Comprehensive Evaluation of Vacrol Oil Combination in Experimental Wound Healing: From Phytochemical Analysis to Functional and Structural Repair
Abstract
1. Introduction
2. Results
2.1. GC–MS Profile Chromatographic Pattern
2.2. Linear Incision Wound Model
2.3. Circular Excision Wound Model
2.4. Histopathological Findings
2.5. Hydroxyproline Content
2.6. In Vitro Enzyme Inhibition Assays
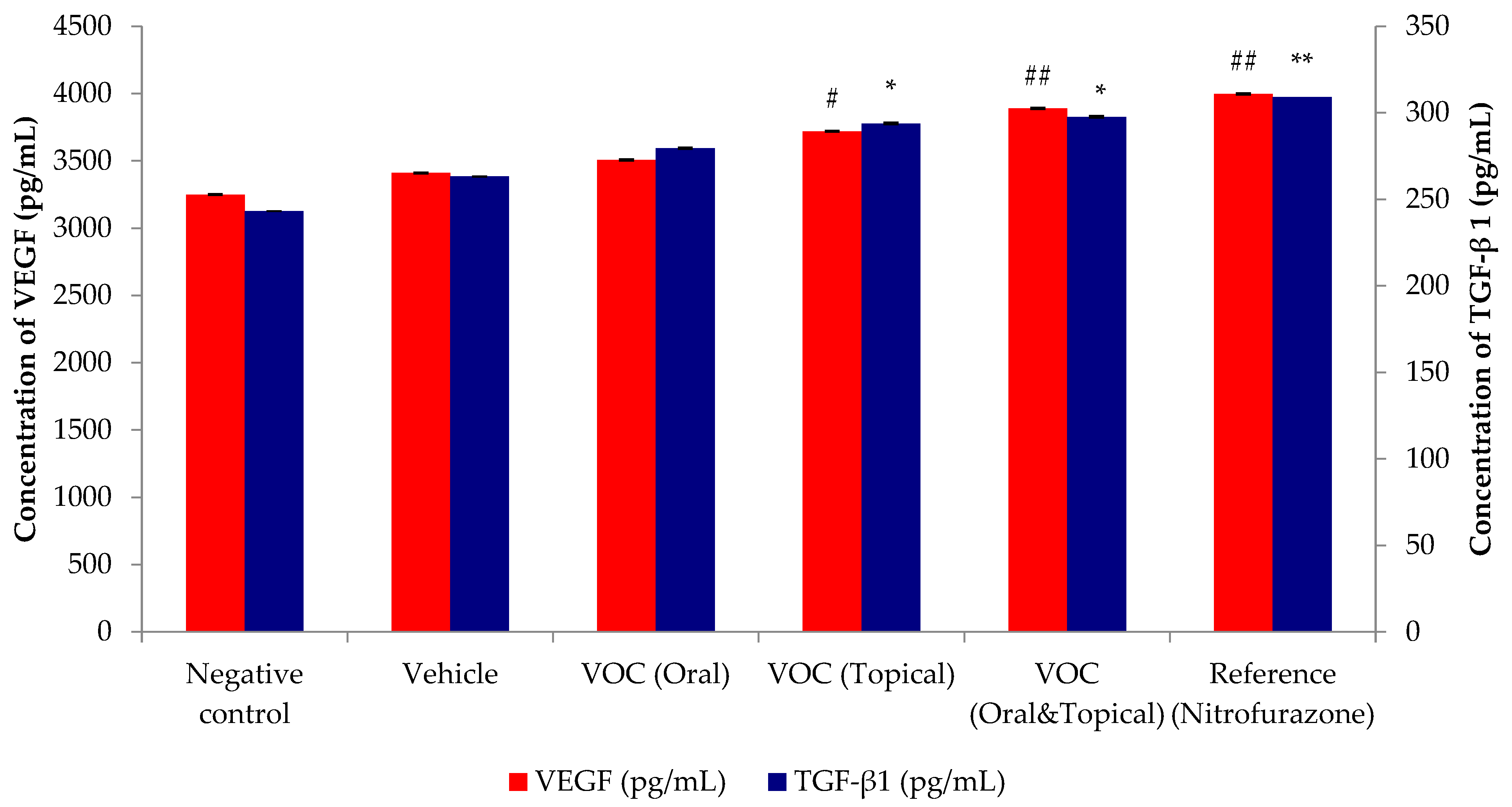
2.7. Effect of VOC on the Healing Growth Factors (VEGF and TGF-β1) of Wound Tissue
3. Discussion
4. Materials and Methods
4.1. Material
4.2. GC-MS Analysis
4.3. In Vivo Experimental Studies
4.3.1. Ethical Approval
4.3.2. Experimental Animals
4.3.3. Animal Treatment and Experimental Groups
4.3.4. Linear Incision Wound Model
4.3.5. Circular Excision Wound Model
4.3.6. Histopathological Examination
4.3.7. Hydroxyproline Assay
4.4. In Vitro Enzyme Inhibition Assays
4.4.1. Hyaluronidase Inhibition Assay
4.4.2. Collagenase Inhibition Assay
4.4.3. Elastase Inhibition Assay
4.4.4. ELISA-Based Measurement of Wound Growth Factor (VEGF and TGF-β1) Responses
4.5. Statistical Analysis
5. Conclusions
6. Limitations and Clinical Translation
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of Variance |
| CaCl2 | Calcium chloride |
| CRM | Certified Reference Material |
| DMSO | Dimethyl sulfoxide |
| ECM | Extracellular matrix |
| ELISA | Enzyme Linked Immunosorbent Assay |
| FALGPA | N-(3-[2-furyl]acryloyl)-Leu-Gly-Pro-Ala |
| GC-MS | Gas Chromatography–Mass Spectrometry |
| H&E | Hematoxylin-Eosin |
| HCl | Hydrochloric acid |
| HRP | Horseradish peroxidase |
| i.p. | Intraperitoneal |
| MAAPV | N-Methoxysuccinyl-Ala-Ala-Pro-Val-p-nitroanilide |
| NaCl | Sodium chloride |
| NaOH | Sodium hydroxide |
| NIST | National Institute of Standards and Technology |
| RT | Retention time |
| SEM | Standard Error of the Mean |
| TGF- β1 | Transforming growth factor beta 1 |
| TMB | 3,3′,5,5′-Tetramethylbenzidine |
| TÜBİTAK | Türkiye Bilimsel ve Teknolojik Araştırma Kurumu |
| VEGF | Vascular endothelial growth factor |
| VOC | Vacrol Oil Combination |
| w/w | Weigh/weight |
References
- Guo, S.; DiPietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef]
- Menke, N.B.; Ward, K.R.; Witten, T.M.; Bonchev, D.G.; Diegelmann, R.F. Impaired wound healing. Clin. Dermatol. 2007, 25, 19–25. [Google Scholar] [CrossRef]
