elicits morphogenic and biochemical variations in callus cultures of sugarcane. IET Nanobiotechnol. 2019, 13, 896–904. [Google Scholar] [CrossRef]
- Mustafa, H.S.; Oraibi, A.G.; Ibrahim, K.M.; Ibrahim, N.K. Influence of silver and copper nanoparticles on physiological characteristics of Phaseolus vulgaris L. in vitro and in vivo. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 834–843. [Google Scholar] [CrossRef]
- Dutta Gupta, S.; Agarwal, A.; Pradhan, S. Phytostimulatory effect of silver nanoparticles (AgNPs) on rice seedling growth: An insight from antioxidative enzyme activities and gene expression patterns. Ecotoxicol. Environ. Saf. 2018, 161, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Caselles, C.; Burgos, L.; Sánchez-Balibrea, I.; Egea, J.A.; Faize, L.; Martín-Valmaseda, M.; Bogdanchikova, N.; Pestryakov, A.; Alburquerque, N. The effect of silver nanoparticle addition on micropropagation of apricot cultivars (Prunus armeniaca L.) in semisolid and liquid media. Plants 2023, 12, 1547. [Google Scholar] [CrossRef]
- Khan, I.; Raza, M.A.; Bin Khalid, M.H.; Awan, S.A.; Raja, N.I.; Zhang, X.; Min, S.; Wu, B.C.; Hassan, M.J.; Huang, L. Physiological and biochemical responses of pearl millet (Pennisetum glaucum L.) seedlings exposed to silver nitrate (AgNO3) and silver nanoparticles (AgNPs). Int. J. Environ. Res. Public Health 2019, 16, 2261. [Google Scholar] [CrossRef]
- Rymen, B.; Sugimoto, K. Tuning growth to the environmental demands. Curr. Opin. Plant Biol. 2012, 15, 683–690. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Zhu, J.K. Thriving under stress: How plants balance growth and the stress response. Dev. Cell 2020, 55, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Grodetskaya, T.A.; Evlakov, P.M.; Fedorova, O.A.; Mikhin, V.I.; Zakharova, O.V.; Kolesnikov, E.A.; Evtushenko, N.A.; Gusev, A.A. Influence of copper Oxide nanoparticles on gene expression of birch clones in vitro under stress caused by phytopathogens. Nanomaterials 2022, 12, 864. [Google Scholar] [CrossRef]
- Niemietz, C.M.; Tyerman, S.D. New potent inhibitors of aquaporins: Silver and gold compounds inhibit aquaporins of plant and human origin. FEBS Lett. 2002, 531, 443–447. [Google Scholar] [CrossRef]
- Fan, X.; Zhou, X.; Chen, H.; Tang, M.; Xie, X. Cross-talks between macro- and micronutrient uptake and signalling in plants. Front. Plant Sci. 2021, 12, 663477. [Google Scholar] [CrossRef]
- Zuverza-Mena, N.; Armendariz, R.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Effects of silver nanoparticles on radish sprouts: Root growth reduction and modifications in the nutritional value. Front. Plant Sci. 2016, 7, 90. [Google Scholar] [CrossRef]
- Yang, J.; Jiang, F.; Ma, C.; Rui, Y.; Rui, M.; Adeel, M.; Cao, W.; Xing, B. Alteration of crop yield and quality of wheat upon exposure to silver nanoparticles in a life cycle study. J. Agric. Food Chem. 2018, 66, 2589–2597. [Google Scholar] [CrossRef]
- Salachna, P.; Byczyńska, A.; Zawadzińska, A.; Piechocki, R.; Mizielińska, M. Stimulatory effect of silver nanoparticles on the growth and flowering of potted oriental lilies. Agronomy 2019, 9, 610. [Google Scholar] [CrossRef]
- Shabala, S. Signalling by potassium: Another second messenger to add to the list? J. Exp. Bot. 2017, 68, 4003–4007. [Google Scholar] [CrossRef] [PubMed]
- Singh, R. Calcium in plant biology: Nutrient and second messenger. Int. J. Biol. Innov. 2020, 2, 31–35. [Google Scholar] [CrossRef]
- Houmani, H.; Corpas, F.J. Can nutrient act as signals under abiotic stress? Plant Physiol. Biochem. 2024, 206, 108313. [Google Scholar] [CrossRef] [PubMed]
- McShan, D.; Ray, P.C.; Yu, H. Molecular toxicity mechanism of nanosilver. J. Food Drug Anal. 2014, 22, 116–127. [Google Scholar] [CrossRef]