- Edwards, J.V.; Howley, P.; Cohen, I.K. In vitro inhibition of human neutrophil elastase by oleic acid–albumin formulations from derivatized cotton wound dressings. Int. J. Pharm. 2004, 284, 1–12. [Google Scholar]
- Atiyeh, B.S.; Costagliola, M.; Hayek, S.N.; Dibo, S.A. Effect of silver on burn wound infection control and healing: Review of the literature. Burns 2007, 33, 139–148. [Google Scholar] [CrossRef]
- Thring, T.S.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 9, 27. [Google Scholar]
- Lodhi, S.; Pawar, R.S.; Jain, A.P.; Singhai, A.K. Wound healing potential of Tephrosia purpurea in rats: Incision, excision and dead space models. J. Ethnopharmacol. 2006, 108, 204–210. [Google Scholar]
- Rasik, A.M.; Shukla, A.; Patnaik, G.K.; Dhawan, B.N.; Kulshrestha, D.K. Wound healing activity of latex of Euphorbia neriifolia Linn. Indian J. Pharmacol. 1996, 28, 107–109. [Google Scholar]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Costa, M.F.; Durço, A.O.; Rabelo, T.K.; Barreto, R.S.S.; Guimarães, A.G. Effects of carvacrol, thymol and essential oils containing such monoterpenes on wound healing: A systematic review. J. Pharm. Pharmacol. 2019, 71, 141–155. [Google Scholar]
- Matluobi, D.; Araghi, A.; Azimi Maragheh, B.F.; Rezabakhsh, A.; Soltani, S.; Khaksar, M.; Siavashi, V.; Feyzi, A.; Saghaei Bagheri, H.; Rahbarghazi, R.; et al. Carvacrol promotes angiogenic paracrine potential and endothelial differentiation of human mesenchymal stem cells at low concentrations. Microvasc. Res. 2018, 115, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Juergens, U.R.; Dethlefsen, U.; Steinkamp, G.; Gillissen, A.; Repges, R.; Vetter, H. Anti-inflammatory activity of 1.8-cineol (eucalyptol) in bronchial asthma: A double-blind placebo-controlled trial. Respir. Med. 2003, 97, 250–256. [Google Scholar]
- Peana, A.T.; D’Aquila, P.S.; Panin, F.; Serra, G.; Pippia, P.; Moretti, M.D.L. Anti-inflammatory activity of linalool and linalyl acetate constituents of essential oils. Phytomedicine 2002, 9, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.A.; Mohammed, S.A.A.; Khan, O.; Ali, H.M. Topical eucalyptol ointment accelerates wound healing and exerts antioxidant and anti-inflammatory effects in rats’ skin burn model. J. Oleo Sci. 2022, 71, 1777–1788. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo Jimenez, B.A.; Awwad, F.; Desgagné-Penix, I. Cinnamaldehyde in focus: Antimicrobial properties, biosynthetic pathway, and industrial applications. Antibiotics 2024, 13, 1095. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.Z.; Sun, M.; Corke, H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J. Agric. Food Chem. 2005, 53, 7749–7759. [Google Scholar] [CrossRef]
- Nisar, M.F.; Khadim, M.; Rafiq, M.; Chen, J.; Yang, Y.; Wan, C.C. Pharmacological properties and health benefits of eugenol: A comprehensive review. Oxidative Med. Cell. Longev. 2021, 2021, 2497354. [Google Scholar]
- Bassolé, I.H.N.; Juliani, H.R. Essential oils in combination and their antimicrobial properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef]
- Rashed, A.N.; Afifi, F.U.; Disi, A.M. Simple evaluation of the wound healing activity of a crude extract of Portulaca oleracea L. (growing in Jordan) in Mus musculus JVI-1. J. Ethnopharmacol. 2003, 88, 131–136. [Google Scholar] [CrossRef]
- Shetty, S.; Udupa, S.L.; Udupa, A.L.; Vollala, V.R. Wound healing activities of bark extract of Jatropha curcas Linn in albino rats. Saudi Med. J. 2006, 27, 1473–1476. [Google Scholar]
- Mukherjee, P.K.; Maity, N.; Nema, N.K.; Sarkar, B.K. Bioactive compounds from natural resources against skin aging. Phytomedicine 2011, 19, 64–73. [Google Scholar] [CrossRef]
- Shukla, A.; Rasik, A.M.; Jain, G.K.; Shankar, R.; Kulshrestha, D.K.; Dhawan, B.N. In vitro and in vivo wound healing activity of asiaticoside isolated from Centella asiatica. J. Ethnopharmacol. 1999, 65, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.K.; Cho, J.J.; Choi, J.D. Anti-elastase and anti-hyaluronidase of phenolic substance from Areca catechu as a new anti-ageing agent. Int. J. Cosmet. Sci. 2001, 23, 341–346. [Google Scholar]
- Buhren, B.A.; Schrumpf, H.; Hoff, N.-P.; Bölke, E.; Hilton, S.; Gerber, P.A. Dose- and time-dependent effects of hyaluronidase on structural cells and the extracellular matrix of the skin. Eur. J. Med. Res. 2020, 25, 60. [Google Scholar] [CrossRef] [PubMed]
- Aya, K.L.; Stern, R. Hyaluronan in wound healing: Rediscovering a major player. Wound Repair Regen. 2014, 22, 579–593. [Google Scholar] [CrossRef]
- Caley, M.P.; Martins, V.L.C.; O’Toole, E.A. Metalloproteinases and wound healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Reiss, M.J.; Han, Y.-P.; Garcia, E.; Goldberg, M.; Yu, H.; Garner, W.L. Matrix metalloproteinase-9 delays wound healing in a murine wound model. Surgery 2009, 147, 295–302. [Google Scholar] [CrossRef]
- Ferrara, N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr. Rev. 2004, 25, 581–611. [Google Scholar] [CrossRef]
- Bao, P.; Kodra, A.; Tomic-Canic, M.; Golinko, M.S.; Ehrlich, H.P.; Brem, H. The role of vascular endothelial growth factor in wound healing. J. Surg. Res. 2009, 153, 347–358. [Google Scholar] [CrossRef]
- Frangogiannis, N. Transforming growth factor beta in tissue fibrosis. J Exp. Med. 2020, 217, e20190103. [Google Scholar] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Hotta, M.; Nakata, R.; Katsukawa, M.; Hori, K.; Takahashi, S.; Inoue, H. Carvacrol, a component of thyme oil, activates PPARα and γ and suppresses COX-2 expression. J. Lipid Res. 2010, 51, 132–139. [Google Scholar] [CrossRef]
- Park, S.H.; Sim, Y.B.; Lee, J.K.; Kim, S.M.; Kang, Y.J.; Jung, J.S.; Suh, H.W. The analgesic effects and mechanisms of orally administered eugenol. Arch. Pharm. Res. 2011, 34, 501–507. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas–liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Lodhi, S.; Singhai, A.K. Wound healing effect of flavonoid rich fraction and luteolin isolated from Martynia annua Linn. on streptozotocin induced diabetic rats. Asian Pac. J. Trop. Med. 2013, 6, 253–259. [Google Scholar] [CrossRef]
- Ehrlich, H.P.; Hunt, T.K. The effects of cortisone and anabolic steroids on the tensile strength of healing wounds. Ann. Surg. 1969, 170, 203–206. [Google Scholar] [CrossRef]
- Morton, J.J.; Malone, M.H. Evaluation of vulnerary activity by an open wound procedure in rats. Arch. Int. Pharmacodyn. Ther. 1972, 196, 117–126. [Google Scholar] [PubMed]
- Nayak, B.S.; Pinto Pereira, L.M. Catharanthus roseus flower extract has wound-healing activity in Sprague Dawley rats. BMC Complement. Altern. Med. 2006, 6, 41. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques, 6th ed.; Churchill Livingstone Elsevier: Philadelphia, PA, USA, 2008. [Google Scholar]
- Woessner, J.F. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch. Biochem. Biophys. 1961, 93, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Choi, E.M. The effects of Areca catechu L extract on anti-aging. Int. J. Cosmet. Sci. 1999, 21, 285–295. [Google Scholar] [CrossRef]