- Taylor, A.F.; Rylott, E.L.; Anderson, C.W.; Bruce, N.C. Investigating the toxicity, uptake, nanoparticle formation and genetic response of plants to gold. PLoS ONE 2014, 9, e93793. [Google Scholar] [CrossRef]
- Pokhrel, L.R.; Dubey, B. Evaluation of developmental responses of two crop plants exposed to silver and zinc oxide nanoparticles. Sci. Total Environ. 2013, 452–453, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Vinković, T.; Novák, O.; Strnad, M.; Goessler, W.; Jurašin, D.D.; Parađiković, N.; Vrček, I.V. Cytokinin response in pepper plants (Capsicum annuum L.) exposed to silver nanoparticles. Environ. Res. 2017, 156, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Falhof, J.; Pedersen, J.T.; Fuglsang, A.T.; Palmgren, M. Plasma membrane H+-ATPase regulation in the center of plant physiology. Mol. Plant 2016, 9, 323–337. [Google Scholar] [CrossRef]
- Noori, A.; Bharath, L.P.; White, J.C. Type-specific impacts of silver on the protein profile of tomato (Lycopersicon esculentum L.). Int. J. Phytorem. 2022, 12, 12–24. [Google Scholar] [CrossRef]
- Janicka-Russak, M.; Kabała, K.; Burzyński, M. Different effect of cadmium and copper on H+-ATPase activity in plasma membrane vesicles from Cucumis sativus roots. J. Exp. Bot. 2021, 63, 4133–4142. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Wang, W.; Wang, P.; Ma, H.; Li, W. The role of reactive oxygen species in regulation of the plasma membrane H+-ATPase activity in Masson pine (Pinus massoniana Lamb.) roots responding to acid stress. Tree Physiol. 2024, 44, tpae083. [Google Scholar] [CrossRef]
- Cvjetko, P.; Zovko, M.; Štefanić, P.P.; Biba, R.; Tkalec, M.; Domijan, A.-M.; Vrček, I.V.; Letofsky-Papst, I.; Šikić, S.; Balen, B. Phytotoxic effects of silver nanoparticles in tobacco plants. Environ. Sci. Pollut. Res. Int. 2018, 25, 5590–5602. [Google Scholar] [CrossRef]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; et al. Recent developments in enzymatic antioxidant defence mechanism in plants with special reference to abiotic stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Zhang, X.; Wuyun, T.; Li, Z.; Chen, L.; Sun, Z.; Li, X.; Niinemets, Ü.; Zhang, L. Sex-specific ozone responses of poplar: Mechanisms of enhanced tolerance of males. Plant Physiol. Biochem. 2025, 223, 109833. [Google Scholar] [CrossRef]
- Glavaš Ljubimir, K.; Domijan, A.M.; Radić Brkanac, S. Phytotoxic action of silver nanoparticles on Lemna minor: Multi-parameter analysis of different physiological processes. Plants 2023, 12, 343. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Shweta; Upadhyay, N.; Singh, J.; Liu, S.; Singh, V.P.; Prasad, S.M.; Chauhan, D.K.; Tripathi, D.K.; Sharma, S. Differential phytotoxic impact of plant mediated silver nanoparticles (AgNPs) and silver nitrate (AgNO3) on Brassica sp. Front. Plant Sci. 2017, 8, 1501. [Google Scholar] [CrossRef]
- Chen, G.; Li, J.; Han, H.; Du, R.; Wang, X. Physiological and molecular mechanisms of plant responses to copper stress. Int. J. Mol. Sci. 2022, 23, 12950. [Google Scholar] [CrossRef]
- Narayan, O.P.; Kumar, P.; Yadav, B.; Dua, M.; Johri, A.K. Sulfur nutrition and its role in plant growth and development. Plant Signal Behav. 2022, 18, 2030082. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Habig, W.H.; Jakoby, W.B. Assay for differentiation of glutathione S-transferases. Methods Enzymol. 1981, 77, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Ernst, O.; Zor, T. Linearization of the Bradford Protein Assay. J. Vis. Exp. 2010, 12, 38. [Google Scholar] [CrossRef]