- Barrantes, E.; Guinea, M. Inhibition of collagenase and metalloproteinases by aloins and aloe gel. Life Sci. 2003, 72, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Melzig, M.F.; Löser, B.; Ciesielski, S. Inhibition of neutrophil elastase activity by phenolic compounds from plants. Pharmazie 2001, 56, 967–970. [Google Scholar] [PubMed]
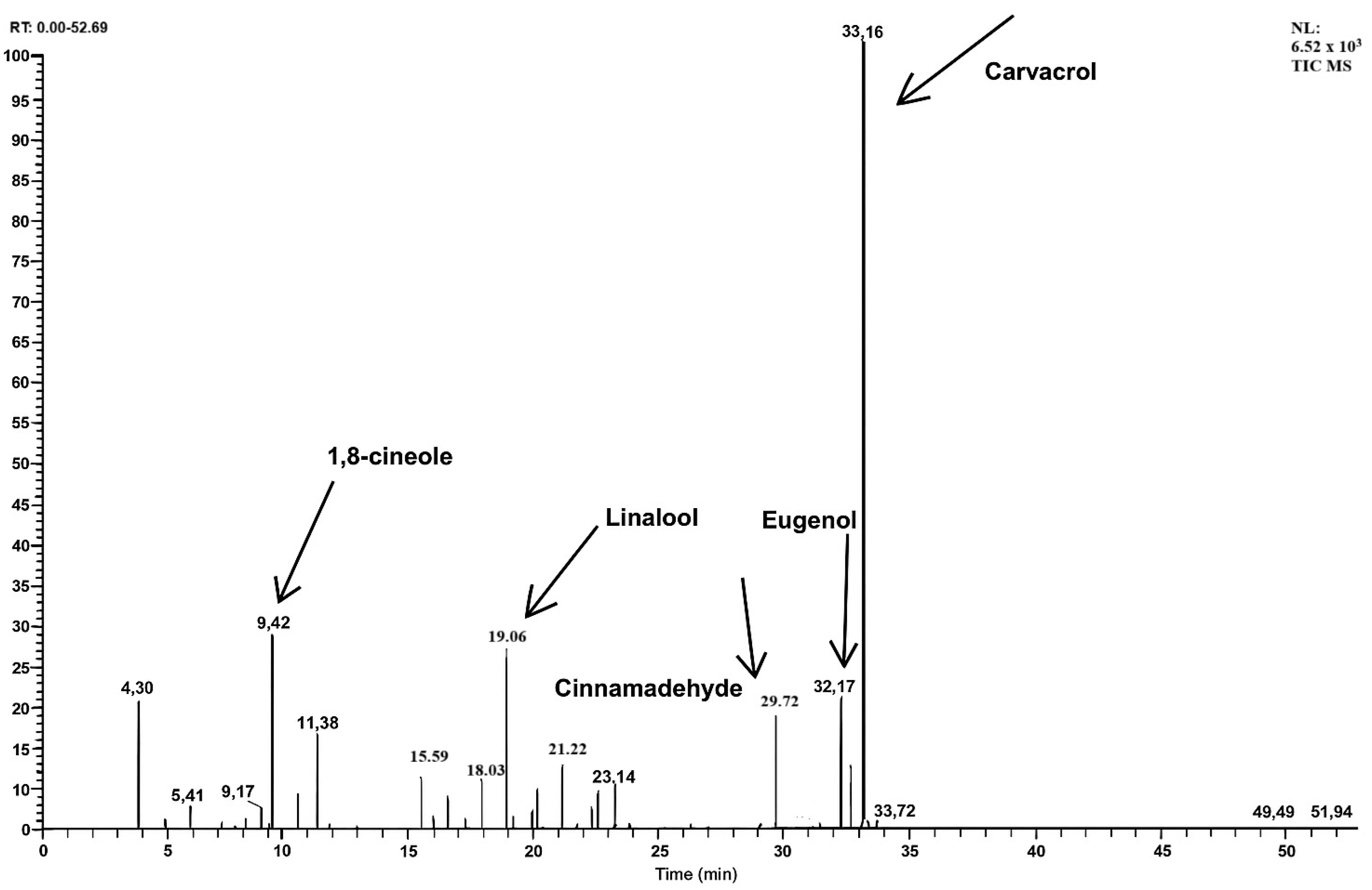


| RT (min) | Compounds | Concentration (%, w/w) |
|---|---|---|
| 4.30 | α-Pinene | 3.5 ± 0.1 |
| 5.30 | Camphene | 0.4 ± 0.1 |
| 6.41 | β-Pinene | 0.7 ± 0.1 |
| 7.65 | Δ-3-Carene | 0.4 ± 0.1 |
| 8.21 | Myrcene | 0.3 ± 0.1 |
| 8.59 | α-Terpinene | 0.3 ± 0.1 |
| 9.17 | Limonene | 0.8 ± 0.1 |
| 9.42 | 1,8-Cineole | 9.7 ± 0.1 |
| 10.64 | δ-Terpinene | 1.1 ± 0.1 |
| 11.38 | p-Cymene | 3.2 ± 0.1 |
| 15.59 | α-Thujone | 1.3 ± 0.1 |
| 16.11 | β-Thujone | 0.4 ± 0.1 |
| 16.68 | Menthone | 0.8 ± 0.1 |
| 18.03 | Camphor | 1.2 ± 0.1 |
| 19.06 | Linalool | 6.3 ± 0.1 |
| 20.07 | Caryophyllene | 0.9 ± 0.1 |
| 20.31 | 4-Terpineol | 1.3 ± 0.1 |
| 21.22 | Menthol | 1.9 ± 0.1 |
| 21.76 | Humulene | 0.3 ± 0.1 |
| 22.44 | Terpinenyl acetate | 0.8 ± 0.1 |
| 22.55 | Fencyl alcohol | 0.6 ± 0.1 |
| 22.65 | Borneol | 0.9 ± 0.1 |
| 23.14 | Bisabolene | 1.1 ± 0.1 |
| 29.71 | Cinnamaldehyde | 4.3 ± 0.1 |
| 32.17 | Eugenol | 4.7 ± 0.1 |
| 32.60 | Thymol | 2.1 ± 0.1 |
| 33.16 | Carvacrol | 50.1 ± 0.1 |
| 51.10 | Hexadecadienoic acid, methyl ester | 0.6 ± 0.1 |
| Experimental Group | Wound Tensile Strength (g) ± S.E.M. (Wound Tensile Strength %) |
|---|---|
| Negative Control | 101.08 ± 4.15 |
| Vehicle (Olive oil) | 140.13 ± 6.82 (38.6) |
| VOC (Oral) | 171.58 ± 7.13 (22.4) |
| VOC (Topical) | 205.21 ± 3.56 (46.4) * |
| VOC (Oral & Topical) | 244.91 ± 3.14 (74.8) *** |
| Reference drug (Nitrofurazone) | 235.04 ± 3.11 (67.7) *** |
| Experimental Group | Wound Area ± SEM (Contraction %) | |||||
|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 10 | |
| Negative Control | 291.23 ± 4.73 | 295.75 ± 4.19 | 274.26 ± 3.98 | 242.51 ± 3.14 | 191.05 ± 3.11 | 105.27 ± 3.62 |
| Vehicle (Olive oil) | 295.83 ± 5.25 | 294.41 ± 5.17 | 255.07 ± 4.11 (6.9) | 210.73 ± 3.57 (13.1) | 164.19 ± 3.05 (14.1) | 75.38 ± 2.61 (28.4) |
| VOC (Oral) | 300.46 ± 4.17 | 290.16 ± 4.49 | 215.22 ± 4.03 (15.6) | 154.26 ± 3.91 (26.8) | 121.08 ± 3.53 (26.3) | 54.76 ± 2.94 (27.4) |
| VOC (Topical) | 293.17 ± 5.94 | 299.53 ± 4.34 | 206.36 ± 3.75 (19.1) | 112.14 ± 3.01 (46.8) * | 94.22 ± 3.01 (42.6) * | 27.51 ± 2.64 (63.5) ** |
| VOC (Oral & Topical) | 298.39 ± 3.39 | 235.34 ± 3.08 (20.1) | 171.15 ± 3.16 (32.9) * | 97.04 ± 2.92 (53.9) ** | 56.09 ± 2.71 (65.8) ** | 00.00 ± 0.00 (100) *** |
| Reference drug (Nitrofurazone) | 297.68 ± 4.86 | 276.33 ± 3.72 (6.1) | 156.29 ± 3.82 (38.7) ** | 71.54 ± 3.28 (66.1) ** | 42.15 ± 2.43 (74.3) ** | 14.70 ± 2.08 (80.5) ** |
| Material | Hydroxyproline Content (µg/mg Tissue) ± S.E.M. |
|---|---|
| Negative Control | 7.65 ± 1.81 |
| Vehicle (Olive oil) | 12.57 ± 2.09 |
| VOC (Oral) | 19.72 ± 1.63 |
| VOC (Topical) | 22.34 ± 1.08 * |
| VOC (Oral & Topical) | 32.06 ± 1.57 ** |
| Reference drug (Nitrofurazone) | 49.51 ± 1.13 *** |
| Material | Concentration (µg/mL) | Hyaluronidase Inhibition (%) ± SEM | Collagenase Inhibition (%) ± SEM | Elastase Inhibition (%) ± SEM |
|---|---|---|---|---|
| VOC | 100 | 71.63 ± 0.52 *** | 9.84 ± 1.53 | 8.26 ± 1.19 |
| Tannic acid | 100 | 83.17 ± 0.34 *** | - | - |
| Epigallocatechin gallate | 100 | - | 49.52 ± 0.98 ** | 67.11 ± 0.92 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Küpeli Akkol, E.; Deliorman Orhan, D. Comprehensive Evaluation of Vacrol Oil Combination in Experimental Wound Healing: From Phytochemical Analysis to Functional and Structural Repair. Plants 2025, 14, 3547. https://doi.org/10.3390/plants14223547
Küpeli Akkol E, Deliorman Orhan D. Comprehensive Evaluation of Vacrol Oil Combination in Experimental Wound Healing: From Phytochemical Analysis to Functional and Structural Repair. Plants. 2025; 14(22):3547. https://doi.org/10.3390/plants14223547
Chicago/Turabian StyleKüpeli Akkol, Esra, and Didem Deliorman Orhan. 2025. "Comprehensive Evaluation of Vacrol Oil Combination in Experimental Wound Healing: From Phytochemical Analysis to Functional and Structural Repair" Plants 14, no. 22: 3547. https://doi.org/10.3390/plants14223547
APA StyleKüpeli Akkol, E., & Deliorman Orhan, D. (2025). Comprehensive Evaluation of Vacrol Oil Combination in Experimental Wound Healing: From Phytochemical Analysis to Functional and Structural Repair. Plants, 14(22), 3547. https://doi.org/10.3390/plants14223547