- Fiorillo, A.; Mattei, M.; Aducci, P.; Visconti, S.; Camoni, L. The Salt Tolerance Related Protein (STRP) mediates cold stress responses and abscisic acid signalling in Arabidopsis thaliana. Front. Plant Sci. 2020, 11, 1251. [Google Scholar] [CrossRef]
- Visconti, S.; D’Ambrosio, C.; Fiorillo, A.; Arena, S.; Muzi, C.; Zottini, M.; Aducci, P.; Marra, M.; Scaloni, A.; Camoni, L. Overexpression of 14-3-3 proteins enhances cold tolerance and increases levels of stress-responsive proteins of Arabidopsis plants. Plant Sci. 2019, 289, 110215. [Google Scholar] [CrossRef]
- Fiorillo, A.; Parmagnani, A.S.; Visconti, S.; Mannino, G.; Camoni, L.; Maffei, M.E. 14-3-3 Proteins and the Plasma Membrane H+-ATPase Are Involved in Maize (Zea mays) Magnetic Induction. Plants 2023, 12, 2887. [Google Scholar] [CrossRef] [PubMed]


| Clone | Treatment (mg/L) | Fresh Weight (g) | Dry Weight (g) | Water Content (%) |
|---|---|---|---|---|
| 0 | 0.39 ± 0.084 b | 0.019 ± 0.004 cd | 95.04 b | |
| 58-861 | AgNPs-cit-GSH 2.5 | 0.45 ± 0.080 b | 0.024 ± 0.58 b | 94.7 b |
| AgNPs-cit-GSH 5 | 0.51 ± 0.12 b | 0.025 ± 0.006 b | 95.1 b | |
| AgNO3 2.5 | 0.28 ± 0.075 c | 0.017 ± 0.005 de | 94 b | |
| AgNO3 5 | 0.41 ± 0.12 b | 0.024 ± 0.007 b | 94.1 b | |
| 0 | 1.04 ± 0.18 a | 0.034 ± 0.006 a | 96.7 a | |
| Poli | AgNPs-cit-GSH 2.5 | 0.44 ± 0.15 b | 0.023 ± 0.008 bc | 94.8 b |
| AgNPs-cit-GSH 5 | 0.27 ± 0.05 c | 0.015 ± 0.003 de | 94.3 b | |
| AgNO3 2.5 | 0.3 ± 0.08 c | 0.015 ± 0.004 e | 95 b | |
| AgNO3 5 | 0.45 ± 0.1 b | 0.018 ± 0.004 de | 95.9 b | |
| Clone: ns | Clone: * | Clone: * | ||
| TRT: *** | TRT: *** | TRT: ** | ||
| Clone × TRT: *** | Clone × TRT: *** | Clone × TRT: * |
| Clone | Treatment (mg/L) | Ag Content (µg/g DW) |
|---|---|---|
| 0 | <LOD | |
| 58-861 | AgNPs-cit-GSH 2.5 | 7.79 ± 2.5 e |
| AgNPs-cit-GSH 5 | 5.05 ± 1.16 f | |
| AgNO3 2.5 | 375.8 ± 203.9 b | |
| AgNO3 5 | 490.4 ± 31.4 b | |
| 0 | <LOD | |
| Poli | AgNPs-cit-GSH 2.5 | 12.72 ± 2.65 d |
| AgNPs-cit-GSH 5 | 21.01 ± 4.2 c | |
| AgNO3 2.5 | 401.96 ± 49.35 b | |
| AgNO3 5 | 774.41 ± 1.14 a | |
| Clone: *** | ||
| TRT: *** | ||
| Clone × TRT: *** |
| Nutrient | Clone | Treatment (TRT) | Clone × TRT |
|---|---|---|---|
| Ca | *** | *** | *** |
| Cu | *** | *** | *** |
| K | *** | *** | *** |
| Mg | *** | *** | *** |
| Mn | *** | *** | ** |
| Na | * | *** | *** |
| S | *** | * | *** |
| Zn | *** | *** | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iori, V.; Gentile, D.; Casentini, B.; Camoni, L.; Fiorillo, A.; Kuzminsky, E.; Venditti, I.; Iannelli, M.A. Sex-Related Differences in Physiological and Biochemical Responses of Populus nigra to Bifunctionalized Silver Nanoparticles and Silver Ions Exposure In Vitro. Plants 2025, 14, 3560. https://doi.org/10.3390/plants14233560
Iori V, Gentile D, Casentini B, Camoni L, Fiorillo A, Kuzminsky E, Venditti I, Iannelli MA. Sex-Related Differences in Physiological and Biochemical Responses of Populus nigra to Bifunctionalized Silver Nanoparticles and Silver Ions Exposure In Vitro. Plants. 2025; 14(23):3560. https://doi.org/10.3390/plants14233560
Chicago/Turabian StyleIori, Valentina, Davide Gentile, Barbara Casentini, Lorenzo Camoni, Anna Fiorillo, Elena Kuzminsky, Iole Venditti, and Maria Adelaide Iannelli. 2025. "Sex-Related Differences in Physiological and Biochemical Responses of Populus nigra to Bifunctionalized Silver Nanoparticles and Silver Ions Exposure In Vitro" Plants 14, no. 23: 3560. https://doi.org/10.3390/plants14233560
APA StyleIori, V., Gentile, D., Casentini, B., Camoni, L., Fiorillo, A., Kuzminsky, E., Venditti, I., & Iannelli, M. A. (2025). Sex-Related Differences in Physiological and Biochemical Responses of Populus nigra to Bifunctionalized Silver Nanoparticles and Silver Ions Exposure In Vitro. Plants, 14(23), 3560. https://doi.org/10.3390/plants14233560







